Abstract
The complement anaphylatoxins C3a, C5a, and C5adesArg play critical roles in the induction of inflammation and the modulation of innate and acquired immune responses after binding to their G protein-coupled receptors, C3aR and C5aR. The role of C5adesArg in inducing cell activation has been often neglected, since the affinity of C5adesArg for C5aR has been reported to be much lower than that of C5a. We have used a novel label-free cellular assay to reassess the potential of C5adesArg to induce activation of transfected and primary immune cells. Our results indicate that physiological levels of C5adesArg induce significant levels of cell activation that are even higher than that achieved by stimulating cells with analogous concentrations of C5a. Such activation was strictly dependent on C5aR, since it was completely abrogated by PMX-53, a C5aR antagonist. Pharmacological inhibition of specific G proteins located downstream of C5aR indicated differential involvement of Gα proteins upon C5aR engagement by C5a or C5adesArg. Further, mass spectrometric characterization of plasma-derived C5a and C5adesArg provided important insight into the post-translational modification pattern of these anaphylatoxins, which includes glycosylation at Asn64 and partial cysteinylation at Cys27. While the context-specific physiological contribution of C5adesArg has to be further explored, our data suggest that C5adesArg acts as a key molecule in the triggering of local inflammation as well as the maintenance of blood surveillance and homeostatic status.
Keywords: anaphylatoxin, C5a, cell activation, complement, photonic crystal biosensors
Introduction
Complement is increasingly recognized as an efficient surveillance system that participates in the clearance of microbes, immune complexes, and cellular debris, and in the modulation of inflammatory, innate, and acquired immune responses. Under normal conditions, complement is activated in response to various pathogen- and damage-associated molecular patterns, immune complexes, and apoptotic cells, but it can also be directly induced by coagulation factors and via a tick-over mechanism that assures constant low-level complement activation in the circulation. Triggering any of the traditional activation pathways (classical, lectin, or alternative) converges in the cleavage of complement components C3 and C5 and culminates in the release of the anaphylatoxins C3a and C5a and the assembly of the terminal complement complex (TCC) (1).
Anaphylatoxins exert their biological functions via the G protein-coupled receptors (GPCR) C3a receptor (C3aR) and C5a receptor (C5aR; CD88), which are present at the surface of several types of myeloid cells as well as tissue cells (2). Carboxypeptidases present in the serum and tissues quickly degrade C3a and C5a by removing their C-terminal arginine (Arg) residue, producing the so-called C3adesArg and C5adesArg anaphylatoxin fragments (3). In the case of C3aR, only C3a (and not C3adesArg) has been reported to bind and activate the receptor (4). In contrast, both C5a and C5adesArg have been shown to trigger activation of C5aR; however, the binding affinity of C5a toward C5aR has been reported to be approximately 100-fold stronger than that of C5adesArg for C5aR (5). Various in vitro assays have indicated a markedly lower potency of C5adesArg (as compared to C5a) with regard to the induction of neutrophil degranulation, chemotaxis, and release of intracellular calcium (6). Taken together, these observations over time shaped the concept of C5adesArg being the product of a quick “deactivation” of the potent C5a in circulation. Historically, however, C5adesArg has been found to induce robust cell responses via C5aR signaling both in vivo and in vitro, yet this activity appears to be dependent on the experimental context and readout as well on the cell type (7–10).
In addition to C5aR, a second anaphylatoxin receptor, C5L2 (GPR77), has been shown to bind both C5a and C5adesArg. Unlike C5aR, however, C5L2 does not seem to couple to G-proteins, and C5adesArg binds with a 20–30 fold higher affinity to C5L2 than to C5aR (5,11). Even though the binding of C3a and C3adesArg to C5L2 has been proposed, their contribution to and significance for the functional activity of this receptor are an ongoing matter of debate. Nearly 12 years after its discovery, C5L2 is still considered an enigmatic molecule. Divergent roles have been suggested for this receptor, ranging from decoy and regulatory to anti- and pro-inflammatory (2), and it is clear that further studies are required to fully elucidate its participation in the modulation of cellular responses. The most recent report addressing molecular mechanisms has indicated that C5L2 is predominantly intracellular in human neutrophils and that it acts by negatively modulating C5a-induced C5aR signaling through the activation of the β-arrestin signaling pathway (12).
Along with their role in promoting inflammation, anaphylatoxins have been shown to modulate the induction of CD4+ T cell immunity by regulating the cytokine profile produced by antigen-presenting cells (13–16). They are also key determinants of the outcome of disorders such as sepsis (17), asthma (18,19), rheumatoid arthritis (20), transplant rejection (21), cancer (22), neurodegenerative (23) and periodontal (24) disorders, among others (25). It is not surprising, therefore, that anaphylatoxin receptors are among the best-investigated targets for therapeutic intervention in complement-mediated diseases (25,26).
For the past 25 years, the measurement of cell activation triggered by anaphylatoxins has relied on techniques such as chemotaxis, degranulation, and calcium release assays. Heterogeneous sources of anaphylatoxins (i.e., recombinantly expressed or derived from plasma-purified C3 and C5) have been explored, often with little regard for potential differences between the native and recombinant proteins, such as glycosylation patterns. Furthermore, the specific evaluation of C5adesArg-induced responses has often been neglected as a consequence of the proposed lower binding affinity of C5adesArg for C5aR when compared to C5a. Importantly, C5a is rapidly and efficiently converted to C5adesArg by serum carboxypeptidases, and most, if not all, of the C5a present in the circulation is found in the desarginated form (27). Therefore, a more holistic and comprehensive assessment of anaphylatoxin signaling is highly desirable, with the goal of generating improved models and assays for research and drug discovery purposes.
Here, we have compared the ability of native anaphylatoxins (derived from human plasma-purified C3 or C5) to induce the activation of transfected and primary cells, by using a new label-free cell assay based on photonic crystal (PC) biosensors. This method employs highly sensitive measurements of changes in binding, morphology (i.e., activation-mediated effects on the cytoskeleton), or adherence of cells and biomolecules in proximity to a titanium oxide biosensor surface. Such events usually occur downstream of several cellular activation pathways, specifically those involving GPCR-derived cell responses. By recording activation signals over time, a kinetic and cell/receptor-specific activation profile can be obtained (28–31). Our data obtained by this novel method and by orthogonal assays provide new evidence for a significant role for C5adesArg in cell activation and point to a new, easy, rapid, and reliable technology for detecting GPCR-mediated cell activation.
Material and Methods
Cell culture
Rat basophil leukemia (RBL-2H3) cells transfected with C3aR, C5aR, or C5L2 were obtained as described elsewhere (5,32). Cells were stably transfected by electroporation, and homogeneous cell populations (confirmed by flow cytometry) were used in this study. Cells were routinely cultured at 37 °C and 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) with GlutaMAX (Life technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Life technologies), 50 units/ml penicillin, 50 μg/ml streptomycin (Life technologies) and 400 μg/ml geneticin (CellGro, Manassas, VA).
Isolation of human polymorphonuclear cells
Blood was collected from healthy donors in the presence of 50 μg/ml lepirudin as anticoagulant in order to maintain complement-activation capacity (33). Human polymorphonuclear (PMN) blood cells were isolated as previously described (34), using Ficoll-Paque gradient centrifugation (GE Healthcare, Pittsburg, PA) followed by a dextran sedimentation step. After hypotonic lysis of residual red blood cells, PMN cells were resuspended in PBS and immediately used in the experiments.
Anaphylatoxins source and characterization
Native human C3a, C3adesArg, C5a, and C5adesArg prepared from plasma-derived C3 and C5, respectively, were purchased from Complement Technology (Tyler, TX). Endotoxin levels were determined to be <0.05 EU/μg protein. Deglycosylation of anaphylatoxins was achieved by treatment with PNGase F (New England Biolabs) under non-denaturing conditions as per manufacture’s instructions.
The identity and relative concentrations of each anaphylatoxin were determined by mass spectrometry (MS) as follows: 5 pmol sample was injected into LC-MS system for MS or MS/MS analysis. After injection, analytes were separated by reversed-phase liquid chromatography with an online ACQUITY UPLC (Waters, Milford, MA, USA) system coupled to MS. The system was equipped with a 1.7 μm UPLC BEH130 C18 column (1.0 μm × 100 mm; Waters), the temperature of which was held at 40 °C. Proteins/peptides were separated at a flow rate of 0.15 mL/min. A linear gradient from 5 to 45% B (0.1% formic acid in acetonitrile) was applied over 8 min (for proteins) or 27 min (for peptides). MS and MS/MS analysis was performed on a SYNAPT G2S mass spectrometer equipped with an electrospray ionization source controlled by MassLynx 4.1 software (Waters). The capillary voltage was set to 3.2 kV, the cone voltage to 25 V and the source temperature to 120 °C. [Glu1]-fibrinopeptide B was used for lock-mass correction with a sampling rate of 30 seconds. Mass spectra were acquired in positive ionization mode over an m/z range of 50–1900 Da at scan rate of 1s by using both MS and MSe methods. Deconvoluted spectra were generated by MaxEnt1 (Waters), and MS/MS spectra were processed by ProteinLynx Global Server 2.51.
For further characterization and identification of post-translational modifications, peptides were generated for each anaphylatoxin by digestion with trypsin or pepsin (1:25 enzyme/protein weight ratio for 18 h at 37 °C). In order to reduce disulfide bonds the sample was incubated for 45 min at room temperature in the presence of 10 mM dithiothreitol (DTT) followed by treatment with 20 mM iodoacetamide (45 min at room temperature) for alkylation of sulfhydryl groups. Remaining iodoacetamide was neutralized by addition of 20 mM DTT.
Cell activation assays
Label-free cell activation assays were performed on a photonic crystal (PC) biosensor instrument (BIND PROFILER; SRU Biosystems, Woburn, MA). Such technology allows for the measurement of an integrated cellular response encompassing a variety of cellular events downstream of the GPCR. Briefly, optical biosensors located at the bottom of a microtiter plate detect changes in dielectric permittivity of attached biomolecules or cells (by measuring the resonant reflection spectrum after illumination by a source of infrared light). In the case of receptor assays, signal shifts may occur due to changes in cell adhesion, cell morphology, or dynamic mass redistribution upon stimulation of the GPCR; the resulting response profile is dependent on the cell type and signaling pathway, among others (28–31). Selected wells of a 384-well BIND CA2 biosensor plate (SRU Biosystems), containing a cell attachment matrix consisting of collagen, fibronectin and ovalbumin (28), were hydrated with 50 μl of deionized water for 30 min at room temperature. The water was tapped out, and 25 μl of complete medium (DMEM + 10% FBS) was added to each well and incubated for 30 min at room temperature. Medium background was detected in each well by scanning the biosensor plate with the BIND PROFILER instrument. RBL cells were plated at a density of 2.5 × 104 cells/well in complete medium and incubated overnight at 37oC and 5% CO2. The plates were then equilibrated at room temperature for 30 min, and background levels of cell attachment were determined with the BIND PROFILER. Serial dilutions of anaphylatoxins (maximum concentration: 100 nM) were prepared in 25 μl of complete medium (unless otherwise specified) and added to the wells. Cell activation signals, measured as peak wavelength values (PWV) in picometers (pm), were recorded over a period of 20 min after analyte addition, and the data were processed and analyzed using EMS software (SRU Biosystems).
PMN cell assays were performed as described above, except that cells were plated at a density of 5.0 × 104 cells/well in complete medium, and cells were incubated at 37oC for only 2 h, to ensure viability.
Where indicated, C5aR was blocked by pre-incubation of the cells with the C5aR antagonist PMX-53 (35). As a control, an inactive linear peptide (PMX-control) was used, in which the last two amino acids (Trp-Arg) were replaced by Ala-dArg. Gc-globulin and the carboxypeptidase inhibitor DL-2-mercaptomethyl-3-guanidinoethylthiopropanoic acid were obtained from Sigma and EMD Biosciences, respectively. G-protein inactivation was achieved by incubation of cells with 0.5 μg/ml Pertussis toxin (EMD Biosciences) or 10 μg/ml Cholera toxin (Sigma) at 37 °C for 15 h.
Flow cytometry
The expression of C3aR and C5aR on human PMN cells was detected with PE-conjugated anti-C3aR (clone hC3aRZ8; BioLegend, San Diego, CA) and FITC-conjugated anti-C5aR (clone 347214; R&D Systems, Minneapolis, MN) antibodies, respectively. The expression of CD11b on human neutrophils was detected by cell staining with FITC-conjugated anti-CD11b (clone CBM1/5; BioLegend). Alternatively, cells were stained with the respective isotypes. All cell preparations showed at least 95% viability, as determined by ViaProbe (BD Biosciences, San Diego, CA) staining. Samples were then analyzed using BD FACSCanto II flow cytometer software (BD Biosciences) and FCS Express (v4.0; De Novo Software).
Results
Detection of anaphylatoxin-induced cell activation using a label-free cell assay
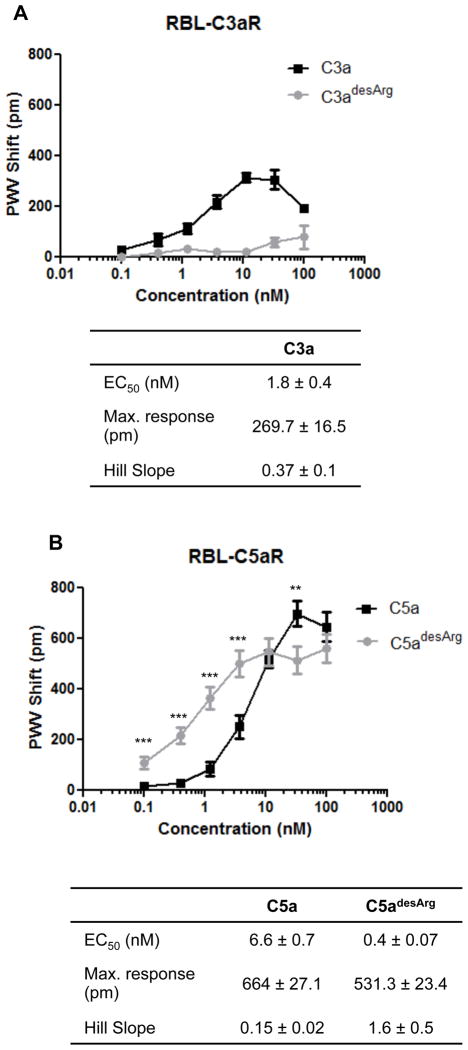
We set out to investigate the role of the anaphylatoxin receptors C3aR, C5aR, and C5L2 in triggering cell activation in response to stimulation by the complement activation fragments C3a, C3adesArg, C5a, and C5adesArg. We used native human serum-derived proteins in order to avoid potential variations related to recombinant proteins, such as folding heterogeneities or missing glycan chains. As expected, C3a, but not C3adesArg, induced dose-dependent activation of C3aR-transfected RBL cells, with a maximal PWV shift of 270±17 pm in response to 10 nM C3a. The calculated EC50 and Hill slope values of 1.8 nM and 0.37, respectively, indicated a strong stimulation of the receptor by C3a (Fig. 1A). Neither C5a nor C5adesArg induced any response in C3aR-transfected cells.
Fig. 1. Detection of anaphylatoxin-induced cell activation using a label-free cell assay.
RBL-C3aR- (A) and RBL-C5aR- (B) transfected cell lines were attached to biosensor plates by incubation in complete medium (DMEM plus 10% FBS) for 16 h at 37°C, and cell activation was monitored in response to increasing concentrations of anaphylatoxins. Tables show calculated EC50, maximum response, and Hill slope values. *P<0.05, **P<0.01, ***P<0.001.
In the case of the C5aR-transfected RBL cells, C5a induced strong cell activation, as evidenced by a PMV shift that reached a maximal value of 664±27 pm in response to 33 nM C5a (Fig. 1B). EC50 and Hill slope values of 6.6 nM and 0.15, respectively, were calculated from the dose-response curve (Fig. 1B). We then determined cell activation profiles in response to the “inactivated” C5a form, i.e. C5adesArg. Surprisingly, C5adesArg triggered substantial cell activation, with a maximal value of 531±23 pm and a stronger EC50 but higher Hill slope value than those of C5a (0.4 vs. 6.6 nM and 1.6 vs. 0.15, respectively) (Fig. 1B). These data suggest that low concentrations of C5adesArg (up to 10 nM) have a greater ability to induce cell activation than do similar levels of C5a.
The same method was used to assess anaphylatoxin-induced activation responses mediated via the C5L2 receptor. However, none of the native anaphylatoxins (C3a, C3adesArg, C5a, or C5adesArg) induced significant activation signals on C5L2-transfected RBL cells (data not shown), indicating that the photonic crystal-based label-free technology may not be suitable for this particular type of non-GPCR-mediated activation event.
Mass spectrophotometric characterization of C5a and C5adesArg
Our observation that C5adesArg induces potent cell activation prompted us to run full MS characterization of C5a and C5adesArg in order to certify the purity and identity of such proteins. The mass spectrum showed three main peaks with 10473, 10592 and 10708 Da for C5a (Fig. 2A; peaks on the right). Evaluation of the C5adesArg sample resulted in similar spectrum of three peaks (10317, 10436 and 10582 Da), yet featuring a consistent difference of 156 Da between the C5a and C5adesArg peaks that corresponds to the mass of one arginine residue (Fig. 2B; peaks on the right).
Figure 2. Mass spectrometry characterization of C5a and C5adesArg.
Deconvoluted data from mass spectrometric analysis of native and deglycosylated C5a (A), C5adesArg (B), and deglycosylated and reduced samples (C,D). Dotted line depicts mass difference between native and deglycosylated samples.
In order to identify the nature of the three distinct peaks, both C5a and C5adesArg were deglycosylated and further analyzed by MS. Removal of oligosaccharides by the PNGase F enzyme resulted in spectra showing two peaks each with 8269 and 8388 Da for C5a (Fig. 2A; peaks on the left) and 8113 and 8232 Da for C5adesArg (Fig. 2B; peaks on the left). The composition of the oligosaccharide chain was subsequently deduced after fragmentation of electrospray-ionized N-glycopeptides at m/z 5096.1 (Supplemental Fig. 1). The resulting spectrum was dominated by a Y-type fragmentation pattern typically observed for glycosidic linkages (36). Our analysis identified the C5a/C5adesArg-associated carbohydrate moiety as a sialylated biantennary glycan with the composition (GlcNAc)4(Man/Gal)5(NeuAc)2 that was attached to Asn64 of the protein (Supplemental Fig. 1). Consistent with previous findings (37), removal of the oligosaccharide chain did not account for major changes in the activity potential (data not shown).
We further determined the identity of the two residual peaks after the deglycosylation of C5a and C5adesArg. The minor peaks (8268 and 8113 Da, respectively) correspond to the expected mass based on the amino acid composition of these proteins (38), whereas both main peaks present a mass adduct of 119 Da. Using MS/MS analysis, the location of this modification could be pinpointed to residue Cys27. While C5a engages six of its cysteines in three disulfide bridges between residues 21–47, 22–54 and 34–55 (39), it also contains a seventh Cys residue at position 27 that is free and therefore prone to association with sulfhydryl-containing molecules in circulation (38). If the adduct involves free plasma cysteine, a phenomenon also known as cysteinylation, this results to a mass increase of 119 Da in the targeted protein (40) that should be sensitive to reductive treatment. Indeed, reduction of the disulfide bonds by DTT in deglycosylated C5a and C5adesArg resulted in a spectrum with one single peak each with mass corresponding to the amino acid sequence (Fig. 2C, D), thereby confirming partial cysteinylation of Cys27 in 2/3 of total C5a and C5adesArg. Importantly, the peak pattern and ratios induced by these post-translational modifications were highly comparable between C5a and C5adesArg, therefore confirming that the only difference between the two proteins used in this study lies in the presence or absence of the C-terminal arginine residue.
C5a- and C5adesArg-induced cell activation is entirely dependent on C5aR
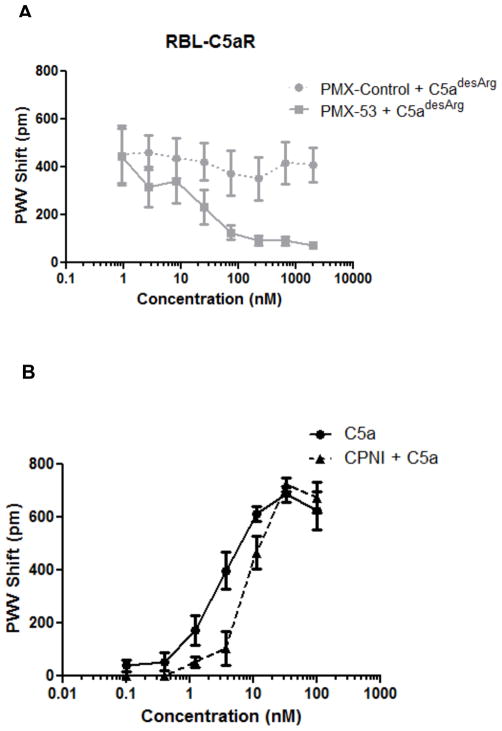
As the MS results confirmed the identity of both C5a and C5adesArg, we then investigated whether the potent cell activation induced by C5adesArg results exclusively from C5aR signaling or may be influenced by additional, yet unidentified mechanisms. For this purpose, we pharmacologically blocked C5aR signaling in C5aR-transfected RBL cells with the C5aR antagonist PMX-53 or an inactive control peptide (PMX-control) (35); we measured cell activation to a defined concentration of agonist (20 nM C5adesArg) after addition of increasing antagonist concentrations. C5adesArg-induced cell activation was inhibited by PMX-53 in a dose-dependent fashion, but not by the PMX-control peptide (Fig. 3A), indicating that the cell response triggered by C5adesArg is entirely dependent on C5aR.
Figure 3. C5a- and C5adesArg-induced cell activation is entirely dependent on C5aR.
A, RBL-C5aR cells attached to biosensor plates were pre-incubated for 1 h with various concentrations of the C5aR antagonist PMX-53 or a control peptide (PMX-Control), and cell activation was assessed after the addition of 20 nM C5adesArg. B, C5a-induced cell activation was assessed in cells that were pre-incubated (for 1 h) with or without 1 mM carboxypeptidase N inhibitor (CPNI).
All the assays described thus far were performed in the presence of medium containing 10% inactivated FBS in order to render the assay conditions more physiologic; similar results were obtained in the presence of 10% inactivated normal human serum (data not shown). To exclude the possibility that carboxypeptidases present in the serum were influencing our results by rapidly converting C5a into C5adesArg, we repeated the assay in the presence of a potent carboxypeptidase inhibitor (2-mercaptomethyl-3-guanidinoethylthiopropionic acid; CPNI). Importantly, C5a induced similar levels of cell activation in the presence or absence of CPNI (Fig. 3B), thereby indicating that the inactivated FBS is devoid of active carboxypeptidases.
Serum potentiates C5a- and C5adesArg-induced cell activation
In order to determine whether anaphylatoxin-induced cell signaling is affected by other factors present in the serum, we assessed cell activation after 2 h of serum starvation. Serum starvation resulted in markedly reduced levels of cell activation induced by either C5a or C5adesArg, which produced maximal values of only 390±26 and 381±20 pm, respectively (Fig. 4A), as compared to 664±27 and 531±23 pm in the presence of serum (Fig. 1B). Notably, C5a and C5adesArg induced similar maximal cell responses in the absence of serum, yet C5adesArg-induced cell activation was associated with lower EC50 and higher Hill slope values than was C5a (0.5 vs. 3 nM and 1.9 vs. 0.4, respectively) (Fig. 4A). These data indicate that factors present in the serum likely potentiate anaphylatoxin-induced cell activation in in vitro assays, either directly or by maintaining cell viability. Importantly, the 2 h of serum starvation did not change the expression levels of C5aR on the transfected RBL cells (data not shown).
Figure 4. The presence of serum potentiates C5a- and C5adesArg- induced cell activation.
A, RBL-C5aR cells were attached to biosensor plates by incubation in complete medium (DMEM plus 10% FBS) for 16 h at 37°C. The medium was replaced by serum-free medium, and cell activation in response to increasing concentrations of C5a or C5adesArg was monitored after 2 h of serum starvation. Calculated EC50, maximum response, and Hill slope values are shown in the table. B, C5a-induced cell activation was assessed in the presence and absence of 10% FBS (FBS + C5a) and 50 nM Gc-globulin (Gc-globulin + C5a). *P<0.05, ***P<0.001.
Correlations between increased C5a-dependent chemotactic activity and the presence of Gc-globulin (also known as vitamin D-binding protein) in serum have been reported (41). We therefore asked whether Gc-globulin might have an impact on C5a-induced cell activation in our model. Pre-incubation of C5aR-transfected RBL cells with 50 nM of Gc-globulin in serum-free medium did not enhance C5a-induced cell activation to the extent we observed in the presence of serum (Fig. 4B), indicating that Gc globulin is not responsible for the observed differences in C5a signaling in the presence and absence of serum.
Human polymorphonuclear cells are activated by C5a and C5adesArg, but not C3a or C3adesArg
We next examined the ability of anaphylatoxins to induce the activation of human primary cells, more specifically blood polymorphonuclear (PMN) cells that express high levels of anaphylatoxin receptors under physiological conditions (Supplemental Fig. 2A). Despite their high expression of C3aR, human PMN cells did not respond to either C3a or C3adesArg (Fig. 5A). In contrast, C5a induced strong cell activation, leading to a maximal PWV shift of 1094±94 pm and EC50 and Hill slope values of 10.3 nM and 0.1, respectively. Interestingly, the C5adesArg-dependent cell response only reached a maximal PWV value of 612±53 pm, almost 2-fold lower than that induced by C5a (Fig. 5B). Yet despite the lower absolute response, the dose-dependent activation was actually stronger in the case of C5adesArg with EC50 and Hill slope values of 4.0 nM and 0.2, respectively. Again, and as we had observed for the C5aR-transfected RBL cell line, low concentrations of C5adesArg (up to 5 nM) were more potent inducers of cell activation than analogous concentrations of C5a. This finding is of particular importance as physiologic plasma concentrations of C5adesArg range from 5 to 10 ng/ml (~1 nM) (42). Therefore, our data point to a constant activation of blood cells by C5adesArg that may be important for the maintenance of basal activation and blood surveillance.
Figure 5. Human neutrophils are activated by C5a and C5adesArg, but not C3a or C3adesArg.
Human polymorphonuclear cells (PMN) were isolated from blood and analyzed for anaphylatoxin receptor activation in response to anaphylatoxin stimulation. A–B, PMN cells in complete medium (DMEM plus 10% FBS) were attached to biosensors, and cell activation was monitored in response to increasing concentrations of C3a (A) or C5a (B). **P<0.05
The label-free cell assay detects the immediate response induced by the triggering of GPCRs by anaphylatoxins. In the case of PMN cells, activation signals were detected as early as 1 s upon addition of 100 nM of either C5a or C5adesArg, with a maximal response obtained after 3–4 min (Supplemental Fig. 2B, left). Similarly, cell activation in response to lower concentrations (1 nM) of C5a or C5adesArg could be detected as early as 1 s, with a maximal response detected after 1 min upon ligand addition (Supplemental Fig. 2B, right). Notably, C5adesArg-induced response is stronger and sustained for longer when compared with the response induced by same concentration (1 nM) of C5a (Supplemental Fig. 2B, right). In order to compare the magnitude of the anaphylatoxin-induced cell activation achieved at later time points, we evaluated the expression levels of CD11b in human PMN cells that had been incubated for 1 h with either C5a or C5adesArg. Physiologic concentrations (1 nM) of both anaphylatoxins were able to induce similar up-regulation of CD11b (Supplemental Fig. 2C). A similar trend was seen in response to higher concentrations (Supplemental Fig. 2C), indicating that C5adesArg, especially at physiologic concentrations, induces substantial activation of PMN cells.
We also verified that C5a- and C5adesArg-induced PMN cell activation was specifically inhibited in the presence of the C5aR antagonist PMX-53, but not in the presence of the control peptide (Fig. 6), thereby confirming the specificity of C5a and C5adesArg signaling via C5aR.
Figure 6. Human neutrophils are activated by C5a and C5adesArg, but not C3a or C3adesArg.
Human polymorphonuclear cells (PMN) in complete medium (DMEM plus 10% FBS) were attached to biosensors. Cells were pre-incubated for 1 h with different concentrations of the C5aR antagonist PMX-53 or the control peptide (PMX-Control), and cell activation was assessed after the addition of 20 nM C5a (A) or C5adesArg (B).
C5a and C5adesArg trigger differential activation of G proteins
The data obtained in both transfected and primary immune cells demonstrated that plasma-derived C5adesArg acts as a potent inducer of C5aR-mediated cell activation at physiologic concentrations. However, they also revealed a distinct concentration-dependent signaling pattern, which prompted us to further investigate the molecular events downstream of C5aR in response to C5a and C5adesArg. Previous studies have shown that in cells such as neutrophils and mast cells, C5aR couples primarily to the pertussis toxin (PTX)-sensitive G protein Gαi2 (43,44). We therefore determined the impact of Gαi inhibition on the promotion of cell activation by C5a and C5adesArg. Indeed, PTX negatively affected C5a- and C5adesArg-induced responses (Fig. 7A). Though not statistically significant, C5a-induced cell responses seemed to be less affected by PTX; while 60–85% of C5adesArg-induced responses were inhibited by PTX, C5a-induced cell responses were inhibited by only 45–65% (Fig. 7A). In order to determine if other pathways may be responsible for the residual C5a-induced response, the influence of Gαs was investigated via inactivation with cholera toxin (CTX). C5adesArg-induced responses were only marginally affected by CTX and the effect was independent of the ligand concentration (Fig. 7B). In contrast, however, CTX affected the cell responses promoted by C5a in a highly distinct manner. While the responses induced by 10–100 nM C5a were slightly decreased by CTX (to a similar extent as for C5adesArg), the responses triggered by low concentrations of C5a (0.1–1.0 nM) were conversely magnified by CTX (Fig. 7B). As such, these data not only indicate that C5a and C5adesArg promote differential activation of G proteins but also point to the involvement of different classes of G proteins depending on the concentrations of the ligands.
Figure 7. C5a and C5adesArg trigger differential activation of G-proteins.
C5aR-transfected RBL cells were pre-incubated for 15 h with pertussis toxin (PTX; 0.5 μg/ml) (A) or cholera toxin (CTX; 100 μg/ml) (B) and cell activation was assessed after the addition of increasing concentrations of C5a or C5adesArg. The results are shown as percentage of inhibition (%) calculated in relation to the maximum activation achieve in response to each individual concentration in the absence of G protein inhibition. Graphs depict mean ± S.E.M from 4 (A) or 7 (B) independent experiments. *P<0.05, **P<0.01, ***P<0.001.
Discussion
Anaphylatoxin-induced inflammatory responses play a crucial role in many physiological and pathophysiological processes, and their therapeutic modulation has moved into the spotlight of complement-related drug discovery efforts (2,25). Yet, while the triggering of cell responses by anaphylatoxin receptors is well appreciated, their quantitation so far have mostly been assessed indirectly by means of calcium release, cell degranulation, oxygen burst, cytokine production, and chemotaxis (5). Although widely employed, such assays have significant disadvantages: They can be time-consuming, require high cell numbers, are restricted by low throughput, and do not reflect integrated cellular responses. We present here the utilization of a new high-throughput label-free cell assay based on PC biosensors that is able to directly detect anaphylatoxin-induced cell activation over time, in a specific and rapid manner, using as few as 2.5×104 cells. Although the detection principle shares similarity with that of surface plasmon resonance, the limited lateral propagation of light and lack of microfluidics render PC biosensors amendable to the analysis of whole cells. Importantly, compared to the orthogonal cell activation assays mentioned above, PC biosensors are able to simultaneously detect a broad range of cellular events downstream of GPCR signaling that lead to changes in cell morphology or adhesion properties. As a consequence, such technology may display a more holistic cellular response that is usually not detected by other conventional assays (28–30).
In the case of C3-derived anaphylatoxins, we detected C3a-induced activation of C3aR-transfected RBL cells (EC50 ~2 nM), but not human PMN cells. Furthermore, no cell activation was induced in response to C3adesArg triggering, neither in RBL or PMN cells nor in the neutrophil migration model. The release of β-glucosaminidase by C3aR-RBL cells in response to C3a stimulation has been reported previously (EC50: 14 nM). Consistent with our data and the effectiveness of the label-free assay, C3a, but not C3adesArg, has been previously described as a potent chemotatic factor for eosinophils, but not neutrophils. Despite the presence of C3aR on human neutrophils, no degranulation or oxygen burst has previously been observed in response to C3a (4,45). While these results support and validate our label-free assay approach, further investigation is clearly required to address the lack of C3aR-related signaling in human neutrophils.
C5L2 stimulation by C3a, C3adesArg, C5a, and C5adesArg did not trigger any response in RBL-C5L2 cells that could be detected by the label-free cell assay. In line with these results, no β-hexosaminidase secretion or intracellular Ca2+ release were detected when RBL-C5L2 cells were activated with 100 nM of C5a, C5adesArg, C3a, or C4a in previous studies (5). Despite the high expression of C5L2 in human neutrophils (12), the presence of these receptors does not account for C5a- or C5adesArg-induced neutrophil activation in our assay, since the cell response was completely abrogated by the C5aR antagonist PMX-53 (35). Since C5L2 has been implicated in the activation of the β-arrestin signaling pathway (12), more suitable assays should be considered to address potential stimulation of cells via C5L2 activation. However, it cannot be excluded that C5L2 may be involved in the lower absolute signal response for C5adesArg on PMN, either by acting as a co-receptor or by internalizing the ligand, as C5L2-induced responses could not be detected by the label-free cellular assay.
Triggering of C5aR by either C5a or C5adesArg induced the activation of both transfected RBL-C5aR cells and human PMN cells. Such responses were specifically inhibited in a dose-dependent fashion by the C5aR antagonist PMX-53 (35), confirming the requirement for C5aR for C5a- and C5adesArg-induced cell responses. Most importantly, we found that physiologic levels of C5adesArg (5–10 ng/ml; around 1 nM) induce substantial cell responses in relation to similar concentrations of C5a, a possibility that might have been overlooked in previous cases due to the lack of assays able to determine integrated cellular responses, or the comparison between C5a and C5adesArg is not even considered in the experimental planning. Of note, it has previously been shown that C5a is entirely converted to C5adesArg within 5 min in the presence of plasma (27), highlighting the importance of C5adesArg over C5a in the circulation. Although C5adesArg is often considered an “inactivation” product of C5a, a natural variation in responses to C5a and C5adesArg have been observed in the past. Both C5a and C5adesArg bear similar efficacy at inducing histamine and leukotriene C4 release by primed basophils (8), calcium mobilization in mouse peritoneal macrophages (46) and neutrophil-mediated platelet aggregation (47). Further, both proteins are able to prime macrophages and enhance their susceptibility to HIV infection (48). Therefore, C5adesArg-induced cell responses are critically dependent on the type of cell and response studied (7–10).
Our data also point to the requirement for serum, mimicking physiological conditions, in order to obtain maximal cell response and reliable activation pattern to anaphylatoxin stimulation. This is of particular importance for designing anaphylatoxin assays to ensure comparability, as many assays described in literature have been performed in a variety of buffer/media conditions. C5a- and C5adesArg-induced cell activation is greatly decreased by serum starvation, highlighting the importance of close-to-physiological conditions for proper cell stimulation. The presence of plasma Gc-globulin has previously been found to correlate with a higher potential for C5a-induced chemotaxis (41). However, the presence of Gc-globulin did not affect the C5a-induced responses in our model, indicating that other serum factors may also contribute to C5aR activity, either by acting as “cofactors” or by generally enhancing the fitness of the cells.
C5a- and C5adesArg-induced responses were inhibited by PTX treatment, consistent with previous observation that C5aR signaling relies on heterotrimeric G-proteins and is mainly achieved by the PTX-sensitive alpha unit Gαi2 (43,44). Further, our data shed new light on the signaling pathways triggered downstream C5aR by showing that not only Gαi but also Gαs proteins may likely be involved in C5a- and C5adesArg-induced cellular responses. The apparent distinct engagement of G proteins induced by C5a and C5adesArg may reflect differential binding of these proteins to the C5aR. Indeed, the interaction between the residues Lys68 in the ligand and Glu199 in the C5aR has been shown to be essential for C5adesArg- but not C5a-induced receptor activation (49). The Gαs protein has been previously implicated in the C5a-dependent release of 5-hydroxy[3H]tryptamine by transfected RBL cells (32). Notably, it is well appreciated that the activation of Gαs proteins counteracts the effects induced by Gαi proteins and vice versa, especially when it comes to the regulation of intracellular levels of cyclic adenosine monophosphate (cAMP) (50). C5a has been previously shown to modulate the intracellular levels of cAMP in negative and positive manners (46,51). Such apparent controversy may rely on the different cell types (which express different classes of Gα proteins) and ligand concentrations used in these studies. Indeed, ligand concentration has been previously shown to determine preferential activation of Gαs versus Gαi proteins (52). While further investigation is required to determine the biological consequence of such differential involvement of Gαi and Gαs proteins upon C5aR engagement by C5a or C5adesArg and whether differential molecular mechanisms are triggered by C5a and C5adesArg during the course of inflammation and homeostasis, our data suggest that constant activation of blood cells by physiologic concentrations of C5adesArg may play a role in blood surveillance as well as the maintenance of blood homeostasis.
Finally, the full MS characterization of C5a and C5adesArg provided additional insight into the post-translational modification pattern of these anaphylatoxins, which includes glycosylation at Asn64 and partial cysteinylation at Cys27. While the 2204 kDa oligosaccharide chain deduced as (GlcNAc)4(Man/Gal)5(NeuAc)2 does not influence the anaphylatoxic activity of C5a and C5adesArg (37), the impact of cysteinylation on the activity of such molecules has not been addressed so far. Increased plasma levels of cysteinylated proteins has been previously associated with aging and irreversible oxidation of plasma proteins (53). Additional studies are required to assess whether the ratio of free vs. cysteinylated Cys27 on C5a and C5adesArg correlates with changes in activity and pathologies.
Supplementary Material
Acknowledgments
We thank Dr. Deborah McClellan for excellent editing of the manuscript.
Abbreviations used in this article
- Arg
arginine
- C3aR
C3a receptor
- C5adesArg
desarginated C5a
- C5aR
C5a receptor
- C5L2
C5a receptor-like 2
- cAMP
cyclic adenosine monophosphate
- CPNI
carboxypeptidase N inhibitor
- CTX
cholera toxin
- GPCR
G protein-coupled receptor
- MS
mass spectrometry
- PC
photonic crystal
- pm
picometer
- PMN
polymorphonuclear cells
- PTX
pertussis toxin
- PWV
peak wavelength value
- RBL
rat basophil leukemia cells
Footnotes
This work was supported by National Institutes of Health grants AI030040, AI068730, EY020633, AI097805, and GM097747 to J.D.L and/or D.R., and British Heart Foundation grant PG/09/018/25279 to P.N.M.
References
- 1.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Köhl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;46:2753–2766. doi: 10.1016/j.molimm.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bokisch VA, Müller-Eberhard HJ. Anaphylatoxin inactivator of human plasma: its isolation and characterization as a carboxypeptidase. J Clin Invest. 1970;49:2427–2436. doi: 10.1172/JCI106462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilken HC, Gotze O, Werfel T, Zwirner J. C3a(desArg) does not bind to and signal through the human C3a receptor. Immunol Lett. 1999;67:141–145. doi: 10.1016/s0165-2478(99)00002-4. [DOI] [PubMed] [Google Scholar]
- 5.Cain SA, Monk PN. The orphan receptor C5L2 has high affinity binding sites for complement fragments C5a and C5a des-Arg(74) J Biol Chem. 2002;277:7165–7169. doi: 10.1074/jbc.C100714200. [DOI] [PubMed] [Google Scholar]
- 6.Monk PN, Scola AM, Madala P, Fairlie DP. Function, structure and therapeutic potential of complement C5a receptors. Br J Pharmacol. 2007;152:429–448. doi: 10.1038/sj.bjp.0707332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scholz W, McClurg MR, Cardenas GJ, Smith M, Noonan DJ, Hugli TE, Morgan EL. C5a-mediated release of interleukin 6 by human monocytes. Clin Immunol Immunopathol. 1990;57:297–307. doi: 10.1016/0090-1229(90)90043-p. [DOI] [PubMed] [Google Scholar]
- 8.Burgi B, Brunner T, Dahinden CA. The degradation product of the C5a anaphylatoxin C5adesarg retains basophil-activating properties. Eur J Immunol. 1994;24:1583–1589. doi: 10.1002/eji.1830240720. [DOI] [PubMed] [Google Scholar]
- 9.Eglite S, Pluss K, Dahinden CA. Requirements for C5a receptor-mediated IL-4 and IL-13 production and leukotriene C4 generation in human basophils. J Immunol. 2000;165:2183–2189. doi: 10.4049/jimmunol.165.4.2183. [DOI] [PubMed] [Google Scholar]
- 10.Werfel T, Oppermann M, Butterfield JH, Begemann G, Elsner J, Gotze O, Zwirner J. The human mast cell line HMC-1 expresses C5a receptors and responds to C5a but not to C5a(desArg) Scand J Immunol. 1996;44:30–36. doi: 10.1046/j.1365-3083.1996.d01-272.x. [DOI] [PubMed] [Google Scholar]
- 11.Okinaga S, Slattery D, Humbles A, Zsengeller Z, Morteau O, Kinrade MB, Brodbeck RM, Krause JE, Choe HR, Gerard NP, Gerard C. C5L2, a nonsignaling C5A binding protein. Biochemistry. 2003;42:9406–9415. doi: 10.1021/bi034489v. [DOI] [PubMed] [Google Scholar]
- 12.Bamberg CE, Mackay CR, Lee H, Zahra D, Jackson J, Lim YS, Whitfeld PL, Craig S, Corsini E, Lu B, Gerard C, Gerard NP. The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. J Biol Chem. 2010;285:7633–7644. doi: 10.1074/jbc.M109.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng Q, Li K, Anderson K, Farrar CA, Lu B, Smith RA, Sacks SH, Zhou W. Local production and activation of complement up-regulates the allostimulatory function of dendritic cells through C3a-C3aR interaction. Blood. 2008;111:2452–2461. doi: 10.1182/blood-2007-06-095018. [DOI] [PubMed] [Google Scholar]
- 14.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, Shapiro VS, Dubyak GR, Heeger PS, Medof ME. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang C, Zhang X, Miwa T, Song WC. Complement promotes the development of inflammatory T-helper 17 cells through synergistic interaction with Toll-like receptor signaling and interleukin-6 production. Blood. 2009;114:1005–1015. doi: 10.1182/blood-2009-01-198283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weaver DJ, Jr, Reis ES, Pandey MK, Köhl G, Harris N, Gerard C, Köhl J. C5a receptor-deficient dendritic cells promote induction of Treg and Th17 cells. Eur J Immunol. 2010;40:710–721. doi: 10.1002/eji.200939333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward PA. Role of the complement in experimental sepsis. J Leukoc Biol. 2008;83:467–470. doi: 10.1189/jlb.0607376. [DOI] [PubMed] [Google Scholar]
- 18.Köhl J, Baelder R, Lewkowich IP, Pandey MK, Hawlisch H, Wang L, Best J, Herman NS, Sproles AA, Zwirner J, Whitsett JA, Gerard C, Sfyroera G, Lambris JD, Wills-Karp M. A regulatory role for the C5a anaphylatoxin in type 2 immunity in asthma. J Clin Invest. 2006;116:783–796. doi: 10.1172/JCI26582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lajoie S, I, Lewkowich P, Suzuki Y, Clark JR, Sproles AA, Dienger K, Budelsky AL, Wills-Karp M. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol. 2010;11:928–935. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuboi N, Ernandez T, Li X, Nishi H, Cullere X, Mekala D, Hazen M, Köhl J, Lee DM, Mayadas TN. Regulation of human neutrophil Fcgamma receptor IIa by C5a receptor promotes inflammatory arthritis in mice. Arthritis Rheum. 2011;63:467–478. doi: 10.1002/art.30141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q, Peng Q, Xing G, Li K, Wang N, Farrar CA, Meader L, Sacks SH, Zhou W. Deficiency of C5aR prolongs renal allograft survival. J Am Soc Nephrol. 2010;21:1344–1353. doi: 10.1681/ASN.2009090977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, Coukos G, Lambris JD. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9:1225–1235. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ager RR, Fonseca MI, Chu SH, Sanderson SD, Taylor SM, Woodruff TM, Tenner AJ. Microglial C5aR (CD88) expression correlates with amyloid-beta deposition in murine models of Alzheimer’s disease. J Neurochem. 2010;113:389–401. doi: 10.1111/j.1471-4159.2010.06595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang M, Krauss JL, Domon H, Hosur KB, Liang S, Magotti P, Triantafilou M, Triantafilou K, Lambris JD, Hajishengallis G. Microbial hijacking of complement-toll-like receptor crosstalk. Sci Signal. 2010;3:ra11. doi: 10.1126/scisignal.2000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ricklin D, Lambris JD. Complement-targeted therapeutics. Nat Biotechnol. 2007;25:1265–1275. doi: 10.1038/nbt1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qu H, Ricklin D, Lambris JD. Recent developments in low molecular weight complement inhibitors. Mol Immunol. 2009;47:185–195. doi: 10.1016/j.molimm.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mueller-Ortiz SL, Wang D, Morales JE, Li L, Chang JY, Wetsel RA. Targeted disruption of the gene encoding the murine small subunit of carboxypeptidase N (CPN1) causes susceptibility to C5a anaphylatoxin-mediated shock. J Immunol. 2009;182:6533–6539. doi: 10.4049/jimmunol.0804207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shamah SM, Cunningham BT. Label-free cell-based assays using photonic crystal optical biosensors. Analyst. 2011;136:1090–1102. doi: 10.1039/c0an00899k. [DOI] [PubMed] [Google Scholar]
- 29.Fang Y, Frutos AG, Verklereen R. Label-free cell-based assays for GPCR screening. Comb Chem High Throughput Screen. 2008;11:357–369. doi: 10.2174/138620708784534789. [DOI] [PubMed] [Google Scholar]
- 30.Scott CW, Peters MF. Label-free whole-cell assays: expanding the scope of GPCR screening. Drug Discov Today. 2010;15:704–716. doi: 10.1016/j.drudis.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Schroder R, Janssen N, Schmidt J, Kebig A, Merten N, Hennen S, Müller A, Blattermann S, Mohr-Andra M, Zahn S, Wenzel J, Smith NJ, Gomeza J, Drewke C, Milligan G, Mohr K, Kostenis E. Deconvolution of complex G protein-coupled receptor signaling in live cells using dynamic mass redistribution measurements. Nat Biotechnol. 2010;28:943–949. doi: 10.1038/nbt.1671. [DOI] [PubMed] [Google Scholar]
- 32.Monk PN, Pease JE, Barker MD. C5a stimulus-secretion coupling in rat basophilic leukaemia (RBL-2H3) cells transfected with the human C5a receptor is mediated by pertussis and cholera toxin-sensitive G proteins. Biochem Mol Biol Int. 1994;32:13–20. [PubMed] [Google Scholar]
- 33.Mollnes TE, Brekke OL, Fung M, Fure H, Christiansen D, Bergseth G, Videm V, Lappegard KT, Köhl J, Lambris JD. Essential role of the C5a receptor in E coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood. 2002;100:1869–1877. [PubMed] [Google Scholar]
- 34.Huber-Lang M, Younkin EM, Sarma JV, Riedemann N, McGuire SR, Lu KT, Kunkel R, Younger JG, Zetoune FS, Ward PA. Generation of C5a by phagocytic cells. Am J Pathol. 2002;161:1849–1859. doi: 10.1016/S0002-9440(10)64461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paczkowski NJ, Finch AM, Whitmore JB, Short AJ, Wong AK, Monk PN, Cain SA, Fairlie DP, Taylor SM. Pharmacological characterization of antagonists of the C5a receptor. Br J Pharmacol. 1999;128:1461–1466. doi: 10.1038/sj.bjp.0702938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Domon B, Costello CE. Structure elucidation of glycosphingolipids and gangliosides using high-performance tandem mass spectrometry. Biochemistry. 1988;27:1534–1543. doi: 10.1021/bi00405a021. [DOI] [PubMed] [Google Scholar]
- 37.Gerard C, Chenoweth DE, Hugli TE. Response of human neutrophils to C5a: a role for the oligosaccharide moiety of human C5ades Arg-74 but not of C5a in biologic activity. J Immunol. 1981;127:1978–1982. [PubMed] [Google Scholar]
- 38.Fernandez HN, Hugli TE. Primary structural analysis of the polypeptide portion of human C5a anaphylatoxin. Polypeptide sequence determination and assignment of the oligosaccharide attachment site in C5a. J Biol Chem. 1978;253:6955–6964. [PubMed] [Google Scholar]
- 39.Fredslund F, Laursen NS, Roversi P, Jenner L, Oliveira CL, Pedersen JS, Nunn MA, Lea SM, Discipio R, Sottrup-Jensen L, Andersen GR. Structure of and influence of a tick complement inhibitor on human complement component 5. Nat Immunol. 2008;9:753–760. doi: 10.1038/ni.1625. [DOI] [PubMed] [Google Scholar]
- 40.Cabras T, Manconi B, Iavarone F, Fanali C, Nemolato S, Fiorita A, Scarano E, Passali GC, Manni A, Cordaro M, Paludetti G, Faa G, Messana I, Castagnola M. RP-HPLC-ESI-MS evidenced that salivary cystatin B is detectable in adult human whole saliva mostly as S-modified derivatives: S-Glutathionyl, S-cysteinyl and S-S 2-mer. J Proteomics. 2012;75:908–913. doi: 10.1016/j.jprot.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Kew RR, Fisher JA, Webster RO. Co-chemotactic effect of Gc-globulin (vitamin D binding protein) for C5a. Transient conversion into an active co-chemotaxin by neutrophils. J Immunol. 1995;155:5369–5374. [PubMed] [Google Scholar]
- 42.Stove S, Welte T, Wagner TO, Kola A, Klos A, Bautsch W, Köhl J. Circulating complement proteins in patients with sepsis or systemic inflammatory response syndrome. Clin Diagn Lab Immunol. 1996;3:175–183. doi: 10.1128/cdli.3.2.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skokowa J, Ali SR, Felda O, Kumar V, Konrad S, Shushakova N, Schmidt RE, Piekorz RP, Nurnberg B, Spicher K, Birnbaumer L, Zwirner J, Claassens JW, Verbeek JS, van RN, Köhl J, Gessner JE. Macrophages induce the inflammatory response in the pulmonary Arthus reaction through G alpha i2 activation that controls C5aR and Fc receptor cooperation. J Immunol. 2005;174:3041–3050. doi: 10.4049/jimmunol.174.5.3041. [DOI] [PubMed] [Google Scholar]
- 44.Sheth B, Banks P, Burton DR, Monk PN. The regulation of actin polymerization in differentiating U937 cells correlates with increased membrane levels of the pertussis-toxin-sensitive G-protein Gi2. Biochem J. 1991;275(Pt 3):809–811. doi: 10.1042/bj2750809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daffern PJ, Pfeifer PH, Ember JA, Hugli TE. C3a is a chemotaxin for human eosinophils but not for neutrophils. I. C3a stimulation of neutrophils is secondary to eosinophil activation. J Exp Med. 1995;181:2119–2127. doi: 10.1084/jem.181.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang S, Krauss JL, Domon H, McIntosh ML, Hosur KB, Qu H, Li F, Tzekou A, Lambris JD, Hajishengallis G. The C5a receptor impairs IL-12-dependent clearance of Porphyromonas gingivalis and is required for induction of periodontal bone loss. J Immunol. 2011;186:869–877. doi: 10.4049/jimmunol.1003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Damerau B, Meyer B, Vogt W. Formation of mixed aggregates of human polymorphonuclear leukocytes and platelets by C5a-desArg. Complement Inflamm. 1991;8:25–32. doi: 10.1159/000463174. [DOI] [PubMed] [Google Scholar]
- 48.Kacani L, Banki Z, Zwirner J, Schennach H, Bajtay Z, Erdei A, Stoiber H, Dierich MP. C5a and C5a(desArg) enhance the susceptibility of monocyte-derived macrophages to HIV infection. J Immunol. 2001;166:3410–3415. doi: 10.4049/jimmunol.166.5.3410. [DOI] [PubMed] [Google Scholar]
- 49.Crass T, Bautsch W, Cain SA, Pease JE, Monk PN. Receptor activation by human C5a des Arg74 but not intact C5a is dependent on an interaction between Glu199 of the receptor and Lys68 of the ligand. Biochemistry. 1999;38:9712–9717. doi: 10.1021/bi990139q. [DOI] [PubMed] [Google Scholar]
- 50.Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M. Cyclic AMP: master regulator of innate immune cell function. Am J Respir Cell Mol Biol. 2008;39:127–132. doi: 10.1165/rcmb.2008-0091TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peng Q, Li K, Wang N, Li Q, Asgari E, Lu B, Woodruff TM, Sacks SH, Zhou W. Dendritic cell function in allostimulation is modulated by C5aR signaling. J Immunol. 2009;183:6058–6068. doi: 10.4049/jimmunol.0804186. [DOI] [PubMed] [Google Scholar]
- 52.Waelbroeck M. Activation of guanosine 5′-[gamma-(35)S]thio-triphosphate binding through M(1) muscarinic receptors in transfected Chinese hamster ovary cell membranes; 1. Mathematical analysis of catalytic G protein activation. Mol Pharmacol. 2001;59:875–885. doi: 10.1124/mol.59.4.875. [DOI] [PubMed] [Google Scholar]
- 53.Rossi R, Giustarini D, Milzani A, le-Donne I. Cysteinylation and homocysteinylation of plasma protein thiols during ageing of healthy human beings. J Cell Mol Med. 2009;13:3131–3140. doi: 10.1111/j.1582-4934.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.