Abstract
Purpose
Refractory ascites (RA) is closely related to a high morbidity and mortality. In this study, we investigated predictors of RA development in patients with hepatitis B virus (HBV)-related cirrhosis who were hospitalized to control ascitic decompensation, and determined predictors for survival in patients who experienced RA.
Materials and Methods
We analyzed 199 consecutive patients with HBV-related cirrhosis who were hospitalized to control ascitic decompensation between January 1996 and December 2008.
Results
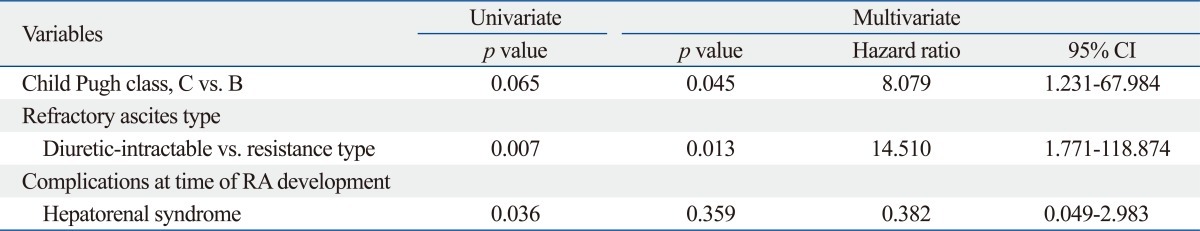
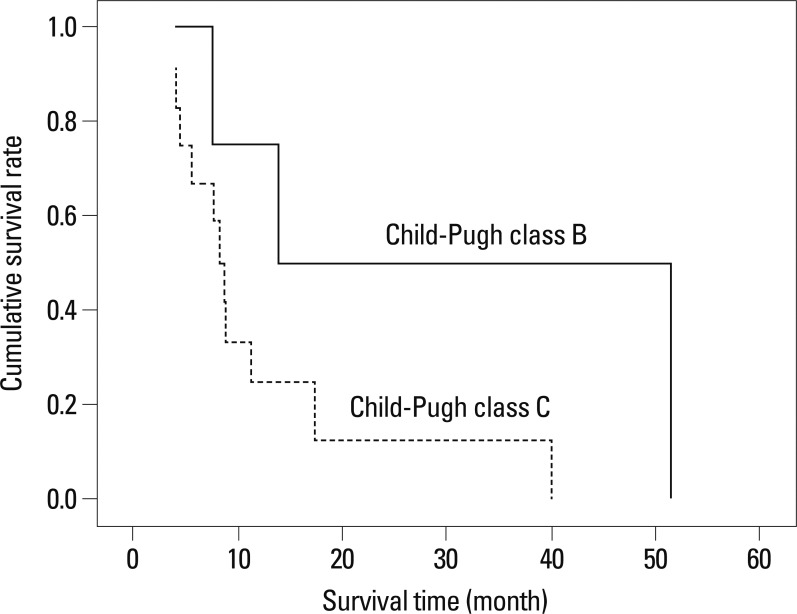
Multivariate analyses showed that only serum potassium at admission predicted RA development independently [p=0.013; hazard ratio (HR), 2.800; 95% confidence interval (CI), 1.166-6.722]. During the follow-up period, 16 (8.0%) patients experienced RA within 4.2 (range, 1.0-39.2) months after admission for controlling ascitic decompensation, and they survived a median of 8.7 (range, 3.9-51.3) months. Child-Pugh class and RA type were identified as independent prognostic factors affecting the survival in patients with RA (p=0.045; HR, 8.079; 95% CI, 1.231-67.984 and p=0.013; HR, 14.510; 95% CI, 1.771-118.874, respectively).
Conclusion
Serum potassium was an independent predictor of RA development in patients with HBV-related cirrhosis who were hospitalized to control ascitic decompensation. After RA development, Child-Pugh class and RA type were independent predictors for survival.
Keywords: Ascites, chronic hepatitis B, cirrhosis, predictor, refractory, survival
INTRODUCTION
Ascitic decompensation is a common major complication of cirrhosis and a sign of advanced liver disease because the prognosis becomes poorer after ascitic decompensation.1-3 Once cirrhosis is clinically confirmed, nearly 60% of patients experience ascitic decompensation within 10 years,4 and eventually ascites becomes resistant to medical treatments in 5-10% of the patients,5 which is called refractory ascites (RA). A significant increase in sodium excretion cannot be achieved because patients with RA either do not respond to a high dose of diuretics or they suffer from side effects related to the use of diuretics, such as hyperkalemia, hyponatremia, hepatic encephalopathy (HE), and renal failure. Thus, RA is closely related to high morbidity and mortality with a 2-year survival rate of less than 50%.6
To date, large volume paracentesis, peritoneo-venous shunt, and transjugular intra-hepatic portosystemic shunt (TIPS), combined with diuretics, have widely been used to control ascites by reducing portal pressure and improving renal sodium excretion in patients with RA, so as to improve their quality of life and help ease the waiting time for liver transplantation.7-9 However, these interventions have failed to significantly improve survival, and the prognosis of RA has been demonstrated to be related more to the severity of underlying liver disease, regardless of treatment modalities. Thus, liver transplantation has been strongly recommended for patients with RA to improve survival.10
Consequently, it is meaningful to investigate predictors of RA development in cirrhotic patients with ascitic decompensation, because these patients will benefit from prompt liver transplantation. However, there have been few investigations identifying predictors of RA development in cirrhotic patients. Thus, the primary aim of our present study was to identify predictors of RA development in patients with hepatitis B virus (HBV)-related cirrhosis who were hospitalized to control ascitic decompensation, and the secondary aim was to determine predictors for the survival of patients who experienced RA during the follow-up period.
MATERIALS AND METHODS
Patient characteristics
This study included 199 consecutive patients with HBV-related cirrhosis who were admitted to Severance Hospital, Yonsei University, Seoul, Korea, for controlling ascitic decompensation between January 1996 and December 2008. The study population had received oral diuretics for a median of 28.2 (range, 0.3-41.4) months before admission. Our study protocol was in compliance with the ethical guidelines of the 1975 Declaration of Helsinki. This retrospective study was approved by the institutional review board of our institute.
We excluded patients with concomitant hepatic decompensation such as HE, gastrointestinal variceal bleeding, or hepatorenal syndrome (HRS) at the time of admission and those with ascites caused by nephrogenic causes, tuberculosis, or malignancy. Patients with intra-abdominal malignancies, including hepatocellular carcinoma (HCC), at the time of admission, and those who underwent liver transplantation during the follow-up period were also excluded from the statistical analysis to exclude the possible confounding effects on the disease course.
We investigated age, gender, Child-Pugh class, past history of HE, comorbid disorders, complications at admission and during hospitalization, antiviral treatment, and performed serologic tests and ascitic fluid examinations. HBV DNA levels were measured using Digene Hybrid Capture assay (Digene, Gaithersburg, MD, USA), which has a lower detection limit of 141500 copies/mL. For patients who developed RA during the follow-up period, we checked also the type of RA, treatment modalities for RA, and amount of albumin infusion.
Diagnosis of ascites and RA
The diagnosis of ascites was made based on physical examination, ultrasonography or computed tomography results, and that of RA according to the International Ascites Club diagnostic criteria5 as follows: "ascites that cannot be mobilized, or early recurrence of ascites which cannot be satisfactorily prevented by medical therapy". Accordingly, there were two types of RA: "diuretic-intractable ascites" which could not be mobilized or its early recurrence could not be prevented because the development of diuretic-induced complications such as hyperkalemia, hypokalemia, hyponatremia, HE, or renal failure precluded the use of an effective diuretic dosage, and "diuretic-resistant ascites", which is induced by failure of a significant increase in sodium excretion because patients do not respond to high doses of diuretics (spironolactone 400 mg/day and furosemide 160 mg/day) and sodium restriction.5
Definitions of liver-related complications
The definitions of liver-related complications were as follows: 1) spontaneous bacterial peritonitis (SBP) was defined as more than 250 polymorphonuclear cells/mm3 in the ascitic fluid with no evidence of secondary peritonitis, accompanied by fever and abdominal pain,2,3 2) renal failure was defined as acute or chronic renal failure, but not HRS,11,12 3) gastrointestinal variceal bleeding was endoscopically confirmed, 4) HE was defined as personality changes, intellectual impairment, and a depressed level of consciousness,13 5) HRS was defined according to the International Ascites Club diagnostic criteria,5 and 6) liver failure was defined as rapid deterioration of liver function, resulting in coagulopathy, usually an international normalized ratio greater than 1.5 and any degree of HE.14
Treatment strategies
Ascites was managed with diuretics or intermittent paracentesis with cautions to prevent the increase of the previous serum creatinine level by more than 50% after ascites management.5 Patients with grade 2 or moderate ascites were treated with salt restriction (Na+ 30-50 mmol, 1600-2400 kcal/day) and a progressive dose of spironolactone alone or with furosemide (starting dose: furosemide 0-40 mg/day, spironolactone 50-100 mg/day; maintenance dose: furosemide 0-160 mg/day, spironolactone 50-400 mg/day). Patients with grade 3 or tense ascites were treated with additional albumin replacement, therapeutic paracentesis, peritoneo-venous shunt, or TIPS.1
RA development and survival analysis
RA development was used as an event to calculate predictors of RA development. For all patients, death was the end-point of follow-up. The overall survival time was calculated from the date of admission for controlling ascitic decompensation to death. Additionally, the survival time from the date of RA diagnosis to death was also calculated in months for patients who experienced RA during the follow-up period, and predictors for survival were also investigated in these patients.
Statistical analyses
Results of continuous variables are presented as means±standard deviation, and those of categorical variables as numbers (%). Student's t-test (or Mann-Whitney U test) and the chi-squared test (or Fisher's exact test) were used to compare quantitative and qualitative variables, respectively. Cumulative probability of RA development and cumulative survival of patients with RA were estimated according to the Kaplan-Meier method. To identify independent predictors of RA development and predictors for survival in patients with RA, variables that achieved a p-value <0.1 in univariate analyses were included in a multivariate analysis, based on a proportional hazards Cox regression model. Hazard ratio (HR) and corresponding 95% confidence intervals (CI) were calculated. All data analyses were conducted using the SPSS software (ver. 16.0; SPSS, Chicago, IL, USA).
RESULTS
Baseline characteristics
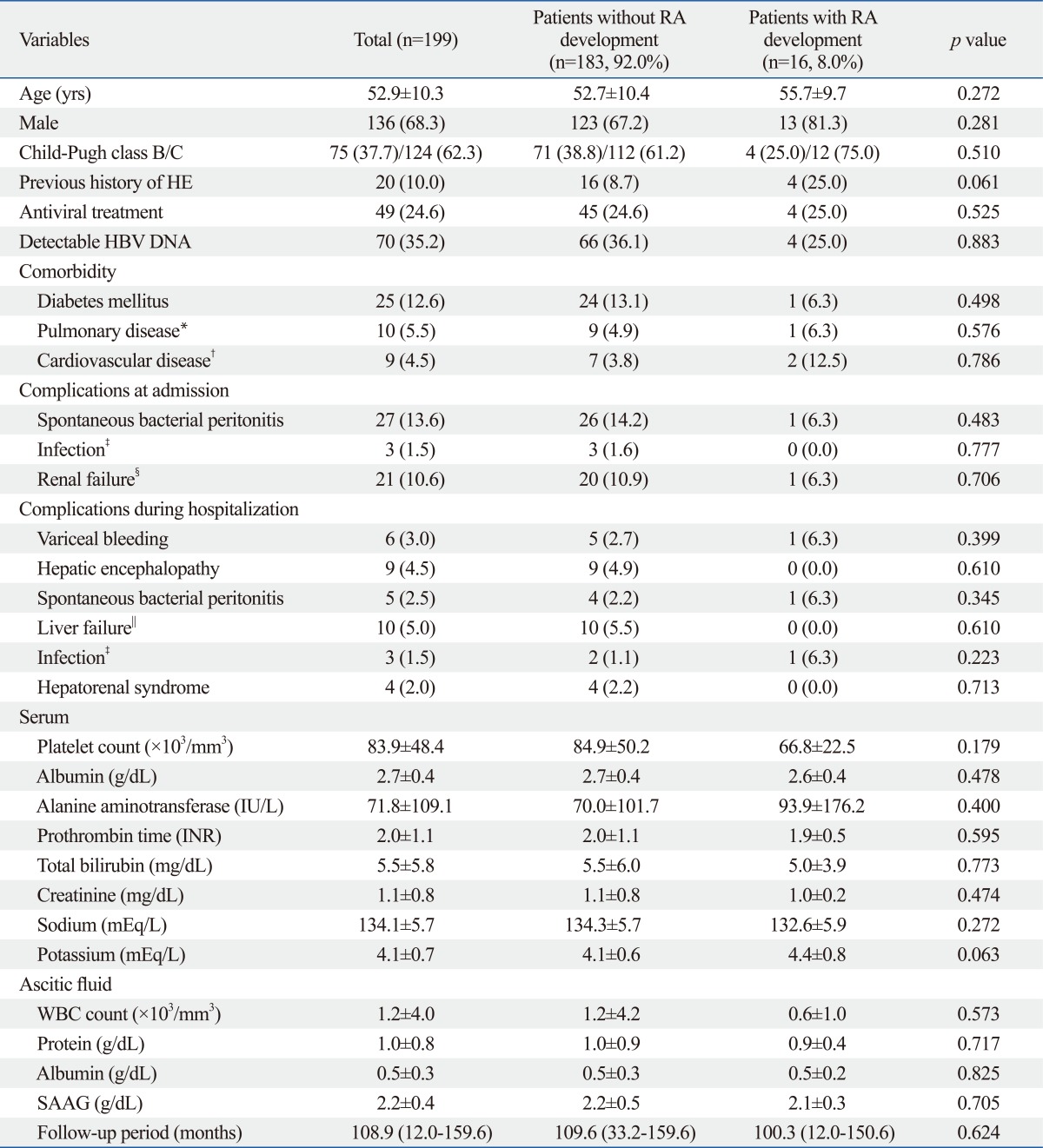
Baseline characteristics of 199 patients hospitalized to control ascitic decompensation are shown in Table 1. Of them, 136 (68.3%) patients were males, and the mean age of all patients was 52.9 years. SBP was the most common complication at admission (n=27, 13.6%), and concomitant infections (pneumonia, urinary tract infection, and sepsis) other than SBP were noted in 3 (1.5%) patients at admission. Seventy (35.2%) patients showed detectable HBV DNA and the median HBV DNA level in these patients was 1094520 (range, 141910-28422420) copies/mL. Of these, 49 patients received antiviral treatment using nucleos(t)ide analogs.
Table 1.
Baseline Characteristics of Patients with HBV-Related Liver Cirrhosis Who Were Hospitalized to Control Ascitic Decompensation at Admission (n=199)
HBV, hepatitis B virus; HE, hepatic encephalopathy; INR, international normalized ratio; WBC, white blood cell; SAAG, serum ascites albumin gradient.
Variables are expressed as mean±SD, median (range), or n (%).
*Pulmonary disease included pulmonary tuberculosis, chronic obstructive pulmonary disease, and asthma.
†Cardiovascular disease included hypertension and congestive heart failure.
‡Infection included pneumonia, urinary tract infection, and sepsis.
§Renal failure was defined as acute or chronic renal failure, but not hepatorenal syndrome.
∥Liver failure was defined as rapid deterioration of liver function results in coagulopathy, usually an INR greater than 1.5 and any degree of HE.
Between patients with and without RA development, there were no significant differences in baseline characteristics. However, the proportion of previous history of HE and serum potassium level had a trend to be higher in patients with RA development (25.0 vs. 8.7%; p=0.061 and 4.4±0.8 vs. 4.1±0.6 mEq/L; p=0.063).
Follow-up and survival
All follow-ups were discontinued on March 30, 2009 with a median follow-up period of 108.9 (range, 12.0-159.6) months after admission for controlling ascitic decompensation. The median survival of all patients was 10.7 (range, 0.3-158.8) months. During the follow-up period, 16 (8.0%) patients experienced RA within 4.2 (range, 1.0-39.2) months (13 patients within 1 year, 1 between 1-2 years, 1 between 2-3 years, and 1 between 3-4 years) and they survived a median of 8.7 (range, 3.9-51.3) months after RA development. The cumulative 1-, 2-, and 3-year survival rates after RA development were 25.0%, 18.8% and 12.5%, respectively. During the study period, 15 (7.5%) patients experienced HCC development.
Predictors of RA development
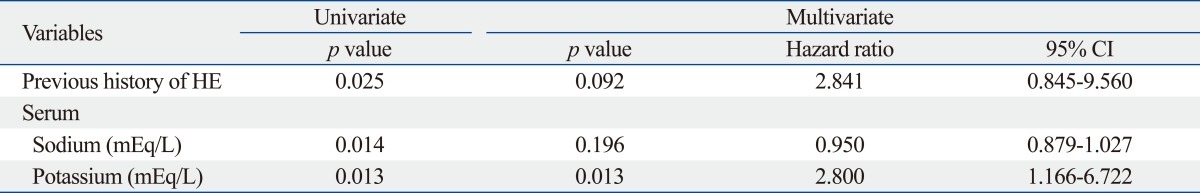
Table 2 shows the univariate and subsequent multivariate analyses that were performed to identify independent predictors of RA development. A previous history of HE, serum sodium, and serum potassium was selected as significant variables in univariate analysis associated to RA development. However, subsequent multivariate analysis using these three variables showed that serum potassium was the only independent predictor of RA development in patients with HBV-related cirrhosis who were hospitalized to control ascitic decompensation (p=0.013; HR, 2.800; 95% CI, 1.166-6.722).
Table 2.
Independent Predictors of RA Development in Patients with HBV-Related Liver Cirrhosis Who Were Hospitalized to Control Ascitic Decompensation (n=199)
HBV, hepatitis B virus; CI, confidence interval; HE, hepatic encephalopathy; RA, refractory ascites.
-2 log likelihood=102.212 (p value=0.002).
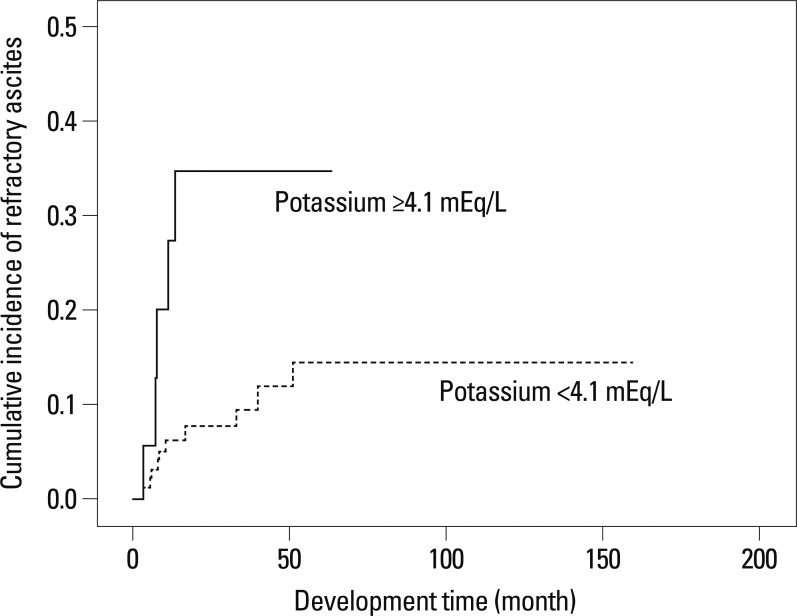
When we selected the mean serum potassium level of our study population (4.1 mEq/L; sensitivity 68.7%, specificity 54.6%, positive predictive value 11.6%, and negative predictive value 95.3%) as a cutoff value, RA development was significantly higher in patients with serum potassium ≥4.1 mEq/L than those with <4.1 mEq/L (log-rank test, p=0.011) (Fig. 1). The 1-, 2-, and 3-year cumulative incidence rates of RA were 81.8%, 100%, and 100%, respectively, in patients with serum potassium ≥4.1 mEq/L whereas they were 38.2%, 60.4%, and 100%, respectively, in those with serum potassium <4.1 mEq/L.
Fig. 1.
Kaplan-Meier estimate of RA development according to serum potassium levels in patients with HBV-related cirrhosis who were hospitalized to control ascitic decompensation. The incidence of RA is significantly higher in patients with serum potassium ≥4.1 mEq/L than in those with <4.1 mEq/L. RA, refractory ascites; HBV, hepatitis B virus.
Clinical characteristics at the time of diagnosis of RA
Sixteen (8.0%) patients developed RA in a median of 4.2 (range, 1.0-39.2) months, regardless of medical therapy or repeated paracentesis after admission for controlling ascitic decompensation. Table 3 shows the clinical characteristics at the time of diagnosis of RA. Their mean age was 55.7 years with a male predominance (n=13, 81.3%). Eleven (68.7%) patients had diuretic-resistant RA, and the other 5 (31.3%) had diuretic-intractable RA. For managing patients with RA, large-volume paracentesis was repeatedly performed for 2 (12.5%) patients, whereas peritoneo-venous shunt and TIPS were performed for 6 (37.5%) and 8 (50.0%) patients, respectively.
Table 3.
Clinical Characteristics of Patients in Whom Refractory Ascites Developed during Follow-Up Period (n=16)
TIPS, transjugular intrahepatic portosystemic shunt; RA, refractory ascites; INR, international normalized ratio; WBC, white blood cell; SAAG, serum ascites albumin gradient; HBV, hepatitis B virus.
Variables are expressed as mean±SD or n (%).
*Portosystemic shunt procedure included peritoneo-venous shunt and transjugular intrahepatic portosystemic shunt.
†Renal failure was defined as acute or chronic renal failure, but not hepatorenal syndrome.
Predictors for survival in patients with RA
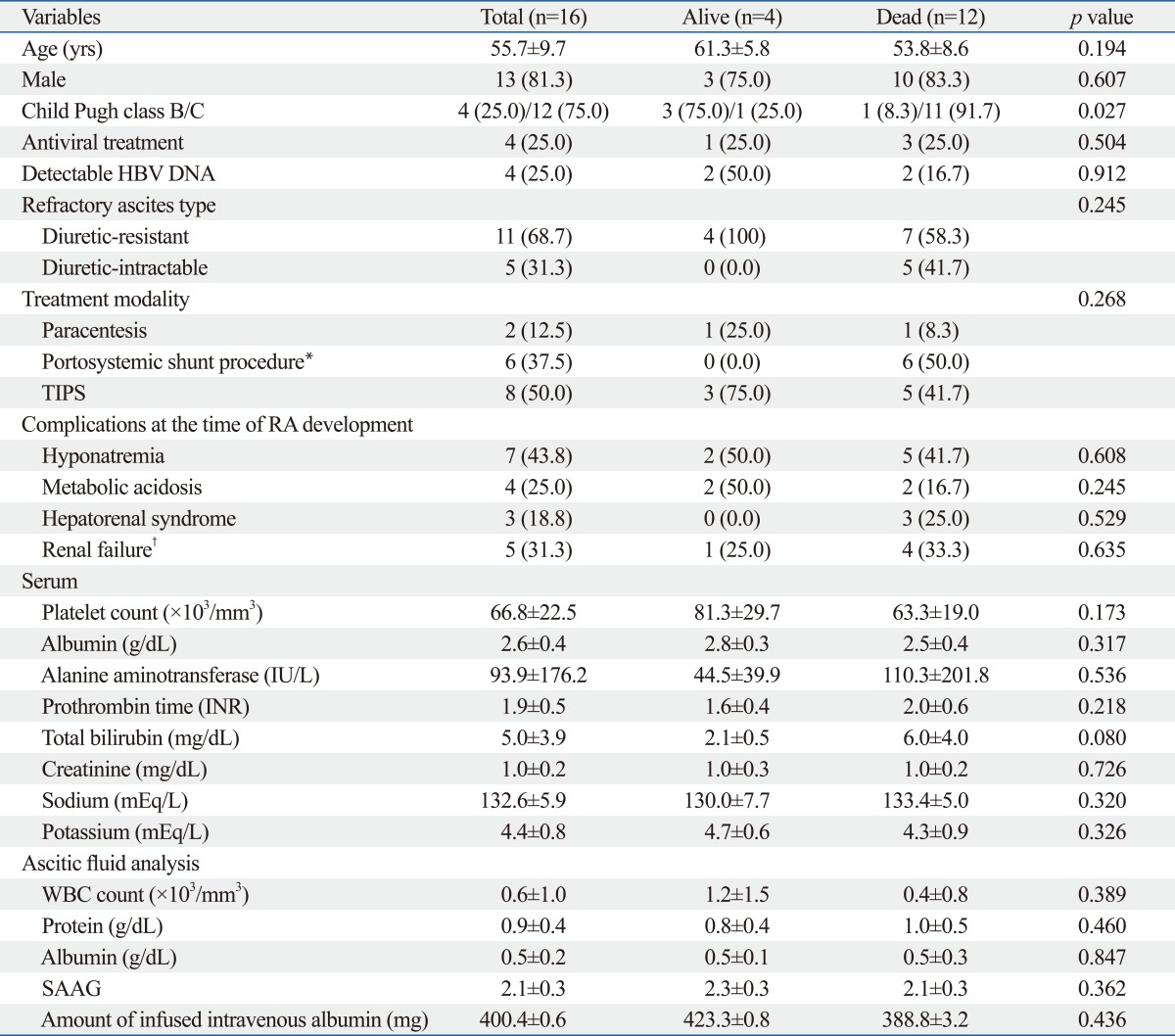
We compared the clinical characteristics at the time of RA diagnosis between 4 patients with RA who were alive (survivors) and 12 who were dead (non-survivors) at the end of follow-up period. There was no significantly different baseline characteristic between survivors and non-survivors except poorer liver function in non-survivors than survivors (Child-Pugh C class, 91.7 vs. 25.0%; p=0.027) (Table 3). All 4 survivors and 7 (58.3%) non-survivors had the diuretic-resistant type of RA.
In a multivariate Cox regression analysis, Child-Pugh class and RA type were found to be independent predictors for survival in patients with RA (p=0.045; HR, 8.079; 95% CI, 1.231-67.984 and p=0.013; HR, 14.510; 95% CI, 1.771-118.874, respectively) (Table 4). The median survival was significantly better in patients in Child-Pugh class B than those in Child-Pugh class C [median 13.9 (range, 7.5-51.3) vs. 8.3 (range, 3.9-39.8) months; log-rank test, p=0.034] (Fig. 2). Patients with the diuretic-resistant type of RA had a significantly better median survival than those with the diuretic-intractable type [median 13.9 (range, 4.1-51.3) vs. 5.6 (range, 3.9-8.7) months; log-rank test, p=0.002] (Fig. 3).
Table 4.
Independent Predictors of Overall Survival in Patients with Refractory Ascites (n=16)
CI, confidence interval; RA, refractory ascites.
-2 log likelihood=40.358 (p value=0.003). Reference values are Child-Pugh class B and diuretic-resistance type.
Fig. 2.
The cumulative survival rates according to Child-Pugh class in patients with RA. The median survival of patients with Child-Pugh B is significantly better than those with Child-Pugh C [median 13.9 months (range, 7.5-51.3) vs. 8.3 months (range, 3.9-39.8); log-rank test, p=0.034]. RA, refractory ascites.
Fig. 3.
The cumulative survival rates according to RA type in patients with RA. The median survival of patients with diuretic-resistant type RA is significantly better than those with diuretic-intractable type RA [median 13.9 months (range, 4.1-51.3) vs. 5.6 months (range, 3.9-8.7); log-rank test, p=0.002]. RA, refractory ascites.
Causes of mortality
Of 183 patients without RA, 157 (85.8%) had died by the end of follow-up. The most common causes of mortality were end-stage liver disease (n=108, 68.8%), followed by variceal bleeding in 28, progression of HCC in 6, septic shock in 12, and pneumonia in 3 patients. Of 16 patients with RA, 12 died by the end of follow-up and their causes of mortality were variceal bleeding in 4 (33.3%), HRS in 3 (25.0%), pneumonia in 3 (25.0%), and septic shock in 2 (16.7%) patients.
DISCUSSION
Refractory ascites is an infrequent condition, and only 5-10% of cirrhotic patients are admitted to a hospital due to severe ascites that does not respond to diuretics or complications that preclude the administration of adequate doses of diuretics.5,15 Among our 199 patients with HBV-related cirrhosis who were hospitalized to control ascitic decompensation, 16 (8.0%) patients developed RA regardless of medical interventions. Recently, a similar incidence of RA (5-10%) among patients with liver cirrhosis was reported.15 Because we calculated survival time only after admission without considering the treatment period for ascites in out-patient clinic, the relatively poorer median survival time of 10.7 months can be explained.
At least 40% of patients with responsive ascites are expected to survive for 5 years.16 In contrast, recent studies reported a poor prognosis after RA development, and the 1- and 2-year survival probabilities of patients with RA were only 50-52% and 30%, respectively, without liver transplantation.10,17 Thus, liver transplantation is recommended as the first and most important management of all cirrhotic patients with RA.10 However, liver transplantation is not a readily available procedure, and even if these patients wait for a long time, they may never reach the top of the transplant list. Repeated large volume paracentesis with albumin infusion, peritoneo-venous shunt, and TIPS are frequently performed to control RA while waiting for a liver transplant, although the efficacy and survival benefit of these treatment modalities are controversial.18-20 Thus, the identification of predictors that can select a subgroup of patients with liver cirrhosis who will experience RA, carries important meaning in establishing the priority of prompt liver transplantation.
In this study, serum potassium level was identified as the only independent predictor of RA development, while a previous history of HE showed borderline significance (p=0.092). Although some studies showed that liver failure was probably sufficient for RA development in patients with Child-Pugh C liver function,10,21 Child-Pugh class in our study was not found to be a significant predictor of RA development even in the univariate analysis. However, because a previous history of HE to indicate decreased liver function with portal hypertension showed borderline statistical significance for predicting RA development in the multivariate analysis, poor liver function seems to affect RA development to a certain extent.
Electrolyte imbalances in patients with liver cirrhosis, such as hyponatremia or hyperkalemia, are associated with severe ascites, as indicated by high prevalence of RA.22,23 Bernardi, et al.24 and Angeli, et al.25 showed a relationship between serum electrolyte levels and responsiveness of ascites to diuretic therapy. They found that patients who do not respond to diuretics have lower serum sodium and higher potassium concentrations than those who do respond to diuretics. Additionally, several reports have proposed that serum potassium level may affect RA development in part.22,26 Similarly, our study showed that the risk of developing RA was significantly related to a higher serum potassium level in patients with ascitic decompensation. Although serum sodium concentration is known to have a significant correlation with the survival of cirrhosis patients awaiting for liver transplantation,23,27 its role in the prediction of RA development has not been clear. In our study, a low sodium level was not found to be a significant predictor of RA development, although it was significant in a univariate analysis (p=0.014).
Underlying liver function, reflected by Child-Pugh class, was an independent predictor for survival in patients with RA. Interestingly, the RA type in our present study was also an independent predictor for survival in these patients. We think that based on the diuretic-resistance type according to the definition of diuretic-intractable ascites, the prognosis became poorer in patients with diuretic-intractable ascites, which might induce diuretic-induced complications. Indeed, in our study the prevalence of diuretic-induced complications was higher than that in the diuretic-resistant type (40-60% vs. 18.2-36.4%). Similarly, Planas, et al.15 showed that 11.4% of cirrhotic patients developed RA during follow-up, which was more frequent in diuretic-intractable ascites patients who developed an electrolyte imbalance (i.e., dilutional hyponatremia, diuretic-induced hypokalemia, or hyperkalemia).
In our study, the cumulative survival rate of patients with RA at 1 year after RA development was 25.0%, which was much lower than previous studies (30-50%).10,17 This result may be attributed to the fact that our study included HBV-related liver cirrhosis only, which did not represent all cases with liver cirrhosis caused by varying etiologies. Indeed, the progression of HCV-related liver cirrhosis can be stopped or delayed with pegylated-interferon plus ribavirin treatment, resulting in an improvement in long-term prognosis. In particular, sustained virological response is known to reduce the likelihood of clinical decompensation and improve survival independently in patients with hepatitis C virus-related chronic liver disease.28 Regarding alcoholic liver cirrhosis, abstinence has been a cornerstone of treatment strategies to improve short-term mortality and the severity of alcoholic liver cirrhosis.29 In contrast, potential viral breakthrough due to the appearance of a mutant HBV strain can be related to disease progression, despite active antiviral treatment in HBV-related liver cirrhosis. Thus, exclusion of HCV- or alcohol-related liver cirrhosis may have aggravated overall prognosis of our study population.
This study has several limitations. First, some variables, which might be important for patients with liver cirrhosis accompanying ascites, such as portal pressure, parameters indicating renal functions (e.g., urine Na+, urine K+, and the glomerular filtration rate), and hormone levels (plasma renin and aldosterone) were not included in our retrospective study. Second, our results cannot be expanded to all patients with liver cirrhosis caused by varying etiologies other than HBV. However, because of different disease courses due to different treatment strategies which are based on etiologies, we focused on HBV-related liver cirrhosis, which is prevalent in South Korea. Third, in spite of significant correlation between hyperkalemia and the use of spironolactone, we failed to unravel the role of hyperkalemia independent of spironolactone, because of heterogenous treatment period and various total cumulative dose of spironolactone in our study population.
In conclusion, we found in the present study that the prognosis became poor after RA development, and that serum potassium level was the only significant predictor of RA development in patients with HBV-related liver cirrhosis who were hospitalized to control ascitic decompensation. Furthermore, we demonstrated that Child-Pugh class and RA type significantly affected survival after RA development. However, future prospective studies should assess whether serum potassium level can be used to establish the priority of liver transplantation in these patients.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Healthcare technology R&D project, Ministry of Health and Welfare, Republic of Korea (A102065).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Kim SU, Han KH, Nam CM, Park JY, Kim do Y, Chon CY, et al. Natural history of hepatitis B virus-related cirrhotic patients hospitalized to control ascites. J Gastroenterol Hepatol. 2008;23:1722–1727. doi: 10.1111/j.1440-1746.2008.05510.x. [DOI] [PubMed] [Google Scholar]
- 2.Lee JM, Han KH, Ahn SH. Ascites and spontaneous bacterial peritonitis: an Asian perspective. J Gastroenterol Hepatol. 2009;24:1494–1503. doi: 10.1111/j.1440-1746.2009.06020.x. [DOI] [PubMed] [Google Scholar]
- 3.Kim SU, Kim do Y, Lee CK, Park JY, Kim SH, Kim HM, et al. Ascitic fluid infection in patients with hepatitis B virus-related liver cirrhosis: culture-negative neutrocytic ascites versus spontaneous bacterial peritonitis. J Gastroenterol Hepatol. 2010;25:122–128. doi: 10.1111/j.1440-1746.2009.05970.x. [DOI] [PubMed] [Google Scholar]
- 4.Ginés P, Quintero E, Arroyo V, Terés J, Bruguera M, Rimola A, et al. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7:122–128. doi: 10.1002/hep.1840070124. [DOI] [PubMed] [Google Scholar]
- 5.Arroyo V, Ginès P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23:164–176. doi: 10.1002/hep.510230122. [DOI] [PubMed] [Google Scholar]
- 6.Forns X, Ginès A, Ginès P, Arroyo V. Management of ascites and renal failure in cirrhosis. Semin Liver Dis. 1994;14:82–96. doi: 10.1055/s-2007-1007300. [DOI] [PubMed] [Google Scholar]
- 7.Martinet JP, Fenyves D, Legault L, Roy L, Dufresne MP, Spahr L, et al. Treatment of refractory ascites using transjugular intrahepatic portosystemic shunt (TIPS): a caution. Dig Dis Sci. 1997;42:161–166. doi: 10.1023/a:1018861827399. [DOI] [PubMed] [Google Scholar]
- 8.Ginès A, Fernández-Esparrach G, Monescillo A, Vila C, Domènech E, Abecasis R, et al. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology. 1996;111:1002–1010. doi: 10.1016/s0016-5085(96)70068-9. [DOI] [PubMed] [Google Scholar]
- 9.Stanley MM, Ochi S, Lee KK, Nemchausky BA, Greenlee HB, Allen JI, et al. Peritoneovenous shunting as compared with medical treatment in patients with alcoholic cirrhosis and massive ascites. Veterans Administration Cooperative Study on Treatment of Alcoholic Cirrhosis with Ascites. N Engl J Med. 1989;321:1632–1638. doi: 10.1056/NEJM198912143212403. [DOI] [PubMed] [Google Scholar]
- 10.Moreau R, Delègue P, Pessione F, Hillaire S, Durand F, Lebrec D, et al. Clinical characteristics and outcome of patients with cirrhosis and refractory ascites. Liver Int. 2004;24:457–464. doi: 10.1111/j.1478-3231.2004.0991.x. [DOI] [PubMed] [Google Scholar]
- 11.Ginès A, Escorsell A, Ginès P, Saló J, Jiménez W, Inglada L, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105:229–236. doi: 10.1016/0016-5085(93)90031-7. [DOI] [PubMed] [Google Scholar]
- 12.Chutaputti A. Management of refractory ascites and hepatorenal syndrome. J Gastroenterol Hepatol. 2002;17:456–461. doi: 10.1046/j.1440-1746.2002.02724.x. [DOI] [PubMed] [Google Scholar]
- 13.O'Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342:273–275. doi: 10.1016/0140-6736(93)91818-7. [DOI] [PubMed] [Google Scholar]
- 14.Adukauskiene D, Dockiene I, Naginiene R, Kevelaitis E, Pundzius J, Kupcinskas L. Acute liver failure in Lithuania. Medicina (Kaunas) 2008;44:536–540. [PubMed] [Google Scholar]
- 15.Planas R, Montoliu S, Ballesté B, Rivera M, Miquel M, Masnou H, et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol. 2006;4:1385–1394. doi: 10.1016/j.cgh.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Salerno F, Borroni G, Moser P, Badalamenti S, Cassarà L, Maggi A, et al. Survival and prognostic factors of cirrhotic patients with ascites: a study of 134 outpatients. Am J Gastroenterol. 1993;88:514–519. [PubMed] [Google Scholar]
- 17.Cárdenas A, Arroyo V. Refractory ascites. Dig Dis. 2005;23:30–38. doi: 10.1159/000084723. [DOI] [PubMed] [Google Scholar]
- 18.Rössle M, Ochs A, Gülberg V, Siegerstetter V, Holl J, Deibert P, et al. A comparison of paracentesis and transjugular intrahepatic portosystemic shunting in patients with ascites. N Engl J Med. 2000;342:1701–1707. doi: 10.1056/NEJM200006083422303. [DOI] [PubMed] [Google Scholar]
- 19.Ginès P, Uriz J, Calahorra B, Garcia-Tsao G, Kamath PS, Del Arbol LR, et al. Transjugular intrahepatic portosystemic shunting versus paracentesis plus albumin for refractory ascites in cirrhosis. Gastroenterology. 2002;123:1839–1847. doi: 10.1053/gast.2002.37073. [DOI] [PubMed] [Google Scholar]
- 20.Quiroga J, Sangro B, Núñez M, Bilbao I, Longo J, García-Villarreal L, et al. Transjugular intrahepatic portal-systemic shunt in the treatment of refractory ascites: effect on clinical, renal, humoral, and hemodynamic parameters. Hepatology. 1995;21:986–994. [PubMed] [Google Scholar]
- 21.Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38:258–266. doi: 10.1053/jhep.2003.50315. [DOI] [PubMed] [Google Scholar]
- 22.Ginès P, Cárdenas A. The management of ascites and hyponatremia in cirrhosis. Semin Liver Dis. 2008;28:43–58. doi: 10.1055/s-2008-1040320. [DOI] [PubMed] [Google Scholar]
- 23.Moini M, Hoseini-Asl MK, Taghavi SA, Sagheb MM, Nikeghbalian S, Salahi H, et al. Hyponatremia a valuable predictor of early mortality in patients with cirrhosis listed for liver transplantation. Clin Transplant. 2011;25:638–645. doi: 10.1111/j.1399-0012.2010.01350.x. [DOI] [PubMed] [Google Scholar]
- 24.Bernardi M, Laffi G, Salvagnini M, Azzena G, Bonato S, Marra F, et al. Efficacy and safety of the stepped care medical treatment of ascites in liver cirrhosis: a randomized controlled clinical trial comparing two diets with different sodium content. Liver. 1993;13:156–162. doi: 10.1111/j.1600-0676.1993.tb00624.x. [DOI] [PubMed] [Google Scholar]
- 25.Angeli P, Wong F, Watson H, Ginès P CAPPS Investigators. Hyponatremia in cirrhosis: results of a patient population survey. Hepatology. 2006;44:1535–1542. doi: 10.1002/hep.21412. [DOI] [PubMed] [Google Scholar]
- 26.Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130:1652–1660. doi: 10.1053/j.gastro.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Heuman DM, Abou-Assi SG, Habib A, Williams LM, Stravitz RT, Sanyal AJ, et al. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology. 2004;40:802–810. doi: 10.1002/hep.20405. [DOI] [PubMed] [Google Scholar]
- 28.Fernández-Rodríguez CM, Alonso S, Martinez SM, Forns X, Sanchez-Tapias JM, Rincón D, et al. Peginterferon plus ribavirin and sustained virological response in HCV-related cirrhosis: outcomes and factors predicting response. Am J Gastroenterol. 2010;105:2164–2172. doi: 10.1038/ajg.2010.294. [DOI] [PubMed] [Google Scholar]
- 29.Kalaitzakis E, Wallskog J, Björnsson E. Abstinence in patients with alcoholic liver cirrhosis: A follow-up study. Hepatol Res. 2008;38:869–876. doi: 10.1111/j.1872-034X.2008.00353.x. [DOI] [PubMed] [Google Scholar]