Abstract
Introduction
Bronchial challenge tests by inhalation of aerosolized methacholine (MCH) are commonly used in the clinical diagnosis of airway hyperresponsiveness (AHR). While the detection of airway narrowing relies on the patient's cooperation performing forced spirometry, body plethysmographic measurements of airway resistance are less depending on the patient's cooperation and do not alter the respiratory tract by maximal maneuvers. Hence we compared both methods concerning their clinical value and correlation during MCH challenges in patients with asthma.
Materials and Methods
Cumulative MCH challenges test, consisting of up to 5 steps, evaluated with body plethysmography on each step were performed in 155 patients with bronchial asthma. Airway responses were recorded at each step of MCH application (Master-Screen Body, Cardinal Health, Höchberg). At the baseline test and after crossing the provocation dose (PD) threshold in body plethysmography (PD+100 sReff), forced expirations were performed and FEV1, FVC, and FEV1 %FVC were measured. Using regression analysis of the airway parameters and taking the MCH dose as the covariate, we could extrapolate to missing spirometric values and interpolate the estimated MCH dose when crossing the PD threshold (PD-20 FEV1) between two consecutive measurements. The administered PD+100 MCH doses for specific airway resistance, sRtot, and sReff were compared with resistance parameters Rtot and Reff, and to PD-20 of FEV1 and FEV1 %FVC.
Results
Regarding sReff we found a mild, moderate, or severe AHR in 114 patients (75%), but only 50 (32%) according to FEV1. A statistical analysis showed strongly linear correlated parameters of airway resistance, but no significant correlation between the results of body plethysmography and forced spirometry
Conclusions
Using MCH challenges, we found specific airway resistance to be the most sensitive parameter to detect AHR. Raw is largely independent of height and gender facilitating the interpretation of measurements carried out longitudinally.
Keywords: bronchial challenge, methacholine, forced spirometry, asthma, airway hyperresponsiveness
Introduction
Airway hyperresponsiveness (AHR) is defined as an excessive response to an aerosolized drug provocation that elicits little or no response in a normal person. This feature, at some point in time, appears to distinguish most patients with asthma, and underlies the rationale for bronchoprovocation testing [1-3]. Bronchial challenge tests by inhalation of aerosolized methacholine (MCH) are recommended by both the American Thoracic Society (ATS) [4] and the European Respiratory Society (ERS) [5]. Differences in the reaction on provocative concentrations or provocative doses of methacholine causing a fall in FEV1 by 20% and/or an increase in sRaw by 100% derived by the dosimeter method or the tidal breathing method are still in intensive discussion [6-8]. Currently, a 1-concentration 4-step dosimeter protocol for MCH testing was developed [9]. The procedure was compared to the ATS standard protocol of five different concentrations [4] modified from the standard procedures of Chai et al [10] and Ryan et al [11].
Detection of airway narrowing in Anglo-American countries commonly relies on forced spirometry (FEV1). A fall in FEV1 ≥20% following MCH inhalation is widely used to determine the degree of AHR. FEV1, however, is largely depending on the subject's cooperation, his compliance, and motivation. In addition inspiration to TLC and forced expiratory maneuvers widen the airway caliber and thus lower respiratory airflow flow resistance [12]. This effect was already described by Nadel and Tierney in 1961 [13]. Slas et al [14] showed that airways inflammation plays an essential role in responses to deep inhalations in healthy subjects and patients with bronchial asthma causing bronchodilatation. Bronchidilatation by deep inhalation may however be of only minor importance in patients with chronic obstructive pulmonary disease (COPD) and increased airway resistance [14].
In contrast, for body plethysmographic measurements of specific airway resistance (sRaw), or specific conductance (sGaw), which have lately been standardized and recommended by the 'Deutsche Atemwegsliga', only quiet breathing is required with a minimum of cooperation. The major part of airways resistance is localized in the first 8 to 12 bronchial generations and less in peripheral lungs. The peripheral lung, however, is incorporated in sRaw or sGaw by their volume related component. An increase of sRaw of ≥200% from baseline value and its absolute value larger than 2.0 kPa · s as well as a fall in sGaw ≥40% from baseline with an absolute value below 0.5 1/(kPa · s) are recommended by both international societies, ATS and ERS, to determine AHR. ATS and ERS consider both spirometry (FEV1) and body plethysmography (sReff and sGeff) for bronchoprovocation testing; comparative studies are, however, lacking.
In the present study, we compared both methods in order to evaluate the concordance of forced spirometry and body plethysmography, concerning their clinical value during MCH challenge testing in patients with confirmed bronchial asthma.
Materials and Methods
Study Population
Cumulative MCH challenges test were performed in 155 patients (82 males, 73 females) with a mean age of 50.4 ± 15.9 years (range 18-84 years), 170 ± 10 cm height (range 154-194 cm) and a mean BMI of 28 ± 6.2 kg/m2 (range 16.8-48.9) during a clinical assessment of patients with chronic cough or suspected mild asthma referred to a 205-bed-center for pulmonology and thoracic surgery prospectively. None of the patients had any cardiac or pulmonary disease other than suspected asthma and none were receiving medication for upper or lower airway disease, other than inhaled short acting bronchodilators. Inclusion criteria: > 18 yr of age, non-smoker, no respiratory infection or exacerbation of asthma within the preceding 6 weeks; FEV1 ≥70% pred and ≥1.5 L, no previous treatment with oral or inhaled steroids. Subjects were interviewed by a pulmonologist, and asked to refrain from using caffeine-containing beverages, and gave informed written consent to the study.
Methacholine Challenges
Methacholine chloride was dissolved in isotonic saline to a final concentration of 16 mg/ml. This standard MCH concentration was used at all provocation steps. The patients were advised to breathe tidal and slow without exceeding a maximal flow of 0.5 L/s. At inhalation after a time of 0.2 s, the nebulizer generated an aerosol bolus for defined periods of time (0.29 s, 0.59 s and 0.94 s). The MCH challenge procedure had the following design: after measuring baseline values and an initial inhalation of aerosolized isotonic saline, maximal 5 provocation steps (0.025, 0.050, 0.100, 0.201, and 0.401 mg) were performed. The challenge test was terminated by administration of the short acting bronchodilator. The APS provocation system (Cardinal Health, Höchberg, Germany) uses the effective and reliable nebulizer from MedicAid with an aerodynamic mass median diameter of particles of MMAD 3.2 μm, which is optimal size for effective sedimentation of the aerosol in the lung. The cumulative inhaled doses after each step of inhalation (0.025, 0.075, 0.175, 0.376, and 0.777 mg MCH) were obtained by taking two breaths with MCH boluses at step one and two, 4 breaths at step 3, 8 breaths at step 4, and 10 breaths at step 5. Classification of AHR was performed in four steps: severe (MCH dose < 0.05 mg), moderate (0.05 mg < MCH dose < 0.20 mg) mild (0.20 < MCH dose < 0.65 mg), and normal responsive (MCH dose > 0.65 mg).
Lung Function Measurements
Specific airway resistance (sRaw), specific conductance (sGaw), airway resistance (Raw), conductance (Gaw) and intrathoraxic gas volume (ITGV) were recorded by body plethysmography (MasterScreen Body, Cardinal Health, Höchberg, Germany). In the combined measurements, body plethysmography was performed first followed by forced spirometry. In both test the patient was sitting in upright position. To allow optimal instruction and motivation in the forced spirometric maneuver, the door of the plethysmograph was opened for this test. According to the recommendations of the manufactures the body plethysmograph was calibrated every morning.
The spirometric test results were compared to the reference equations of the ECCS [15]. Classification of body plethysmography based on the current recommendations of Criee at al [16]. At rest, after saline inhalation and after each step of MCH inhalation body plethysmography was measured 2 minutes after MCH inhalation, with determination of Reff, sReff, Rtot, sRtot, sGeff and sGtot, and ITGV [17]. An increase of sRaw of ≥200% from 100% baseline value and its absolute value larger than 2.0 kPa · s or a fall in sGaw ≥40% and its absolute value lower than 0.5 1/(kPa · s) are recommended as thresholds to determine airways hyperresponsiveness. At baseline and after crossing the PD threshold in body plethysmography (PD+100 Raw, sRaw,) forced spirometric maneuvers were performed and FEV1, FVC, and FEV1 %FVC were determined and the PD-20 FEV1 extrapolated.
Data Analysis
Because the challenge needed to be stopped as soon as patients showed significant symptoms based on airway narrowing and assessed via PD+100 sRaw, most spirometric datasets were left incomplete with some parameters not reaching the diagnostic threshold PD-20 FEV1. Utilizing the regression analysis of the recorded airway resistance parameters taking the metacholine dose as the covariate, we could extrapolate to missing spirometric values and interpolate the estimated metacholine dose when crossing the threshold between two consecutive measurements.
Regression was done in a simple 3-way process of transforming data into linear space, doing linear regression and back transformation, where linear regression was performed by built-in statistical functions of an Oracle© 10 g XE database. Quality checking was done by calculating the mean goodness-of-fit for each parameter which yielded the following results: Using regression analysis of the recorded airway parameters taking the MCH dose as the covariate we could extrapolate to missing values and interpolate the estimated MCH dose when crossing the threshold of -20% for FEV1, between two consecutive measurements. The regression analysis resulted in high values for the 'goodness of fit' (0.83 > r2 < 0.95). The required PD+100 MCH doses for airway, specific airway resistance (Rtot, Reff, sRtot, and sReff), and specific conductance (sGaw) were compared to PD-20 of FEV1.
Results
Lung Function Parameters of Patients Included in the Study
Under baseline conditions, the mean values of respiratory parameters of males with mild asthma were in the normal range for sReff (mean 0.77 ± 0.036 kPa · s, range 0.26-1.6), FEV1 (3.51 ± 0.90 L, range 0.95-5.55, or 95% according ECCS reference values) and sGeff (mean 1.53 ± 0.63 1/kPa · s) (Table 1). The mean baseline values for females corresponded to ECCS reference values for FEV1 (2.45 ± 0.60 L, range 1.23-4.16 L, or 95.5% predicted) and to plethysmographically measured sReff (0.835 ± 0.306 kPa · s, range 0.12-1.65) and sGeff (mean 1.37 ± 0.63 1/kPa · s).
Table 1.
Baseline respiratory parameters of patients with mild asthma included in the study.
| FEV1 (L/s) |
FEV1% FVC (%) |
Reff (kPa/L · s) |
sReff (kPa · s) |
Rtot (kPa/L · s) |
sRtot (kPa · s) |
sGeff (1/kPa · s) |
sGtot (1/kPa · s) |
|
|---|---|---|---|---|---|---|---|---|
| Males, n = 82 | ||||||||
| MW | 3.51 | 82.2 | 0.19 | 0.77 | 0.22 | 0.87 | 1.53 | 1.29 |
| SD | ± 0.91 | ± 7.60 | ± 0.07 | ± 0.36 | ± 0.07 | ± 0.34 | ± 0.62 | ± 0.41 |
| Med | 3.47 | 83.2 | 0.19 | 0.71 | 0.22 | 0.80 | 1.41 | 1.26 |
| Females, n = 73 | ||||||||
| MW | 2.45 | 85.0 | 0.26 | 0.84 | 0.29 | 0.92 | 1.40 | 1.19 |
| SD | ± 0.62 | ± 7.06 | ± 0.10 | ± 0.31 | ± 0.09 | ± 0.28 | ± 0.63 | ± 0.47 |
| Med | 2.36 | 85.40 | 0.26 | 0.79 | 0.28 | 0.90 | 1.12 | 1.11 |
Classification of Airway Hyperresponsiveness
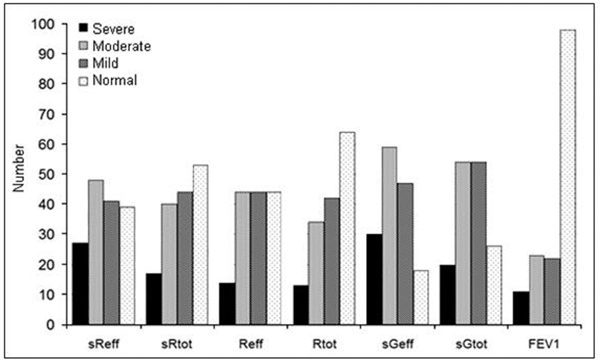
According to PD+100 sReff, 25 patients suffered from severe AHR, in 47 a moderate AHR was found, in 41 patients a mild airway AHR, and in another 41 patients normal responses with an increase in sReff < 2.0 kPa/L · s or < 100%. So a total of 113 patients (75%) showed positive MCH challenges based on sReff (Figure 1).
Figure 1.
Number of patients with MCH responses, classified as severe, moderate, mild, and normal, based on PD+100 for sReff, sRtot, Reff, Rtot, PD-40 for sGeff and sGRtot and PD-20 for FEV1.
Regarding PD-40 sGeff with a decrease of 40% as the threshold value, 28 patients had severe, 58 patients moderate, and 45 mild AHR, whereas only 19 patients had normal responses, so 88% of the patients had positive test results by sGeff.
Taking a fall in FEV1 > 20% of baseline value (PD-20 FEV1) as a criterion, only 6 patients had severe AHR, 23 had moderate responses, 21 had mild responses, and the majority of 104 patients had no response. So, 50 from the 154 patients (32%) had a positive test result with FEV1 (Figure 2).
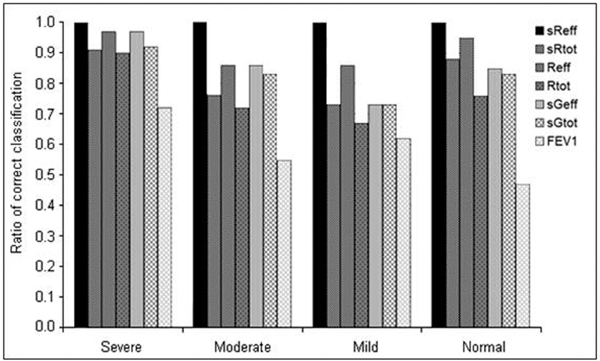
Figure 2.
Accuracy of responses classified as severe, moderate, mild and normal, based on PD+100 for sReff, sRtot, Reff, Rtot, PD-40 for sGeff and sGRtot, and PD-20 for FEV1.
The mean MCH-dose for diagnosis of severe AHR was 0.026 ± 0.012 mg MCH. This dose was inhaled after the first or second MCH-inhalation step. Moderate AHR was diagnosed after step 3 or 4, at a mean cumulative dose of 0.122 ± 0.046 mg MCH. The mean dose for a mild AHR was 0.356 ± 0.122 mg MCH.
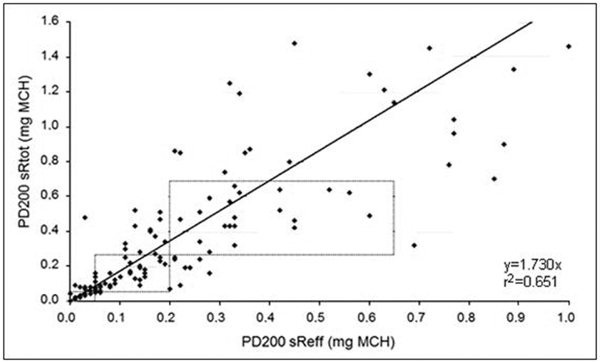
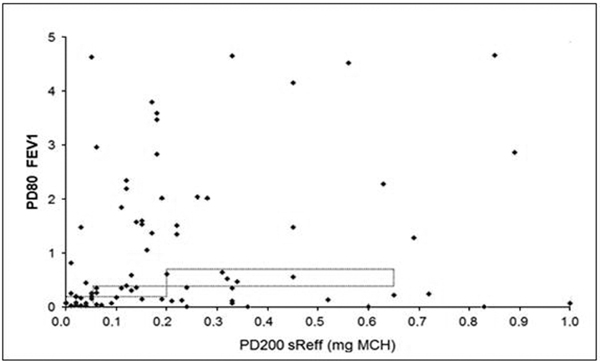
Statistical analysis revealed strongly linearly correlated parameters of airway resistance, but no significant relationship between the test results of body plethysmography and forced spirometry. PD+100 sReff and PD+100 sRtot significantly correlated in patients with severe, moderate and mild degree of AHR (Figure 3). For PD-20 FEV1 and PD+100 sReff no significant correlation could be found (Figure 4). Significant accuracy of PD+100 sReff and PD-40 sGeff could be stated.
Figure 3.
Correlation of PD+100 sRtot and PD+100 sReff in cumulative MCH challenge testing inpatients with mild asthma.
Figure 4.
Correlation of PD+100 sReff and of PD-20 FEV1 in cumulative MCH challenge tests in patients with mild asthma.
Coinciding positive or negative results in Reff and sReff were found in 95% of the MCH tests, sReff and sRtot matched in 88% of the patients, 85% matched in sReff and sGeff, but only 47% coinciding responses were obtained in sReff and FEV1.
Discussion
The concordance of forced spirometry and tidal breathing recording, studied by body plethysmography, was investigated by bronchial challenge tests in patients with chronic cough or suspected mild asthma. In cumulative MCH challenges, we found specific airway resistance (sReff) to be the most useful parameter in the detection of AHR. The measurement of specific airway resistance (sRaw) by body plethysmography is largely independent of patient's cooperation, facilitating the interpretation of measurements. Furthermore, higher MCH doses were necessary before FEV1, as a key parameter of forced spirometry, showed a significant decrease above 20%. Although most practitioners only measure FEV1, reliance upon this measurement alone can lead to both false negative and false positive test results.
Goldstein et al [18] reported that sensitivity of a MCH challenge determined by FEV1 alone was only 60%, but increased to 97% after the assessments of FVC, sGaw, and thoracic gas volume (TGV) were added to the analysis. This result is in accordance with the known axial heterogeneity of the response of airways of different caliber to bronchoactive agents. Furthermore, if patients not fully inhale to total lung capacity (TLC), a fall in FEV1 can occur and cause a false positive result. Generally, the sensitivity of a positive MCH challenge test for the diagnosis of asthma is 85%. A positive predictive value is more limited, as false positive results may be seen in patients with allergic rhinitis, cystic fibrosis, congestive heart failure, COPD, and bronchitis. [1,19]. However, patients with allergic rhinitis, cystic fibrosis, heart failure, or COPD were not included in this study.
Impact of Deep Inspirations during Forced Expiratory Maneuvers on MCH-Testing
Deep breaths effectively modulate airway caliber. In healthy subjects, deep inhalations result in bronchodilatation and reduce pharmacologically induced airways obstruction [20]. On the other hand, avoidance of maximal maneuvers enhances reactions of bronchoconstrictor agents [21]. Taking deep breaths before bronchial challenge tests diminshes obstructive responses as a consequence [22,23]. Therefore, deep inspirations during forced expiratory maneuvers provide an effective protection against airways obstruction. However, the beneficial effects of deep inspirations are impaired in asthma [24,25] and during acute exacerbations of asthma. In these clinical settings deep inspirations may even enhance obstructive responses [26]. In chronic obstructive pulmonary disease (COPD), bronchodilatory effects of deep inspirations are effectively reduced, which may depend on the degree of parenchymal damage [27]. Airway inflammation is a typical feature of asthma and COPD, although the composition of inflammatory cells is different [28,29]. Inflammatory processes induce airway remodelling and thus increasing thickness of airway walls and reducing the elasticity of the airways [30]. The result would be a reduced strain transmission from lung parenchyma to the airway walls and diminished effects of stretching the airways [31].
Slats et al [14] demonstrated that the bronchodilatory effects of deep inspirations in asthma are related to inflammatory cell counts in bronchial walls and smooth muscle layers, whereas in moderate COPD this relationship could not be found. They conclude that the physiologic protection against narrowing of the airways by deep inspirations is impaired in asthma and COPD, but depends on different mechanisms.
Changes in Airway Resistance During Quiet Breathing
Using body plethysmography for the assessment of airway resistance responses, measurements are performed during quiet breathing and are not masked by the bronchodilatory responses of deep inhalations or positive intraluminal pressure in the airways. In addition, the results are largely independent of patients' cooperation. There was a close correlation between Reff and Rtot, the two parameters for airway resistance, which are calculated from different approaches, and the PD+100 of both parameters. In addition, specific airway resistance sReff and sRtot, including lung volume and changes in lung volume during airway obstruction, closely correlate. Since Raw and intrathoracic gas volume (ITGV) both increase during acute airway obstruction, the largest response rates can be recorded in sReff and sRtot. A close correlation was also found between specific airway resistance and specific conductance, sGaw, the reciprocal value of specific resistance. In further investigations, PD sGaw should be adjusted to PD+100 sReff.
Clinical Relevance of Non-Specific Challenge Testing
AHR is an important defining feature of asthma and is a manifestation of reversible airflow obstruction due to smooth muscle contraction. AHR represents an exaggerated constrictor response to a variety of physical, chemical, or environmental stimuli. AHR can be quantified by the dose response to pharmacologic agents such as methacholine or histamine, causing a 20% decrease in FEV1 from baseline. While AHR is not specific for asthma, patients with asthma typically demonstrate AHR to much lower doses (e.g., 10- to 100-fold) of these agents than normal or allergic individuals. The single, most typical abnormality in many patients with asthma is AHR. As a result, the assessment of changes in AHR using bronchoprovocation testing may be preferable to the reliance upon subjective changes in symptoms alone. This is particularly relevant when asthma control requires a complex, costly, and potentially toxic therapeutic regimen. Thus, an appropriate bronchoprovocation testing is of high clinical relevance. Indications for bronchoprovocation testing include not only the accurate diagnosis of asthma, but also the assessment of the response to asthma therapy, and, less commonly, the identification of triggers for cases involving environmental or occupational asthma. The measurement of AHR by bronchoprovocation testing is potentially useful for several reasons: failure to show AHR argues against the diagnosis of asthma, AHR may be the sole objective evidence of airway dysfunction, AHR is quantitatively associated with the presence and severity of disease, the occurrence of AHR in an asymptomatic person may help predict the future development of asthma, the degree of AHR in a symptomatic person can have prognostic and potentially therapeutic implications, the periodicity of asthma exists in parallel with changes in the degree of AHR [32,33]. A false negative FEV1 in patients with asthma may have serious consequences for the patient due to undertreatment. Thus, recording responses of airway resistance by measurements performed during quiet breathing, which are not masked by the bronchodilatory responses of deep inspirations may increase the diagnostic impact of bronchoprovocation.
Summary and Conclusion
The authors compared forced spirometry and body plethysmography in order to evaluate the concordance of the forced respiratory maneuvers and measurements of airway resistance during normal breathing in MCH challenges. PD+100 Raw and PD-40 Gaw for the following plethysmographically measured parameters Rtot, Reff, sRtot, sReff, sGtot, and sGeff, are closely correlated with cumulative MCH-challenge tests. Our results did not show a correlation of PD+100 sReff and PD-20 FEV1. The reason for the different responses might be found in the location of the plethymographically measured airway resistance and the flow reduction measured in forced spirometric maneuvers. From our results, we would recommend sReff and sGaw as the reliable parameters for classification of AHR. Additional investigations on healthy subjects and patients with asthma and COPD should be performed to compare the sensitivity and specificity of body plethysmography and forced spirometry for MCH-challenge tests.
Conflicts of interest
The authors declare that they have no competing interests.
References
- Crapo RO, Casaburi R, Coates AL. et al. Guidelines for methacholine and exercise challenge testing - 1999. The official statement of the American Thoracic Society adopted by the ATS Board of Directors. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- Juniper EF, Frith PA, Hargreave FE. Airway responsiveness to histamine and methacholine: Relationship to minimum treatment to control symptoms of asthma. Thorax. 1981;36:575–579. doi: 10.1136/thx.36.8.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockroft DW, Killian DN, Mellon JA. et al. Bronchial reactivity to inhaled histamine: A method and clinical survey. Clin Allergy. 1977;7:235. doi: 10.1111/j.1365-2222.1977.tb01448.x. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society. Guidelines for methacholine and exercise challenge testing. Am J Respir Crit Care Med. 1999;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- Sterk PJ, Fabbri LM, Quanjer PH. Airway responsiveness: standardized challenge testing with pharmacological, physical and sensitizing stimuli in adults. Eur Respir J. 1993;6:53–83. doi: 10.1183/09041950.053s1693. [DOI] [PubMed] [Google Scholar]
- Smith L, McFadden ER Jr. Bronchial hyperresponsiveness revisited. Ann Allergy Asthma Immunol. 1995;74:454–469. [PubMed] [Google Scholar]
- Cockroft DW, Davis BE, Todd AC. et al. Methacholine challenge: comparison of two methods. Chest. 2005;127:839–844. doi: 10.1378/chest.127.3.839. [DOI] [PubMed] [Google Scholar]
- Prieto L, Lopez V, Llusar R, Rocio Rojas RN, Marin J. Differences in the response to methacholine between the tidal breathing and dosimeter methods. Influence of the dose of bronchoconstrictor agent delivered to the mouth. Chest. 2008;134:699–703. doi: 10.1378/chest.08-0093. [DOI] [PubMed] [Google Scholar]
- Merget R, Jörres RA, Heinze E, Haufs MG, Taeger D, Bruning Th. Development of a 1-concentration-4-step dosimeter protocol for methacholine testing. Respir Med. 2009;103(4):607–613. doi: 10.1016/j.rmed.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Chai H, Farr RS, Froehlich LA, Mathison DA, McLean JA, Rosenthal RR. Standardization of bronchial inhalation challenge procedures. J Allergy Clin Immunol. 1975;56:323–327. doi: 10.1016/0091-6749(75)90107-4. [DOI] [PubMed] [Google Scholar]
- Ryan G, Dolovich MB, Obminski G, Cockroft DW, Juniper E, Hargreave FE. Standardization of inhalation provocation tests: influence of nebulizer output, particle size, and method of inspiration. J Appl Physiol. 1981;67:156–161. doi: 10.1016/0091-6749(81)90012-9. [DOI] [PubMed] [Google Scholar]
- Cockroft DW, Davis BE. The bronchoprotective effect of inhaling methacholine by using total lung capacity inspirations has a marked influence on the interpretation of test results. J Allergy Clin Immunol. 2006;117:1244–1248. doi: 10.1016/j.jaci.2006.02.038. [DOI] [PubMed] [Google Scholar]
- Nadel JA, Tierney DF. Effect of a previous deep inspiration on airway resistance. J Appl Physiol. 1961;16:717–719. doi: 10.1152/jappl.1961.16.4.717. [DOI] [PubMed] [Google Scholar]
- Slats AM, Janssen K, van Schadewijk A, van der Plas DT, Schot R, van der Aardweg JG, de Jongste JC, Hiemstra PS, Mauad T, Rabe KF, Sterk PJ. Bronchial inflammation and airway responses to deep inspiration in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:121–128. doi: 10.1164/rccm.200612-1814OC. [DOI] [PubMed] [Google Scholar]
- Quanjer P, Tammeling GJ, Cotes JE, Pederson OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report of the working party standardization of lung functions tests. European Community for Steel and Coal. Eur Respir J. 1993;6(Suppl 16) [Google Scholar]
- Camarri B, Eastwood PR, Cecins NM, Thompson PJ, Jenkins S. Six minutes walk distance in healthy subjects aged 55-75 years. Respir Med. 2006;100:658–665. doi: 10.1016/j.rmed.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Criée CP, Berdel D, Heise D, Jörres RA, Kardos P, Köhler D, Leupold W, Magnussen H, Marek W, Merget R, Mitfessel H, Rasche K, Rolke M, Smith HJ, Sorichter S, Worth H. Empfehlungen zur Ganzkörperplethysmographie. München, Orlando: Dustri-Verlag Dr. Karl Feistle; 2009. [Google Scholar]
- Goldstein MF, Pacana SM, Dvorin DJ, Dunsky EH. Retrospective analysis of methacholine inhalation challenges. Chest. 1994;105:1082–1088. doi: 10.1378/chest.105.4.1082. [DOI] [PubMed] [Google Scholar]
- Goldstein MF, Veza BA, Dunsky EH, Belecanech GA, Haralabatos JC. Comparisons of peak diurnal expiratory flow variation, postdilatator FEV1 responses, and methacholine inhalation challenges in the evaluation of suspected asthma. Chest. 2001;119:1001–1010. doi: 10.1378/chest.119.4.1001. [DOI] [PubMed] [Google Scholar]
- Scichilone N, Permutt S, Togias A. The lack of the bronchoprotective and not the bronchodilatatory ability is associated with airway hyperresponsiveness. Am J Respir Crit Care Med. 2001;163:413–419. doi: 10.1164/ajrccm.163.2.2003119. [DOI] [PubMed] [Google Scholar]
- Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J Clin Invest. 1995;96:2393–2403. doi: 10.1172/JCI118296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsali T, Permutt S, Laube B, Scichilone N. Potent bronchoprotective effect of deep inspiration and its absence in asthma. J Appl Physiol. 2000;89:711–720. doi: 10.1152/jappl.2000.89.2.711. [DOI] [PubMed] [Google Scholar]
- Scichilone N, Kapsali T, Permutt S, Togias A. Deep inspiration-induced bronchoprotection is stronger than bronchodilatation. Am J Respir Crit Care Med. 2009;162:910–916. doi: 10.1164/ajrccm.162.3.9907048. [DOI] [PubMed] [Google Scholar]
- Jensen A, Atileh H, Suki B, Ingenito EP, Lutchen KR. Selected contribution: airway caliber in healthy and asthmatic subjects: effects of bronchial challenge and deep inspiration. J Appl Physiol. 2001;91:506–515. doi: 10.1152/jappl.2001.91.1.506. [DOI] [PubMed] [Google Scholar]
- Salome CM, Thorpe CW, Dipa C, Brown NJ, Berend N, King GG. Airway re-narrowing following deep inspiration in asthmatic and non asthmatic subjects. Eur Respir J. 2003;22:62–68. doi: 10.1183/09031936.03.00117502. [DOI] [PubMed] [Google Scholar]
- Lim TK, Ang SM, Rossing TH, Ingenito EP, Ingram RH Jr. The effects of deep inhalation on maximal expiratory flow during intensive treatment of spontaneous asthmatic episodes. Am Rev Respir Dis. 1989;140:340–343. doi: 10.1164/ajrccm/140.2.340. [DOI] [PubMed] [Google Scholar]
- Fraishter RD. Airway hysteresis in normal subjects and individuals with chronic airflow obstruction. J Appl Physiol. 1985;58:1505–1510. doi: 10.1063/1.336084. [DOI] [PubMed] [Google Scholar]
- Jeffrey PK. Remodelling and inflammation of the bronchi in asthma and chronic obstructive pulmonary disease. Proceedings American Thoracic Society. 2004;1:176–183. doi: 10.1513/pats.200402-009MS. [DOI] [PubMed] [Google Scholar]
- Fabbri LM, Romagnoli M, Corbetta L, Casoni G, Busljetic K, Turato G, Ligabue G, Ciaccia A, Saetta M, Papi A. Differences in airway inflammation in patients with fixed airflow obstruction due to asthma and or chronic obstructive pulmonary desease. Am J Respir Crit Care Med. 2003;167:418–424. doi: 10.1164/rccm.200203-183OC. [DOI] [PubMed] [Google Scholar]
- Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodelling in asthma. J Clin Invest. 1999;104:1001–1006. doi: 10.1172/JCI8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklem PT. A theoretical analysis of the effects of airway smooth muscle load on airway narrowing. Am J Respir Crit Care Med. 1996;153:83–89. doi: 10.1164/ajrccm.153.1.8542167. [DOI] [PubMed] [Google Scholar]
- Laprise C, Laviolette M, Boutet M, Boulet LP. Asymptomatic airway responsiveness: relationship with airway inflammation and remodelling. Eur Respir J. 1999;14:63–73. doi: 10.1034/j.1399-3003.1999.14a12.x. [DOI] [PubMed] [Google Scholar]
- Weiss ST, van Natta ML, Zeiger RS. Relationship between increased airway responsiveness and asthma severity in the childhood asthma management program. Am J Respir Crit Care Med. 2000;162:50–56. doi: 10.1164/ajrccm.162.1.9811005. [DOI] [PubMed] [Google Scholar]