Abstract
Purpose
Cancer stem cells have recently been thought to be closely related to tumor development and reoccurrence. It may be a promising way to cure malignant glioma by using glioma stem cell-targeted dendritic cells as a tumor vaccine. In this study, we explored whether pulsing dendritic cells with antigens of glioma stem cells was a potent way to induce specific cytotoxic T lymphocytes and anti-tumor immunity.
Materials and Methods
Cancer stem cells were cultured from glioma cell line U251. Lysate of glioma stem cells was obtained by the repeated freezing and thawing method. Dendritic cells (DCs) were induced and cultured from the murine bone marrow cells, the biological characteristics were detected by electron microscope and flow cytometry. The DC vaccine was obtained by mixing DCs with lysate of glioma stem cells. The DC vaccine was charactirizated through the mixed lymphocyte responses and cell killing experiment in vitro. Level of interferon-γ (IFN-γ) in the supernatant was checked by ELISA.
Results
After stimulation of lysate of glioma stem cell, expression of surface molecules of DC was up-regulated, including CD80, CD86, CD11C and MHC-II. DCs pulsed with lysate of glioma stem cells were more effective than the control group in stimulating original glioma cells-specific cytotoxic T lymphocytes responses, killing glioma cells and boosting the secretion of IFN-γ in vitro.
Conclusion
The results demonstrated DCs loaded with antigens derived from glioma stem cells can effectively stimulate naive T cells to form specific cytotoxic T cells, kill glioma cells cultured in vitro.
Keywords: Glioma, cancer stem cell, dendritic cell, vaccine
INTRODUCTION
Malignant glioma is a common primary brain tumor. Currently, malignant glioma is treated by surgical removal combined with radiochemotherapies. However, patient survival rates remain unsatisfactory.1 Dendritic cell (DC)-based tumor vaccines are gaining interest for treating malignant glioma because of encouraging results obtained from basic studies as well as phase I and II clinical trials. However, DC-based vaccines do not completely eradicate tumor cells and prevent recurrence.2,3 Current disadvantages of tumor vaccines may be attributed to limited target specificities. Recent studies revealed that numerous tumors, including glioma, contain a small number of cells with stem cell characteristics. Known as cancer stem cells, these cells are closely related to tumor development and reoccurrence.4-6 In animal models, studies have also found that cancer stem cells possess higher immunogenicities compared with those of tumor cells, and can induce stronger immune response.7 However, whether human glioma stem cells can induce the same immune response is still unknown. In this study, we prepared a new tumor vaccine by loading DCs with human glioma stem cell lysates. We then studied the capacity of the vaccine to activate naive T-cells for targeted killing of glioma cells in vitro, followed by analysis of the associated mechanisms.
MATERIALS AND METHODS
Animals and cell line
The human glioma cell line U251 was purchased from the Shanghai Institute for Biological Sciences, Chinese Academy of Sciences. Specific pathogen-free grade 6-8 week-old male C57BL/6 mice were purchased from the Center of Laboratory Animals, Wuhan University, which were maintained in a virus-free environment in accordance with the Laboratory Animal Resources Commission standards. All aspects of the studies requiring animal experimentation were approved by the Wuhan University Institutional Animal Care and Use Committee. Every effort was made to minimize both the animal suffering and the number of animals used.
Glioma stem cell culture and lysate preparation
Approximately 1×106 U251 cells in the logarithmic growth phase were suspended in DMEM/F12 (Hyclone, Logan, UT, USA) medium supplemented with 10 ng/mL LIF (Millipore, Bedford, MA, USA), 20 ng/mL epidermal growth factor (EGF, Peprotech, Rocky Hill, NJ, USA), 20 ng/mL FGF (Peprotech, Rocky Hill, NJ, USA) and 20 µL/mL B27 (Gibco, Grand Island, NY, USA) supplement. U251 cells were incubated at 37℃ in an atmosphere of 5% CO2 and 95% relative humidity. Cells were observed daily for growth status and medium was exchanged every 48 h or according to medium acidity. After 5-7 d incubation, spheroids were collected and dissociated into single cells with 0.25% trypsin. Trypsinization was stopped by adding a serum-containing medium and cells were rinsed three times with phosphate-buffered saline (PBS). Cells were cultured in the described medium and passaged weekly. CD133+ cells were isolated by fluorescence-activated cell sorting (FACS) (EPICS ALTRA II, Beckman, Fullerton, CA, USA) and passaged under the same conditions for up to 4 weeks. A portion of the spheroids were analyzed for monoclonality, cell surface marker expression and differentiation potential. Cell concentration was adjusted to 1000 cells/mL and colony formation was assessed.8 Spheroids were cultured on sterile glass cover slips pretreated with 10% polylysine for 2 h in DMEM/F12 medium at 37℃. The glioma stem cell marker, nestin and CD133 expression were then observed by immunofluorescence. Spheroids were also incubated in DMEM/F12 supplemented with 10% fetal bovine serum (FBS, Gibco, Grand Island, NY, USA) for 5-10 d followed by microtube-associated protein 2 (MAP2, Sigma, St. Louis, MO, USA) and glial fibrillary acidic protein (GFAP, Sigma, St. Louis, MO, USA) expression analysis by immunofluorescence to identify differentiated phenotypes. Cells were also lysed under sterile conditions by three cycles of immersion in liquid nitrogen for 1 min followed by incubation in a water bath at 37℃ for 3 min. Lysates were assayed for protein concentration using the bicinchoninic acid method (BCA Protein Assay Kit, Pierce, Rockford, IL, USA) and stored at -80℃ until use.
Heat treatment of U251 cells
U251 cells were heat-treated using a method described below. Cells were collected at the logarithmic growth phase and cultured at various temperature-time combinations (43, 44 or 45℃; 1, 2, 3 or 4 h). Then, cells were transferred to a fresh medium supplemented with 2% FBS and cultured at 37℃ for 12 h. In order to test the effect of heat treatment, cells were analyzed by anti-annexin V-FITC/propidium iodide (PI) double-staining (AnnexinV-FITC Kit, Bender, Burlingame, CA, USA) to determine apoptosis rates. Briefly, samples were adjusted to a concentration of 2×105-3×105 cells/mL and rinsed twice with cold PBS. Cells were then suspended in 190 µL binding buffer and thoroughly mixed with 5 µL anti-annexin V-FITC followed by incubation in the dark at room temperature for 10 min. 5 µL PI was added to the cell suspensions followed by incubation for a further 15 min and then flow cytometric analysis.
Dendritic cell isolation and culture
DCs were isolated from the bone marrow of 20 C57BL/6 mice.9 Femurs and tibias were harvested under sterile conditions and bone marrow cells were collected and treated with a erythrocyte lysis buffer. The remaining cells were suspension cultured in medium supplemented with 10 ng/mL recombinant murine granulocyte macrophage-colony stimulating factor (Peprotech, Rocky Hill, NJ, USA) and 5 ng/mL recombinant murine interleukin-4 (rmIL-4, Peprotech, Rocky Hill, NJ, USA) with daily changes of half medium volumes. On day 6, 1 µg/mL lipopolysaccharide (LPS, Sigma, St. Louis, MO, USA) was used to stimulate DC maturation. DCs were harvested on days 6 and 7 followed by scanning and transmission electron microscopy (SEM and TEM, respectively, Hitachi/TM-1000, Tokyo, Japan) examinations of cell morphology. Cell surface markers CD11c, CD80, CD86 and MHC-II (antibodies were purchased from eBioscience, San Diego, CA, USA) were also analyzed by flow cytometry.
T cell isolation and preparation
Spleens were harvested from C57BL/6 mice under sterile conditions and mechanically homogenized into suspensions followed by treatment with an erythrocyte lysis buffer. Cell suspensions were adjusted to 1×108 cells/mL and transferred to a nylon fiber column (Wako, Osaka, Japan), then incubated in the dark at 37℃ for 1 h. Freshly prepared warm medium was added to the column and the effluent was collected after becoming slightly turbid. The effluent was centrifuged and the cell pellet resuspended in RPMI-1640 medium (Gibco, Grand Island, NY, USA) supplemented with 100 U/mL recombinant murine interleukin-2 (rmIL-2, eBioscience, San Diego, CA, USA) and cultured in six well plates.
Dendritic cell antigen loading
DCs were combined with glioma stem cell lysates and cultured for 24 h in six well plates, as described by Ashley, et al.10 Antigen-loaded DCs were termed BGS-DCs (brain glioma stem cell pulsed DCs). Cell surface markers CD80, CD86, CD11C and MHC-II were analyzed by flow cytometry. DCs were also mixed with heat-treated U251 cells (U251 : DC ratio: 3 : 1, determined by preliminary testing) and cultured in 96 well plates for 24 h. These DCs were referred to as heat-treated U251 cell pulsed DCs (HT-DC). HT-DC phenotypes were analyzed by flow cytometry. As controls, LPS and PBS replaced tumor antigen treatments and were designated as LPS-DCs and PBS-DCs, respectively.
T-cell proliferation assay
Naive T-cells (2×104 cells/well) were co-cultured with BGS-DCs, HT-DCs, LPS-DCs or PBS-DCs at various ratios (1 : 5, 1 : 10, 1 : 20, 1 : 40 and 1 : 80) in 96 well plates. The culture medium was supplemented with 100 U/mL rmIL-2. After 5 d, T-cell proliferation was assayed using a cell counting kit-8 (CCK-8, Dojindo, Kumamoto, Japan) to determine the optimal DC: T-cell ratio for subsequent experiments. Each assay group contained ≥4 replicates.
Targeted killing of glioma cells by DC-activated T cells
U251 cells in the logarithmic growth phase were seeded in 96 well plates and co-cultured with T-cells and various DC combinations (BGS-DCs, HT-DCs, LPS-DCs and PBS-DCs) at three effector: target ratios (20 : 1, 40 : 1 and 80 : 1). After incubation for 72 h, 100 µL aliquots of conditioned medium were collected. Interferon-γ (IFN-γ) levels were analyzed by an ELISA Kit (R&D, Minneapolis, MN, USA) according to the manufacturer's instructions. The remaining medium was discarded, then 100 µL PBS and 10 µL CCK-8 reagent was added to each well. Optical density (OD) was measured and converted to kill rate using the formula: kill rate (%)=(1-ODexperimental group/ODPBS group). Each assay group contained ≥4 replicates.
Statistical analyses
Data were expressed as the mean±standard deviation and analyzed by SPSS 13.0 (SPSS, Chicago, IL, USA). Statistical analysis was performed using a nonparametric Kruskal-Wallis test for T-cell proliferation, killing rate, IFN-γ level. For DC surface marker comparisons, a Student t-test was used. Statistical significance was accepted at p<0.05.
RESULTS
Glioma stem cell identification
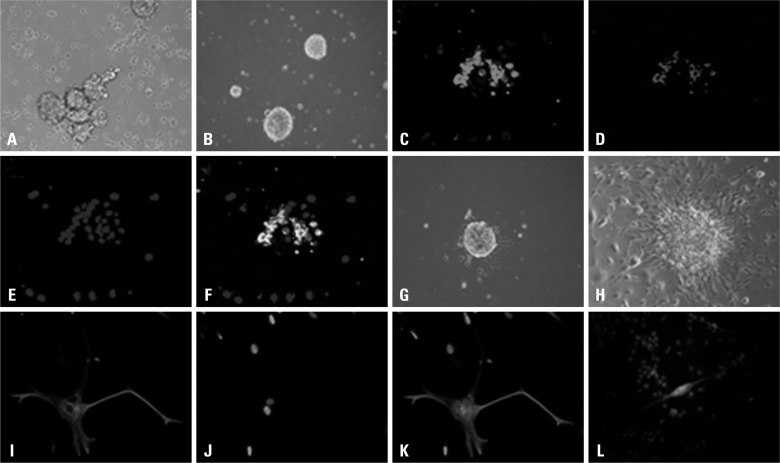
Twelve hours after culture in serum-free medium, a small number of cells adhered and the majority formed suspended aggregates. After 48 h, a large number of cell agglomerates with various sizes and shapes were observed (Fig. 1A). Cells were passaged after 7 d and new spheroids formed, which became increasingly regular in shape with passaging. CD133+ cells were isolated by FACS and cultured in serum-free medium at 1×103 cells/mL. Isolated single cells were also observed to form spheroids (Fig. 1B). Immunofluorescence staining revealed that spheroids contained abundant nestin and CD133 double-positive cells (Fig. 1C-F). After a 4 h culture in a FBS-containing medium, spheroids adhered and initiated differentiation. After 24 h, a small number of synapses extended outward from spheroids (Fig. 1G). With further culture, an increasing number of synapses extended radially and cells with irregular polygon shapes and abundant synapses began to migrate out from spheroids (Fig. 1H). Cells were confluent after 7-10 d culture. Immunofluorescence staining revealed that a small number of cells were strongly GFAP+ (Fig. 1I, J and K), while other cells were MAP2+ with an outward radiation of synapses from spheroids (Fig. 1L). A portion of heterogeneous cells were double-positive for both GFAP and MAP2.
Fig. 1.
Glioma stem cell isolation and differentiation. (A) One week in suspension culture. (B) Spheroid formations. (C-F) Glioma stem cell double-staining with anti-nestin-FITC and anti-CD133-PE with nuclei DAPI stained. (G) Spheroids after 12 h differentiation. (H) Spheroids after 1 week differentiation. (I, J and K) Differentiated cells staining positive with anti-GFAP-CY3 with nuclei DAPI stained. (L) Staining of differentiated cells with anti-MAP2-FITC (a small number of cells stained positive, nuclei labeled with PI). PI, propidium iodide.
Dendritic cell isolation
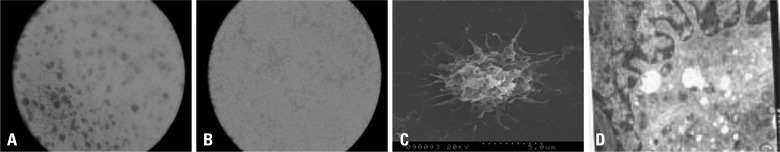
Cells extracted from mouse bone marrow were small and able to form colonies after a 3 d culture (Fig. 2A). Cells and colonies enlarged with additional culture (Fig. 2B). Following LPS stimulation, cells developed abundant dendritic processes, a mature DC characteristic (Fig. 2C and D). Flow cytometric analysis revealed that mature cells expressed significantly higher levels of DC surface markers including CD11c, MHC-II, CD80 and CD86 compared with those of immature cells (Table 1).
Fig. 2.
DC culture and identification. (A) DCs after 2 d culture (B) DCs after 5 d culture (C) Scanning electron micrograph showing DCs with dendritic processes (D) Transmission electron micrograph revealing inner DC structures. Cells contained dendritic processes and folds. Nuclei are darkly stained and located toward one side with irregular morphologies (generally lobular). Cytoplasm contains abundant mitochondria, endosomes, endoplasmic reticula and Golgi apparatuses. However, only a small number of lysosomes are observed.
Table 1.
Flow Cytometric Analysis of the Expression of Surface Markers on the Immature and Mature DCs (mean±standard, %)
Increased expression of cell surface markers on DCs matured by LPS stimulation.
*p<0.05.
†p<0.01.
U251 cell apoptosis rate after heat treatment
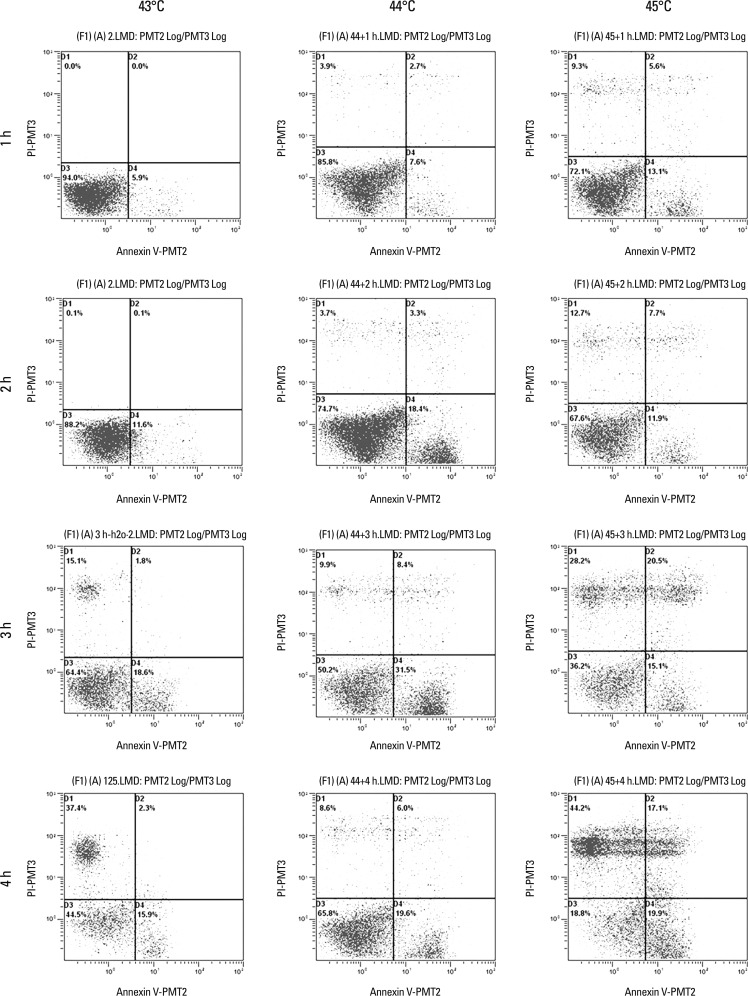
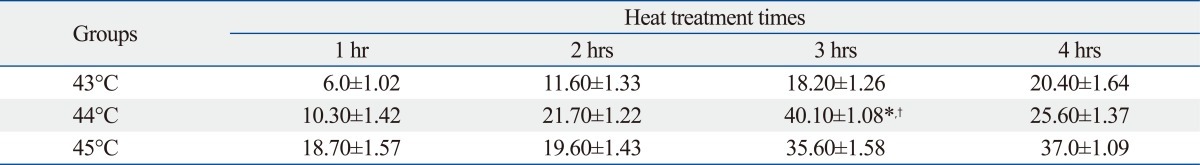
An apoptosis rate of 40.10±1.08% was observed in cells treated at 44℃ for 3 h, which was significantly higher compared with those of other temperature-time combinations (*p<0.01, †p<0.05) (Fig. 3) (Table 2). So 44℃ for 3 h was the best heat treated condition for subsequent experiments.
Fig. 3.
Flow cytometric analysis of the expression of surface markers on the DCs pulsed with heat-treated U251 or glioma stem cell lysate.
Table 2.
Flow Cytometric Analysis of Apoptotic Rate of Heat Treatment U251 Cells by Annexin V-FITC/PI Method (mean±standard, %)
PI, propidium iodide.
44℃ for 3 h vs. 44℃ for 1, 2, or 4 h: *p<0.01; 44℃ for 3 h vs. other temperatures for 1, 2, 3, or 4 h: †p<0.05.
Antigen-loaded DC phenotypes
DCs were incubated with glioma stem cell lysates or heat-treated U251 cells for 24 h, as described elsewhere.10 HT-DCs expressed significantly lower levels of DC surface markers compared with those of BGS-DCs (*p<0.05, †p<0.01) (Table 3).
Table 3.
Flow Cytometric Analysis of the Expression of Surface Markers on the DCs Pulsed with Heat-Treated U251 or Glioma Stem Cell Lysate (mean±standard, %)
Compared with DCs stimulated with thermally treated U251 cells (HT-DCs), DCs stimulated with lysate of glioma stem cells (BGS-DCs) expressed significantly higher levels of CD11c, CD80, CD86, and MHC-II.
*p<0.05.
†p<0.01.
Stimulation of T-cell proliferation by antigen-loaded DCs
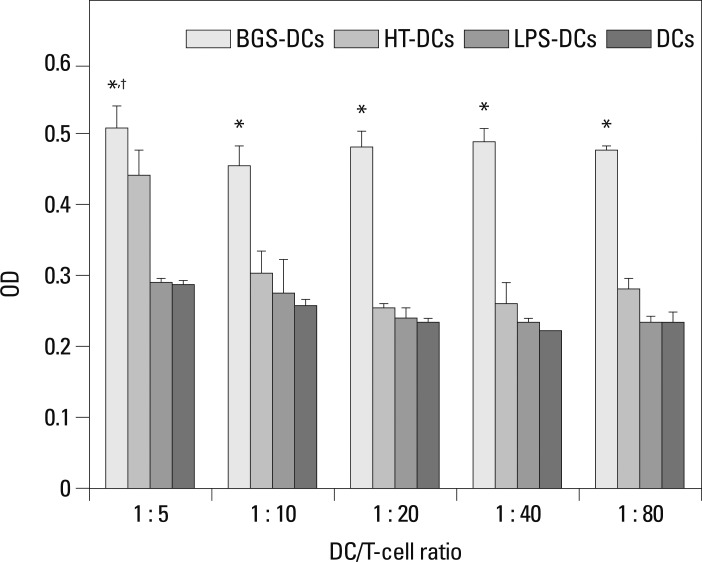
Various vaccine combinations were co-cultured with T-cells for 5 d. At the same DC : T-cell ratio, BGS-DC-stimulated T-cell proliferation was the highest compared with those of other DCs (HT-DCs, LPS-DCs and PBS-DCs) (*p<0.01) (Fig. 4). In BGS-DCs group, at the DC : T-cell ratio of 1 : 5, T-cell proliferation was the highest compared with other DC : T-cell ratios (†p<0.01) (Fig. 4). So the DC : T-cell ratio of 1 : 5 was optimal for subsequent experiments. T-cell stimulation by LPS-DCs and PBS-DCs demonstrated similar results (p>0.05).
Fig. 4.
Stimulation of T-cell proliferation by various antigen-loaded DCs by CCK-8 method. At the same DC/T-cell ratio, OD in BGS-DCs group was higher than in HT-DCs group (*p<0.01). In the BGS-DCs group, OD was highest at the DC/T-cell ratio of 1 : 5 (1 : 5 vs. 1 : 10, 1 : 20, 1 : 40, or 1 : 80: †p<0.05).
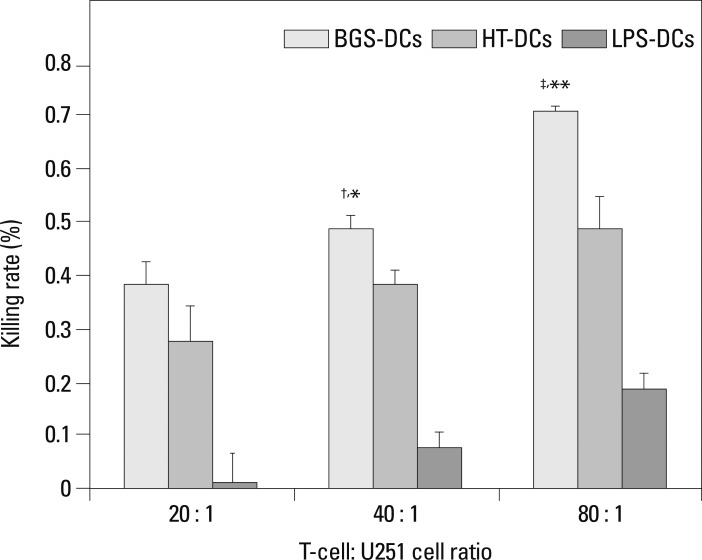
Targeted killing of U251 cells by DC-activated T-cells
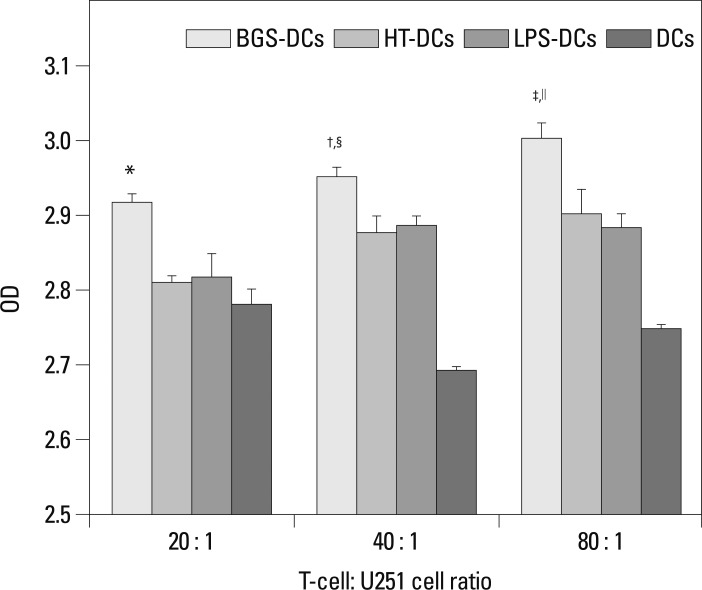
Various antigen-loaded DCs were co-cultured with T-cells and U251 cells for 72 h. At an effector : target ratio of 20 : 1, there was no difference between BGS-DCs and HT-DCs (p>0.05). At an effector : target ratio of 40 : 1 and 80 : 1, wells that contained BGS-DCs showed a significantly lower mean OD, indicating a higher kill rate compared with those of HT-DCs (†p<0.01, ‡p<0.01) (Fig. 5). In addition, kill rate increased with increasing effector : target ratios (Fig. 5). ELISA results revealed that IFN-γ levels increased as the effector : target ratio increased in BGS-DCs groups (†p<0.01, ∥p<0.01) (Fig. 6). At the same effector : target ratio, wells with BGS-DCs contained higher IFN-γ levels (*p<0.01, §p<0.05, ‡p<0.01) (Fig. 6).
Fig. 5.
Targeted killing of U251 cells by vaccine-induced tumor-specific cytotoxic T-cells by CCK-8. At T-cell : U251 cell ratio of 20 : 1, BGS-DCs vs. HT-DCs: p>0.05; At T-cell : U251 cell ratio of 40 : 1, BGS-DCs vs. HT-DCs: †p<0.01; At T-cell : U251 cell ratio of 80 : 1, BGS-DCs vs. HT-DCs: ‡p<0.01. In BGS-DCs group, 20 : 1 vs. 40 : 1: *p<0.01, 40 : 1 vs. 80 : 1: **p<0.01. CCK, cell counting kit.
Fig. 6.
Stimulation of IFN-γ secretion of T-cell by various antigen-loaded DCs by ELISA. The level of IFN-γ was detected in the supernatant of medium. The following are the results after statistical analysis. At T-cell : U251 cell ratio of 20 : 1, BGS-DCs vs. HT-DCs, LPS-DCs or DCs: *p<0.01. At T-cell : U251 cell ratio of 40 : 1, BGS-DCs vs. other groups: §p<0.05; At T-cell : U251 cell ratio of 80 : 1, BGS-DCs vs. other groups: ‡p<0.01. In BGS-DCs group, 20 : 1 vs. 40 : 1: †p<0.01, 40 : 1 vs. 80 : 1: ∥p<0.01, OD increased as DC : T-cell ratio increased.
DISCUSSION
DCs are potent "professional" antigen-presenting cells, which are responsible for capturing and presenting antigens for initiation of T-cell immune responses. DCs also play pivotal roles in the regulation and monitoring of the immune system.11 In vitro, DC stimulation with tumor antigens may induce tumor-specific T-cells that generate an effective anti-tumor immune response. Thus far, various antigens have been used to prepare DC-based vaccines against glioma such as tumor polypeptides and lysates, RNA, cDNA, as well as fusion and apoptotic cells.12 Although various degrees of success have been achieved, DC-based vaccines could not completely eradicate glioma. This observation may be due to DC apoptosis and tumors lacking targeted antigens.13 Recent studies demonstrate that numerous tumors contain a small number of "side population" cells with stem cell characteristics. These cells are known as cancer stem cells and are closely related to tumor occurrence, progression, metastasis, recurrence, drug resistance and immune evasion.14-16 Therefore, tumor stem cell eradiation is essential for complete cancer clearance. Cancer stem cells have been identified in glioma, which provide new targets for active vaccination.17,18 Therefore, DCs loaded with glioma stem cell antigens may be used to stimulate T-cells for a tumor-specific cytotoxicity against glioma cells. Studies have found that heat treatment of tumor cells up-regulates heat-shock protein expression and results in enhanced immunogenicity. Thus, we used DCs pulsed with heat-treated U251 cells as a control group.
Over the past decade, there is accumulating evidence for the presence of cancer stem cell-like progenitors in malignant glioma.19-22 To date, identification and purification of brain glioma stem cells (BGS) depends mainly on neural stem cell-like biological properties and cell surface markers. Similar to normal neural stem cells (NSCs), BGS can form neurospheres, possess the capacity of self-renewal and multilineage differentiation, express CD133 and nestin, and not express neural differentiation markers.19,20,23,24 In our study, neurospheres from U251 cells showed CD133+ and nestin+ are capable of being passaged repeatedly in the serum-free medium, and differentiated into neuron-like cells (MAP2+ CD133-) and astrocyte-like cells (GFAP+ CD133-) in the medium with FBS. It is reported that, unlike NSCs, BGS can reform spheres even after the induction of differentiation, and are capable of forming tumors in vivo. Furthermore, differentiated cells are heterogeneous and are similar to the origin tumor cells. In addition, common tumor cells have no capacity of unlimited proliferation and differentiation.19-24 These were also consistent with our findings. As a cancer cell line, U251 cells do not contain NSCs. So, our findings indicated that U251 cell line contained cancer stem cells. We succeeded in isolating brain glioma stem cells following methods by Singh, et al.17,20
In our study, we prepared lysates of glioma stem cells by freeze-thaw cycles for use in antigen-loaded DC-based vaccines. Freeze-thaw cycling can effectively recover whole cell antigens and allow DC vaccines to present optimal target cell antigens that may lead to tumor-specific targeting of glioma cells including glioma stem cells. We also isolated highly pure DCs at a high efficiency and relatively low cost using a method adapted from Inaba, et al.9 After culture with glioma stem cell lysates, BGS-DCs expressed significantly higher levels of MHC-II, CD11C, CD80 and CD86, compared with those of control groups. Increased expression of these cell surface markers reveals that glioma stem cells contain unknown antigens with strong immunogenicities, which are recognized by DCs. This observation indicates that BGS-DCs may effectively stimulate naive T-cell activation. Antigen-loaded DC and naive T-cell co-culturing demonstrated that BSG-DCs significantly stimulate T-cell proliferation compared with those of HT-DCs, PBS-DCs and LPS-DCs co-cultures. Moreover, T-cell proliferation improves as the number of DCs increases. T-cells and U251 glioma cells were then co-cultured with various antigen-loaded DCs to compare the capacities of the DC-based vaccines to kill glioma cells. BGS-DCs co-cultures demonstrated significantly less cell survival compared with those of HT-DCs, LPS-DCS and PBS-DC co-cultures. This result indicates that BGS-DCs effectively induce cytotoxic T-cells that can kill tumor cells. Collectively, these results confirm that glioma stem cells contain tumor-specific antigens that are targetable and can be used for the development of active vaccines against glioma.
Previous studies have found that glioma stem cells express higher levels of MHC-II, CD80 and CD86 compared with glioma cells.7 Consequently, glioma stem cells may be more easily recognized by immune cells. EGF may play an important role in regulating glioma stem cell immunogen. EGF increases HLA I, HLA II and NF-κB expression in breast cancer cells, which up-regulates CD80 and CD86.25-28 EGF is an essential component in culturing glioma stem cells. Thus, the lysates of glioma stem cells strongly stimulate DCs to express co-stimulatory molecules. Following stimulation by antigens, DCs present antigens via two mechanisms for immunoeffector cell activation. First, CD4+ T-cell activation subsequently activates CD8+ T-cells and second, direct activation of resting CD8+ T-cells. Both mechanisms induce tumor-specific cytotoxicity T-cells that can kill tumor cells. Moreover, activated T-cells secrete IFN-γ that enhances the anti-tumor effect of tumor necrosis factor and stimulates DCs to express MHC-II. In our study, BGS-DCs expressed highly MHC-II and T-cells stimulated by BGS-DCs produced higher amounts of IFN-γ compared with those of HT-DCs, LPS-DCs, and PBS-DCs. Therefore, MHC-II and IFN-γ may mutually enhance anti-tumor effects of vaccines.
We used human glioma cells and murine DCs and T cells in the study; therefore, differences between species might affect the experimental result. At the T-cell : U251 cell ratio of 20 : 1, 40 : 1 and 80 : 1, the kill rate was respectively (1.5±0.055%), (7.6±0.034%), and (18.7±0.034%) in the LPS-DCs group, and was respectively (38.5±0.043%), (48.8±0.028%), and (70.9±0.010%) in the BGS-DCs group. The effect different species might exist, but if it did, it had a small effect on the results. Therefore we were able to demonstrate that DC vaccines loaded with lysate of glioma stem cells had a stronger antitumor effect than those loaded with heat-treated glioma cells.
In conclusion, compared with DCs loaded with antigens of glioma cell, DCs loaded with antigens derived from glioma stem cells more effectively stimulate naive T-cells to form tumor-specific cytotoxic T-cells that kill glioma cells cultured in vitro. These observations provide preclinical evidence for active vaccination against malignant glioma using DC-based vaccines and are an experimental basis for further studies.
ACKNOWLEDGEMENTS
The study was supported by funds from the National Natural Science Research Foundation of China (NSFC: 30772223).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Ohgaki H. Epidemiology of brain tumors. Methods Mol Biol. 2009;472:323–342. doi: 10.1007/978-1-60327-492-0_14. [DOI] [PubMed] [Google Scholar]
- 2.Fecci PE, Mitchell DA, Archer GE, Morse MA, Lyerly HK, Bigner DD, et al. The history, evolution, and clinical use of dendritic cell-based immunization strategies in the therapy of brain tumors. J Neurooncol. 2003;64:161–176. doi: 10.1007/BF02700031. [DOI] [PubMed] [Google Scholar]
- 3.Wheeler CJ, Black KL. Dendritic cell vaccines and obstacles to beneficial immunity in glioma patients. Curr Opin Mol Ther. 2005;7:35–47. [PubMed] [Google Scholar]
- 4.Gao JX. Cancer stem cells: the lessons from pre-cancerous stem cells. J Cell Mol Med. 2008;12:67–96. doi: 10.1111/j.1582-4934.2007.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao XH, Ping YF, Bian XW. Contribution of cancer stem cells to tumor vasculogenic mimicry. Protein Cell. 2011;2:266–272. doi: 10.1007/s13238-011-1041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inagaki A, Soeda A, Oka N, Kitajima H, Nakagawa J, Motohashi T, et al. Long-term maintenance of brain tumor stem cell properties under at non-adherent and adherent culture conditions. Biochem Biophys Res Commun. 2007;361:586–592. doi: 10.1016/j.bbrc.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 7.Pellegatta S, Poliani PL, Corno D, Menghi F, Ghielmetti F, Suarez-Merino B, et al. Neurospheres enriched in cancer stem-like cells are highly effective in eliciting a dendritic cell-mediated immune response against malignant gliomas. Cancer Res. 2006;66:10247–10252. doi: 10.1158/0008-5472.CAN-06-2048. [DOI] [PubMed] [Google Scholar]
- 8.Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, et al. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- 9.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashley DM, Faiola B, Nair S, Hale LP, Bigner DD, Gilboa E. Bone marrow-generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors. J Exp Med. 1997;186:1177–1182. doi: 10.1084/jem.186.7.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 12.Gu JH, Li G. Dendritic cell-based immunotherapy for malignant glioma. Neurosci Bull. 2008;24:39–44. doi: 10.1007/s12264-008-1107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monti P, Leone BE, Zerbi A, Balzano G, Cainarca S, Sordi V, et al. Tumor-derived MUC1 mucins interact with differentiating monocytes and induce IL-10highIL-12low regulatory dendritic cell. J Immunol. 2004;172:7341–7349. doi: 10.4049/jimmunol.172.12.7341. [DOI] [PubMed] [Google Scholar]
- 14.Sagar J, Chaib B, Sales K, Winslet M, Seifalian A. Role of stem cells in cancer therapy and cancer stem cells: a review. Cancer Cell Int. 2007;7:9. doi: 10.1186/1475-2867-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soltanian S, Matin MM. Cancer stem cells and cancer therapy. Tumour Biol. 2011;32:425–440. doi: 10.1007/s13277-011-0155-8. [DOI] [PubMed] [Google Scholar]
- 16.Dey M, Ulasov IV, Tyler MA, Sonabend AM, Lesniak MS. Cancer stem cells: the final frontier for glioma virotherapy. Stem Cell Rev. 2011;7:119–129. doi: 10.1007/s12015-010-9132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 18.Rogers LR, Wicha M. Therapeutic approaches to target cancer stem cells. In: Rajasekhar VK, Vemuri MC, editors. Regulatory Networks in Stem Cells. New York, NY, USA: Humana Press; 2009. pp. 545–560. [Google Scholar]
- 19.Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 21.Tabatabai G, Weller M. Glioblastoma stem cells. Cell Tissue Res. 2011;343:459–465. doi: 10.1007/s00441-010-1123-0. [DOI] [PubMed] [Google Scholar]
- 22.Lathia JD, Venere M, Rao MS, Rich JN. Seeing is believing: are cancer stem cells the Loch Ness monster of tumor biology? Stem Cell Rev. 2011;7:227–237. doi: 10.1007/s12015-010-9194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rebetz J, Tian D, Persson A, Widegren B, Salford LG, Englund E, et al. Glial progenitor-like phenotype in low-grade glioma and enhanced CD133-expression and neuronal lineage differentiation potential in high-grade glioma. PLoS One. 2008;3:e1936. doi: 10.1371/journal.pone.0001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prestegarden L, Svendsen A, Wang J, Sleire L, Skaftnesmo KO, Bjerkvig R, et al. Glioma cell populations grouped by different cell type markers drive brain tumor growth. Cancer Res. 2010;70:4274–4279. doi: 10.1158/0008-5472.CAN-09-3904. [DOI] [PubMed] [Google Scholar]
- 25.Bernard DJ, Courjal F, Maurizis JC, Bignon YJ, Chollet P, Plagne R. Effect of epidermal growth factor in HLA class I and class II transcription and protein expression in human breast adenocarcinoma cell lines. Br J Cancer. 1992;66:88–92. doi: 10.1038/bjc.1992.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun L, Carpenter G. Epidermal growth factor activation of NF-kappaB is mediated through IkappaBalpha degradation and intracellular free calcium. Oncogene. 1998;16:2095–2102. doi: 10.1038/sj.onc.1201731. [DOI] [PubMed] [Google Scholar]
- 27.Zhao J, Freeman GJ, Gray GS, Nadler LM, Glimcher LH. A cell type-specific enhancer in the human B7.1 gene regulated by NF-kappaB. J Exp Med. 1996;183:777–789. doi: 10.1084/jem.183.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Liu Z, Jiang S, Cortesini R, Lederman S, Suciu-Foca N. T suppressor lymphocytes inhibit NF-kappa B-mediated transcription of CD86 gene in APC. J Immunol. 1999;163:6386–6392. [PubMed] [Google Scholar]