Abstract
Purpose
The repopulating lymphocytes after allogeneic hematopoietic stem cell transplantation have an important role not only on the prevention of serious infections in the early transplantation period, but also on the killing of residual leukemic cells by graft-versus-leukemia effect. The aim of this study was to analyze the impact of lymphocyte recovery after allogeneic stem cell transplantation in children with hematologic malignancies.
Materials and Methods
We evaluated 69 children transplanted for acute lymphoblastic leukemia (ALL) (n=34), acute myeloid leukemia (AML) (n=26), chronic leukemia (n=7) and juvenile myelomonocytic leukemia (n=2) between 1996 and 2008 at the Chonnam National University Hospital, Korea. The patients were grouped based on absolute lymphocyte counts (ALC) <500/µL or ≥500/µL at D+21 and D+30 after transplant.
Results
Patients with a High ALC at D+21 and D+30 had a faster neutrophil and platelet engraftment. The High at D+30 group had a better 5 year overall survival (71% vs. 53%, p=0.043) and event-free survival (72% vs. 53%, p=0.065) than the Low at D+30 group. The incidence of grade II-IV acute and chronic graft-versus-host disease (GVHD), and relapse rate did not differ by the ALC counts. However, the Low at D+30 group had a significantly increased risk for transplant-related mortality (p=0.019). The univariate analysis showed that the factors associated with decreased survival were a Low ALC at D+30, patients with high risk ALL, and grade II-IV aGVHD in patients with ALL and AML.
Conclusion
Early posttransplant serial lymphocyte measurement would be a simple but useful method for predicting transplant outcomes.
Keywords: Absolute lymphocyte count, allogeneic stem cell transplantation, children, leukemia
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (HSCT) is often the only curative treatment for children with high risk or relapsed leukemia.1,2 The rapid hematopoietic reconstitution, as evidenced by early neutrophil engraftment, can be predicted by the number of CD34+ cells infused and an increase in the immature reticulocyte early posttransplant.3-5 A recent study reported that a higher absolute lymphocyte count (ALC) on day 30 was associated with faster hematopoietic recovery.6
The repopulating lymphocytes after allogeneic HSCT play an important role not only in the prevention of serious infections during the early transplantation period, but also in killing the residual leukemic cells by graft-versus-leukemia (GVL) effect. Thus, they may affect the outcome of the transplant by influencing the rate of relapse and the transplant-related mortality (TRM).7-9 Several studies have shown that early lymphocyte recovery after allogeneic HSCT was associated with decreased relapse, better survival, and reduced TRM rates in adult patients with myeloid and lymphoid leukemias.6,10,11 A recent study reported that early lymphocyte recovery post-HSCT was associated with significant GVL effects without an increase of graft-versus-host disease (GVHD) in children with acute lymphoblastic leukemia (ALL).12
Natural killer (NK) cells are the first lymphocytes to recover during the early post-transplant period; they can mediate cytotoxicity without prior sensitization and play a pivotal role in the GVL effect and innate host defenses.13-16 NK cells are major components of the ALC at D+30, and a higher ALC at D+21 or +30 has been associated with an improved transplant outcome in patients with myeloid and lymphoid leukemia.6,10,11 The ALC at D+30 has been considered a surrogate for NK cell recovery. The aim of this study was to analyze the impact of lymphocyte recovery after HSCT on predicting the survival, relapse, TRM, and GVHD in children with hematological malignancies.
MATERIALS AND METHODS
Patients
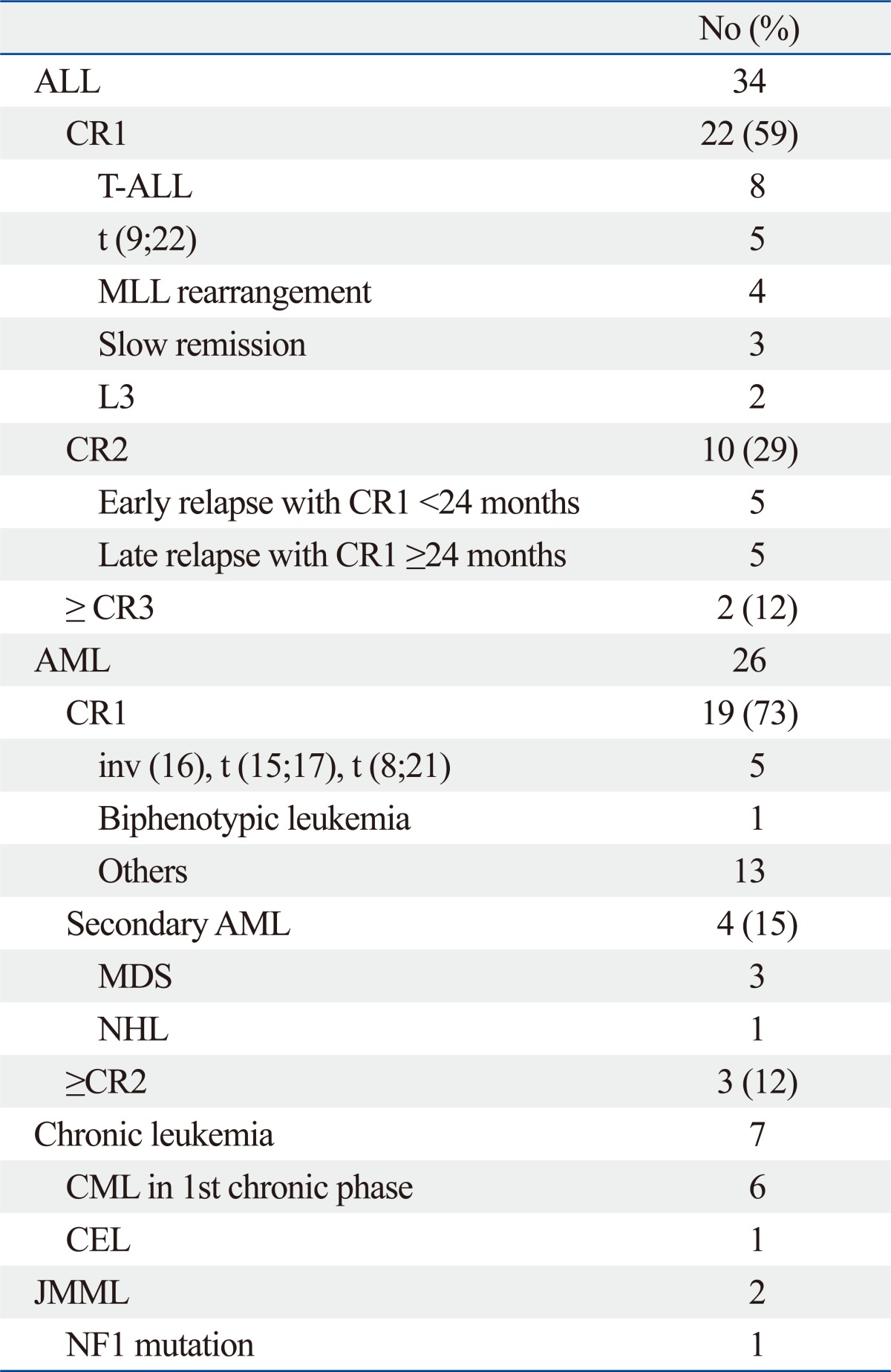
We retrospectively evaluated 69 children who had received allogeneic HSCT for ALL [n=34: Complete remission 1 (CR1), 22; CR2, 10; >CR2, 2], acute myeloid leukemia (AML) (n=26: CR1, 19; secondary AML, 4; >CR1, 3), chronic leukemia (n=7: chronic myeloid leukemia, 6; chronic eosinophilic leukemia, 1) and juvenile myelomonocytic leukemia (n=2) at the Chonnam National University Hospital between January 1996 and March 2008. Three patients with AML who received a 2nd allogeneic HSCT following autotransplants were included (Table 1). The data for the D+21 and +30 leukocyte counts, both the total and differential counts, were collected from the medical records and hospital electronic database. All patients survived at least 21 days after the HSCT. The patients were divided into two groups based on the ALC <500/µL or ≥500/µL to assess the effects of the ALC at D+21 and +30 on survival, frequency of relapse and GVHD. The effects were analyzed in relation to several levels of ALC including 200/µL, 300/µL, 400/µL and 500/µL; however, the cutoff value of 500/µL was chosen based on preliminary data (data not shown). The patients were considered at high risk if they had: ALL ≥CR2, AML ≥CR2 or secondary AML; all other patients were considered to be at standard risk.
Table 1.
Disease Status of 69 Patients That Underwent Stem Cell Transplantation
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CEL, chronic eosinophilic leukemia; CML, chronic myelogenous leukemia; CR, complete remission; JMML, juvenile myelomonocytic leukemia; MDS, myelodysplastic syndrome; NHL, non-Hodgkin's lymphoma.
Conditioning regimens
Pretransplant conditioning regimes were either total body irradiation (TBI)-based (n=41), or non-TBI based (n=28). Sixty four patients received conventional myeloablative conditioning: cytarabine (Ara-C) 3.0 gm/m2 twice daily for 3 days+cyclophosphamide (Cy) 45 mg/kg/day for 2 days+ TBI 200 cGY twice daily for 3 days, 21; Cy 60 mg/kg/day for 2 days+TBI 200 cGY twice daily for 3 days, 19; busulfan (Bu) 4 mg PO or 3.2 mg/kg/dose i.v. in divided doses daily for 4 days+Cy 60 mg/kg/day for 2 days, 18; Bu for 4 days+melphalan (Mel) 60 mg/m2/day for 3 days, 4; Bu for 4 days+Cy for 2 days+Mel 140 mg/m2/day for 1 day, 1; Bu for 4 days+Cy for 2 days+VP-16 60 mg/kg/day for 1 day, 1. Five patients received non-myeloablative conditioning.
Stem cell source
The stem cell sources were as follows: bone marrow (BM), 46; peripheral blood stem cell (PBSC), 10; umbilical cord blood (UCB), 12; BM+PBSC, 1. Matched sibling donors were available in 26, while unrelated donors in 43.
GVHD prophylaxis
The combination of cyclosporine (CsA)+methotrexate (MTX) was used mainly for related transplants, and the combination of tacrolimus (Tac)+MTX for unrelated transplants. CsA was used intravenously at 2.5 mg/kg twice daily, starting on day -1, and 1.5 mg/kg was given twice daily thereafter. The CsA dose was titrated to maintain plasma levels between 100-200 ng/mL. The Tac was continuously infused at a dose of 0.03 mg/kg/day starting from day -1 and titrated to maintain plasma levels within the 5-15 ng/mL range. Both CsA and Tac were used orally when patients could tolerate oral medication. The MTX dose was 15 mg/m2 intravenously on day 1 and 10 mg/m2 on day 3, 6 and 11. Some patients received a combination of Tac+mycophenolate mofetil (Table 2). Acute GVHD (aGVHD) was treated using methylprednisolone at a dose of 1 to 2 mg/kg/day as soon as the diagnosis was confirmed.
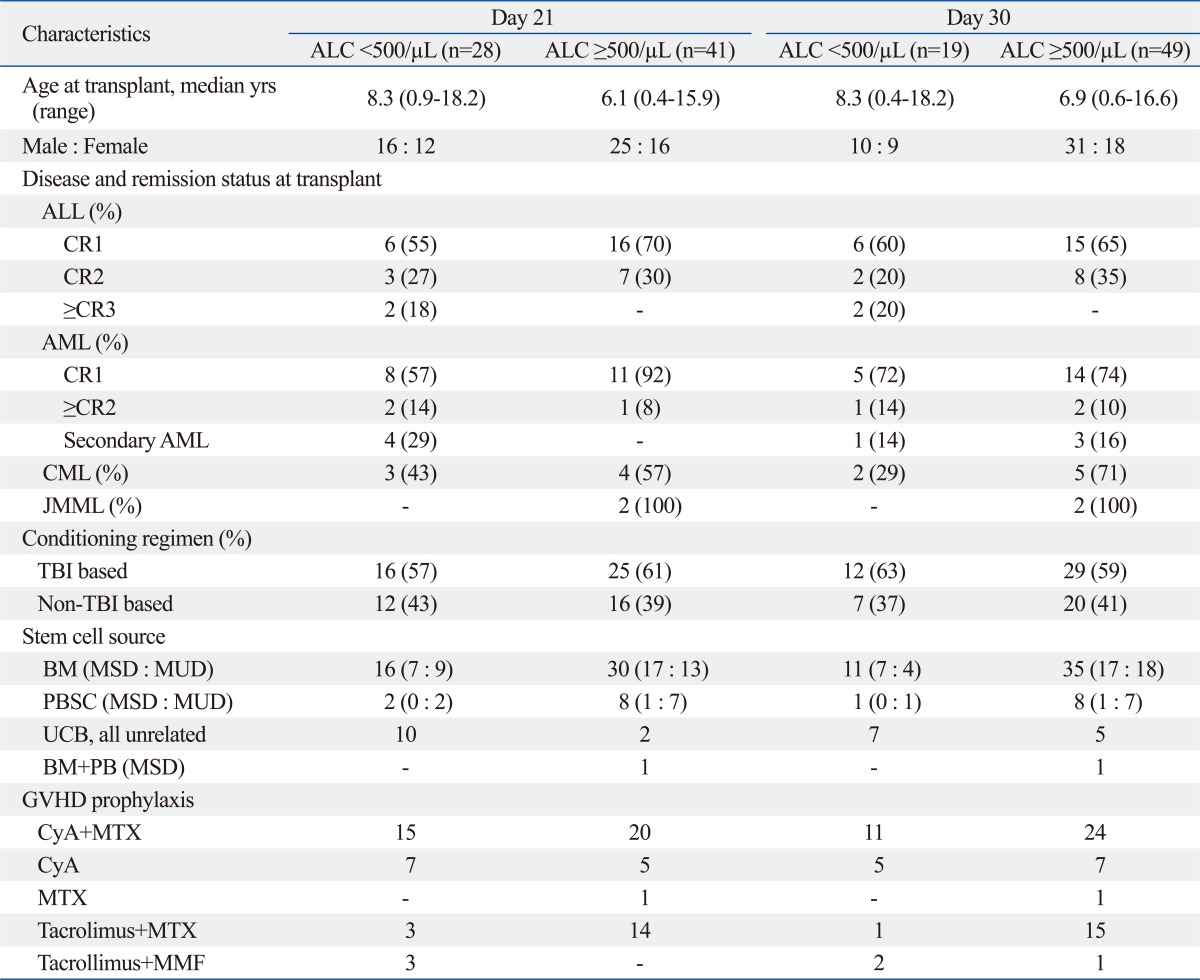
Table 2.
Clinical Characteristics Based on the ALC at D+21 and D+30
ALC, absolute lymphocyte count; ALL, acute lymphoblastic leukemia; CR, complete remission; AML, acute myelogenous leukemia; BM, bone marrow; CML, chronic myelogenous leukemia; CyA cyclosporine A; GVHD, graft-versus-host disease; JMML, juvenile myelomonocytic leukemia; MMF, mycophenolate mofetil; MSD, matched sibling donor; MTX, methotrexate; MUD, matched unrelated donor; PBSC, peripheral blood stem cell; TBI, total body irradiation; UCB, umbilical cord blood.
Hematological engraftment
Neutrophil engraftment was defined as the first day with an absolute neutrophil count (ANC) ≥500/µL for three consecutive days. Platelet engraftment was defined as a platelet count of ≥20000/µL for seven consecutive days independent of transfusions.
Supportive care
All patients were treated in a single room with high-efficiency particulate air filters. All patients received oral fluconazole or itraconazole for fungal prophylaxis, as well as ciprofloxacin and acyclovir, from the beginning of the conditioning regimen. Patients with neutropenic fever were treated with broad-spectrum antibiotics and amphotericin B or other antifungal agents sequentially over the next 72 hr, according to the patient's response to therapy. Intravenous immunoglobulins were used every other week from D-1 until D+90, and once a month to D+180 thereafter. Granulocyte colony-stimulating factor 5 µg/kg/day was used from D+0 until neutrophil engraftment. The prophylaxis for hepatic veno-occlusive disease (VOD) was ursodeoxycholic acid and dopamine. Liposomal prostaglandin E1 1 µg/kg/day was added in unrelated transplants. All patients received total parenteral nutrition after the HSCT until oral intake could be tolerated.
End points
Relapse or death after the transplantation was the primary end point. Remission and relapse were defined by conventional criteria. The other objective end point was the development of GVHD. TRM was defined as the time from HSCT until death from infection, GVHD, graft failure, or any other causes unrelated to the underlying disease. Acute and chronic GVHD (cGVHD) was defined according to the conventional criteria.17,18 Prognostic variables examined for survival and relapse were patient age, gender, conditioning regimen (TBI vs. non-TBI), risk group (standard vs. high risk), donor type (related vs. unrelated), aGVHD, cGVHD, stem cell source (BM vs. PBSC vs. UCB), ALC at D+21 and ALC at D+30. These factors were evaluated for all patients, and also separately for patients with ALL and AML.
Statistical analysis
The χ2 test was used to compare nominal variables and the t-test was used to compare numerical variables between the two groups. The overall survival (OS) and event-free survival (EFS) were analyzed using the Kaplan-Meier method. The log rank test was used to analyze the differences between the two groups. The cumulative incidence of relapse and the TRM was estimated. p values less than 0.05 were considered statistically significant. The Cox proportional hazards model was used to examine the impact of multiple prognostic factors on survival. Factors with a p value less than 0.1 by univariate analysis were subsequently evaluated by multivariate analysis. All statistical analyses were performed using SPSS version 17.0 for Windows (IBM, Chicago, IL, USA).
Ethics statement
This study was approved by the Institutional Review Board of Chonnam National University Hwasun Hospital (approved number 2011-37). Informed consent was acquired from all subjects.
RESULTS
Patient characteristics
Sixty-nine children with leukemia who received HSCT were identified for this study. The patients included 41 males and 28 females with a median age of 7.1 years (range, 0.4-18.2) at transplant. The patient characteristics are shown in Table 1 and 2. The majority of patients (50/69, 72%) had a standard risk at the time of the transplant. The proportion of patients in CR1 was 59% (22/34) for ALL and 73% (19/26) for AML. The median follow-up was 26 months (range, 1-134). The patients were grouped based on ALC <500/µL or ≥500/µL at D+21 and +30 after the transplant: Low at D+21 (n=28) vs. High at D+21 (n=41); Low at D+30 (n=19) vs. High at D+30 (n=49). Patient characteristics at both D+21 and D+30 were not significantly different between the two groups in regard to age at transplant, gender, remission status, conditioning regimen, stem cell sources and GVHD prophylaxis.
Engraftment
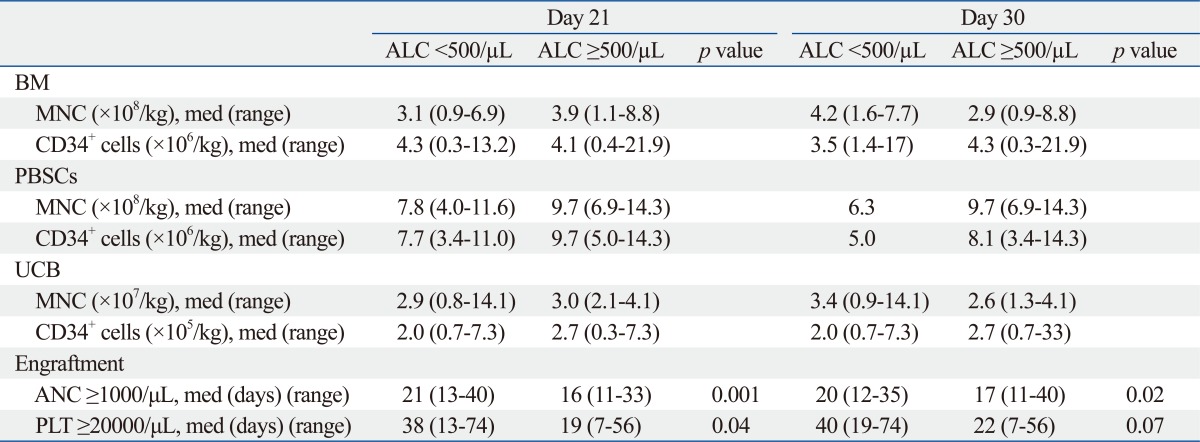
The numbers of infused stem cells, by the same stem cell sources, were not statistically different between the two groups (Table 3). There was no engraftment failure. The median time to neutrophil and platelet engraftment for all patients was 17.5 days (range, 11-40) and 25 days (range, 7-74), respectively. Patients with a High ALC at D+21 and D+30 had faster neutrophil and platelet engraftment: the median days to neutrophil engraftment (>1000/µL) was D+16 for High at D+21 vs. D+21 for Low at D+21 (p=0.001); and D+17 for High at D+30 vs. D+20 for Low at D+30 (p=0.02). The median time for platelet engraftment (>20000/µL) was D+19 for High at D+21 vs. D+38 for Low at D+21 (p=0.04); and D+22 for High at D+30 vs. D+40 for Low at D+30 (p=0.07) (Table 3).
Table 3.
Infused Stem Cell Number and Engraftment Kinetics Based on the ALC
ALC, absolute lymphocyte count; ANC, absolute neutrophil count; BM, bone marrow; MNC, mononuclear cells; med, median; PBSC, peripheral blood stem cells; PLT, platelets; UCB, umbilical cord blood.
GVHD and TRM
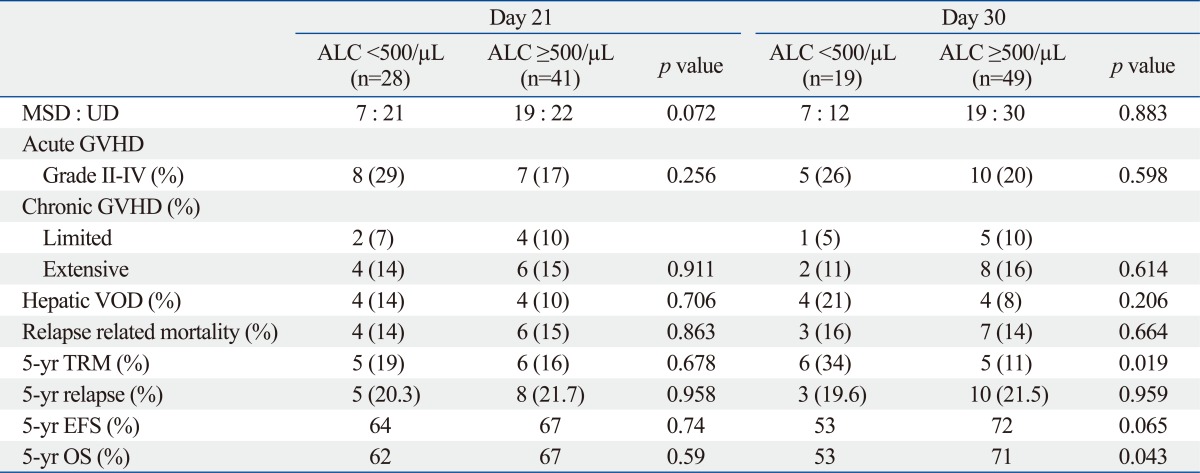
The frequency of aGVHD, cGVHD and hepatic VOD did not differ between the two groups. Twelve out of 69 (17%) patients died of non-relapse causes, with a cumulative incidence of TRM of 18.5%. The causes of death were as follows: aGVHD, 4; cGVHD, 3; acute respiratory distress syndrome, 3; invasive pulmonary aspergillosis, 1; bleeding, 1. The Low at D+30 group had a significantly increased risk for TRM compared to the High at D+30 group (34% vs. 11%, p=0.019) (Table 4).
Table 4.
Post-Transplant Complications and Survival Based on the ALC at D+21 and D+30
ALC, absolute lymphocyte count; GVHD, graft-versus-host disease; EFS, event-free survival; MSD, matched sibling donor; OS, overall survival; TRM, transplant-related mortality; UD, unrelated donor; VOD, veno-occlusive disease.
Relapse and survival
Thirteen patients relapsed with a cumulative incidence of 21.3%. The median time to relapse was 5.4 months (range, 2.2-12.4). The relapse rate did not differ between the ALC groups. The relapse rate was not dependent on the age at transplant, donor type, stem cell source, risk status and the development of aGVHD or cGVHD.
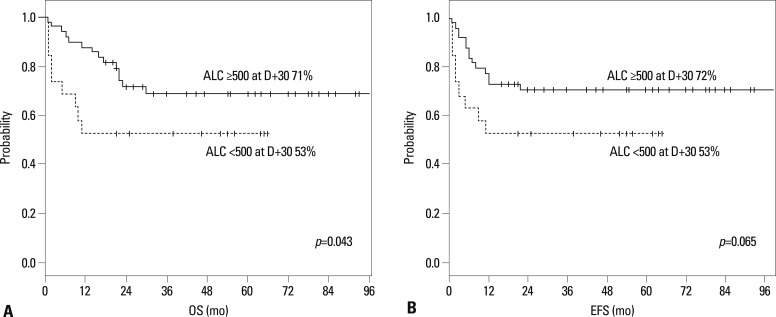
The 5-year Kaplan-Meier OS and EFS for the 69 patients were 64% and 65%, respectively. The 5-year Kaplan-Meier OS and EFS did not differ between the two groups according to the D+21 ALC. However, a High at D+30 had a significantly higher 5-year OS than a Low at D+30 (71% vs. 53%, p=0.043) due to higher TRM in the latter group. In addition, a High at D+30 had a tendency of better EFS than the Low at D+30 (72% vs. 53%, p=0.065) (Table 4) (Fig. 1).
Fig. 1.
Overall survival (A) and event-free survival (B) of 69 consecutive children according to ALC at D+30 after HSCT. ALC, absolute lymphocyte counts; HSCT, hematopoietic stem cell transplantation; OS, overall survival; EFS, event-free survival.
Prognostic factors on survival
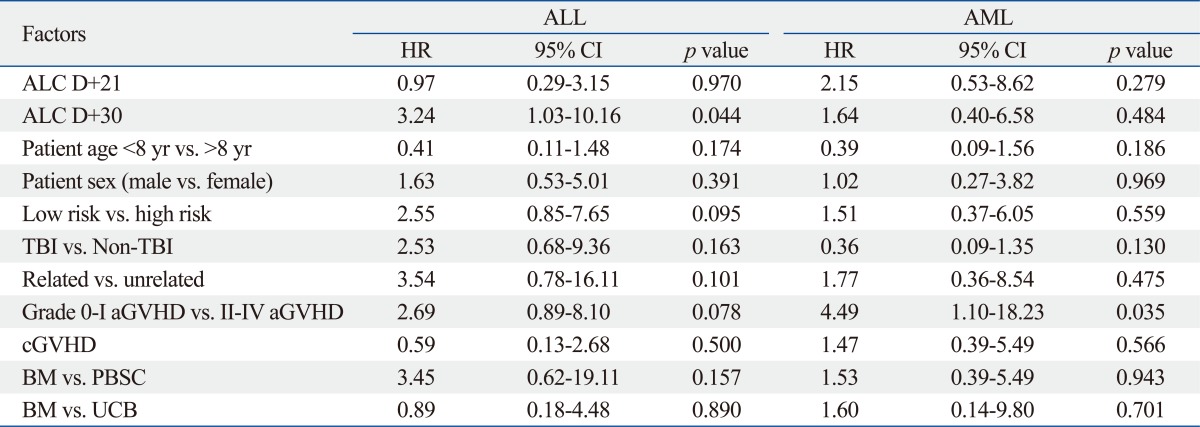
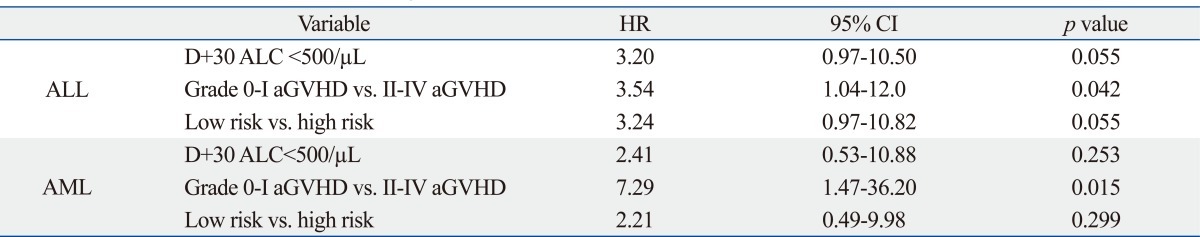
As of July 2010, 23 out of 69 patients died [9/19 (47.4%) in the Low at D+30 vs. 14/49 (28.6%) in the High at D+30, p=0.142]. Factors associated with survival were evaluated separately for the ALL and AML patients. In univariate analysis, factors associated with decreased survival were as follows: a Low ALC at D+30 [hazard ratio (HR) 3.24, p=0.044], high risk (HR 2.55, p=0.095), and grade II-IV aGVHD (HR 2.69, p=0.078) in the ALL patients; grade II-IV aGVHD (HR 4.49, p=0.035) in the AML patients (Table 5). Grade II-IV aGVHD was the only variable with a significant impact on survival by the multivariate Cox regression analysis (Table 6).
Table 5.
Factors Associated with Overall Survival by the Univariate Analysis
ALC, absolute lymphocyte count; ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; BM, bone marrow; GVHD, graft-versus-host disease; HR, hazard ratio; PBSC, peripheral blood stem cell; TBI, total body irradiation; UCB, umbilical cord blood.
Table 6.
Results from the Multivariate Analysis of Factors Associated with Overall Survival
ALC, absolute lymphocyte count; ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; GVHD, graft-versus-host disease; HR, hazard ratio.
DISCUSSION
The present results showed that earlier lymphocyte recovery greater than 500/µL on D+30 was associated with faster myeloid and platelet engraftment, and better survival without increasing the incidence of GVHD. These findings are consistent with other observations which implicate the importance of early lymphocyte recovery as a significant prognostic factor for the outcome after allo-HSCT.6,10,19 It has been shown that patients with prompt lymphocyte recovery who had more than the median total lymphocyte counts early post-transplant also had a more favorable outcome; i.e., decreased GVHD, less leukemic relapse, and lower TRM, thus, resulting in significantly improved survival.6,20
A recent study on 102 adult patients with myeloid leukemias (AML, 54; chronic myeloid leukemia 38; myelodysplastic syndrome, 10) showed a relationship between the ALC at D+30 and the transplant outcome.10 The patients were divided into three subgroups according to ALC at D+30: low (<200/µL), 18; intermediate (200-1000/µL), 67; high (≥1000/µL), 17. A higher ALC at D+30 had a significant impact on a lower TRM, increased relapse-free survival (RFS), and improved survival when analyzed as a continuous variable in the multivariate analysis. In addition, a high ALC at D+30, a high CD34+ cell dose, and absence of grade II-IV aGVHD were the independent factors with an impact on RFS by multivariate analysis. The OS was 91%, 60%, and 36% in the high, intermediate, and low ALC groups, respectively.
There is only one published report evaluating the importance of the ALC in the pediatric population. Ishaqi, et al.12 reported a significant GVL effect in 136 children with ALL. Two groups were identified based on the ALC cutoff value of 300/µL at D+21 and +30. Patients with a lower ALC at D+21 had a greater risk of relapse (HR 5.3, p=0.002) and had a lower 3-year EFS (42% vs. 66%, p=0.02). Patients with a lower ALC at D+30 had an increased risk of relapse (HR 2.2, p=0.01) and had an inferior 3-year EFS (30% vs. 57%, p=0.0001). A higher ALC at D+21 and +30 was not associated with an increased frequency of aGVHD, cGVHD, and TRM. Neutrophil engraftment did not differ between the two groups.
However, there are several differences between Ishaqi's study and the current study. First, patients with a High ALC at D+21 and D+30 had a more rapid neutrophil and platelet engraftment in this study, while Ishaqi's study showed no difference in neutrophil engraftment. Second, the cut-off value for the ALC in this study was 500/µL, which differs from the 300/µL in Ishaqi's report. The 500/µL value was chosen as a cutoff point after analyzing the outcome for several levels of ALC: 200/µL, 300/µL, 400/µL and 500/µL. Third, a Low at D+30 was associated with a higher TRM (72% vs. 53%, p=0.065). Fourth, there was no difference in the relapse rate between the ALC groups in this study. The reasons for these discrepancies between the two studies are not clear, but the differences in the study populations and ALC levels used might explain the different results.
In this study, however, the more rapid lymphocyte recovery did not translate into a decrease in the relapse rate. A better NK cell recovery on D+30 has been reported to be associated with an improved survival, decreased aGVHD, and prognostic impact on relapse, which might be facilitated by NK cells-mediated GVL effect.21 A recent report showed that the transplant outcome was dependent not on CD3+ T cell count, but on absolute NK cell count.20 Their effects on disease relapse were observed only in the subset of adult patients with myeloid malignancies, and not with lymphoid leukemia. Because ALL cells are less susceptible to NK cell cytotoxicity than AML cells, this might reflect a NK cell-associated effect.20 However this continues to be debated; other reports have suggested that early lymphocyte recovery after HSCT was associated with a significant GVL effect in adults and children with ALL.11,12 Clinical data from haploidentical HSCT showed that NK cells are associated with favorable effects in both adult and pediatric high-risk leukemia, attributing to killer immunoglobulin-like receptors and CD94/NKG2A.22 Whether NK cell recovery is directly associated with a better prognostic impact on relapse or whether it is a surrogate for some other effect associated with immune recovery is not known for certain. Nevertheless, these results suggest a possible role for NK cells in HSCT. NK cells have traditionally been defined as expressing CD56 with or without CD16, without expressing T cell markers.23,24 CD56+ cells have been used with other markers for assessment of immune reconstitution after HSCT.13 In the current study, CD56+ NK cells were not assessed routinely at D+21 or D+30.
The enumeration of the lymphocyte count after transplantation can easily be done in routine daily practice. It has practical benefits as it may identify the high risk patients of relapse who could be appropriate candidates for early interventions or aggressive approaches to improve transplant outcome. In addition, early lymphocyte recovery may also identify a risk group for non-relapse mortality from infections and GVHD, and graft failure.25-27 There is increasing evidence that prompt lymphocyte recovery has significant effects on the outcomes of patients with hematological malignancies after not only autologous and allogeneic HSCT, but also chemotherapy.28
The number of CD34+ cells in the hematopoietic grafts has been shown to correlate with rapid reconstitution of hematopoietic function after HSCT. However, there are several limitations to using CD34 as a marker for stem cells. CD34+ cells are very heterogeneous, and various cells with limited self-renewal ability continue to express this antigen.29 Also, one study reported that some hematopoietic stem cells do not express CD34.30 Therefore, many other markers, such as immature reticulocyte counts and ALC,3,4,6 have been used to predict engraftment. The current study showed that patients with a higher ALC at D+21 and +30 had a more rapid neutrophil and platelet engraftment, suggesting that early lymphocyte recovery may serve as a surrogate for hematopoietic recovery.6,10,11
In conclusion, early lymphocyte recovery might be a surrogate for neutrophil and platelet engraftment, and a higher ALC D+30 was associated with a decreased TRM and better survival rate. If the usefulness of early ALC recovery after pediatric HSCT is reproduced in various disease groups and transplant settings, ALC measurement should widely be used as a prognostic variable, thus serving as a basis for tailored-treatment strategies to improve the transplant outcome in patients who fail to achieve sufficient ALC in the early transplant period. Further studies incorporating larger number of children and longer follow-up with serial NK cell enumeration are warranted.
ACKNOWLEDGEMENTS
This study was supported by a grant (CRI09086-1) of the Chonnam National University Hospital Research Institute of Clinical Medicine, and Environmental Health Center for Childhood Leukemia and Cancer.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Smith AR, Baker KS, Defor TE, Verneris MR, Wagner JE, Macmillan ML. Hematopoietic cell transplantation for children with acute lymphoblastic leukemia in second complete remission: similar outcomes in recipients of unrelated marrow and umbilical cord blood versus marrow from HLA matched sibling donors. Biol Blood Marrow Transplant. 2009;15:1086–1093. doi: 10.1016/j.bbmt.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diaz MA, Gonzalez-Vicent M, Ramirez M, Sevilla J, Lassaletta A, Perez A, et al. Allogeneic cord blood transplantation in children with hematological malignancies: a long-term follow-up single-center study. Pediatr Hematol Oncol. 2009;26:165–174. doi: 10.1080/08880010902773040. [DOI] [PubMed] [Google Scholar]
- 3.Zubair A, Zahrieh D, Daley H, Schott D, Gribben JG, Freedman A, et al. Early neutrophil engraftment following autologous BMT provides a functional predictor of long-term hematopoietic reconstitution. Transfusion. 2003;43:614–621. doi: 10.1046/j.1537-2995.2003.00369.x. [DOI] [PubMed] [Google Scholar]
- 4.Noronha JF, De Souza CA, Vigorito AC, Aranha FJ, Zulli R, Miranda EC, et al. Immature reticulocytes as an early predictor of engraftment in autologous and allogeneic bone marrow transplantation. Clin Lab Haematol. 2003;25:47–54. doi: 10.1046/j.1365-2257.2003.00486.x. [DOI] [PubMed] [Google Scholar]
- 5.Molina JR, Sanchez-Garcia J, Torres A, Alvarez MA, Serrano J, Casaño J, et al. Reticulocyte maturation parameters are reliable early predictors of hematopoietic engraftment after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:172–182. doi: 10.1016/j.bbmt.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Savani BN, Mielke S, Rezvani K, Montero A, Yong AS, Wish L, et al. Absolute lymphocyte count on day 30 is a surrogate for robust hematopoietic recovery and strongly predicts outcome after T cell-depleted allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:1216–1223. doi: 10.1016/j.bbmt.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta J, Powles R, Kulkarni S, Treleaven J, Singhal S. Induction of graft-versus-host disease as immunotherapy of leukemia relapsing after allogeneic transplantation: single-center experience of 32 adult patients. Bone Marrow Transplant. 1997;20:129–135. doi: 10.1038/sj.bmt.1700859. [DOI] [PubMed] [Google Scholar]
- 8.Cornelissen JJ, Carston M, Kollman C, King R, Dekker AW, Löwenberg B, et al. Unrelated marrow transplantation for adult patients with poor-risk acute lymphoblastic leukemia: strong graft-versus-leukemia effect and risk factors determining outcome. Blood. 2001;97:1572–1577. doi: 10.1182/blood.v97.6.1572. [DOI] [PubMed] [Google Scholar]
- 9.Remberger M, Mattsson J, Hentschke P, Aschan J, Barkholt L, Svennilson J, et al. The graft-versus-leukaemia effect in haematopoietic stem cell transplantation using unrelated donors. Bone Marrow Transplant. 2002;30:761–768. doi: 10.1038/sj.bmt.1703735. [DOI] [PubMed] [Google Scholar]
- 10.Le Blanc K, Barrett AJ, Schaffer M, Hägglund H, Ljungman P, Ringdén O, et al. Lymphocyte recovery is a major determinant of outcome after matched unrelated myeloablative transplantation for myelogenous malignancies. Biol Blood Marrow Transplant. 2009;15:1108–1115. doi: 10.1016/j.bbmt.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar S, Chen MG, Gastineau DA, Gertz MA, Inwards DJ, Lacy MQ, et al. Lymphocyte recovery after allogeneic bone marrow transplantation predicts risk of relapse in acute lymphoblastic leukemia. Leukemia. 2003;17:1865–1870. doi: 10.1038/sj.leu.2403055. [DOI] [PubMed] [Google Scholar]
- 12.Ishaqi MK, Afzal S, Dupuis A, Doyle J, Gassas A. Early lymphocyte recovery post-allogeneic hematopoietic stem cell transplantation is associated with significant graft-versus-leukemia effect without increase in graft-versus-host disease in pediatric acute lymphoblastic leukemia. Bone Marrow Transplant. 2008;41:245–252. doi: 10.1038/sj.bmt.1705891. [DOI] [PubMed] [Google Scholar]
- 13.Kook H, Goldman F, Padley D, Giller R, Rumelhart S, Holida M, et al. Reconstruction of the immune system after unrelated or partially matched T-cell-depleted bone marrow transplantation in children: immunophenotypic analysis and factors affecting the speed of recovery. Blood. 1996;88:1089–1097. [PubMed] [Google Scholar]
- 14.Niederwieser D, Gastl G, Rumpold H, Marth C, Kraft D, Huber C. Rapid reappearance of large granular lymphocytes (LGL) with concomitant reconstitution of natural killer (NK) activity after human bone marrow transplantation (BMT) Br J Haematol. 1987;65:301–305. doi: 10.1111/j.1365-2141.1987.tb06857.x. [DOI] [PubMed] [Google Scholar]
- 15.Jiang YZ, Barrett AJ, Goldman JM, Mavroudis DA. Association of natural killer cell immune recovery with a graft-versus-leukemia effect independent of graft-versus-host disease following allogeneic bone marrow transplantation. Ann Hematol. 1997;74:1–6. doi: 10.1007/s002770050246. [DOI] [PubMed] [Google Scholar]
- 16.Sconocchia G, del Principe D, Barrett AJ. Non-classical antileukemia activity of early recovering NK cells after induction chemotherapy and HLA-identical stem cell transplantation in myeloid leukemias. Leukemia. 2006;20:1632–1633. doi: 10.1038/sj.leu.2404300. [DOI] [PubMed] [Google Scholar]
- 17.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 19.Kim DH, Kim JG, Sohn SK, Sung WJ, Suh JS, Lee KS, et al. Clinical impact of early absolute lymphocyte count after allogeneic stem cell transplantation. Br J Haematol. 2004;125:217–224. doi: 10.1111/j.1365-2141.2004.04891.x. [DOI] [PubMed] [Google Scholar]
- 20.Savani BN, Mielke S, Adams S, Uribe M, Rezvani K, Yong AS, et al. Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia. 2007;21:2145–2152. doi: 10.1038/sj.leu.2404892. [DOI] [PubMed] [Google Scholar]
- 21.Ruggeri L, Mancusi A, Burchielli E, Aversa F, Martelli MF, Velardi A. Natural killer cell alloreactivity in allogeneic hematopoietic transplantation. Curr Opin Oncol. 2007;19:142–147. doi: 10.1097/CCO.0b013e3280148a1a. [DOI] [PubMed] [Google Scholar]
- 22.Moretta A, Pende D, Locatelli F, Moretta L. Activating and inhibitory killer immunoglobulin-like receptors (KIR) in haploidentical haemopoietic stem cell transplantation to cure high-risk leukaemias. Clin Exp Immunol. 2009;157:325–331. doi: 10.1111/j.1365-2249.2009.03983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 24.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 25.Chakrabarti S, Brown J, Guttridge M, Pamphilon DH, Lankester A, Marks DI. Early lymphocyte recovery is an important determinant of outcome following allogeneic transplantation with CD34+ selected graft and limited T-cell addback. Bone Marrow Transplant. 2003;32:23–30. doi: 10.1038/sj.bmt.1704082. [DOI] [PubMed] [Google Scholar]
- 26.Lee KH, Choi SJ, Lee JH, Lee JS, Kim WK, Lee KB, et al. Prognostic factors identifiable at the time of onset of acute graft-versus-host disease after allogeneic hematopoietic cell transplantation. Haematologica. 2005;90:939–948. [PubMed] [Google Scholar]
- 27.Savani BN, Rezvani K, Mielke S, Montero A, Kurlander R, Carter CS, et al. Factors associated with early molecular remission after T cell-depleted allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood. 2006;107:1688–1695. doi: 10.1182/blood-2005-05-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrett AJ, Savani BN. Does chemotherapy modify the immune surveillance of hematological malignancies? Leukemia. 2009;23:53–58. doi: 10.1038/leu.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donaldson C, Denning-Kendall P, Bradley B, Hows J. The CD34(+)CD38(neg) population is significantly increased in haemopoietic cell expansion cultures in serum-free compared to serum-replete conditions: dissociation of phenotype and function. Bone Marrow Transplant. 2001;27:365–371. doi: 10.1038/sj.bmt.1702810. [DOI] [PubMed] [Google Scholar]
- 30.Verfaillie CM, Almeida-Porada G, Wissink S, Zanjani ED. Kinetics of engraftment of CD34(-) and CD34(+) cells from mobilized blood differs from that of CD34(-) and CD34(+) cells from bone marrow. Exp Hematol. 2000;28:1071–1079. doi: 10.1016/s0301-472x(00)00506-3. [DOI] [PubMed] [Google Scholar]