Abstract
Purpose
Esophageal candidiasis (EC) is the most frequent opportunistic fungal infection in immunocompromised host. However, we have found EC in healthy individuals through esophagogastroduodenoscopy (EGD). The aim of this study was to determine the prevalence and risk factors for EC in healthy individuals.
Materials and Methods
We retrospectively reviewed the medical records of 281 patients who had been incidentally diagnosed with EC. We also conducted age and sex matched case control study to identify the risk factor for EC.
Results
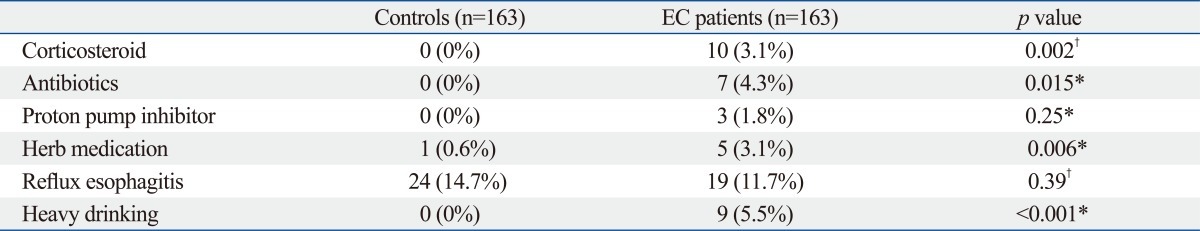
The prevalence of EC was 0.32% (281/88125). The most common coexisting EGD finding was reflux esophagitis (49/281, 17.4%). An antifungal agent was prescribed in about half of EC, 139 cases (49.5%). Follow-up EGD was undertaken in 83 cases (29.5%) and 20 cases of candidiasis was persistently found. Case control study revealed EC were more often found in user of antibiotics (p=0.015), corticosteroids (p=0.002) and herb medication (p=0.006) as well as heavy drinking (p<0.001).
Conclusion
The prevalence of EC was 0.32% (281/88125) in Korea. Use of antibiotics, corticosteroids and herb as well as heavy drinking were significant risk factors for EC in healthy individuals.
Keywords: Candidiasis, healthy, prevalence, risk factors
INTRODUCTION
Esophageal candidiasis (EC) is one of the most common opportunistic infections in patients with impaired cellular immunity, such as human immunodeficiency virus (HIV) infection.1 However, we have sometimes come across EC in healthy individuals without HIV infection.2-6 When EC has been found in healthy individuals, predisposing medical conditions have often been identified.2-6 Broad-spectrum antibiotics may eliminate certain bacteria that inhibit fungal growth, thereby enhancing Candida overgrowth.2 Soon after the introduction of H2-receptor antagonists, some isolated cases of digestive Candida were reported.3 In recent years, proton-pump inhibitors have become widely used, and some reports link omeprazole use with the development of EC.4 Esophageal disease, such as noninfectious esophagitis or achalasia may favor the development of EC.5,6 Some studies represented that corticosteroid and variable cytotoxic drugs are also possible risk factors of EC.7
However, except for HIV infection, there are few data to prove a causative effect with EC,1 and the exact prevalence and risk factors of EC have never been reported in healthy individuals. The aim of this study was to investigate the prevalence and clinical characteristics of EC in non-HIV infected people and predisposing risk factors of EC in healthy individuals.
MATERIALS AND METHODS
Retrospective study
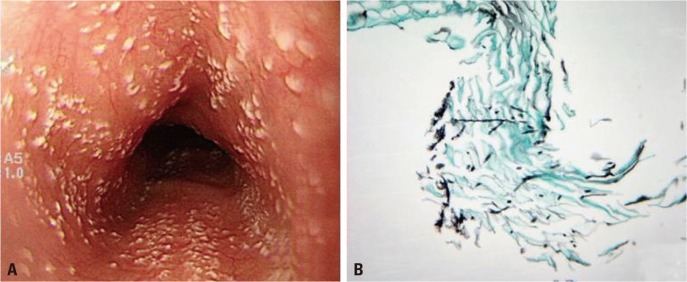
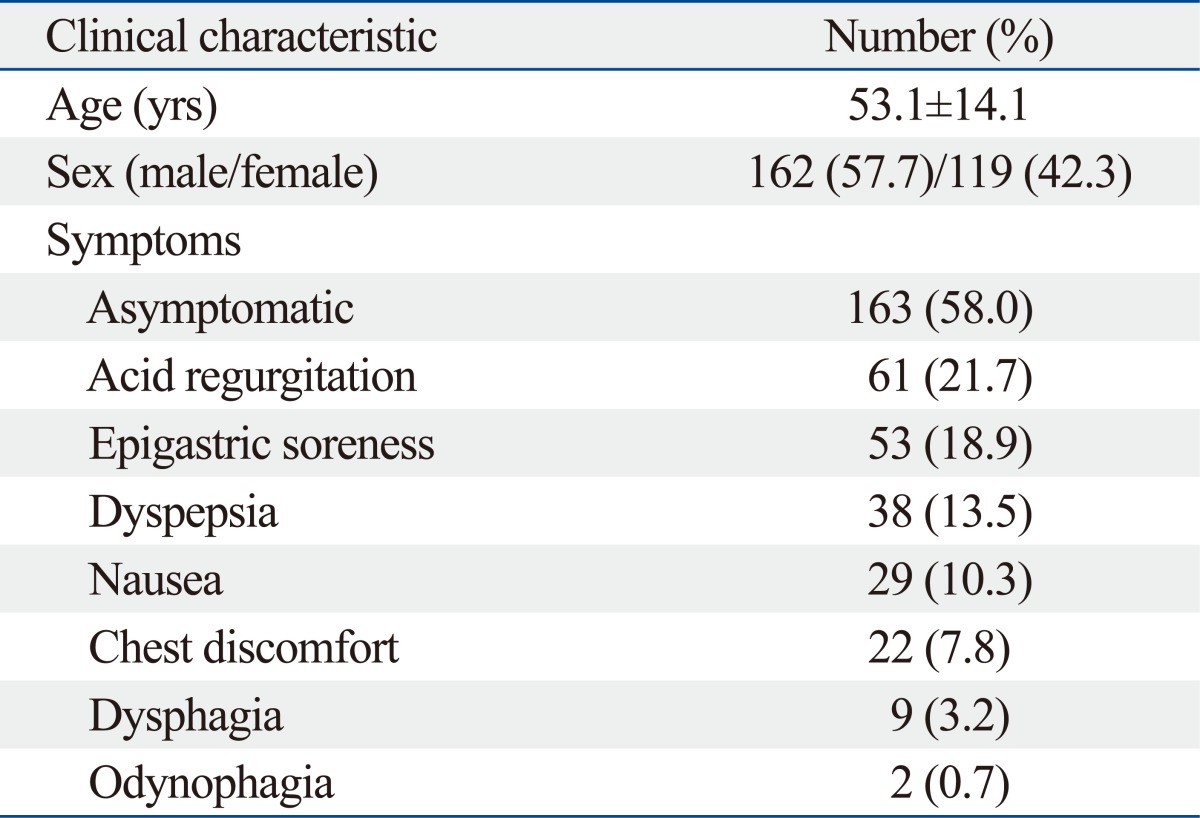
We performed a retrospective study from July 2005 through April 2011 at the Dongguk University Ilsan Hospital. EC had been diagnosed when whitish exudates or plaques were endoscopically identified (Fig. 1A) or yeast forms, candida pseudohyphae, were histopathologically documented in tissue samples taken by biopsy (Fig. 1B). For exclusion of any misdiagnosis of EC endoscopically, two different endoscopists (JH Choi, YJ Lim) interpreted endoscopic findings of whitish plaques and EC cases that come to agreement and were finally enrolled in this investigation.
Fig. 1.
Esophageal candidiasis. (A) Endoscopic finding; multiple whitish plaques were identified. (B) Histopathologic finding; esophageal mucosa containing candida spores and pseudohyphae were noted (Grocott methenamine silver stain, ×400).
We retrospectively analyzed medical records of 281 subjects who had been diagnosed with EC. Insufficient information was additionally surveyed through the telephone. Age, sex, proton pump inhibitor, antibiotics, heavy drinking, herb medication, corticosteroid (oral or inhaler) use, coexisting endoscopic finding, concomitant disease, and symptoms were investigated. We also investigated clinical course including treatment and persistence of EC on follow-up esophagogastroduodenoscopy (EGD). Follow-up EGD was performed about one third of EC patients (83 cases, 29.5%) and EGD was performed annually for routine health examination.
Case control study
We performed a case control study to evaluate the risk factors for EC towards 163 asymptomatic EC. The control group was formed by healthy examinees without a diagnosis of EC and received endoscopy immediately before and after every case of EC. Each control was selected within the limit ±5 years and same sex. The following putative risk factors were antibiotics, herb medication, proton pump inhibitor, corticosteroid, reflux esophagitis, and heavy drinking. The use of antibiotics, corticosteroids, herb medication and proton pump inhibitor within 30 days prior to the EC diagnosis were asked. Heavy drinking was defined when drinking occurred over at least three times a week and more than 80 mg at one time. The Institutional Review Board of Dongguk University Ilsan Hospital approved this study.
Statistics analysis
Results were expressed as mean±standard deviation for continuous variable (age), or the number (percentage) for categorical variable (sex, coexisting EGD finding, concomitant disease, proton pump inhibitors, steroids, antibiotics, herb, heavy drinking, reflux esophagitis). Chi-square tests were performed for comparison of categorical variables (sex, corticosteroid, proton pump inhibitors, steroids, antibiotics, herb, heavy drinking, reflux esophagitis) and Student t-tests were performed for age as a continuous variable. Multivariate analysis was done to evaluate the risk factors of EC in this case control study. A p-value of <0.05 was considered to be statistically significant. Statistical analyses were performed using SPSS version 12 (SPSS Inc., Chicago, IL, USA).
RESULTS
Retrospective study
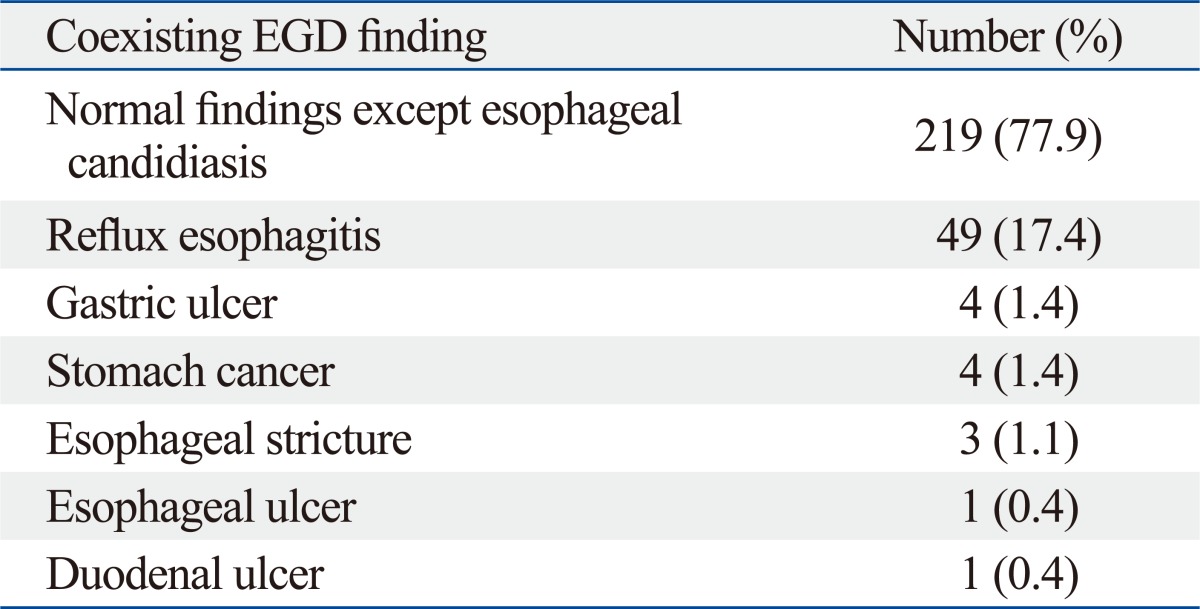
Clinical characteristics of immunocompetent patients (Table 1)
Table 1.
Clinical Characteristic in 281 Patients with Esophageal Candidiasis
During 5 years, 88125 EGDs were performed. Two hundred and a eighty-one cases (281/88125, 0.32%) were finally included with EC. The clinical characteristics of the 281 patients are shown in Table 1. All patients with EC enrolled in this investigation had a negative HIV serology. The mean age was 53.1±14.1 years and males were predominant (162/281, 57.7%). More than half of the patients were asymptomatic (163/281, 58.0%). One hundred eighteen (118/281, 42.0%) patients had variable gastrointestinal symptoms. Acid regurgitation was the most common symptom (61/281, 21.7%). Epigastric soreness (53/281, 18.9%), dyspepsia (38/281, 13.5%) and nausea (29/281, 10.3%) were also found. Only 33 patients (33/281, 11.7%) represented typical esophageal symptoms such as dysphagia, odynophagia or chest discomfort.
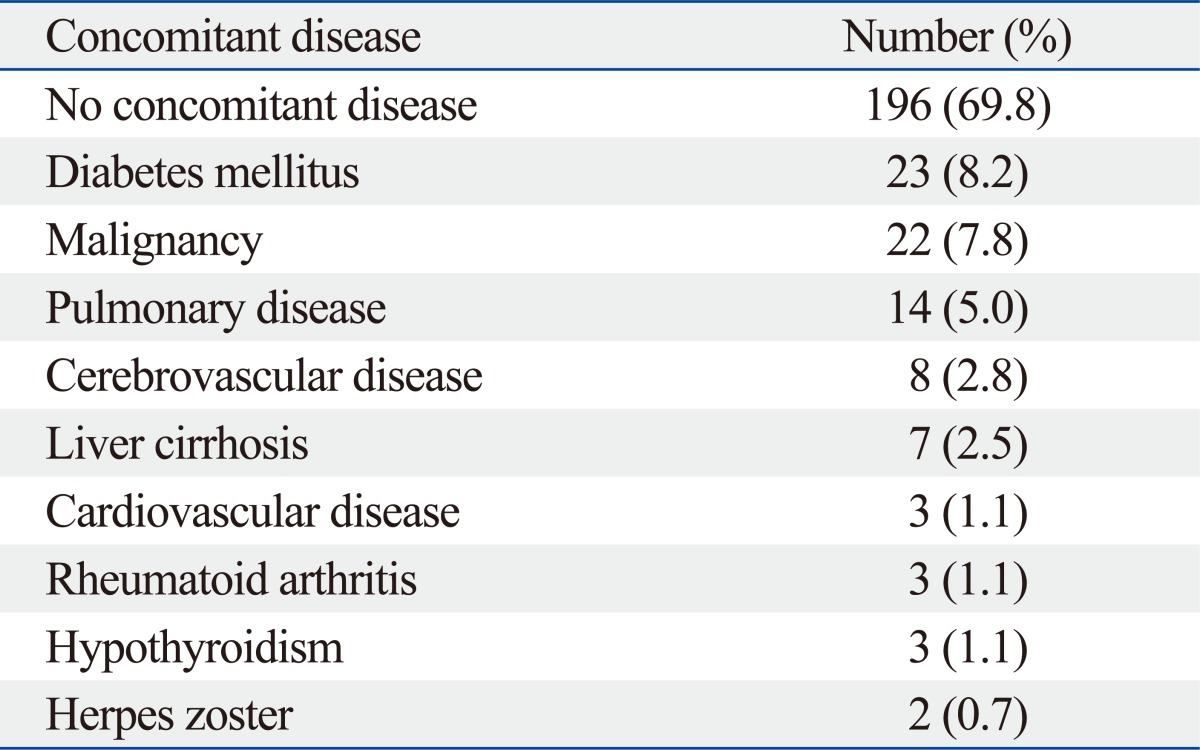
Coexisting EGD finding (Table 2)
Table 2.
Coexisting EGD Findings of Esophageal Candidiasis
EGD, esophagogastroduodenoscopy.
Reflux esophagitis is the most common coexisting endoscopic finding (49/281, 17.4%). In addition, gastric ulcer (4/281, 1.4%), stomach cancer (4/281, 1.4%), esophageal stricture (3/281, 1.1%), esophageal ulcer (1/281, 0.4%) and duodenal ulcer (1/281, 0.4%) were also found.
Concomitant disease (Table 3)
Table 3.
Concomitant Disease of Esophageal Candidiasis
Both underlying commodity and immunocompromised conditions such as HIV antibody had been evaluated when EC was found in EGD. All EC patients in this investigation were negative for HIV antibody. There were no underlying disease in 69.8% (196/281). Diabetes mellitus (23/281, 8.2%) and malignancy (22/281, 7.8%) were the most common concomitant diseases. The other accompanying diseases were pulmonary disease (14/281, 5.0%), cerebrovascular disease (8/281, 2.8%), liver cirrhosis (7/281, 2.5%), cardiovascular disease (3/281, 1.1%), rheumatoid arthritis (3/281, 1.1%), hypothyroidism (3/281, 1.1%) and herpes zoster (2/281, 0.7%). Pulmonary disease included chronic obstructive pulmonary disease, interstitial pulmonary fibrosis and pulmonary tuberculosis. Cerebrovascular disease included cerebral hemorrhage and cerebral infarction. Cardiovascular disease included heart failure, angina pectoris and myocardial infarction.
Clinical course
An antifungal agent was prescribed in 139 cases (49.5%). Fluconazole, nystatin, or itraconazole were respectively prescribed in 99/139 (71.2%), 31/139 (22.3%), and 9/139 (6.5%). Follow-up EGD had been undertaken in 83 cases (83/281, 29.5%) for annual health examination and candidiasis had been persistently found in 20 cases (20/83, 24.1%). Among these twenty cases, 12 cases had been found despites the previous use of antifungal agents. There were no severe complications of EC such as esophageal bleeding, perforation or systemic dissemination in our study population.
Case control study
Risk factors for EC (Table 4)
Table 4.
Risk Factors for Esophageal Candidiasis
EC, esophageal candidiasis.
*p value from chi-square test.
†p value from Fisher's exact test.
Risk factors for EC are summarized in Table 4. In this study, recent use of antibiotics (p=0.015), corticosteroids (p=0.002), herbal medication (p=0.006) and heavy drinking (p<0.001) were significant risk factors for EC. However, the use of a proton pump inhibitor (p=0.25) was not associated with EC. Interestingly, reflux esophagitis was the most coexisting EGD finding, but, there was no statistically relationship with EC (p=0.39). In this study, 67 patients (67/163, 41.1%) had at least one significant risk factor such as use of antibiotics, corticosteroid and herb medication as well as heavy drinking.
DISCUSSION
Since the early 1980s, most studies of EC have involved HIV infected patients.1 However, the frequent use of screening endoscopy has led to more frequent diagnosis of EC in healthy individuals.8,9 In Korea, the exact prevalence of EC and predisposing risk factors are not fully identified. We performed a retrospective case control study to evaluate the prevalence and clinical characteristics of EC and to determine the independent risk factors of EC in healthy individuals.
The development of EC is a two-step process consisting of colonization of the esophagus and subsequent invasion of the epithelial layer.10,11 It is already well accepted that Candida are known to colonize the esophagus of 20% of healthy adults.10,11 Once colonization has been established, impaired cellular immunity permits invasion of the epithelial layer, such as HIV infection.10,11 Proton pump inhibitors, H2-receptor antagonists, and prior vagotomy are thought to increase the risk of EC, which elevated gastric pH and alters the colonization of the esophagus by oral cavity bacteria and yeast.3,4 Antibiotics may predispose immunocompetent patients to fungal infection by allowing overgrowth and colonization of the candida species.2 Administered corticosteroids predispose one to infection by suppressing both lymphocyte and granulocyte function.7 EC is also seen in the setting of functional or mechanical obstruction of the esophagus, with stasis and excessive growth of the fungus.4,5 However, the exact mechanism of invasion in a healthy individuals has not been fully elucidated.10,11
The main findings of our study was that the prevalence of EC was less observed (0.32%, 281/88125) comparing with the data reported other countries by Underwood, et al.12 (0.71%, 18/2527) and by Naito, et al.13 (1.17%, 41/3501). Most of all, EC can be discovered in healthy individuals without apparent predisposing risk factors and symptoms. We identified that more than two-thirds of EC (76.2%, 214/281) may occur with no putative risk factors and more than half was asymptomatic (58%, 163/281).
We found that antibiotics (p=0.015), corticosteroids (p=0.002), heavy drinking (p<0.001) and herb medication (p=0.006) were independent risk factors for EC. However, unlike the findings of previous reports, proton pump inhibitor (p=0.25) was not associated with EC. Interestingly, reflux esophagitis was the most common concomittent disease but there we no statistically relationship with EC (p=0.39). We did not contain diabetes as potential risk factor in this study. Previously, diabetes is considered as risk factor for EC because of impaired immunity and stasis of esophageal contents.14 However, most of the cases of diabetes-related EC reported have been associated with chronic poor glycemic control (hyperglycemia more than 2 years) and combined with secondary factors, such as other factors causing an immunocompromised state.14 Diabetes was found in 23 patients in this investigation and were healthy individuals and under good glycemic control.
We found that about more than half of the patients with EC, about 58% (163/281), had no gastrointestinal symptoms. A similar ratio was reported in previous studies. One report by Naito, et al.13 reported that among 3501 patients undergoing routine EGD in their study, 41 were found to have EC (1.17%), and about two-thirds of those patients had no symptoms. In the present study, symptomatic EC was found in 42% (118/281) of the population. They complained of variable gastrointestinal symptoms including epigastric discomfort, dyspepsia, nausea and so on. It is questionable whether various gastrointestinal symptoms may be associated with incidental finding of EC. Among them, only 11.7% (33/281) had classic symptoms of infectious esophagitis, such as dysphagia, odynophagia and chest discomfort. This investigation suggests that most EC patients are asymptomatic and typical esophageal symptoms are not common.
It is questionable whether asymptomatic EC in health examinee has a clinical significance. Most patients with EC did not complain of any esophageal symptoms and cause serious complications. About three forth of the cases of EC spontaneously disappeared while one forth of EC were persistently found at follow-up endoscopy. Moreover, more than half of persistent EC previously had antifungal agents. We were not able to confirm resistant EC to antifungal agent in this investigation. But, this finding suggests the possibility that EC can easily relapse in spites of antifungal agent and EC can be spontaneously regressed.15,16
Although most cases of the EC may remain silent and invasion of esophageal wall is usually limited to the superficial epithelium, extensive tissue necrosis and ulceration resulting in esophageal perforation was possible.17 There have been eight previous reported cases of esophageal perforation associated with Candida infection, most of these predominantly occurs in immunocompromised hosts, such as transplanted or leukemia patients.17,18 It is possible that neutropenia, irradiation and chemotherapy such as methotrexate may have led to mucosal disruption, thereby facilitating deeper invasion of the esophagus by Candida.17,18 In immunocompetent hosts, chronic alcohol consumption and long-standing gastroesophageal reflux may increase the risk of transmural invasive Candida infection and esophageal perfration.17,19 Esophageal perforation should be considered in patients with pre-existing esophagitis, unexplained fever, pleuritic chest pain and pleural effusion.19
Our study has limitations. First, it is a retrospective study, which can lead to an underestimation of the exact incidence of risk factors. Second, all cases of EC in this investigation had not been documented through culture and pathology. In spite of agreement of experts about diagnosis, the possibility of misdiagnosing other rare condition such as eosinophillic esophagitis cannot be excluded.20
In conclusion, EC is a rare condition among healthy individuals in Korea (0.32%, 281/88125) and more than half of those with EC was asymptomatic (58%, 163/281). Use of antibiotics, corticosteroids, heavy drinking and herb medication were significant risk factors for EC in healthy individuals. EC can be discovered in patients without apparent predisposing risk factors. Further prospective studies are needed to elucidate the mechanisms of transition from colonization to infection.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Laine L, Bonacini M. Esophageal disease in human immunodeficiency virus infection. Arch Intern Med. 1994;154:1577–1582. [PubMed] [Google Scholar]
- 2.Thapa BR, Kumar L. Candida esophagitis after antibiotic use. Indian J Pediatr. 1989;56:296–299. doi: 10.1007/BF02726633. [DOI] [PubMed] [Google Scholar]
- 3.Kochhar R, Talwar P, Singh S, Mehta SK. Invasive candidiasis following cimetidine therapy. Am J Gastroenterol. 1988;83:102–103. [PubMed] [Google Scholar]
- 4.Karmeli Y, Stalnikowitz R, Eliakim R, Rahav G. Conventional dose of omeprazole alters gastric flora. Dig Dis Sci. 1995;40:2070–2073. doi: 10.1007/BF02208680. [DOI] [PubMed] [Google Scholar]
- 5.Weerasuriya N, Snape J. Oesophageal candidiasis in elderly patients: risk factors, prevention and management. Drugs Aging. 2008;25:119–130. doi: 10.2165/00002512-200825020-00004. [DOI] [PubMed] [Google Scholar]
- 6.Baehr PH, McDonald GB. Esophageal infections: risk factors, presentation, diagnosis, and treatment. Gastroenterology. 1994;106:509–532. doi: 10.1016/0016-5085(94)90613-0. [DOI] [PubMed] [Google Scholar]
- 7.Simon MR, Houser WL, Smith KA, Long PM. Esophageal candidiasis as a complication of inhaled corticosteroids. Ann Allergy Asthma Immunol. 1997;79:333–338. doi: 10.1016/S1081-1206(10)63024-4. [DOI] [PubMed] [Google Scholar]
- 8.Phaosawasdi K, Rice P, Lee B. Primary and secondary Candida esophagitis. IMJ Ill Med J. 1986;169:361–365. [PubMed] [Google Scholar]
- 9.Mathieson R, Dutta SK. Candida esophagitis. Dig Dis Sci. 1983;28:365–370. doi: 10.1007/BF01324956. [DOI] [PubMed] [Google Scholar]
- 10.Hoshika K, Iida M, Mine H. Esophageal Candida infection and adherence mechanisms in the nonimmunocompromised rabbit. J Gastroenterol. 1996;31:307–313. doi: 10.1007/BF02355017. [DOI] [PubMed] [Google Scholar]
- 11.Vermeersch B, Rysselaere M, Dekeyser K, Rasquin K, De Vos M, Elewaut A, et al. Fungal colonization of the esophagus. Am J Gastroenterol. 1989;84:1079–1083. [PubMed] [Google Scholar]
- 12.Underwood JA, Williams JW, Keate RF. Clinical findings and risk factors for Candida esophagitis in outpatients. Dis Esophagus. 2003;16:66–69. doi: 10.1046/j.1442-2050.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- 13.Naito Y, Yoshikawa T, Oyamada H, Tainaka K, Morita Y, Kogawa T, et al. Esophageal candidiasis. Gastroenterol Jpn. 1988;23:363–370. doi: 10.1007/BF02779203. [DOI] [PubMed] [Google Scholar]
- 14.Tamura Y, Araki A, Chiba Y, Ishimaru Y, Ishimaru Y, Horiuchi T, et al. A case of type 2 diabetes mellitus in an elderly patient with rapid attenuation of insulin secretion that resembled fulminant type 1 DM but with incomplete beta cell damage. Endocr J. 2006;53:633–637. doi: 10.1507/endocrj.k06-008. [DOI] [PubMed] [Google Scholar]
- 15.Rex JH, Walsh TJ, Sobel JD, Filler SG, Pappas PG, Dismukes WE, et al. Infectious Diseases Society of America. Practice guidelines for the treatment of candidiasis. Clin Infect Dis. 2000;30:662–678. doi: 10.1086/313749. [DOI] [PubMed] [Google Scholar]
- 16.Vazquez JA. Invasive oesophageal candidiasis: current and developing treatment options. Drugs. 2003;63:971–989. doi: 10.2165/00003495-200363100-00004. [DOI] [PubMed] [Google Scholar]
- 17.Cascio A, Barone M, Micali V, Iaria C, Delfino D, David A, et al. On a fatal case of Candida krusei pleural empyema in a pregnant woman with spontaneous esophagus perforation. Mycopathologia. 2010;169:451–455. doi: 10.1007/s11046-010-9277-6. [DOI] [PubMed] [Google Scholar]
- 18.Tran HA, Vincent JM, Slavin MA, Grigg A. Esophageal perforation secondary to angio-invasive Candida glabrata following hemopoietic stem cell transplantation. Clin Microbiol Infect. 2003;9:1215–1218. doi: 10.1111/j.1469-0691.2003.00762.x. [DOI] [PubMed] [Google Scholar]
- 19.Al-Shawwa B, D'Andrea L, Quintero D. Candida esophageal perforation and esophagopleural fistula: a case report. J Med Case Rep. 2008;2:209. doi: 10.1186/1752-1947-2-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanakala V, Lamb CA, Haigh C, Stirling RW, Attwood SE. The diagnosis of primary eosinophilic oesophagitis in adults: missed or misinterpreted? Eur J Gastroenterol Hepatol. 2010;22:848–855. doi: 10.1097/MEG.0b013e32832c7709. [DOI] [PubMed] [Google Scholar]