Abstract
Pentraxin 3 (PTX3) is a soluble pattern recognition receptor which is classified as a long-pentraxin in the pentraxin family. It is known to play an important role in innate immunity, inflammatory regulation, and female fertility. PTX3 is synthesized by specific cells, primarily in response to inflammatory signals. Among these various cells, neutrophils have a unique PTX3 production system. Neutrophils store PTX3 in neutrophil-specific granules and then the stored PTX3 is released and localizes in neutrophil extracellular traps (NETs). Although certain NET components have been identified, such as histones and anti-microbial proteins, the detailed mechanisms by which NETs localize, as well as capture and kill microbes, have not been fully elucidated. PTX3 is a candidate diagnostic marker of infection and vascular damage. In severe infectious diseases such as sepsis, the circulating PTX3 concentration increases greatly (up to 100 ng/mL, i.e., up to 100-fold of the normal level). Even though it is clearly implied that PTX3 plays a protective role in sepsis and certain other disorders, the detailed mechanisms by which it does so remain unclear. A proteomic study of PTX3 ligands in septic patients revealed that PTX3 forms a complex with certain NET component proteins. This suggests a role for PTX3 in which it facilitates the efficiency of anti-microbial protein pathogen clearance by interacting with both pathogens and anti-microbial proteins. We discuss the possible relationships between PTX3 and NET component proteins in the host protection afforded by the innate immune response. The PTX3 complex has the potential to be a highly useful diagnostic marker of sepsis and other inflammatory diseases.
Keywords: PTX3, pentraxin, diagnosis, protein complex, anti-microbial protein, host-protection
Introduction
The release of neutrophil extracellular traps (NETs), first reported in 2004 (Brinkmann, 2004), is one of the anti-microbial actions of neutrophils. NETs are mesh-like structures that contain DNA as a backbone, with anti-microbial proteins attached (Amulic and Hayes, 2011). NETs trap microbes and form an anti-microbial-protein-rich microenvironment (Medina, 2009).
Pentraxin 3 (PTX3) was reported as one of the NET component proteins (Jaillon et al., 2007). PTX3 is a member of pentraxin family and mainly acts as a soluble pattern recognition receptor (PRR) in the innate immune response (Bottazzi et al., 2010). In NETs, PTX3 may participate in microbial recognition by facilitating the trapping of microbes. The circulating PTX3 level is known to be increased in certain diseases, and PTX3 may predominantly play a critical role in host protection. Interestingly, proteomic identification of the circulating PTX3 interacting proteins revealed that PTX3 formed a complex with NET component proteins (Daigo et al., 2012). This finding implies that the NET component proteins are active in pathogen recognition and clearance by tethering with each other in NETs and bloodstream. PTX3 appears to be a key tethering molecule to enhance the actions of NETs component proteins. In this review, we will discuss the host-protective roles of PTX3 in relation to NETs component proteins.
NETs
Source, expression, and function
Neutrophils are the major player in the innate immune system response against microbial pathogen invasion. One of the anti-microbial activities of neutrophils is the extrusion of NETs (Brinkmann, 2004). NETs are formed upon the activation of neutrophils by factors such as IL-8, lipopolysaccharide (LPS), phorbol 12-myristate 13-acetate (PMA), bacteria, fungi, and activated platelets (Brinkmann, 2004; Clark et al., 2007; Fuchs et al., 2007). Neutrophil death as a result of the extrusion of NETs is called “NETosis,” which is a cell death pathway distinct from apoptosis or necrosis (Brinkmann and Zychlinsky, 2007; Steinberg and Grinstein, 2007). The release of NETs has also been reportedly observed without cell death (Yipp et al., 2012). Extracellular formations of this type are also observed in basophils and eosinophils (Schorn et al., 2012). NETs are mesh-like structures that consist of cellular DNA, along with bactericidal proteins, that reside in neutrophil granules and the nucleus. These proteins are connected to DNA fibers, and form a specialized microenvironment which facilitates the capture and killing of bacteria.
The NET component proteins
Using a proteomic approach, Urban et al. identified 24 NET-associated proteins (Urban et al., 2009). These proteins are; nuclear components such as core histones; granular components such as neutrophil elastase (ELANE), lactotransferrin (LTF), cathepsin G (CTSG), myeloperoxidase (MPO), proteinase 3 (PRTN3), azurocidin 1 (AZU1), lysozyme C (LYZ), neutrophil defensins, and cytoplasmic proteins. In other proteins, histone H1, bactericidal permeability-increasing protein (BPI), pentraxin 3 (PTX3), and cathelicidin anti-microbial peptide (CAMP) are also defined as NET component proteins (Brinkmann, 2004; Jaillon et al., 2007; Lauth et al., 2009). Essentially all of these proteins possess anti-microbial activity.
PTX3
Genome
Breviario et al. identified PTX3 as one of the IL-1β -induced genes in human umbilical vein endothelial cells (HUVECs) (Breviario et al., 1992). The human PTX3 gene is located on chromosome 3q band 25, consists of 1861 base pairs, and is translated into 381 amino acids (Breviario et al., 1992). PTX3 belongs to the pentraxin family, which included the acute phase proteins C-reactive protein (CRP) and serum amyloid P-component (SAP). As PTX3 has a longer N-terminal domain, it is classified as a member of the long-pentraxin subfamily. Unlike the more common short pentraxins CRP and SAP, the PTX3 gene is highly conserved across species (Garlanda et al., 2005). The PTX3 gene consists of three exons, among which the first and second exons encode the signal sequence peptide and the N-terminal domain, and the third exon encodes the C-terminal domain. In the promoter region of the PTX3 gene, a number of potential enhancer binding sequences (Pu-1, AP1, NF-κB, SP1, and NF-IL6) are located (He et al., 2007).
Structure
After the processing of the signal sequence of the translated 1–17 amino acids, the mature PTX3 consists of two domains, i.e., the N-terminal domain (18–178 a.a.) and C-terminal domain (179–381 a.a.). The PTX3 C-terminal domain is a pentraxin-like domain, which is conserved among the pentraxin family with pentraxin signature (His-x-Cys-x-Ser/Thr-Trp-x-Ser). An N-linked glycosylation site (Asn220) is located in the C-terminal domain. In contrast to the C-terminal domain, the PTX3 N-terminal domain is a unique sequence unrelated to other proteins. The PTX3 protein forms an octamer via the inter-molecule disulfide bonds (Inforzato et al., 2008, 2010). Briefly, the N-terminal domain participates in the organization of a tetramer, and the C-terminal domain participates in the dimerization of the tetramer. Interestingly, the N-terminal tetramer formation has two states; a tetramer via the inter-disulfide bonds or non-covalent dimerization of the inter-disulfide-bonded dimer. This results in the asymmetric form of the full-length PTX3 (Inforzato et al., 2010).
Expression pattern
PTX3 mRNA expression is induced by primary inflammatory signals in certain cells, such as myeloid dendritic cells (Doni et al., 2003), peripheral blood leukocytes (Alles et al., 1994), mononuclear macrophages/phagocytes (Alles et al., 1994; Goodman et al., 2000), vascular endothelial cells (Breviario et al., 1992; Lee et al., 1993), smooth muscle cells (Klouche et al., 2004), fibroblasts (Lee et al., 1993; Goodman et al., 2000), adipocytes (Abderrahim-Ferkoune et al., 2003), glial cells (Polentarutti et al., 2000), cumulus oophorus cells (Salustri et al., 2004), mesangial cells (Nauta et al., 2005), and synovial cells (Luchetti et al., 2000). Transcriptional activation of PTX3 in response to the pro-inflammatory cytokines TNFα and IL-1β is regulated by NF-κB binding site in the PTX3 promoter (Altmeyer et al., 1995; Basile et al., 1997). Other pathways also regulate PTX3 expression in a cell- and signal-dependent manner. In detail, please refer to the excellent reviews cited (He et al., 2007; Ortega-Hernandez et al., 2009; Deban et al., 2011; Inforzato et al., 2011).
The characteristic PTX3 expression pattern is observed in neutrophils. In mature neutrophils, the PTX3 protein is abundantly present in granules, but PTX3 mRNA expression is not detected. In contrast, PTX3 mRNA expression is observed in progenitor neutrophils, such as promyelocytes and myelocytes/metamyelocytes (Jaillon et al., 2007). As PTX3 protein expression is observed in both neutrophil precursors and mature neutrophils, it is considered that the PTX3 protein is produced during the course of neutrophil maturation and mature neutrophils store it for use-on-demand. Immunostaining revealed that PTX3 is present in neutrophil granules and that it colocalizes with lactoferrin (Jaillon et al., 2007; Savchenko et al., 2011), suggesting that PTX3 localizes to specific granules. The stored PTX3 in neutrophils is released upon E. coli, S. aureus or zymosan stimulation, as well as PMA, ionomycin or TNFα treatment (Jaillon et al., 2007; Savchenko et al., 2011; Daigo et al., 2012). PTX3 release is not induced by IL-1β or latex bead stimulation (Jaillon et al., 2007). The released PTX3 localizes to NETs and plays a non-redundant role in pathogen resistance. Thus, PTX3 in neutrophils plays a distinctive role in the innate immune response due to its rapid secretion, as well as by its unique pattern of ready-to-use expression and storage.
Circulating levels
As the pentraxins CRP and SAP are well-known acute phase proteins, PTX3 may also be an acute phase biomarker. Under physiological conditions, the circulating PTX3 level is as low as approximately 2 ng/mL (Yamasaki et al., 2009). Recently, many studies on the circulating PTX3 level in clinical trials have been reported. These reports indicate that the PTX3 levels are significantly increased in certain infectious, cardiovascular, kidney, and female reproductive system diseases as well as other disorders (summarized in Table 1). In most cases, the PTX3 level correlates with both the severity and survivability of the disorder. In these diseases, the increases can reach up to 10~100 times the control level in severe inflammatory and infectious diseases such as sepsis. In the case of sepsis, the plasma PTX3 dramatically increases to a level of up to 100 ng/mL (Muller et al., 2001) and the increase correlates with mortality (Mauri et al., 2010).
Table 1.
Circulating PTX3 levels measurements in clinical trials.
| Disease category | Diseases | PTX3 concentration and significance | References |
|---|---|---|---|
| Physiological level | 2.00 (1.95, 2.04)a | Yamasaki et al., 2009 | |
| Infectious diseases | Systemic inflammatory response syndrome (SIRS) | SIRS: 28.0 ± 5.6 Control: 1.04 ± 0.09b p < 0.005 | Muller et al., 2001 |
| Pulmonary tuberculosis (TB) | TB: 3.21 | Azzurri et al., 2005 | |
| Control: 0.98c p < 0.0001 | |||
| Sepsis | Sepsis: 26 (1, 202) | Hill et al., 2009 | |
| Control: 6 (1, 12)d p < 0.001 | |||
| Febrile in the intensive/medium care unit (ICU/MC) or ward | In ICU/MC: 44.4 (13.6, 105.9) | De Kruif et al., 2010 | |
| In ward: 14.2 (7.01, 25.1) | |||
| Control: 2.30 (1.66, 3.67)d p = 0.01 | |||
| Bacteremia | Non-survivor: 44.8 (10.7, 69.4) | Huttunen et al., 2011 | |
| Survivor: 6.4 (3.4, 13.5)d p < 0.001 | |||
| Cardiovascular diseases | Unstable angina pectoris (UAP) | UAP: 6.09 (4.34–7.85) | Inoue et al., 2007 |
| Control: 2.30 (2.03–2.55)e p = 0.00003 | |||
| Chronic heart failure (CHF) | CHF: 3.06 (2.38, 4.23) | Kotooka et al., 2008 | |
| Control: 1.91 (1.35, 2.60)d p = 0.001 | |||
| Cardiac event: 6.0 (4.3, 9.3) | Ishino et al., 2008 | ||
| Event-free: 3.2 (2.0, 5.5)d p < 0.001 | |||
| Heart failure (HF) | Cardiac event: 6.22 (5.59) | Suzuki et al., 2008 | |
| Event-free: 2.99 (2.95)d p < 0.001 | |||
| HF: 3.28 (1.51, 2.90) | Matsubara et al., 2011 | ||
| Non-HF: 2. 18 (1.51, 2.90)d p < 0.001 | |||
| Coronary artery disease (CAD) | CAD with inflammatory rheumatic disease (IRD): 1.96 ± 0.98 | Hollan et al., 2010 | |
| Control: 1.21 ± 0.59b p < 0.001 | |||
| Aortic valve stenosis (AS) | AS: 3.5 ± 1.9 | Naito et al., 2010 | |
| Control: 2.1 ± 0.8b p < 0.05 | |||
| Acute coronary syndrome (ACS) | ACS: 1.73 ± 0.82 | Ustundag et al., 2011 | |
| Control: 0.50 ± 0.39b p < 0.001 | |||
| ACS: 0.36 (0.225, 1.39) | Kume et al., 2011 | ||
| Control: 0.015 (0, 0.06)f p < 0.0001 | |||
| Hypertension | Anti-hypertensive mediation | Parlak et al., 2012 | |
| Pre-treatment: 35.25 ± 5.45 | |||
| Post-treatment: 0.14 ± 0.19b p < 0.0001 | |||
| Acute ischemic strokes | Non-survivor: 18.0 (8.2, 26.1) | Ryu et al., 2012 | |
| Survivor: 6.4 (3.4, 11.8)d p < 0.001 | |||
| Giant cell arteritis (GCA) | GCA: 23.31 ± 4.06 | Baldini et al., 2012 | |
| Control: 3.97 ± 0.28g p < 0.003 | |||
| Kidney diseases | Hemodialysis (HD) | HD: 3.03 ± 1.81 | Malaponte et al., 2007 |
| Uremic patients: 2.34 ± 1.19 | |||
| Control 1.03 ±0.4b p < 0.001 | |||
| HD: 1.87 (1.34, 2.50) | Xu et al., 2011 | ||
| Control: 1.11 (0.86, 1.51)d p < 0.001 | |||
| Renal transplant patients: 5.78 (1.09–20.36) | Argani et al., 2012 | ||
| HD group: 1.65 (0.24–7.89)h p = 0.0001 | |||
| Chronic kidney disease (CKD) | Stage 5 CKD: 5.7 (0.9, 64.3) | Tong et al., 2007 | |
| Stage 3 to 4 CKD: 2.2 (0.4, 16.0) | |||
| Control: 1.8 (0.1, 9.1)d p < 0.001 | |||
| Stage 5 CKD: 5.3 (1.0, 58.0) | Suliman et al., 2008 | ||
| Control: 1.8 (0.1, 9.2)d p < 0.001 | |||
| CKD: 7.7 (1.8, 32.9) | Yilmaz et al., 2010 | ||
| Control: 1.3 (0.1, 2.7)f p < 0.001 | |||
| CKD: 3.80 ± 2.35 | Nishi et al., 2011 | ||
| Control: 2.15 ± 0.93b p < 0.0001 | |||
| CKD with periodontitis: 6.3380 ± 2.74875 | Pradeep et al., 2012 | ||
| CKD: 5.4100 ± 2.65296 | |||
| Healthy: 1.8350 ± 0.75977b p = 0.000 | |||
| Female reproductive system diseases | Preeclampsia (PE) | PE: 13.8 (3.9, 32.3) | Cetin et al., 2006 |
| Control: 2.2 (1.2, 3.8)d p < 0.001 | |||
| PE: 22.64 (18.56, 26.34) | Hamad et al., 2012 | ||
| Control: 13.17 (8.55, 16.54)d p < 0.001 | |||
| Pelvic inflammatory disease (PID) | PID: 9.3 ± 1.01 | Chang et al., 2011 | |
| Control: 2.27 ± 0.12b p < 0.001 | |||
| Polycystic ovary syndrome (PCOS) | PCOS: 1.0 ± 3.6 | Aydogdu et al., 2012 | |
| Control: 0.8 ± 0.8b p = 0.021 | |||
| Others | Severe Psoriasis (sP) | sP: 2.84 ± 0.94 | Bevelacqua et al., 2006 |
| Control: 1.22 ± 0.47b p < 0.0001 | |||
| Ulcerative colitis (UC) and crohn's disease (CD) | Active UC: 8.22 ± 5.48 | Kato et al., 2008 | |
| Active CD: 5.80 ± 3.59 | |||
| Control: 1.76 ± 1.02b p < 0.05 | |||
| Obesity | Obesity: 0.99 ± 0.09 | Miyaki et al., 2010 | |
| Control: 0.63 ± 0.05g p < 0.01 | |||
| Central obesity in abdominal obesity patients | Central obesity: 3.00 ± 2.61 | Shim et al., 2010 | |
| Control: 1.33 ± 0.81b p < 0.01 | |||
| Severe traumatic brain injury (TBI) | non-survivors 9.95 (6.42) | Gullo Jda et al., 2011 | |
| Survivors 5.46 (4.87)b μg/mL p < 0.001 | |||
| Obstructive sleep apnea (OSA) | Moderate-to severe OSA: 2.36 (1.79, 2.98) | Kasai et al., 2011 | |
| Control: 1.53 (1.14, 2.04)f p < 0.01 | |||
| Schizophrenia (SZ) | SZ with the metabolic syndrome: 388.2 (504.1) | Beumer et al., 2012 | |
| SZ: 430.4 (523.0) | |||
| Control: 213.6 (524.0)d pg/mL p < 0.001 |
PTX3 concentrations are shown in ng/mL, unless indicated.
Geometrical mean (confidence interval).
Mean ± SD.
Geometrical mean.
Median (interquartile range).
Mean (95% confidence interval).
Median (25th percentile, 75th percentile).
Mean ± SEM.
Median (Minimum-Maximum).
Although not included in Table 1, there are other infectious diseases, such as severe dengue virus infection (Mairuhu et al., 2005) and meningococcal disease (Sprong et al., 2009), in which the PTX3 levels are also increased. The PTX3 plasma concentration is increased in patients with acute myocardial infarction (Peri et al., 2000). During pregnancy, the serum PTX3 level slightly increases as the pregnancy progresses (Larsson et al., 2011). A higher PTX3 level is observed in preeclampsia (Cetin et al., 2006; Rovere-Querini et al., 2006). Finally, the serum PTX3 level is reported to be a biomarker for lung carcinoma (Diamandis et al., 2011). Thus, the circulating PTX3 level increases non-specifically in various infections and inflammatory disorders. For the purpose of diagnostic measurement, the dynamics of the PTX3 complex, such as the NET component proteins should be monitored (more details are discussed below).
Function
PTX3 has been postulated to play a variety of roles in innate immunity, inflammatory regulation, and female fertility (Bottazzi et al., 2006; Garlanda et al., 2009; Inforzato et al., 2011; Cieslik and Hrycek, 2012). PTX3-knockout and transgenic mice studies have indicated that the predominant role of PTX3 occurs in host protection in the case of lung injury, infection, vascular damage, as well as certain other disorders (summarized in Table 2). Briefly, the resistance against pathogens such as Aspergillus fumigatus, Paracoccidioides brasiliensis, and Klebsiella pneumoniae has been reported (Garlanda et al., 2002; Diniz et al., 2004; Soares et al., 2006). In addition to its anti-pathogenic activity, PTX3 also has been shown to play a role in protecting against severe inflammatory reactions in animal models of sepsis (Dias et al., 2001), seizure-induced neurodegeneration (Ravizza et al., 2001) and acute myocardial infarction (Salio et al., 2008). In addition, PTX3 participates in extracellular matrix deposition. PTX3 is involved in the organization of hyaluronan in the viscoelastic matrix of cumulus oophorus (Scarchilli et al., 2007). It is considered that these functions of PTX3 are exhibited synergistically along with the binding of specific ligands (the details are provided in section “Ligands”).
Table 2.
Responses to certain disorders in PTX3-knockout and PTX3-transgenic mice.
| Category | Experiment summary | Result summary | References |
|---|---|---|---|
| Lung injury | Murine hepatitis virus strain 1 (MHV-1) infection | Causing greater severity of acute lung injury (ALI)a | Han et al., 2012 |
| Ventilator-induced lung injury (VILI) | Faster development of VILIb | Real et al., 2012 | |
| LPS instillation | Causing greater severity of ALIa | Han et al., 2011 | |
| Vascular damage | Coronary artery ligation and reperfusion | Worsen heart damagea | Salio et al., 2008 |
| Atherogenic diet feed | Increased atherosclerotic lesion area in PTX3 and ApoE-double KO mice | Norata et al., 2009 | |
| Ischemia and reperfusion of the superior mesenteric artery | Prevent tissue injury and mortalitya | Souza et al., 2009 | |
| Increased tissue injury and mortalityb | Souza et al., 2002 | ||
| Infection | LPS-induced endotoxemia | Increased survival ratiob | Dias et al., 2001 |
| CLP-induced sepsis | Increased survival ratiob | Dias et al., 2001 | |
| Pulmonary infection by Aspergillus fumigatus | Decreased survival ratioa | Garlanda et al., 2002 | |
| Pulmonary infection by Klebsiella pneumoniae | Faster lethality by a high inoculum administrationb | Soares et al., 2006 | |
| Delayed lethality by a mid-to-low inoculum administrationb | Soares et al., 2006 | ||
| Murine cytomegalovirus (MCMV) infection | More susceptible to MCMV infectiona | Bozza et al., 2006 | |
| Influenza virus infection | More susceptible to influenza virus infectiona | Reading et al., 2008 | |
| Others | Fas-deficient (lpr) C57BL/6 (B6) mice with mild lupus-like autoimmunity | Aggravate autoimmune lung disease in PTX3-KO B6Lpr mice | Lech et al., 2011 |
| Kidney ischemia reperfusion injury | Less kidney injury and inflammationa | Chen et al., 2012 | |
| Subcutaneous injection of Matrigel containing FGF2 and/or TSG-6 | Abolishing of vascularization inhibition in PTX-KO mice | Leali et al., 2012 | |
| Rolling interaction of PMNs in the mesenteric venules | Increased rolling interaction frequencya | Deban et al., 2010 | |
| Sexual system | Subfertilea | Varani et al., 2002 | |
| Kainate-induced seizures | More widespread seizure-related neuronal damage in the forebrain of PTX3-KO mice | Ravizza et al., 2001 |
PTX3-knockout mouse study.
PTX3-transgenic mouse study.
Of note, among the studies in PTX3-knockout and transgenic mice, there are some reports of an opposite effect of PTX3 on host-protection. In an intestinal ischemia and reperfusion model, Souza et al. reported an increased injury and lethality in the PTX3-transgenic mice that seemed to be associated with elevation of the TNFα concentration and aggravation of the inflammatory response (Souza et al., 2002). They also reported the suppression of tissue injury and lethality after ischemic and reperfusion in PTX3-knockout mice. PTX3 administration to these PTX3-knockout mice reversed this suppression (Souza et al., 2009). Other groups have also reported an adverse effect of PTX3 in acute ischemic lung injury (Chen et al., 2012) and ventilator-induced lung injury (Real et al., 2012). In the case of Klebsiella pneumoniae infection, faster lethality was observed when a higher inoculum was administered to PTX3-transgenic mice, but the lethality was conversely delayed when a middle or low inoculum was administered (Soares et al., 2006). Taking these bi-phasic functions of PTX3 in host-defense into account, more detailed accounts of the disease-specific mechanisms of PTX3 need to be elucidated to achieve useful clinical applications.
Ligands
The multiple host-protective functions of PTX3 arise from the capacity for the recognition and binding to ligands. The reported PTX3 ligands are classified as follows: (1) complement components; (2) Fungi, bacteria, microbial components, and viruses; (3) selectin P; (4) extracellular matrix proteins and (5) growth factors (Presta et al., 2007; Mantovani et al., 2008; Deban et al., 2009; Moalli et al., 2011). Some of these ligands bind to PTX3 in a PTX3-domain specific manner, while others require full-length PTX3 for binding (Deban et al., 2009; Bottazzi et al., 2010).
PTX3 binds to certain select complement components, such as C1q (Inforzato et al., 2006), C4b-binding proteins (Braunschweig and Józsi, 2011), ficolins (Ma et al., 2009; Gout et al., 2011), mannose-binding lectin 2 (MBL) (Ma et al., 2011), factor H (Deban et al., 2008; Kopp et al., 2012), factor H-like protein 1 (Kopp et al., 2012) and factor H-related protein 1 (Kopp et al., 2012) for the regulation of the complement pathways in the innate immune response. The interaction of PTX3 and C1q elicits a dual consequence in the classical complement pathway. When C1q binds to immobilized PTX3, the classical complement pathway is activated; however, the binding of C1q to PTX3 in the fluid phase inhibits complement activation (Nauta et al., 2003). PTX3 can also activate the lectin pathway by binding to the ficolins and MBL. PTX3 enhances complement deposition by ficolin-2 on the Aspergillus fumigatus surface (Ma et al., 2009), and PTX3-MBL binding enhanced C4 and C3 deposition as well as the phagocytosis of Candida albicans (Ma et al., 2011). PTX3 is not only involved in complement activation, but also acts as a complement inhibitor to regulate excessive complement activation by binding to C4b-binding proteins and factor H. Please refer to the review by Doni et al. for more detail (Doni et al., 2012).
In the protection afforded against infection, PTX3 recognizes certain fungi, bacteria, microbial moieties, and viruses. PTX3 binds to microbial pathogens such as Pseudomonas aeruginosa (Garlanda et al., 2002), Salmonella typhimurium (Garlanda et al., 2002), Aspergillus fumigatus (Garlanda et al., 2002), and Paracoccidioides brasiliensis (Diniz et al., 2004). PTX3-knockout mice are susceptible to invasive pulmonary aspergillosis due to inappropriate Th1 and Th2-helper-cell-mediated resistance (Garlanda et al., 2002). Macrophages from PTX3-transgenic mice exhibit improved phagocytosis of Paracoccidioides brasiliensis as well as an enhancement of the production of nitric oxide (NO) (Diniz et al., 2004). PTX3 also binds to outer membrane protein A from Klebsiella pneumoniae (KpOmpA) in order to modulate the inflammatory response triggered by KpOmpA (Jeannin et al., 2005). PTX3 binds to cytomegalovirus and influenza virus type A for the inhibition of infection (Bozza et al., 2006; Reading et al., 2008). Upon binding to influenza virus, PTX3 exerts anti-viral activity by the inhibition of hemagglutination, the neutralization of virus infectivity and the inhibition of viral neuraminidase (Reading et al., 2008).
As an inflammatory modulator, PTX3 binds to selectin P. The N-linked glycosidic moiety of PTX3 contributes to the binding of selectin P, and this binding dampens neutrophil recruitment at the sites of inflammation (Deban et al., 2010). Importantly, in a model of acid-induced acute lung injury, both exogenous PTX3 and endogenously released PTX3 administration suppress neutrophil recruitment (Deban et al., 2010). This suggests a negative feedback role of PTX3 that dampens the excessive neutrophil recruitment via selectin P.
PTX3 takes part in extracellular matrix formation by binding to TNFα-induced protein 6 (TNFAIP6 or TSG-6) and inter-α-trypsin inhibitor (Iα I) (Salustri et al., 2004; Scarchilli et al., 2007; Ievoli et al., 2011). PTX3-knockout mice exhibit a defect in female fertility because of the defects in ovulation (Varani et al., 2002) and the organization of the cumulus oophorus extracellular matrix (Salustri et al., 2004). The PTX3-TSG-6 and PTX3-Iα I binding events are considered to be essential for the organization of hyaluronan in the viscoelastic matrix of cumulus oophorus (Inforzato et al., 2011; Moalli et al., 2011).
PTX3 binding to fibroblast growth factor 2 (FGF-2) regulates endothelial cell proliferation and angiogenesis, smooth muscle cell (SMC) activation, and intima thickening after arterial injury (Rusnati et al., 2004; Camozzi et al., 2005). PTX3-FGF2 binding can inhibit the proliferation and chemotactic activity of FGF2 in SMCs by interfering with the interaction of the FGF2 and FGF receptors (Camozzi et al., 2005).
Taking these results, it is clear that the protective effects of PTX3 are realized in coordination with specific PTX3 ligands. Therefore, we carried out a proteome-wide identification of PTX3 ligands and complexes in septic patient serum and plasma. PTX3 and its complex component proteins were immunoprecipitated by anti-PTX3 antibody-crosslinked magnetic beads, and the isolated fractions were subjected to shotgun proteomics analysis for label-free relative quantitation via spectral counting (Daigo et al., 2012). The identified proteins included the known PTX3 ligands such as C1q, ficolins, TSG-6, and Iα I, as mentioned above. Additionally, the ficolin-binding proteins of mannan-binding lectin serine protease 1 and 2 (MASP1 and MASP2) (Ma et al., 2009), and the TSG-6 binding proteins of the versican core protein (VCAN) and thrombospondin-1 (THBS1) (Salustri et al., 2004) were included in the proteins that were identified. As these proteins were identified in pooled normal human plasma with artificially spiked recombinant PTX3, these appear to be stable circulating PTX3 complexes. Nevertheless, the disease-specific dynamics of these binding levels need to be investigated further, as do the specific functions of these PTX3 complexes in sepsis.
NET component proteins as PTX3 ligands: a newly recognized protective role
In the effort to identify the PTX3 ligand in septic patient fluids, a novel finding is that the NET component proteins were included (Daigo et al., 2012) (Table 3). A detailed investigation revealed that azurocidin 1 (AZU1) and myeloperoxidase (MPO) directly bind to PTX3. AZU1 and MPO belong to the NET component proteins (Urban et al., 2009) and exert bactericidal activity (Watorek, 2003; Klebanoff, 2005). AZU1 preferably binds to the PTX3 N-terminal domain, with a pattern of calcium ion dependency. In contrast to AZU1, MPO binds to both the PTX3 N-terminal and C-terminal domains, and does not require calcium ions. Further investigation of the PTX3-AZU1 interaction revealed that the AZU1 binding affinity to PTX3 was 22 ± 7.6 nM, and that AZU1 and PTX3 are partially co-localized in NETs (Daigo et al., 2012).
Table 3.
List of the NET component proteins and proteins belonging to the PTX3 complex.
| NET component proteins | Proteomic identification of PTX3 complex in sepsis | ||
|---|---|---|---|
| Cellular localization | Protein name | Gene name | |
| Granules | Neutrophil elastase | ELANE | |
| Lactotransferrin | LTF | ||
| Azurocidin | AZU1 | Yes | |
| Cathepsin G | CTSG | ||
| Myeloperoxidase | MPO | Yes | |
| Proteinase 3 | PRTN3 | ||
| Lysozyme C | LYZ | ||
| Neutrophil defensin 1 and 3 | DEFA1 and 3 | Yes (DEFA1) | |
| Pentraxin 3 | PTX3 | Target protein | |
| Bactericidal permeability-increasing protein | BPI | ||
| Cathelicidin anti-microbial peptide | CAMP | ||
| Nucleus | Histone H1 | H1F0 | |
| Histone H2A | H2A | Yes | |
| Histone H2B | H2B | ||
| Histone H2B-like | H2B | ||
| Histone H3 | H3 | Yes | |
| Histone H4 | H4 | Yes | |
| Myeloid cell nuclear differentiation antigen | MNDA | ||
| Cytoplasm | S100 calcium-binding protein A8 | S100A8 | |
| S100 calcium binding protein A9 | S100A9 | ||
| S100 calcium-binding protein A12 | S100A12 | ||
| Cytoskeleton | Actin (beta and/or gamma 1) | ACTB, ACTG1 | Yes |
| Myosin-9 | MYH9 | Yes | |
| Alpha-actinin (1 and/or -4) | ACTN1, ACTN4 | ||
| Plastin-2 | LCP1 | ||
| Cytokeratin-10 | KRT10 | Yes | |
| Peroxisomal | Catalase | CAT | |
| Glycolytic enzymes | Alpha-enolase | ENO1 | Yes |
| Transketolase | TKT | Yes | |
From these results, it is suggested that PTX3 may enhance the bactericidal efficiency of AZU1 and MPO in terms of both pathogen recognition and AZU1 and MPO binding (Figure 1). The mechanism by which PTX3 localizes in NETs has not yet been determined, but it is possible that PTX3 localization arises from an interaction with histones or the basic proteins AZU1, MPO, and defensin, along with a simultaneous association between these basic proteins and DNA (Figure 1). It is not clear at present whether the PTX3-AZU1 and PTX3-MPO binding events in the bloodstream take place within or outside of NETs. Either or even both of these are possible, and these complexes may be active in pathogen recognition and also involved in clearance. In septic patients, the plasma levels of AZU1 are increased, but do not significantly correlate with mortality (Berkestedt et al., 2010). As useful biomarkers of sepsis not yet available (Pierrakos and Vincent, 2010), the binding levels of PTX3-AZU1 and PTX3-MPO in septic plasma have the important potential to fulfill this purpose.
Figure 1.
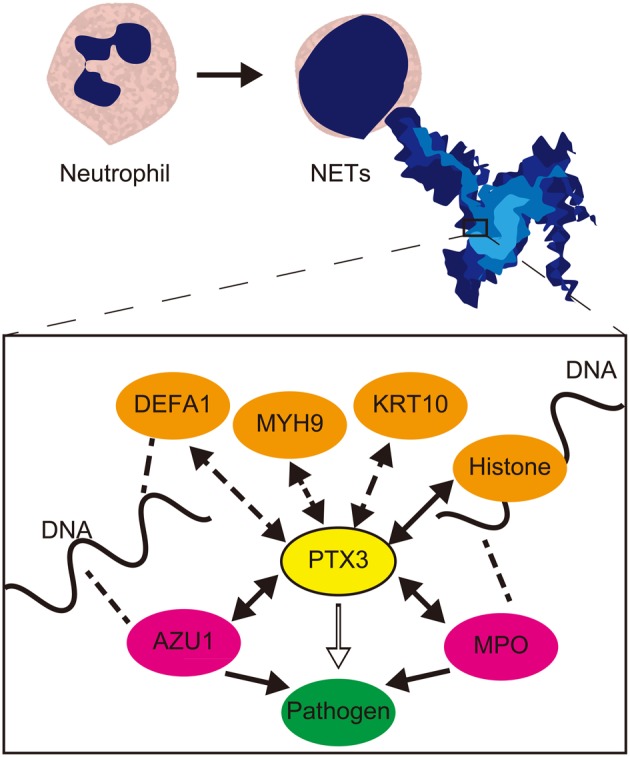
Schematic relationship and role of PTX3 and NET component proteins in pathogen recognition and clearance. NET component proteins which were identified as PTX3 complex (Daigo et al., 2012) are associated PTX3 in NETs. Among these, the confirmed direct interaction of AZU1 and MPO to PTX3, and formerly reported histone-PTX3 interaction (Garlanda et al., 2005) are designated by two-way arrows. These bindings facilitate pathogen clearance efficiency of AZU1 and MPO. The pathogen recognition and anti-pathogenic action are designated by open arrow and closed arrow in box, respectively. Two-way arrows with dashed lines designate other potential interactions to PTX3. The indirect association to DNA via histone or basic proteins such as DEFA1, AZU1, and MPO, which DNA associations are designated by dashed lines, maintains PTX3 localization in NETs. PTX3, pentraxin 3; DEFA1, neutrophil defensin 1; MYH9, Myosn-9; KRT10, Cytokeratin-10; AZU1, azurocidin 1; MPO: myeloperoxidase.
Conclusion
Recent proteomic investigation of the circulating PTX3 complex components has revealed new and pivotal roles of PTX3 in the innate immune response, along with a pattern of binding to the NET component proteins. In NETs, PTX3 brings the NET component proteins into close proximity with the pathogens that PTX3 capture in order to enhance pathogen clearance. Also, in the bloodstream, PTX3 forms a complex with bactericidal proteins for the recognition and clearance of pathogens. These activities of PTX3 in concert contribute to the host-protective effect. In addition, the dynamic changes that occur in PTX3 and its complex proteins may become specific biomarker for severe inflammatory diseases.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by Japan Grants-in-Aid for Scientific Research 20221010 from the Ministry of Education, Culture, Sports, Science and Technology, and collaborative research of the University of Tokyo and JSR Corporation. We thank Dr. Kevin Boru of Pacific Edit for review of the manuscript.
References
- Abderrahim-Ferkoune A., Bezy O., Chiellini C., Maffei M., Grimaldi P., Bonino F., et al. (2003). Characterization of the long pentraxin PTX3 as a TNFalpha-induced secreted protein of adipose cells. J. Lipid Res. 44, 994–1000 10.1194/jlr.M200382-JLR200 [DOI] [PubMed] [Google Scholar]
- Alles V. V., Bottazzi B., Peri G., Golay J., Introna M., Mantovani A. (1994). Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood 84, 3483–3493 [PubMed] [Google Scholar]
- Altmeyer A., Klampfer L., Goodman A. R., Vilcek J. (1995). Promoter structure and transcriptional activation of the murine TSG-14 gene encoding a tumor necrosis factor/interleukin-1-inducible pentraxin protein. J. Biol. Chem. 270, 25584–25590 10.1074/jbc.270.43.25584 [DOI] [PubMed] [Google Scholar]
- Amulic B., Hayes G. (2011). Neutrophil extracellular traps. Curr. Biol. 21, R297–R298 10.1016/j.cub.2011.03.021 [DOI] [PubMed] [Google Scholar]
- Argani H., Ghorbanihaghjo A., Panahi G., Rashtchizadeh N., Safa J., Meimand S. M. (2012). Serum Fetuin-A and Pentraxin3 in hemodialysis and renal transplant patients. Clin. Biochem. 45, 775–779 10.1016/j.clinbiochem.2012.04.011 [DOI] [PubMed] [Google Scholar]
- Aydogdu A., Tasci I., Tapan S., Basaran Y., Aydogan U., Meric C., et al. (2012). High plasma level of long Pentraxin 3 is associated with insulin resistance in women with polycystic ovary syndrome. Gynecol. Endocrinol. 28, 722–725 10.3109/09513590.2011.652719 [DOI] [PubMed] [Google Scholar]
- Azzurri A., Sow O. Y., Amedei A., Bah B., Diallo S., Peri G., et al. (2005). IFN-gamma-inducible protein 10 and pentraxin 3 plasma levels are tools for monitoring inflammation and disease activity in Mycobacterium tuberculosis infection. Microbes Infect. 7, 1–8 10.1016/j.micinf.2004.09.004 [DOI] [PubMed] [Google Scholar]
- Baldini M., Maugeri N., Ramirez G. A., Giacomassi C., Castiglioni A., Prieto-Gonzalez S., et al. (2012). Selective up-regulation of the soluble pattern-recognition receptor pentraxin 3 and of vascular endothelial growth factor in giant cell arteritis: relevance for recent optic nerve ischemia. Arthritis Rheum. 64, 854–865 10.1002/art.33411 [DOI] [PubMed] [Google Scholar]
- Basile A., Sica A., D'Aniello E., Breviario F., Garrido G., Castellano M., et al. (1997). Characterization of the promoter for the human long pentraxin PTX3. Role of NF-kappaB in tumor necrosis factor-alpha and interleukin-1beta regulation. J. Biol. Chem. 272, 8172–8178 10.1074/jbc.272.13.8172 [DOI] [PubMed] [Google Scholar]
- Berkestedt I., Herwald H., Ljunggren L., Nelson A., Bodelsson M. (2010). Elevated plasma levels of antimicrobial polypeptides in patients with severe sepsis. J. Innate Immun. 2, 478–482 10.1159/000317036 [DOI] [PubMed] [Google Scholar]
- Beumer W., Drexhage R. C., De Wit H., Versnel M. A., Drexhage H. A., Cohen D. (2012). Increased level of serum cytokines, chemokines and adipokines in patients with schizophrenia is associated with disease and metabolic syndrome. Psychoneuroendocrinology 37, 1901–1911 10.1016/j.psyneuen.2012.04.001 [DOI] [PubMed] [Google Scholar]
- Bevelacqua V., Libra M., Mazzarino M. C., Gangemi P., Nicotra G., Curatolo S., et al. (2006). Long pentraxin 3: a marker of inflammation in untreated psoriatic patients. Int. J. Mol. Med. 18, 415–423 [PubMed] [Google Scholar]
- Bottazzi B., Bastone A., Doni A., Garlanda C., Valentino S., Deban L., et al. (2006). The long pentraxin PTX3 as a link among innate immunity, inflammation, and female fertility. J. Leukoc. Biol. 79, 909–912 10.1189/jlb.1005557 [DOI] [PubMed] [Google Scholar]
- Bottazzi B., Doni A., Garlanda C., Mantovani A. (2010). An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu. Rev. Immunol. 28, 157–183 10.1146/annurev-immunol-030409-101305 [DOI] [PubMed] [Google Scholar]
- Bozza S., Bistoni F., Gaziano R., Pitzurra L., Zelante T., Bonifazi P., et al. (2006). Pentraxin 3 protects from MCMV infection and reactivation through TLR sensing pathways leading to IRF3 activation. Blood 108, 3387–3396 10.1182/blood-2006-03-009266 [DOI] [PubMed] [Google Scholar]
- Braunschweig A., Józsi M. (2011). Human pentraxin 3 binds to the complement regulator c4b-binding protein. PLoS ONE 6:e23991 10.1371/journal.pone.0023991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breviario F., D'Aniello E. M., Golay J., Peri G., Bottazzi B., Bairoch A., et al. (1992). Interleukin-1-inducible genes in endothelial cells. Cloning of a new gene related to C-reactive protein and serum amyloid P component. J. Biol. Chem. 267, 22190–22197 [PubMed] [Google Scholar]
- Brinkmann V. (2004). Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 10.1007/978-1-61779-527-5_7 [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Zychlinsky A. (2007). Beneficial suicide: why neutrophils die to make NETs. Nat. Rev. Microbiol. 5, 577–582 10.1038/nrmicro1710 [DOI] [PubMed] [Google Scholar]
- Camozzi M., Zacchigna S., Rusnati M., Coltrini D., Ramirez-Correa G., Bottazzi B., et al. (2005). Pentraxin 3 inhibits fibroblast growth factor 2-dependent activation of smooth muscle cells in vitro and neointima formation in vivo. Arterioscler. Thromb. Vasc. Biol. 25, 1837–1842 10.1161/01.ATV.0000177807.54959.7d [DOI] [PubMed] [Google Scholar]
- Cetin I., Cozzi V., Pasqualini F., Nebuloni M., Garlanda C., Vago L., et al. (2006). Elevated maternal levels of the long pentraxin 3 (PTX3) in preeclampsia and intrauterine growth restriction. Am. J. Obstet. Gynecol. 194, 1347–1353 10.1016/j.ajog.2005.11.018 [DOI] [PubMed] [Google Scholar]
- Chang C. C., Wang P. H., Su P. H., Lin D. B., Ying T. H., Yang S. F., et al. (2011). Significant elevation of plasma pentraxin 3 in patients with pelvic inflammatory disease. Clin. Chem. Lab. Med. 49, 1655–1660 10.1515/CCLM.2011.650 [DOI] [PubMed] [Google Scholar]
- Chen J., Matzuk M. M., Zhou X. J., Lu C. Y. (2012). Endothelial pentraxin 3 contributes to murine ischemic acute kidney injury. Kidney Int. 82, 1195–1207 10.1038/ki.2012.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik P., Hrycek A. (2012). Long pentraxin 3 (PTX3) in the light of its structure, mechanism of action and clinical implications. Autoimmunity 45, 119–128 10.3109/08916934.2011.611549 [DOI] [PubMed] [Google Scholar]
- Clark S. R., Ma A. C., Tavener S. A., McDonald B., Goodarzi Z., Kelly M. M., et al. (2007). Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 13, 463–469 10.1038/nm1565 [DOI] [PubMed] [Google Scholar]
- Daigo K., Yamaguchi N., Kawamura T., Matsubara K., Jiang S., Ohashi R., et al. (2012). The proteomic profile of circulating pentraxin 3 (PTX3) complex in sepsis demonstrates the interaction with azurocidin 1 and other components of neutrophil extracellular traps. Mol. Cell Proteomics 11, M111 015073. 10.1074/mcp.M111.015073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deban L., Bottazzi B., Garlanda C., De La Torre Y. M., Mantovani A. (2009). Pentraxins: multifunctional proteins at the interface of innate immunity and inflammation. Biofactors 35, 138–145 10.1002/biof.21 [DOI] [PubMed] [Google Scholar]
- Deban L., Jaillon S., Garlanda C., Bottazzi B., Mantovani A. (2011). Pentraxins in innate immunity: lessons from PTX3. Cell Tissue Res. 343, 237–249 10.1007/s00441-010-1018-0 [DOI] [PubMed] [Google Scholar]
- Deban L., Jarva H., Lehtinen M. J., Bottazzi B., Bastone A., Doni A., et al. (2008). Binding of the long pentraxin PTX3 to factor H: interacting domains and function in the regulation of complement activation. J. Immunol. 181, 8433–8440 [DOI] [PubMed] [Google Scholar]
- Deban L., Russo R. C., Sironi M., Moalli F., Scanziani M., Zambelli V., et al. (2010). Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat. Immunol. 11, 328–334 10.1038/ni.1854 [DOI] [PubMed] [Google Scholar]
- De Kruif M. D., Limper M., Sierhuis K., Wagenaar J. F., Spek C. A., Garlanda C., et al. (2010). PTX3 predicts severe disease in febrile patients at the emergency department. J. Infect. 60, 122–127 10.1016/j.jinf.2009.11.010 [DOI] [PubMed] [Google Scholar]
- Diamandis E. P., Goodglick L., Planque C., Thornquist M. D. (2011). Pentraxin-3 is a novel biomarker of lung carcinoma. Clin. Cancer Res. 17, 2395–2399 10.1158/1078-0432.CCR-10-3024 [DOI] [PubMed] [Google Scholar]
- Dias A. A., Goodman A. R., Dos Santos J. L., Gomes R. N., Altmeyer A., Bozza P. T., et al. (2001). TSG-14 transgenic mice have improved survival to endotoxemia and to CLP-induced sepsis. J. Leukoc. Biol. 69, 928–936 [PubMed] [Google Scholar]
- Diniz S. N., Nomizo R., Cisalpino P. S., Teixeira M. M., Brown G. D., Mantovani A., et al. (2004). PTX3 function as an opsonin for the dectin-1-dependent internalization of zymosan by macrophages. J. Leukoc. Biol. 75, 649–656 10.1189/jlb.0803371 [DOI] [PubMed] [Google Scholar]
- Doni A., Garlanda C., Bottazzi B., Meri S., Garred P., Mantovani A. (2012). Interactions of the humoral pattern recognition molecule PTX3 with the complement system. Immunobiology 217, 1122–1128 10.1016/j.imbio.2012.07.004 [DOI] [PubMed] [Google Scholar]
- Doni A., Peri G., Chieppa M., Allavena P., Pasqualini F., Vago L., et al. (2003). Production of the soluble pattern recognition receptor PTX3 by myeloid, but not plasmacytoid, dendritic cells. Eur. J. Immunol. 33, 2886–2893 10.1002/eji.200324390 [DOI] [PubMed] [Google Scholar]
- Fuchs T. A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., et al. (2007). Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176, 231–241 10.1083/jcb.200606027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C., Bottazzi B., Bastone A., Mantovani A. (2005). Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu. Rev. Immunol. 23, 337–366 10.1146/annurev.immunol.23.021704.115756 [DOI] [PubMed] [Google Scholar]
- Garlanda C., Hirsch E., Bozza S., Salustri A., De Acetis M., Nota R., et al. (2002). Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature 420, 182–186 10.1038/nature01195 [DOI] [PubMed] [Google Scholar]
- Garlanda C., Maina V., Cotena A., Moalli F. (2009). The soluble pattern recognition receptor pentraxin-3 in innate immunity, inflammation and fertility. J. Reprod. Immunol. 83, 128–133 10.1016/j.jri.2009.05.006 [DOI] [PubMed] [Google Scholar]
- Goodman A. R., Levy D. E., Reis L. F., Vilcek J. (2000). Differential regulation of TSG-14 expression in murine fibroblasts and peritoneal macrophages. J. Leukoc. Biol. 67, 387–395 [DOI] [PubMed] [Google Scholar]
- Gout E., Moriscot C., Doni A., Dumestre-Perard C., Lacroix M., Perard J., et al. (2011). M-ficolin interacts with the long pentraxin PTX3: a novel case of cross-talk between soluble pattern-recognition molecules. J. Immunol. 186, 5815–5822 10.4049/jimmunol.1100180 [DOI] [PubMed] [Google Scholar]
- Gullo Jda S., Bertotti M. M., Silva C. C., Schwarzbold M., Diaz A. P., Soares F. M., et al. (2011). Hospital mortality of patients with severe traumatic brain injury is associated with serum PTX3 levels. Neurocrit. Care 14, 194–199 10.1007/s12028-010-9462-y [DOI] [PubMed] [Google Scholar]
- Hamad R. R., Eriksson M. J., Berg E., Larsson A., Bremme K. (2012). Impaired endothelial function and elevated levels of pentraxin 3 in early-onset preeclampsia. Acta Obstet. Gynecol. Scand. 91, 50–56 10.1111/j.1600-0412.2011.01238.x [DOI] [PubMed] [Google Scholar]
- Han B., Haitsma J. J., Zhang Y., Bai X., Rubacha M., Keshavjee S., et al. (2011). Long pentraxin PTX3 deficiency worsens LPS-induced acute lung injury. Intensive Care Med. 37, 334–342 10.1007/s00134-010-2067-2 [DOI] [PubMed] [Google Scholar]
- Han B., Ma X., Zhang J., Zhang Y., Bai X., Hwang D. M., et al. (2012). Protective effects of long pentraxin PTX3 on lung injury in a severe acute respiratory syndrome model in mice. Lab. Invest. 92, 1285–1296 10.1038/labinvest.2012.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Han B., Liu M. (2007). Long pentraxin 3 in pulmonary infection and acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 292, L1039–L1049 10.1152/ajplung.00490.2006 [DOI] [PubMed] [Google Scholar]
- Hill A. L., Lowes D. A., Webster N. R., Sheth C. C., Gow N. A., Galley H. F. (2009). Regulation of pentraxin-3 by antioxidants. Br. J. Anaesth. 103, 833–839 10.1093/bja/aep298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollan I., Bottazzi B., Cuccovillo I., Forre O. T., Mikkelsen K., Saatvedt K., et al. (2010). Increased levels of serum pentraxin 3, a novel cardiovascular biomarker, in patients with inflammatory rheumatic disease. Arthritis Care Res. (Hoboken) 62, 378–385 10.1002/acr.20094 [DOI] [PubMed] [Google Scholar]
- Huttunen R., Hurme M., Aittoniemi J., Huhtala H., Vuento R., Laine J., et al. (2011). High plasma level of long pentraxin 3 (PTX3) is associated with fatal disease in bacteremic patients: a prospective cohort study. PLoS ONE 6:e17653. 10.1371/journal.pone.0017653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ievoli E., Lindstedt R., Inforzato A., Camaioni A., Palone F., Day A. J., et al. (2011). Implication of the oligomeric state of the N-terminal PTX3 domain in cumulus matrix assembly. Matrix Biol. 30, 330–337 10.1016/j.matbio.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inforzato A., Baldock C., Jowitt T. A., Holmes D. F., Lindstedt R., Marcellini M., et al. (2010). The angiogenic inhibitor long pentraxin PTX3 forms an asymmetric octamer with two binding sites for FGF2. J. Biol. Chem. 285, 17681–17692 10.1074/jbc.M109.085639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inforzato A., Jaillon S., Moalli F., Barbati E., Bonavita E., Bottazzi B., et al. (2011). The long pentraxin PTX3 at the crossroads between innate immunity and tissue remodelling. Tissue Antigens 77, 271–282 10.1111/j.1399-0039.2011.01645.x [DOI] [PubMed] [Google Scholar]
- Inforzato A., Peri G., Doni A., Garlanda C., Mantovani A., Bastone A., et al. (2006). Structure and function of the long pentraxin PTX3 glycosidic moiety: fine-tuning of the interaction with C1q and complement activation. Biochemistry 45, 11540–11551 10.1021/bi0607453 [DOI] [PubMed] [Google Scholar]
- Inforzato A., Rivieccio V., Morreale A. P., Bastone A., Salustri A., Scarchilli L., et al. (2008). Structural characterization of PTX3 disulfide bond network and its multimeric status in cumulus matrix organization. J. Biol. Chem. 283, 10147–10161 10.1074/jbc.M708535200 [DOI] [PubMed] [Google Scholar]
- Inoue K., Sugiyama A., Reid P. C., Ito Y., Miyauchi K., Mukai S., et al. (2007). Establishment of a high sensitivity plasma assay for human pentraxin3 as a marker for unstable angina pectoris. Arterioscler. Thromb. Vasc. Biol. 27, 161–167 10.1161/01.ATV.0000252126.48375.d5 [DOI] [PubMed] [Google Scholar]
- Ishino M., Takeishi Y., Niizeki T., Watanabe T., Nitobe J., Miyamoto T., et al. (2008). Risk stratification of chronic heart failure patients by multiple biomarkers: implications of BNP, H-FABP, and PTX3. Circ. J. 72, 1800–1805 [DOI] [PubMed] [Google Scholar]
- Jaillon S., Peri G., Delneste Y., Fremaux I., Doni A., Moalli F., et al. (2007). The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J. Exp. Med. 204, 793–804 10.1084/jem.20061301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannin P., Bottazzi B., Sironi M., Doni A., Rusnati M., Presta M., et al. (2005). Complexity and complementarity of outer membrane protein A recognition by cellular and humoral innate immunity receptors. Immunity 22, 551–560 10.1016/j.immuni.2005.03.008 [DOI] [PubMed] [Google Scholar]
- Kasai T., Inoue K., Kumagai T., Kato M., Kawana F., Sagara M., et al. (2011). Plasma pentraxin3 and arterial stiffness in men with obstructive sleep apnea. Am. J. Hypertens. 24, 401–407 10.1038/ajh.2010.248 [DOI] [PubMed] [Google Scholar]
- Kato S., Ochiai M., Sakurada T., Ohno S., Miyamoto K., Sagara M., et al. (2008). Increased expression of long pentraxin PTX3 in inflammatory bowel diseases. Dig. Dis. Sci. 53, 1910–1916 10.1007/s10620-007-0075-z [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. (2005). Myeloperoxidase: friend and foe. J. Leukoc. Biol. 77, 598–625 10.1189/jlb.1204697 [DOI] [PubMed] [Google Scholar]
- Klouche M., Peri G., Knabbe C., Eckstein H. H., Schmid F. X., Schmitz G., et al. (2004). Modified atherogenic lipoproteins induce expression of pentraxin-3 by human vascular smooth muscle cells. Atherosclerosis 175, 221–228 10.1016/j.atherosclerosis.2004.03.020 [DOI] [PubMed] [Google Scholar]
- Kopp A., Strobel S., Tortajada A., Rodriguez De Cordoba S., Sanchez-Corral P., Prohaszka Z., et al. (2012). Atypical hemolytic uremic syndrome-associated variants and autoantibodies impair binding of factor h and factor h-related protein 1 to pentraxin 3. J. Immunol. 189, 1858–1867 10.4049/jimmunol.1200357 [DOI] [PubMed] [Google Scholar]
- Kotooka N., Inoue T., Aoki S., Anan M., Komoda H., Node K. (2008). Prognostic value of pentraxin 3 in patients with chronic heart failure. Int. J. Cardiol. 130, 19–22 10.1016/j.ijcard.2007.07.168 [DOI] [PubMed] [Google Scholar]
- Kume N., Mitsuoka H., Hayashida K., Tanaka M. (2011). Pentraxin 3 as a biomarker for acute coronary syndrome: comparison with biomarkers for cardiac damage. J. Cardiol. 58, 38–45 10.1016/j.jjcc.2011.03.006 [DOI] [PubMed] [Google Scholar]
- Larsson A., Palm M., Helmersson J., Axelsson O. (2011). Pentraxin 3 values during normal pregnancy. Inflammation 34, 448–451 10.1007/s10753-010-9252-x [DOI] [PubMed] [Google Scholar]
- Lauth X., Von Kockritz-Blickwede M., McNamara C. W., Myskowski S., Zinkernagel A. S., Beall B., et al. (2009). M1 protein allows group A streptococcal survival in phagocyte extracellular traps through cathelicidin inhibition. J. Innate Immun. 1, 202–214 10.1159/000203645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leali D., Inforzato A., Ronca R., Bianchi R., Belleri M., Coltrini D., et al. (2012). Long pentraxin 3/tumor necrosis factor-stimulated gene-6 interaction: a biological rheostat for fibroblast growth factor 2-mediated angiogenesis. Arterioscler. Thromb. Vasc. Biol. 32, 696–703 10.1161/ATVBAHA.111.243998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lech M., Rommele C., Kulkarni O. P., Susanti H. E., Migliorini A., Garlanda C., et al. (2011). Lack of the long pentraxin PTX3 promotes autoimmune lung disease but not glomerulonephritis in murine systemic lupus erythematosus. PLoS ONE 6:e20118 10.1371/journal.pone.0020118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. W., Lee T. H., Vilcek J. (1993). TSG-14, a tumor necrosis factor- and IL-1-inducible protein, is a novel member of the pentaxin family of acute phase proteins. J. Immunol. 150, 1804–1812 [PubMed] [Google Scholar]
- Luchetti M. M., Piccinini G., Mantovani A., Peri G., Matteucci C., Pomponio G., et al. (2000). Expression and production of the long pentraxin PTX3 in rheumatoid arthritis (RA). Clin. Exp. Immunol. 119, 196–202 10.1046/j.1365-2249.2000.01110.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mairuhu A. T., Peri G., Setiati T. E., Hack C. E., Koraka P., Soemantri A., et al. (2005). Elevated plasma levels of the long pentraxin, pentraxin 3, in severe dengue virus infections. J. Med. Virol. 76, 547–552 10.1002/jmv.20397 [DOI] [PubMed] [Google Scholar]
- Malaponte G., Libra M., Bevelacqua Y., Merito P., Fatuzzo P., Rapisarda F., et al. (2007). Inflammatory status in patients with chronic renal failure: the role of PTX3 and pro-inflammatory cytokines. Int. J. Mol. Med. 20, 471–481 [PubMed] [Google Scholar]
- Mantovani A., Garlanda C., Doni A., Bottazzi B. (2008). Pentraxins in innate immunity: from C-reactive protein to the long pentraxin PTX3. J. Clin. Immunol. 28, 1–13 10.1007/s10875-007-9126-7 [DOI] [PubMed] [Google Scholar]
- Matsubara J., Sugiyama S., Nozaki T., Sugamura K., Konishi M., Ohba K., et al. (2011). Pentraxin 3 is a new inflammatory marker correlated with left ventricular diastolic dysfunction and heart failure with normal ejection fraction. J. Am. Coll. Cardiol. 57, 861–869 10.1016/j.jacc.2010.10.018 [DOI] [PubMed] [Google Scholar]
- Mauri T., Bellani G., Patroniti N., Coppadoro A., Peri G., Cuccovillo I., et al. (2010). Persisting high levels of plasma pentraxin 3 over the first days after severe sepsis and septic shock onset are associated with mortality. Intensive Care Med. 36, 621–629 10.1007/s00134-010-1752-5 [DOI] [PubMed] [Google Scholar]
- Ma Y. J., Doni A., Hummelshoj T., Honore C., Bastone A., Mantovani A., et al. (2009). Synergy between ficolin-2 and pentraxin 3 boosts innate immune recognition and complement deposition. J. Biol. Chem. 284, 28263–28275 10.1074/jbc.M109.009225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y. J., Doni A., Skjoedt M. O., Honore C., Arendrup M., Mantovani A., et al. (2011). Heterocomplexes of mannose-binding lectin and the pentraxins PTX3 or serum amyloid P component trigger cross-activation of the complement system. J. Biol. Chem. 286, 3405–3417 10.1074/jbc.M110.190637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina E. (2009). Neutrophil extracellular traps: a strategic tactic to defeat pathogens with potential consequences for the host. J. Innate Immun. 1, 176–180 10.1159/000203699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaki A., Maeda S., Yoshizawa M., Misono M., Sasai H., Shimojo N., et al. (2010). Is pentraxin 3 involved in obesity-induced decrease in arterial distensibility? J. Atheroscler. Thromb. 17, 278–284 [DOI] [PubMed] [Google Scholar]
- Moalli F., Jaillon S., Inforzato A., Sironi M., Bottazzi B., Mantovani A., et al. (2011). Pathogen recognition by the long pentraxin PTX3. J. Biomed. Biotechnol. 2011, 830421 10.1155/2011/830421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller B., Peri G., Doni A., Torri V., Landmann R., Bottazzi B., et al. (2001). Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit. Care Med. 29, 1404–1407 [DOI] [PubMed] [Google Scholar]
- Naito Y., Tsujino T., Akahori H., Ohyanagi M., Mitsuno M., Miyamoto Y., et al. (2010). Increase in tissue and circulating pentraxin3 levels in patients with aortic valve stenosis. Am. Heart J. 160, 685–691 10.1016/j.ahj.2010.06.031 [DOI] [PubMed] [Google Scholar]
- Nauta A. J., Bottazzi B., Mantovani A., Salvatori G., Kishore U., Schwaeble W. J., et al. (2003). Biochemical and functional characterization of the interaction between pentraxin 3 and C1q. Eur. J. Immunol. 33, 465–473 10.1002/immu.200310022 [DOI] [PubMed] [Google Scholar]
- Nauta A. J., De Haij S., Bottazzi B., Mantovani A., Borrias M. C., Aten J., et al. (2005). Human renal epithelial cells produce the long pentraxin PTX3. Kidney Int. 67, 543–553 10.1111/j.1523-1755.2005.67111.x [DOI] [PubMed] [Google Scholar]
- Nishi K., Imamura T., Kitamura K., Ogawa T., Fujimoto S., Kakitsubata Y., et al. (2011). Associations of plasma pentraxin 3 and monocyte chemoattractant protein-1 concentrations with cardiovascular disease in patients with chronic kidney disease. Ren. Fail. 33, 398–404 10.3109/0886022X.2011.568136 [DOI] [PubMed] [Google Scholar]
- Norata G. D., Marchesi P., Pulakazhi Venu V. K., Pasqualini F., Anselmo A., Moalli F., et al. (2009). Deficiency of the long pentraxin PTX3 promotes vascular inflammation and atherosclerosis. Circulation 120, 699–708 10.1161/CIRCULATIONAHA.108.806547 [DOI] [PubMed] [Google Scholar]
- Ortega-Hernandez O. D., Bassi N., Shoenfeld Y., Anaya J. M. (2009). The long pentraxin 3 and its role in autoimmunity. Sem. Arthritis Rheum. 39, 38–54 10.1016/j.semarthrit.2008.03.006 [DOI] [PubMed] [Google Scholar]
- Parlak A., Iyisoy A., Aydogan U., Cakir E., Saglam K. (2012). The effect of valsartan and nebivolol treatment on ADMA and pentraxin-3 levels in hypertensive patients. Med. Hypotheses 79, 294–298 10.1016/j.mehy.2012.05.011 [DOI] [PubMed] [Google Scholar]
- Peri G., Introna M., Corradi D., Iacuitti G., Signorini S., Avanzini F., et al. (2000). PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation 102, 636–641 10.1161/01.CIR.102.6.636 [DOI] [PubMed] [Google Scholar]
- Pierrakos C., Vincent J. L. (2010). Sepsis biomarkers: a review. Crit. Care 14, R15 10.1186/cc8872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polentarutti N., Bottazzi B., Di Santo E., Blasi E., Agnello D., Ghezzi P., et al. (2000). Inducible expression of the long pentraxin PTX3 in the central nervous system. J. Neuroimmunol. 106, 87–94 [DOI] [PubMed] [Google Scholar]
- Pradeep A. R., Kathariya R., Arjun Raju P., Sushma Rani R., Sharma A., Raghavendra N. M. (2012). Risk factors for chronic kidney diseases may include periodontal diseases, as estimated by the correlations of plasma pentraxin-3 levels: a case-control study. Int. Urol. Nephrol. 44, 829–839 10.1007/s11255-011-9997-7 [DOI] [PubMed] [Google Scholar]
- Presta M., Camozzi M., Salvatori G., Rusnati M. (2007). Role of the soluble pattern recognition receptor PTX3 in vascular biology. J. Cell. Mol. Med. 11, 723–738 10.1111/j.1582-4934.2007.00061.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza T., Moneta D., Bottazzi B., Peri G., Garlanda C., Hirsch E., et al. (2001). Dynamic induction of the long pentraxin PTX3 in the CNS after limbic seizures: evidence for a protective role in seizure-induced neurodegeneration. Neuroscience 105, 43–53 10.1016/S0306-4522(01)00177-4 [DOI] [PubMed] [Google Scholar]
- Reading P. C., Bozza S., Gilbertson B., Tate M., Moretti S., Job E. R., et al. (2008). Antiviral activity of the long chain pentraxin PTX3 against influenza viruses. J. Immunol. 180, 3391–3398 [DOI] [PubMed] [Google Scholar]
- Real J. M., Spilborghs G. M., Morato-Marques M., De Moura R. P., Negri E. M., Camargo A. A., et al. (2012). Pentraxin 3 accelerates lung injury in high tidal volume ventilation in mice. Mol. Immunol. 51, 82–90 10.1016/j.molimm.2012.02.113 [DOI] [PubMed] [Google Scholar]
- Rovere-Querini P., Antonacci S., Dell'Antonio G., Angeli A., Almirante G., Cin E. D., et al. (2006). Plasma and tissue expression of the long pentraxin 3 during normal pregnancy and preeclampsia. Obstet. Gynecol. 108, 148–155 10.1097/01.AOG.0000224607.46622.bc [DOI] [PubMed] [Google Scholar]
- Rusnati M., Camozzi M., Moroni E., Bottazzi B., Peri G., Indraccolo S., et al. (2004). Selective recognition of fibroblast growth factor-2 by the long pentraxin PTX3 inhibits angiogenesis. Blood 104, 92–99 10.1182/blood-2003-10-3433 [DOI] [PubMed] [Google Scholar]
- Ryu W. S., Kim C. K., Kim B. J., Kim C., Lee S. H., Yoon B. W. (2012). Pentraxin 3: a novel and independent prognostic marker in ischemic stroke. Atherosclerosis 220, 581–586 10.1016/j.atherosclerosis.2011.11.036 [DOI] [PubMed] [Google Scholar]
- Salio M., Chimenti S., De Angelis N., Molla F., Maina V., Nebuloni M., et al. (2008). Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation 117, 1055–1064 10.1161/CIRCULATIONAHA.107.749234 [DOI] [PubMed] [Google Scholar]
- Salustri A., Garlanda C., Hirsch E., De Acetis M., Maccagno A., Bottazzi B., et al. (2004). PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development 131, 1577–1586 10.1242/dev.01056 [DOI] [PubMed] [Google Scholar]
- Savchenko A. S., Inoue A., Ohashi R., Jiang S., Hasegawa G., Tanaka T., et al. (2011). Long pentraxin 3 (PTX3) expression and release by neutrophils in vitro and in ulcerative colitis. Pathol. Int. 61, 290–297 10.1111/j.1440-1827.2011.02651.x [DOI] [PubMed] [Google Scholar]
- Scarchilli L., Camaioni A., Bottazzi B., Negri V., Doni A., Deban L., et al. (2007). PTX3 interacts with inter-alpha-trypsin inhibitor: implications for hyaluronan organization and cumulus oophorus expansion. J. Biol. Chem. 282, 30161–30170 10.1074/jbc.M703738200 [DOI] [PubMed] [Google Scholar]
- Schorn C., Janko C., Latzko M., Chaurio R., Schett G., Herrmann M. (2012). Monosodium urate crystals induce extracellular DNA traps in neutrophils, eosinophils, and basophils but not in mononuclear cells. Front. Immunol. 3:277 10.3389/fimmu.2012.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim B. J., Jeon H. K., Lee S. J., Kim S. S., Park M. Y., Lee D. H., et al. (2010). The relationship between serum pentraxin 3 and central obesity in ST-segment elevation myocardial infarction patients. Korean Circ. J. 40, 308–313 10.4070/kcj.2010.40.7.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares A. C., Souza D. G., Pinho V., Vieira A. T., Nicoli J. R., Cunha F. Q., et al. (2006). Dual function of the long pentraxin PTX3 in resistance against pulmonary infection with Klebsiella pneumoniae in transgenic mice. Microbes Infect. 8, 1321–1329 10.1016/j.micinf.2005.12.017 [DOI] [PubMed] [Google Scholar]
- Souza D. G., Amaral F. A., Fagundes C. T., Coelho F. M., Arantes R. M., Sousa L. P., et al. (2009). The long pentraxin PTX3 is crucial for tissue inflammation after intestinal ischemia and reperfusion in mice. Am. J. Pathol. 174, 1309–1318 10.2353/ajpath.2009.080240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza D. G., Soares A. C., Pinho V., Torloni H., Reis L. F., Teixeira M. M., et al. (2002). Increased mortality and inflammation in tumor necrosis factor-stimulated gene-14 transgenic mice after ischemia and reperfusion injury. Am. J. Pathol. 160, 1755–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprong T., Peri G., Neeleman C., Mantovani A., Signorini S., Van Der Meer J. W., et al. (2009). Pentraxin 3 and C-reactive protein in severe meningococcal disease. Shock 31, 28–32 10.1097/SHK.0b013e31817fd543 [DOI] [PubMed] [Google Scholar]
- Steinberg B. E., Grinstein S. (2007). Unconventional roles of the NADPH oxidase: signaling, ion homeostasis, and cell death. Sci. STKE 2007, pe11. 10.1126/stke.3792007pe11 [DOI] [PubMed] [Google Scholar]
- Suliman M. E., Yilmaz M. I., Carrero J. J., Qureshi A. R., Saglam M., Ipcioglu O. M., et al. (2008). Novel links between the long pentraxin 3, endothelial dysfunction, and albuminuria in early and advanced chronic kidney disease. Clin. J. Am. Soc. Nephrol. 3, 976–985 10.2215/CJN.03960907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Takeishi Y., Niizeki T., Koyama Y., Kitahara T., Sasaki T., et al. (2008). Pentraxin 3, a new marker for vascular inflammation, predicts adverse clinical outcomes in patients with heart failure. Am. Heart J. 155, 75–81 10.1016/j.ahj.2007.08.013 [DOI] [PubMed] [Google Scholar]
- Tong M., Carrero J. J., Qureshi A. R., Anderstam B., Heimburger O., Barany P., et al. (2007). Plasma pentraxin 3 in patients with chronic kidney disease: associations with renal function, protein-energy wasting, cardiovascular disease, and mortality. Clin. J. Am. Soc. Nephrol. 2, 889–897 10.2215/CJN.00870207 [DOI] [PubMed] [Google Scholar]
- Urban C. F., Ermert D., Schmid M., Abu-Abed U., Goosmann C., Nacken W., et al. (2009). Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 5:e1000639 10.1371/journal.ppat.1000639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustundag M., Orak M., Guloglu C., Sayhan M. B., Alyan O., Kale E. (2011). Comparative diagnostic accuracy of serum levels of neutrophil activating peptide-2 and pentraxin-3 versus troponin-I in acute coronary syndrome. Anadolu Kardiyol. Derg. 11, 588–594 10.5152/akd.2011.160 [DOI] [PubMed] [Google Scholar]
- Varani S., Elvin J. A., Yan C., Demayo J., Demayo F. J., Horton H. F., et al. (2002). Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol. Endocrinol. 16, 1154–1167 [DOI] [PubMed] [Google Scholar]
- Watorek W. (2003). Azurocidin – inactive serine proteinase homolog acting as a multifunctional inflammatory mediator. Acta Biochim. Pol. 50, 743–752 [PubMed] [Google Scholar]
- Xu Y., Ding X., Zou J., Liu Z., Jiang S., Xu S., et al. (2011). Plasma pentraxin 3 is associated with cardiovascular disease in hemodialysis patients. Ren. Fail. 33, 998–1004 10.3109/0886022X.2011.618969 [DOI] [PubMed] [Google Scholar]
- Yamasaki K., Kurimura M., Kasai T., Sagara M., Kodama T., Inoue K. (2009). Determination of physiological plasma pentraxin 3 (PTX3) levels in healthy populations. Clin. Chem. Lab. Med. 47, 471–477 10.1515/CCLM.2009.110 [DOI] [PubMed] [Google Scholar]
- Yilmaz M. I., Carrero J. J., Martin-Ventura J. L., Sonmez A., Saglam M., Celik T., et al. (2010). Combined therapy with renin-angiotensin system and calcium channel blockers in type 2 diabetic hypertensive patients with proteinuria: effects on soluble TWEAK, PTX3, and flow-mediated dilation. Clin. J. Am. Soc. Nephrol. 5, 1174–1181 10.2215/CJN.01110210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yipp B. G., Petri B., Salina D., Jenne C. N., Scott B. N., Zbytnuik L. D., et al. (2012). Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 18, 1386–1393 10.1038/nm.2847 [DOI] [PMC free article] [PubMed] [Google Scholar]


