Abstract
Purpose
Acute kidney injury (AKI) caused by hypothyroidism-induced rhabdomyolysis is a rare and potentially life-threatening syndrome. The aim of this study was to investigate the clinical characteristics of such patients.
Materials and Methods
We retrospectively analyzed five patients treated at the Second Affiliated Hospital of Chongqing Medical University with AKI secondary to hypothyroidism-induced rhabdomyolysis from January 2006 to December 2010.
Results
Of the five cases reviewed (4 males, age range of 37 to 62 years), adult primary hypothyroidism was caused by amiodarone (1 case), chronic autoimmune thyroiditis (1 case), and by uncertain etiologies (3 cases). All patients presented with facial and lower extremity edema. Three patients presented with weakness, while two presented with blunted facies and oliguria. Only one patient reported experiencing myalgia and proximal muscle weakness, in addition to fatigue and chills. Creatine kinase, lactate dehydrogenase, and renal function normalized after thyroid hormone replacement, except in two patients who improved through blood purification.
Conclusion
Hypothyroidism should be considered in patients presenting with renal impairment associated with rhabdomyolysis. Moreover, further investigation into the etiology of the hypothyroidism is warranted.
Keywords: Acute kidney injury, hypothyroidism, rhabdomyolysis
INTRODUCTION
Rhabdomyolysis is a potentially life-threatening syndrome characterized by muscle necrosis and the release of intracellular muscle contents into the circulation. The etiology of rhabdomyolysis can be classified into traumatic and nontraumatic causes. The latter is associated with alcohol and drug abuse, seizures, strenuous exercise, muscle hypoperfusion, hyperthermia, electrolyte disturbances, diabetic coma, severe infection and hypothyroidism,1 among others. Among hospitalized patients, nontraumatic rhabdomyolysis occurs with a prevalence five times that of traumatic causes.2 Nontraumatic rhabdomyolysis is elusive; it often occurs without symptoms and is diagnosed when creatine kinase (CK) levels are elevated in the initial stages of the disease. Acute kidney injury (AKI) and severe electrolyte derangements are life threatening, and are accompanied by extreme elevations in CK levels. Alarmingly, 13% to over 50% of patients with rhabdomyolysis develop AKI.3 While hypothyroidism is a definite cause of rhabdomyolysis, AKI due to hypothyroidism-induced rhabdomyolysis has rarely been reported. We report five cases of AKI secondary to hypothyroidism-induced rhabdomyolysis. We propose that hypothyroidism should be considered in patients presenting with renal impairment associated with rhabdomyolysis and emphasize that the causes of hypothyroidism warrant further investigation.
MATERIALS AND METHODS
We retrospectively analyzed five cases of AKI secondary to hypothyroidism-induced rhabdomyolysis treated from January 2006 to December 2010 at the Second Affiliated Hospital of Chongqing Medical University. All patients fulfilled the following Acute Kidney Injury Network proposed diagnostic criteria for AKI: an abrupt (within 48 hours) reduction in kidney function defined by an absolute increase in serum creatinine of ≥26.4 µmol/L (0.3 mg/dL), a ≥50% increase in serum creatinine (1.5-fold from baseline), or a reduction in urine output (<0.5 mL/kg/h for >6 h).4 The diagnosis of rhabdomyolysis was primarily based upon the presence of muscle injury and elevated levels of CK, lactate dehydrogenase (LDH), and plasma and/or urine myoglobin.
RESULTS
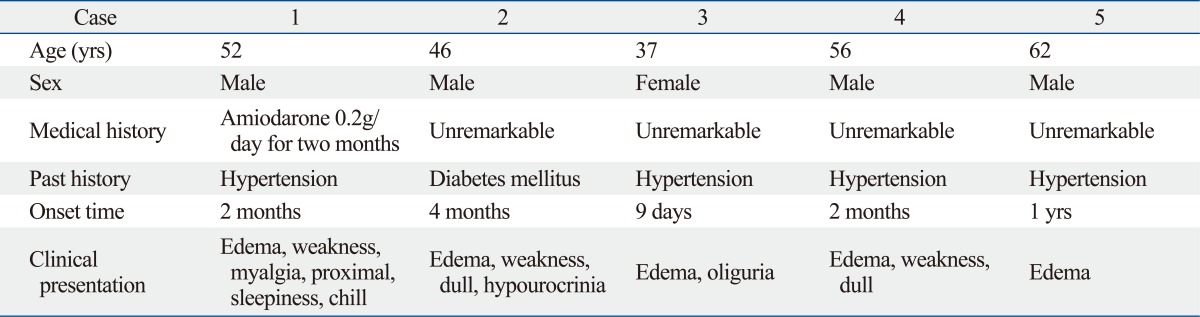
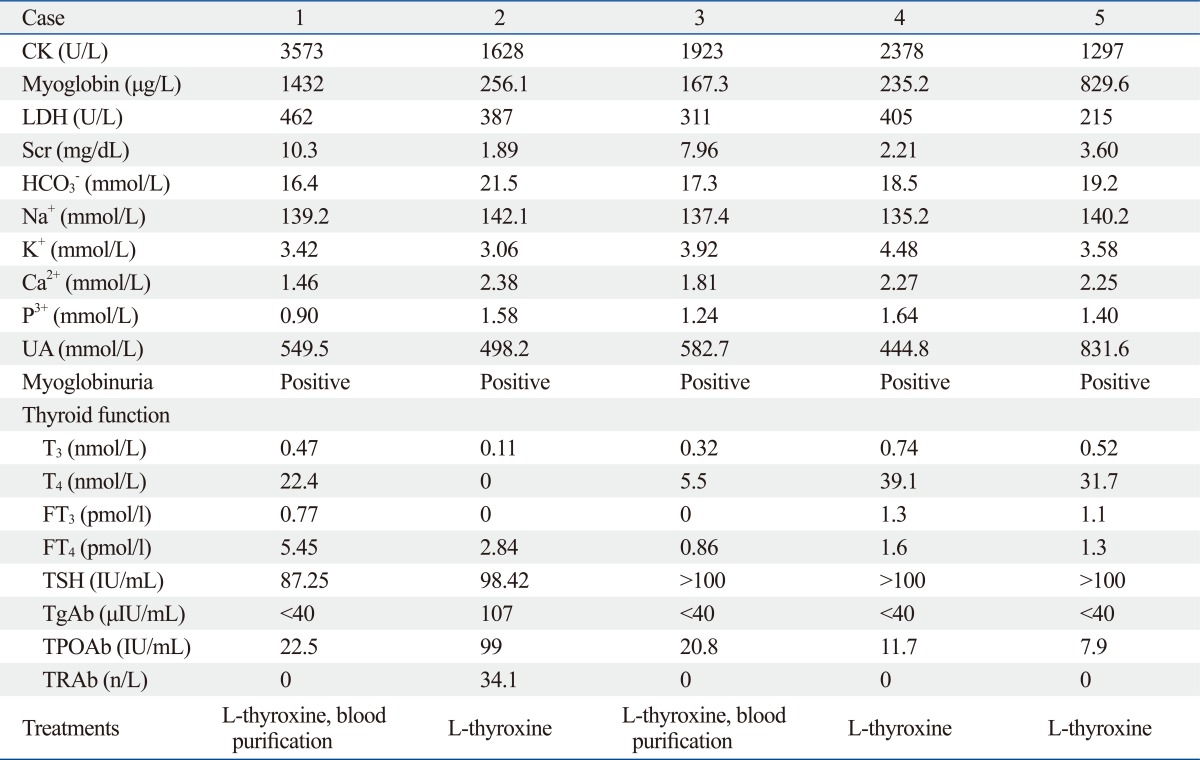
Patient demographics are described in Table 1 and 2. None of the patients had a history of renal disease. Of the five cases reviewed (4 males, age range of 37 to 62 years), adult primary hypothyroidism was caused by amiodarone (1 case), chronic autoimmune thyroiditis (1 case), and by uncertain etiologies (3 cases). Four patients presented with hypertension. One patient presented with diabetes mellitus without diabetic coma. In all cases, facial and lower extremity edema was present. Three patients presented with weakness, and two patients had blunted facies and oliguria. One patient had complications of myalgia and proximal muscle weakness, fatigue, and chills. AKI was diagnosed in all patients with a median serum creatinine of 3.60 mg/dL (range 1.89-10.3 mg/dL) and hyperuricemia, without proteinuria and hematuria. All patients with normal levels of serum sodium exhibited clinical evidence of rhabdomyolysis (elevated CK, LDH, and myoglobinuria). Hypocalcemia was found in two patients. Immunological surveys included rheumatic factor, antinuclear antibody, immunoglobulin G/A/M, complement 3/4, anti-dsDNA antibody, anti-mitochondrial antibody, anti-smooth muscle antibody, anti-neutrophil cytoplasmic antibody, and anti-extractable nuclear antigen panels, all of which were within normal limits. All patients had low total or free thyroxine (FT4) and high thyroid stimulating hormone (TSH) levels. Both serum anti-thyroglobulin antibody (TgAb) and thyroid peroxidase antibody (TPOAb) were negative in all patients except for one. Thyroid gland ultrasound tests failed to demonstrate goiter in any patient.
Table 1.
Patient Demographics
Table 2.
Laboratory Values, Treatment Methods
CK, creatine kinase; LDH, lactate dehydrogenase; TSH, thyroid stimulating hormone; TPOAb, thyroid peroxidase antibody; TgAb, antithyroglobulin antibody; TRAb, anti thyroid hormone receptor antibody; UA, uric acid.
Normal reference levels: CK, 38-174 U/L; myoglobin, 0-70 g/L; LDH, 50-245 U/L; Scr, <1.5 mg/dL; HCO3-, 22.0-30.0 mmol/L; Na+ 135-145 mmol/L; K+ 3.5-5.5 mmol/L; Ca2+ 2.12-2.92 mmol/L; P3+, 0.87-1.42 mmol/L; UA, 90-420 mmol/L; Thyroid function: T3, 0.92-2.79 nmol/L; T4, 58.1-140.6 nmol/L; FT3, 3.5-6.5 pmol/L; FT4, 11.48-22.7 pmol/L; TSH, 0.35-5.5 µIU/mL; TgAb, <40 IU/Ml; TPOAb, <35 IU/Ml; TRAb, <13 IU/L.
As part of the treatment management (in addition to early diuresis, alkalinization of the urine, and withdrawal of suspect medications such as amiodarone), two patients required effective renal replacement therapy to correct fluid, electrolyte, acid-base abnormalities and to remove products of muscle breakdown. Furthermore, all patients received thyroid hormone replacement therapy (L-thyroxine 25 ug/d initial dose, increased to 50 µg/d and 100 µg/d after one and two weeks respectively). Dosages were adjusted according to thyroid function until normalization. When renal function returned to normal and the patient was ready for discharge, dialysis was discontinued.
DISCUSSION
Until now, AKI secondary to hypothyroidism-induced rhabdomyolysis has rarely been reported. Rhabdomyolysis is associated with a wide variety of causes. The pathogenesis of rhabdomyolysis is related to direct sarcolemmic injury (e.g., trauma) or depletion of ATP within myocytes, leading to an unregulated leakage of extracellular calcium ions into the intracellular space.5 Sarcoplasmic calcium is strictly regulated by a series of energy-dependent ion pumps, such as Na+/K+ ATPase and Ca2+ ATPase in the sarcolemma, as well as in other intracellular membranes, that maintain low calcium levels when muscle is at rest and that allow for its increase when actin-myosin binding and muscle contraction is necessary. Regardless of the underlying mechanism, injury to the muscle leads to an increase in sarcoplasmic calcium and persistent contraction. Ultimately, cell protease is activated, followed by subsequent muscle fiber necrosis and release of potassium, phosphates, myoglobin, CK, and uric acid into the extracellular space and bloodstream.6 Hypothyroidism is an uncommon, nontraumatic cause of rhabdomyolysis, the exact etiology of which remains unclear. Thyroid hormone deficiency in glycogenolysis and mitochondrial oxidative metabolism has been proposed as a possible explanation.7
In our cases, the diagnoses of rhabdomyolysis and AKI were based on abnormal laboratory results of elevated serum CK and creatinine, as well as urinary and serum myoglobin. CK is considered the biochemical gold standard for diagnosing rhabdomyolysis. Although there is no established exact serum CK threshold, a concentration five times the upper limit of the normal (i.e., 1000 U/L) is commonly used.8 CK values are generally considered predictive of the likelihood of developing AKI. While a level of 5000 U/L or greater has been linked to renal failure,9 this has not been shown to correlate with the severity of renal failure.1 Indeed, AKI has been shown to develop after the diagnosis of rhabdomyolysis in patients with higher peak serum CK levels at admission.10 As is, there is insufficient evidence for the use of urine myoglobin as a predictor of AKI in patients with suspected rhabdomyolysis, particularly if serum concentrations are high and urine levels are low.11 Low myoglobin clearance may indicate a higher risk for developing AKI, or may be an early marker for kidney dysfunction.12
Hypothyroidism has been deemed a principal cause of rhabdomyolysis and AKI. Although hyponatremia is a common electrolyte derangement in hypothyroidism, especially with kidney derangements, in our study hyponatremia-induced rhabdomyolysis was ruled out by normal levels of serum sodium. In one representative case from our study, adult primary hypothyroidism was caused by amiodarone. Amiodarone is a potent anti-arrhythmic and iodine-rich drug. Its administration may lead to disturbances in thyroid hormone metabolism and/or overt gland dysfunction.13 Each molecule of amiodarone contains two iodine atoms, which constitute 37.5% of its mass. Hence, a patient taking a 200 mg daily dose of amiodarone ingests 75 mg of organic iodine each day. The daily free iodine amount that is subsequently metabolized is 20-40 times higher than the intake thereof in the general population. Amiodarone has a half-life of approximately 100 days, mainly due to its storage in adipose tissue. Its toxicities may persist or even occur after its discontinuation.14 Iodine intake, baseline TSH, and autoimmune thyroiditis may affect the thyroid, even resulting in the development of amiodarone-induced hypothyroidism (AIH). Irrespective of iodine intake, the incidence of AIH ranges from 1-32%.15 AIH generally occurs relatively early (3-12 months) after starting treatment with amiodarone.16
Various theories have been proposed to explain the pathogenesis of AIH. Failing to avoid the Wolff-Chaikoff effect may be the most likely pathogenic mechanism. The clinical presentation of AIH is usually subtle; one patient presented with facial edema, weakness, myalgia, proximal muscle weakness, fatigue, and chills after 2 months of amiodarone use. Moreover, there was no difference in the clinical manifestations of AIH in this patient compared to those with hypothyroidism from other causes. The laboratory features of AIH are similar to those in patients with other causes of primary hypothyroidism. The diagnosis is confirmed by a high TSH concentration (usually above 20 mIU/L) in combination with low total thyroxine or FT4. Triiodothyronine (T3) concentration alone is not considered useful or reliable for diagnosing AIH. It is essential to carefully evaluate patients before and during amiodarone therapy. Careful thyroid gland examination and baseline TSH, FT4, and TPOAb measurements are necessary. Serum TSH and FT4 should be measured after 3 months of therapy and semiannually every 6 months thereafter.
Chronic autoimmune thyroiditis is the leading cause of primary hypothyroidism in iodine-sufficient areas. Clinically, patients may present with or without goiter. The incidence of the disease is particularly high in middle aged or older women. Both types of anti-thyroid antibodies are usually detected; the detection of only one type is rare.17 Additionally, it is rare to find a middle-aged male with chronic autoimmune thyroiditis. Usually an aggravating factor such as the use of lipid-lowering drugs, alcohol, exercise, or chronic renal failure can be identified. In present study, three patients with rhabdomyolysis due to hypothyroidism involved no additional precipitating factor, and the cause of hypothyroidism was uncertain.
Patients with very severe and longstanding hypothyroidism are more likely to develop concurrent complications, such as muscle injury inducing rhabdomyolysis, which is implicated as a significant cause of AKI. Although the exact mechanisms by which rhabdomyolysis impairs glomerular filtration rate (GFR) are unclear. Some evidence suggests that the mechanisms of renal damage may include: 1) intrarenal vasoconstriction and ischemia; 2) direct and indirect ischemic tubule injury; and 3) tubular obstruction. Hypothyroidism may aggravate renal ischemia, as hypothyroidism exerts some hemodynamic effects for reducing cardiac output, increasing systemic and renal vascular resistance, and reducing GFR.18 Accordingly, a significant correlation between TSH and Scr has been found; the greater the impairment in thyroid function was, the more commonly renal failure occurred.19
The typical symptoms of rhabdomyolysis include muscle pain, weakness, and myoglobinuria. Even so, this classic triad occurs in only 10% of patients, and more than half lack clinical evidence of muscle pain or weakness,20 whether traumatic or nontraumatic. Nontraumatic rhabdomyolysis due to hypothyroidism is clinically obscure. In our study, approximately one year was the longest onset time in which a patient presented with facial edema. Furthermore, although patients may be asymptomatic for an extended period, the disease can later present at a serious stage (case 2, 9 days). Therefore, it is essential to attenuate the severity of rhabdomyolysis by early detection of the underlying cause and comorbid conditions and by prompt therapeutic management. Active thyroid hormone replacement therapy and blood purification are imperative.
In conclusion, any patient presenting with unexplained AKI, accompanied by myalgia, weakness and elevated serum muscle enzymes should be evaluated for hypothyroidism-induced rhabdomyolysis. In addition, the etiology of hypothyroidism should be determined. To avoid AIH development, thyroid function should be carefully monitored either before or after administration of amiodarone.
ACKNOWLEDGEMENTS
This study was supported by a grant from Chongqing Municipal health bureau No.2011-2-143 (to L.T.).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Bagley WH, Yang H, Shah KH. Rhabdomyolysis. Intern Emerg Med. 2007;2:210–218. doi: 10.1007/s11739-007-0060-8. [DOI] [PubMed] [Google Scholar]
- 2.Visweswaran P, Guntupalli J. Rhabdomyolysis. Crit Care Clin. 1999;15:415–428. doi: 10.1016/s0749-0704(05)70061-0. [DOI] [PubMed] [Google Scholar]
- 3.Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361:62–72. doi: 10.1056/NEJMra0801327. [DOI] [PubMed] [Google Scholar]
- 4.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannoglou GD, Chatzizisis YS, Misirli G. The syndrome of rhabdomyolysis: pathophysiology and diagnosis. Eur J Intern Med. 2007;18:90–100. doi: 10.1016/j.ejim.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Khan FY. Rhabdomyolysis: a review of the literature. Neth J Med. 2009;67:272–283. [PubMed] [Google Scholar]
- 7.Kisakol G, Tunc R, Kaya A. Rhabdomyolysis in a patient with hypothyroidism. Endocr J. 2003;50:221–223. doi: 10.1507/endocrj.50.221. [DOI] [PubMed] [Google Scholar]
- 8.Cervellin G, Comelli I, Lippi G. Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clin Chem Lab Med. 2010;48:749–756. doi: 10.1515/CCLM.2010.151. [DOI] [PubMed] [Google Scholar]
- 9.Brown CV, Rhee P, Chan L, Evans K, Demetriades D, Velmahos GC. Preventing renal failure in patients with rhabdomyolysis: do bicarbonate and mannitol make a difference? J Trauma. 2004;56:1191–1196. doi: 10.1097/01.ta.0000130761.78627.10. [DOI] [PubMed] [Google Scholar]
- 10.de Meijer AR, Fikkers BG, de Keijzer MH, van Engelen BG, Drenth JP. Serum creatine kinase as predictor of clinical course in rhabdomyolysis: a 5-year intensive care survey. Intensive Care Med. 2003;29:1121–1125. doi: 10.1007/s00134-003-1800-5. [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez-Capote K, Balion CM, Hill SA, Cleve R, Yang L, El Sharif A. Utility of urine myoglobin for the prediction of acute renal failure in patients with suspected rhabdomyolysis: a systematic review. Clin Chem. 2009;55:2190–2197. doi: 10.1373/clinchem.2009.128546. [DOI] [PubMed] [Google Scholar]
- 12.Wu AH, Laios I, Green S, Gornet TG, Wong SS, Parmley L, et al. Immunoassays for serum and urine myoglobin: myoglobin clearance assessed as a risk factor for acute renal failure. Clin Chem. 1994;40:796–802. [PubMed] [Google Scholar]
- 13.Padmanabhan H. Amiodarone and thyroid dysfunction. South Med J. 2010;103:922–930. doi: 10.1097/SMJ.0b013e3181e90500. [DOI] [PubMed] [Google Scholar]
- 14.Basaria S, Cooper DS. Amiodarone and the thyroid. Am J Med. 2005;118:706–714. doi: 10.1016/j.amjmed.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 15.Harjai KJ, Licata AA. Effects of amiodarone on thyroid function. Ann Intern Med. 1997;126:63–73. doi: 10.7326/0003-4819-126-1-199701010-00009. [DOI] [PubMed] [Google Scholar]
- 16.Wiersinga WM. Towards an animal model of amiodarone-induced thyroid dysfunction. Eur J Endocrinol. 1997;137:15–17. doi: 10.1530/eje.0.1370015. [DOI] [PubMed] [Google Scholar]
- 17.Kostoglou-Athanassiou I, Ntalles K. Hypothyroidism-new aspects of an old disease. Hippokratia. 2010;14:82–87. [PMC free article] [PubMed] [Google Scholar]
- 18.Ardalan MR, Ghabili K, Mirnour R, Shoja MM. Hypothyroidism-induced rhabdomyolysis and renal failure. Ren Fail. 2011;33:553–554. doi: 10.3109/0886022X.2011.569109. [DOI] [PubMed] [Google Scholar]
- 19.Baajafer FS, Hammami MM, Mohamed GE. Prevalence and severity of hyponatremia and hypercreatininemia in short-term uncomplicated hypothyroidism. J Endocrinol Invest. 1999;22:35–39. doi: 10.1007/BF03345476. [DOI] [PubMed] [Google Scholar]
- 20.Huerta-Alardin AL, Varon J, Marik PE. Bench-to-bedside review: Rhabdomyolysis--an overview for clinicians. Crit Care. 2005;9:158–169. doi: 10.1186/cc2978. [DOI] [PMC free article] [PubMed] [Google Scholar]