Abstract
Objective
Oxygen therapy is used for the treatment of various diseases, but prolonged exposure to high concentrations of O2 is also associated with formation of free radicals and oxidative damage.
Methods
In the present study we compared α-ketoglutarate dehydrogenase (KGDH) activity and mitochondrial oxidative damage in the hearts of guinea pigs after long-term (17 and 60 h) oxygenation with 100% normobaric O2 and with partially negatively (O2 neg) or positively (O2 posit) ionized oxygen.
Results
Inhalation of O2 led to significant loss in KGDH activity and thiol group content and accumulation of bityrosines. Inhalation of O2 neg was accompanied by more pronounced KGDH inhibition, possibly due to additional formation of protein-lipid conjugates. In contrast, O2 posit prevented loss in KGDH activity and diminished mitochondrial oxidative damage.
Conclusions
These findings suggest that oxygen treatment is associated with impairment of heart energy metabolism and support the view that inhalation of O2 posit optimizes the beneficial effects of oxygen therapy.
Keywords: oxygenation, reactive oxygen species, α-ketoglutarate dehydrogenase, heart, oxidative damage
Introduction
Oxygen therapy is applied for the treatment of various diseases and clinical conditions, including myocardial infarction [1]. The beneficial effects of increased oxygenation of plasma and body tissues are related to the stimulation of angiogenesis, anti-inflammatory response, and immune function [2]. On the other hand, it is well known that exposure to pure O2 is toxic and could cause serious tissue damage. Although the molecular mechanism by which hyperoxia affects body tissues is not completely understood, there is accumulating evidence that reactive oxygen/nitrogen species (ROS/RNS) are important factors mediating both beneficial and adverse effects of increased oxygenation [2,3]. Antioxidant supplementation was used to prevent negative impacts of oxygenation therapy, however, with contradictory results. While some studies have shown protective effects of exogenously administered antioxidants [4,5] others have failed to demonstrate prevention against oxidative damage [6]. Recent studies from our laboratories have suggested that the adverse effects of classical oxygenation therapy on lungs are reduced when inhaled oxygen is partially ionized [7]. Long-term inhalation of partially positively ionized oxygen was associated with better surfactant activity, milder inflammatory response, and lower oxidative stress than inhalation of O2 or partially negatively ionized oxygen.
The aim of present study was to compare the role of long-term classical oxygenation with molecular oxygen and oxygenation with partially ionized oxygen in oxidative damage to the guinea pig heart. For this purpose, we measured the markers of lipid and protein modifications: conjugated dienes, thiol group content, bityrosines, and lysine conjugates with lipid peroxidation-end products in cardiac mitochondria. Furthermore, to evaluate the role of mitochondria in oxygenation-induced oxidative stress, the enzyme activity of α-ketoglutarate dehydrogenase (KGDH), a key component of citric acid cycle was measured after different oxygenation treatments.
Material and methods
Animals and Oxygenation Treatment
All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by The US National Institute of Health (NIH publication NO 85-23, revised 1996), and the ethical guidelines of the Jessenius Faculty of Medicine, Comenius University in Martin.
Forty male Trik guinea pigs (supplied by IEP SAS, Dobra Voda, Slovakia) weighing 250-350 g were used in the experiments. For oxygenation experiments, guinea pigs were placed in a sealed metabolic chamber and exposed for a period of 17 and 60 h to 100% molecular (O2 mol), partially negatively charged, · O2 -, (O2 neg) or partially positively charged, · O2 +, (O2 posit) oxygen as described previously [7]. The O2 concentration was monitored periodically by an oxygen analyzer (Permolyt 3, Veb Junkalor, Germany). Partial and unipolar ionization of medical O2 was induced as described previously [8] between two electrodes with 15 kV using plasma chamber Oxygen Ion 3000/Dr. Engler (CStronic GmBH, Austria) which is certified as medical therapeutic device. The control group inhaled atmospheric air.
Preparation of Cardiac Mitochondria and Determination of KGDH Activity
At the end of oxygenation treatment, a lethal dose of anesthetics was injected intraperitoneally. Hearts were excised, immediately washed with physiological solution, and stored at -80°C until used. Mitochondrial fractions were isolated from thawed cardiac homogenates by differential centrifugation and sonication in water bath. KGDH activity was determined by monitoring NAD+ reduction at 340 nm at 25°C [9]. Mitochondria (50 μg protein/ml) were incubated in medium containing 25 mM KH2PO4, 5 mM MgCl2, 0.5 mM EDTA, 0.1% Triton X-100, 40 mM rotenone, 2.5 mM α-ketoglutarate, 0.1 mM coenzyme A, 0.2 mM thiamine diphosphate and 1 mM NAD+ at pH 7.25.
Determination of Lipid and Protein Oxidative Damage
Formation of conjugated dienes was estimated from the absorbance ratio A233 nm/A215 nm of mitochondria (0.02 mg protein per ml) dispensed in 10 mM phosphate buffer containing 1% Lubrol [10]. Total thiol group content was determined spectrophotometrically at 412 nm (ε = 13.6 mM-1cm-1) as described previously [10]. All fluorescence measurements were performed in solution containing 0.05 mg of mitochondrial protein per ml, 10 mM HEPES and 100 mM KCl, pH = 7.0 at room temperature on a PerkinElmer LS 55 spectrophotofluorimeter, as shown previously [12]. The emission spectra of bityrosine, a product of tyrosine oxidation, were recorded in a range of 380-440 nm (5 nm slit width) at the excitation wavelength of 325 nm (5 nm). The emission spectra (425-480 nm, 5 nm) of lysine conjugates with LPO-end products were recorded at excitation of 365 nm (5 nm).
Statistical Analysis
Data are expressed as means ± SE. One-way analysis of variance with post-hoc comparisons by a Student-Neuman-Keuls test was carried out to test for differences among groups. A value of p < 0.05 was considered to be statistically significant.
Results
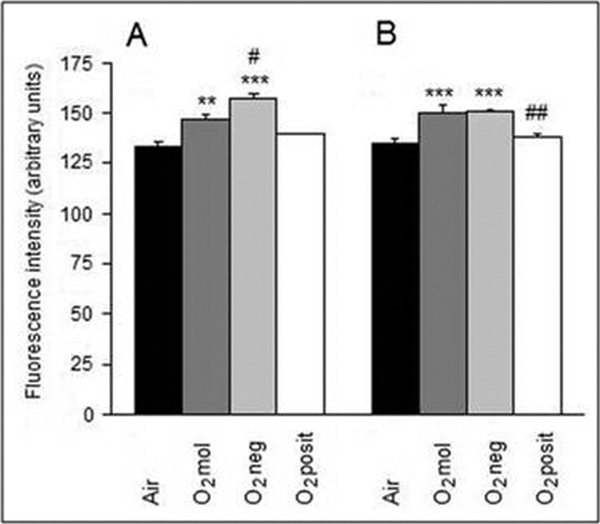
Protein oxidative damage was assessed by the measurements of bityrosines and thiol group content. As shown in Figure 1, 17- and 60-hour inhalations of O2 mol or O2 neg were associated with a significant accumulation of protein bityrosines. When compared with the control guinea pigs, which inhaled atmospheric air, the 17- and 60-hour O2 mol treatments increased bityrosines by 10.1% (p < 0.01) and 10.9% (p < 0.001), and the O2 neg treatments increased bityrosines by 18.0% (p < 0.001) and 11.3% (p < 0.001), respectively (Figure 1). On the other hand, bityrosine content was unchanged by inhalation of O2 posit.
Figure 1.
Effects of 17 h (A) and 60 h (B) oxygenation treatments on bityrosine formation in guinea pig cardiac mitochondria. Values are means ± SE of 5 experiments. **P < 0.01, ***P < 0.001 - significantly different compared with the airtreated group; # P < 0.05, ## P < 0.01 - significantly different when compared with the O2-treated group.
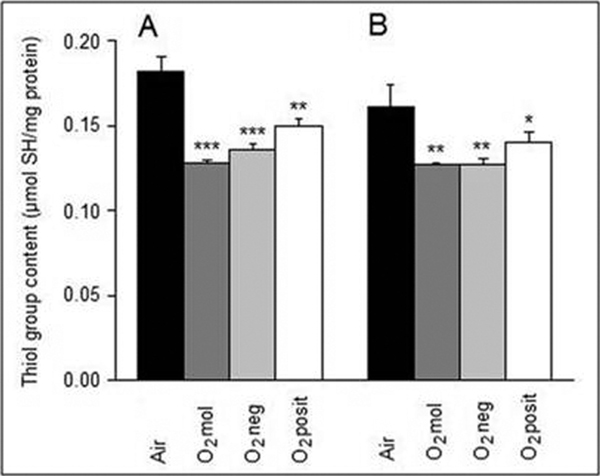
All kinds of oxygenatio n treatments used decreased thet hiol group content, but to a different extend (Figure 2). The O2 mol inhalation caused loss of thiols by 29.7% (p < 0.01) and 21.1% (p < 0.01) after 17- and 60-hour treatment, respectively. The O2 neg inhalation resulted in decreases of thiols by 25.3 % (p < 0.0 1) and 21.1 % (p < 0.0 1). Changes in O2 posit treated anim als were lower (17.6%, p < 0.0 1 and 13.0 %, p < 0.0 5), respectively.
Figure 2.
Thiol group content in guinea pig cardiac mitochondria subjected to 17 h (A) and 60 h (B) oxygenation treatments. Values are as means ± SE of 5 experiments. *P < 0.05, **P < 0.01, ***P < 0.001 - significantly different compared with the air-treated group.
To determine whether oxygenation treatments lead to increased oxidative damage to lipids, we measured the formation of conjugated dienes. While inhalation of molecular O2 was not accompanied by elevated lipid peroxidation, inhalation of partially negatively charged O2 caused significant changes (Table 1). When compared with the control guinea pigs, 17- and 60-hour O2 neg treatment increased conjugated dienes by 18.4 and 11.9%, respectively. In contrast, O2 posit treatment prevented lipid peroxidation, after 17-hour inhalation the level of conjugated dienes was significantly lower not only when compared with the animals which inhaled O2, but also those which inhaled atmospheric air. In order to determine whether lipid peroxidation products (LPO) can contribute to protein lesions fluorescence spectra of conjugates formed from LPO end-products and proteins were measured. As expected, a significant rise in LPO product-protein conjugates was observed only in mitochondria isolated from O2 neg treated guinea pigs (Table 1).
Table 1.
Lipid and protein oxidative damage.
| Conjugated dienes A233 nm/A215 nm | Protein-LPO (arbitrary units) | |
|---|---|---|
| 17 h inhalation of | ||
| Air | 0.266 ± 0.004 | 84.6 ± 1.3 |
| O2mol | 0.249 ± 0.014 | 79.4 ± 1.3 |
| O2neg | 0.315 ± 0.002**### | 102.0 ± 2.1*### |
| O2posit | 0.215 ± 0.011**# | 87.4 ± 3.1 |
| 60 h inhalation of | ||
| air | 0.277 ± 0.001 | 79.8 ± 4 |
| O2mol | 0.265 ± 0.009 | 87.6 ± 5.6 |
| O2neg | 0.310 ± 0.009*## | 101.8 ± 3.2*# |
| O2posit | 0.236 ± 0.011 | 74.4 ± 2.1 |
Values are given as means ± SE of 5 experiments. *p < 0.05, **p < 0.01 -
significantly different compared with the air treated group; #p < 0.05, ##p < 0.01,
###p < 0.001- significantly different compared with the O2 treated group.
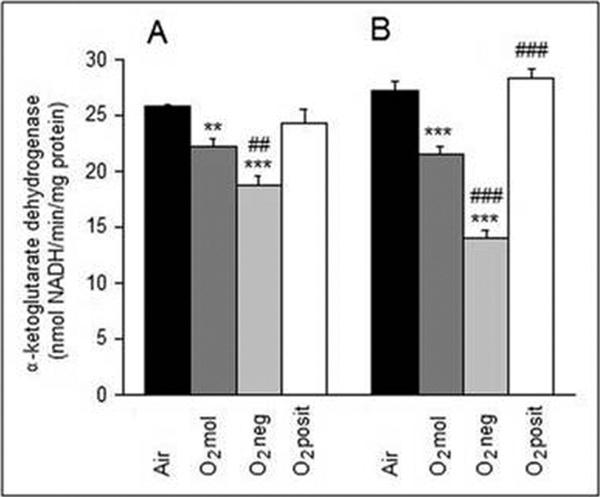
The effects of oxygen treatments on mitochondrial function were evaluated by measuring the activity of the KGDH complex. As shown in Figure 3, individual oxygen treatments affected enzyme activity differently. Compared with the control guinea pigs, the activity was 13.7% (p < 0.001) and 21.2% (p < 0.001) lower in animals, which inhaled molecular O2 for 17- and 60- hours, respectively. Inhalation of O2 neg was associated with a more pronounced change in the activity, it decreased by 27.3% (p < 0.001) and 48.2% (p < 0.001) of the control level after 17- and 60-hour treatments, respectively. Moreover, these activities were also significantly lower when compared with the O2 treatment. In contrast, there were no significant changes in KGDH activity between the guinea pigs inhaling air and O2 posit.
Figure 3.
Effects of 17 h (A) and 60 h (B) oxygenation treatments on KGDH activity in guinea pig cardiac mitochondria. Values are means ± SE of 5 experiments. **P < 0.01, ***P < 0.001 - significantly different compared with the airtreated group. ## P < 0.01, ### P < 0.001 - significantly different compared with the O2-treated group.
Discussion
In this study, long-term oxygenation was used as a model for the determination of ROS-induced oxidative damage. In addition to classical oxygenation, we studied the effects of inhalation of oxygen which was partially ionized in a high-voltage plasma chamber [8,11]. Previous experimental and clinical studies have demonstrated beneficial effects of this therapy in comparison with inhalation of molecular oxygen [7,8,11,12]. Our results demonstrate that 17- and 60-hour inhalation of 100% oxygen is accompanied by increased oxidative damage to lipids and proteins and by loss in KGDH activity in mitochondria of the guinea pig heart. Free radical-induced oxidative damage and enzyme inhibition was even more pronounced when inhaled oxygen was partially negatively charged. On the other hand, when inhaled oxygen was partially positively charged, structural and functional changes were lowered or completely eliminated.
Our study shows that oxygenation treatment increases oxidative stress in mitochondria, which are known to be a major intracellular source of ROS/RNS and a vulnerable target of their damaging effects [13]. Accumulation of mitochondrial oxidative damage is in agreement with earlier study showing that a rise in partial pressure of O2 markedly increases H2 O2 formation in isolated cardiac mitochondria [14]. To further understand the mechanism of oxygenation-induced mitochondrial injury, we examined the KGDH enzyme activity. This enzyme is essential for mitochondrial energy metabolism and other biological function, because it catalyses a critical step in the citric acid cycle that limits the amount of NADH available for respiratory chain [15] and regulates the level of the essential metabolite α-ketoglutarate [16]. KGDH is known to be highly vulnerable to oxidative damage; it has been found to be inhibited by various ROS/RNS including H2 O2 [17,18]. Our study shows that a rise in partial pressure of O2 is associated with KGDH inhibition. The possible mechanisms by which inhalation of O2 mol could inactivate KGDH include oxidation of thiol groups or bityrosine formation, which were found to be significantly changed during the treatment. Analysis of KGDH composition revealed 32 moles of thiol groups per mol of enzyme and the absence of disulfide bridges [19]. It has been shown that thiol groups of the enzyme play a critical role in substrate binding and thiol-disulfide exchange can affect the activity [20]. There are no data in the literature on bityrosine formation in KGDH exposed to ROS, but nitration of tyrosine residues was shown to inhibit its activity [21]. It is then possible that other oxidative tyrosine lesions, including bityrosine formation, might also cause inhibition of KGDH.
In O2 neg treated guinea pigs inhibition of KGDH was much more pronounced than in O2 mol treated animals, although the changes in the contents of thiol groups and bityrosines were similar. These data suggest additional mechanism of KGDH inhibition during O2 neg treatment. In contrast to the other treatments, inhalation of partially negatively charged oxygen was accompanied by a significant rise in conjugated dienes, the markers of LPO., Reactive aldehydes, such as malondialdehyde and 4-hydroxynonenal (HNE), are generated during LPO and these compounds are potentially highly toxic for various cell components, particularly for proteins. Humphries and Szweda [22] have shown that incubation of cardiac mitochondria with HNE results in selective inhibition of KGDH and pyruvate dehydrogenase. We assessed a degree of modification of protein lysine residues by reactive aldehydes by measuring their fluorescence spectra. Our results show that the accumulation of fluorescent lysine derivatives occurs only in mitochondria from animals that inhaled O2 neg. Thus, an indirect protein modification, mediated by LPO, might be the possible cause of a greater loss of KGDH activity in O2 neg compared with O2mol-treated animals. Since KGDH is a rate-limiting enzyme of oxidative phosphorylation, its inhibition is likely associated with impairment of heart energy metabolism and subsequently heart function. In this respect, our findings that inhalation of O2-enriched with positively charged O2 is accompanied by preservation of KGDH activity is of considerable importance. Diminished oxidative lesions in mitochondrial lipids and proteins suggest that elimination of ROS/RNS is a likely mechanism of O2pos protective effects. These data suggest that O2pos oxygenation can be an alternative to antioxidant supplementation, whose efficiency to prevent oxidative damage caused by oxygen therapy is not unequivocal.
In conclusion, our study provides evidence that oxygenation treatment is associated with oxidative stress and loss of α-ketoglutarate dehydrogenase activity in mitochondria of the guinea pig heart. These adverse effects were prevented when inhaled oxygen was partially positively charged. Our findings support the view that ionized oxygenation optimizes the effect of oxygen therapy.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgements
This work was partially supported by grants VEGA 1/0027/08 and VVCE APVV 0064/07 from the Ministry of Education and Science of the Slovak Republic.
References
- Dekleva M, Neskovic A, Vlahovic A, Putnikovic B, Beleslin B, Ostojic M. Adjunctive effect of hyperbaric oxygen treatment after thrombolysis on left ventricular function in patients with acute myocardial infarction. Am Heart J. 2004;148:E14. doi: 10.1016/j.ahj.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Thom SR. Oxidative stress is fundamental to hyperbaric oxygen therapy. J Appl Physiol. 2009;106:988–995. doi: 10.1152/japplphysiol.91004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colantuono G, Tiravanti EA, Di Venosa N, Cazzato A, Rastaldo R, Cagiano R, D'Agostino D, Federici A, Fiore T. Hyperoxia confers myocardial protection in mechanically ventilated rats through the generation of free radicals and opening of mitochondrial ATP-sensitive potassium channels. Clin Exp Pharmacol Physiol. 2008;35:64–71. doi: 10.1111/j.1440-1681.2007.04745.x. [DOI] [PubMed] [Google Scholar]
- Dundar K, Topal T, Ay H, Oter S, Korkmaz A. Protective effects of exogenously administrated or endogenously produced melatonin on hyperbaric oxygen-induced oxidative stress in the rat brain. Clin Exp Pharmacol Physiol. 2005;32:926–930. doi: 10.1111/j.1440-1681.2005.04286.x. [DOI] [PubMed] [Google Scholar]
- Brozmanova M, Plevkova J, Bartos V, Plank L, Javorka M, Tatar M. The interaction of dietary antioxidant vitamins and oxidative stress on cough reflex in guinea-pigs after long term oxygen therapy. J Physiol Pharmacol. 2006;57(Suppl 4):45–54. [PubMed] [Google Scholar]
- Bader N, Bosy-Westphal A, Koch A, Rimbach G, Weimann A, Poulsen HE, Muller MN. Effect of hyperbaric oxygen and vitamin C and E supplementation on biomarkers of oxidative stress in healthy men. Br J Nutr. 2007;98:826–833. doi: 10.1017/S0007114507744380. [DOI] [PubMed] [Google Scholar]
- Calkovska A, Engler I, Mokra D, Drgova A, Sivonova M, Tatarkova Z, Calkovsky V, Brozmanova M, Tatar M. Differences in oxidative status, lung function, and pulmonary surfactant during long-term inhalation of medical oxygen and partially ionized oxygen in guinea pigs. J Physiol Pharmacol. 2008;59(Suppl 6):173–181. [PubMed] [Google Scholar]
- Engler I, Atzmuller Ch, Donic V, Hainschwang W, Jutka K, Steinhäusler F. Application of reactive oxygen species and biotonometry as a new approach to cancer. Folia Biol. in press .
- Nulton-Persson AC, Szweda LI. Modulation of mitochondrial function by hydrogen peroxide. J Biol Chem. 2001;276:23357–23361. doi: 10.1074/jbc.M100320200. [DOI] [PubMed] [Google Scholar]
- Kaplan P, Babusikova E, Lehotsky J, Dobrota D. Free radical-induced protein modification and inhibition of Ca2+-ATPase of cardiac sarcoplasmic reticulum. Mol Cell Biochem. 2003;248:41–47. doi: 10.1023/A:1024145212616. [DOI] [PubMed] [Google Scholar]
- Engler I. Ionisierter Sauerstoff. Uelzen, ML Verlag. 1988.
- Pohl P, Engler-Plörel S, Engler I. Inhalationstherapie mit IO2Th bei patienten mit chronisch progredienter multipler sklerose. Erfahrungsheilkunde. 1992;1:46–48. [Google Scholar]
- Hool LC. Reactive oxygen species in cardiac signaling: from mitochondria to plasma membrane ion channels. Clin Exp Pharmacol Physiol. 2006;33:146–151. doi: 10.1111/j.1440-1681.2006.04341.x. [DOI] [PubMed] [Google Scholar]
- Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu KF, Blass JP. The α-ketoglutarate dehydrogenase complex. Ann NY Acad Sci. 1999;893:61–78. doi: 10.1111/j.1749-6632.1999.tb07818.x. [DOI] [PubMed] [Google Scholar]
- Harrison AP, Pierzynowski SG. Biological effects of 2-oxoglutarate with particular emphasis on the regulation of protein, mineral and lipid absorption/metabolism, muscle performance, kidney function, bone formation and cancerogenesis, all viewed from a healthy ageing perspective state of the art-review article. J Physiol Pharmacol. 2008;59:91–106. [PubMed] [Google Scholar]
- Tretter L, Adam-Vizi V. Inhibition of Krebs cycle enzymes by hydrogen peroxide: a key role of α-ketoglutarate dehydrogenase in limiting NADH production under oxidative stress. J Neurosci. 2000;20:8972–8979. doi: 10.1523/JNEUROSCI.20-24-08972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegate MAB, Humphries KM, Szweda LI. Reversible inhibition of α-ketoglutarate dehydrogenase by hydrogen peroxide: glutathionylation and protection of lipoic acid. Biochemistry. 2008;47:473–478. doi: 10.1021/bi7017464. [DOI] [PubMed] [Google Scholar]
- Koike K, Hamada M, Tanaka N, Otsuka K-I, Ogasahara K, Koike M. Properties and subunit composition of the pig heart 2-oxoglutarate dehydrogenase. J Biol Chem. 1973;249:3836–3842. [PubMed] [Google Scholar]
- Bunik VI, Buneeva OA, Gomazkova VS. Regulation of alpha-ketoglutarate dehydrogenase cooperative properties in substrate binding by thiol-disulfide exchange. Biochem Int. 1990;21:873–881. [PubMed] [Google Scholar]
- Park LCH, Zhang H, Sheu KF, Calingasan NY, Kristal BS, Lindsay JG, Gibson GE. Metabolic impairment induces oxidative stress, compromises inflammatory responses, and inactivates a key mitochondrial enzyme in microglia. J Neurochem. 1999;72:1948–1958. doi: 10.1046/j.1471-4159.1999.0721948.x. [DOI] [PubMed] [Google Scholar]
- Humphries KM, Szweda LI. Selective inactivation of α-ketoglutarate dehydrogenase and pyruvate dehydrogenase: reaction of lipoic acid with 4-hydroxy2-nonenal. Biochemistry. 1998;37:15835–15841. doi: 10.1021/bi981512h. [DOI] [PubMed] [Google Scholar]