Abstract
Genetic suppression of insulin/insulin-like growth factor signaling (IIS) can extend longevity in worms, insects, and mammals. In laboratory mice, mutations with the greatest, most consistent, and best documented positive impact on lifespan are those that disrupt growth hormone (GH) release or actions. These mutations lead to major alterations in IIS but also have a variety of effects that are not directly related to the actions of insulin or insulin-like growth factor I. Long-lived GH-resistant GHR-KO mice with targeted disruption of the GH receptor gene, as well as Ames dwarf (Prop1df) and Snell dwarf (Pit1dw) mice lacking GH (along with prolactin and TSH), are diminutive in size and have major alterations in body composition and metabolic parameters including increased subcutaneous adiposity, increased relative brain weight, small liver, hypoinsulinemia, mild hypoglycemia, increased adiponectin levels and insulin sensitivity, and reduced serum lipids. Body temperature is reduced in Ames, Snell, and female GHR-KO mice. Indirect calorimetry revealed that both Ames dwarf and GHR-KO mice utilize more oxygen per gram (g) of body weight than sex- and age-matched normal animals from the same strain. They also have reduced respiratory quotient, implying greater reliance on fats, as opposed to carbohydrates, as an energy source. Differences in oxygen consumption (VO2) were seen in animals fed or fasted during the measurements as well as in animals that had been exposed to 30% calorie restriction or every-other-day feeding. However, at the thermoneutral temperature of 30°C, VO2 did not differ between GHR-KO and normal mice. Thus, the increased metabolic rate of the GHR-KO mice, at a standard animal room temperature of 23°C, is apparently related to increased energy demands for thermoregulation in these diminutive animals. We suspect that increased oxidative metabolism combined with enhanced fatty acid oxidation contribute to the extended longevity of GHR-KO mice.
Keywords: growth hormone, aging, calorie restriction, dwarf mice, metabolism
INTRODUCTION: GROWTH HORMONE-RELATED MOUSE MUTANTS
Studies of hypopituitary, growth hormone (GH) deficient, and GH-resistant mice provided evidence that deletion of GH signals can produce an impressive extension of longevity (Brown-Borg et al., 1996; Flurkey et al., 2001; Coschigano et al., 2003). Mice lacking GH or GH receptors show numerous symptoms of delayed aging, are partially protected from age-related diseases, and outlive their normal siblings by 30–65% depending on genetic background, sex, and diet composition (reviewed in Bartke, 2011; Bartke, 2012; Brown-Borg and Bartke, 2012). Candidate mechanisms linking the absence of GH signals with extension of longevity include altered expression of numerous genes related to glucose homeostasis, protein synthesis, lipogenesis, lipolysis, and energy metabolism (Tsuchiya et al., 2004; Al-Regaiey et al., 2005; Papaconstantinou et al., 2005; Masternak and Bartke, 2007). Apparently, anti-aging effects of reduced GH signaling involve metabolic adjustments of which some resemble those that mediate the effects of calorie restriction (CR) on aging and longevity (Tsuchiya et al., 2004; Al-Regaiey et al., 2005; Bonkowski et al., 2009).
In this brief review, we will discuss metabolic characteristics of GH-deficient and GH-resistant mice which are likely to represent mechanisms of their extended longevity. Metabolic characteristics of other long-lived mutants, gene knockouts and transgenics as well as phenotypes of mice from strains with different longevity are outside the scope of this article, and the reader is referred to other reviews (Brown-Borg, 2006; Chen et al., 2010; Yuan et al., 2011).
ROLE OF IMPROVED INSULIN SIGNALING
Improved action of insulin on carbohydrate homeostasis is among the key metabolic alterations in long-lived GH-related mutants. GH receptor disrupted GHR-KO mice with profound GH resistance (Zhou et al., 1997), GH releasing hormone disrupted (GHRH-KO) mice with isolated GH deficiency (Alba and Salvatori, 2004), and hypopituitary Ames (Prop1df) and Snell (Pit1dw) dwarf mice with deficiency of GH, along with prolactin and thyrotropin (Bartke, 2011; Brown-Borg and Bartke, 2012; Bartke et al., in press), have reduced insulin levels and enhanced insulin sensitivity (Zhou et al., 1997; Bonkowski et al., 2006; Bartke, 2011; Bartke, 2012; Brown-Borg and Bartke, 2012; Spong and Bartke, unpublished). Since hypoinsulinemia promotes insulin sensitivity and vice versa, it could be debated which of these characteristics is primary and which is secondary. However, available evidence suggests that reduction in GH signals affects both the secretion and actions of insulin. GH and insulin-like growth factor I (IGF-I), a key mediator of GH action, promote development and secretory function of insulin-producing beta cells in the islets of Langerhans in the pancreas. Islet volume is reduced in GHR-KO mice (Guo et al., 2005), and the number of large islets is reduced in Ames dwarf mice (Parsons et al., 1995). Insulin sensitivity is negatively regulated by GH by a variety of mechanisms including reduced adiponectin levels, enhanced mammalian target of rapamycin (mTOR) signaling, and alterations in serum lipid profiles as well as ectopic fat accumulation. Many of these effects of GH on insulin signaling are mediated by enhanced inhibitory (serine) phosphorylation of insulin receptor substrate 1 (IRS-1; Aguirre et al., 2002; Ishizuka et al., 2004; Adochio et al., 2009). All of these mechanisms appear to be involved in improving insulin sensitivity in GH-related mouse mutants (Al-Regaiey et al., 2005; Wang et al., 2006; Bonkowski et al., 2009; List et al., 2011).
ROLE OF ADIPOSE TISSUE AND ITS PRODUCTS IN THE METABOLIC PROFILE OF GH-RELATED MUTANTS
We have recently obtained evidence that enhanced insulin sensitivity of long-lived GHR-KO mice is due to the altered secretory profile of intra-abdominal (“visceral”) adipose tissue and, in particular, to enhanced adiponectin secretion by these fat depots. It is well documented that adiponectin is an important insulin sensitizer. In comparison to normal mice, GHR-KO mutants have increased levels of adiponectin in the epididymal fat and in peripheral circulation (Al-Regaiey et al., 2005; List et al., 2011). To assess the impact of altered secretory activity of visceral fat on insulin signaling, we have compared the impact of removing most of this tissue on insulin and glucose tolerance in these mutants versus normal mice. We removed as much of the epididymal and perinephric (retroperitoneal) fat pads as was possible without endangering blood supply to the testes and the adrenals. In normal mice this resulted in significant improvements in insulin and glucose tolerance (Masternak et al., 2012) as expected from previous studies in this and other species (Shi et al., 2007; Muzumdar et al., 2008). Plasma adiponectin levels were not altered, indicating that in these animals circulating adiponectin is derived primarily from subcutaneous fat, or that other fat depots readily compensate for the consequences of removing visceral fat. In sharp contrast to these findings, visceral fat removal in GHR-KO mice reduced circulating adiponectin levels and reduced, rather than enhanced, tolerance to injected insulin or glucose (Masternak et al., 2012). Apparently, visceral fat is a major source of adiponectin in these animals and visceral fat-derived adiponectin importantly contributes to or perhaps accounts for enhanced insulin sensitivity of GHR-KO mice. In addition to differences in the levels of adiponectin, the levels of interleukin 6 (IL-6), which promotes insulin resistance, are reduced in both epididymal and perinephric fat of GHR-KO as compared to normal mice (Masternak et al., 2012). Altered IL-6 levels may have also contributed to the differential impact of visceral fat removal on insulin sensitivity in GHR-KO versus normal mice.
INTERACTIONS OF CALORIE RESTRICTION AND GH-RELATED MUTATIONS
Association of reduced insulin levels and enhanced insulin sensitivity with extension of longevity was shown in a comparison of GH-related mutants (GHR-KO, GHRH-KO, Prop1df, Pit1dw) with their normal siblings and in studies of the interaction of some of these “longevity genes” with CR (Masternak et al., 2009). Strikingly, CR improves insulin signaling in Ames dwarf mice, in which it also extends longevity (Bartke et al., 2001; Masternak et al., 2009), but has no such effect in GHR-KO mice or in GHRH-KO males in which effects of CR on longevity are absent or minimal (Bonkowski et al., 2006, 2009; Spong, Salvatori, and Bartke, unpublished). Moreover, longevity is not enhanced in transgenic mice overexpressing a GH antagonist in which insulin levels are not suppressed (Coschigano et al., 2003). It deserves emphasis that a reduction in insulin levels and enhancement of insulin sensitivity are among the most consistently observed responses to CR in different mammalian species ranging from mice and rats to non-human primates and humans (Fontana et al., 2004; Anderson and Weindruch, 2012).
In contrast to the strong association of improved insulin signaling with extended longevity in GH-related mutants, several mutations affecting events “downstream” from GH and/or IGF-I receptors are long-lived and insulin resistant (Kurosu et al., 2005; Selman et al., 2009). Further work, including examination of insulin signaling at different stages of life history will be needed to reconcile these findings but possible explanations include the well-documented opposite effects of GH and IGF-I on insulin signaling, as well as a possibility that insulin resistance may mimic some of the effects of hypoinsulinemia by protecting the cells from excessive insulin stimulation (Taguchi et al., 2007; Selman et al., 2009).
INFLAMMATION MARKERS AND METABOLIC ADJUSTMENTS
In addition to influencing glucose homeostasis, suppression of GH signaling promotes β oxidation of fatty acids. Fatty acid oxidation is promoted by the direct or indirect actions of peroxisome proliferator activator receptor α (PPARα), PPARγ coactivator 1α (PGC1α), fibroblast growth factor 21 (FGF-21), adiponectin, and AMP-activated protein kinase (AMPK) – and GH negatively regulates the expression or activation of each of these factors (Al-Regaiey et al., 2005; Masternak and Bartke, 2007; Bonkowski et al., 2009; Louis et al., 2010). Increases in the levels of adiponectin and activation of AMPK in GH-resistant and GH-deficient animals also reduce pro-inflammatory signals by inhibiting nuclear factor kappa B (NFκB) signaling (Salminen et al., 2011; Masternak and Bartke, 2012). The resulting shift in the balance of pro- and anti-inflammatory cytokines constitutes yet another potential mechanism of enhancing insulin sensitivity (Salminen et al., 2011). Association of an altered balance of pro- and anti-inflammatory markers with shifts in carbohydrate and lipid homeostasis in long-lived GH-related mutants can thus be related to the involvement of the same mediators of GH action in the control of inflammation and metabolism.
MITOCHONDRIAL FUNCTION AND OXIDATIVE METABOLISM
Enhanced hepatic expression of PGC1α and reduced serum lipid levels in GH-resistant mice (Al-Regaiey et al., 2005; List et al., 2011) suggest alterations in the number and function of mitochondria. PGC1α is a key regulator of mitochondrial biogenesis, and mitochondrial utilization of fatty acids as a metabolic fuel has a major impact on lipid homeostasis and circulating lipid levels.
There is little information on the number or morphology of mitochondria in long-lived GH-related mutants, while available data suggest lack of major changes in mitochondrial density in the liver or muscle of GHR-KO mice (Westbrook et al., unpublished). In Ames dwarf mice, generation of reactive oxygen species (ROS) by the skeletal muscle mitochondria is reduced, suggesting improved mitochondrial efficiency (Brown-Borg, 2006).
We are using indirect calorimetry to study the impact of GH signaling on energy metabolism. Twenty-four hour recordings of oxygen consumption and carbon dioxide output revealed that oxygen consumption (VO2) per gram of body weight is significantly increased and respiratory quotient (RQ) significantly reduced in Ames dwarf and GHR-KO mice (Westbrook et al., 2009).
These differences were present whether the animals were fed ad libitum or fasted during the recording (Westbrook et al., 2009). Moreover, similar differences between GHR-KO and normal mice were detected after exposing the animals to a prolonged period of caloric restriction or every-other-day-feeding (Westbrook et al., unpublished). Interestingly, opposite changes (reduced VO2 and increased RQ) were seen in giant PEPCK-bGH transgenic mice which are hypersomatotropic, hyperinsulinemic, insulin resistant, and short-lived (Bartke, 2003; Westbrook et al., 2009). The increase of VO2 in GHR-KO and Ames dwarf mice was apparently not due to expressing the data per unit of body mass, because differences between mutant and normal animals were, if anything, magnified when the data were recalculated per unit of lean body mass (as determined by DEXA in age- and sex-matched mice; Westbrook, 2012).
Detecting this increase in VO2 was not anticipated particularly in Ames dwarf mice which are hypothyroid and hypothermic and have reduced spontaneous locomotor activity (Bartke, 2011; Bartke, 2012; Brown-Borg and Bartke, 2012). Moreover, VO2 was reported to be reduced in Snell dwarf mice which phenotypically resemble the Ames dwarfs (Benedict and Lee, 1936). We suspected that the increase of VO2 in GH-related mutants could reflect increased energy expenditure for thermogenesis needed to compensate for increased heat loss. Increased radiation of heat would be expected in these diminutive animals because of the increased body surface to mass ratio. To test the validity of this explanation, we have compared VO2 in GHR-KO and normal mice at a thermoneutral ambient temperature of 30°C. Under these conditions, VO2 of the mutants greatly declined from the values measured at lower temperature and no longer differed from the normal animals (Westbrook et al., unpublished). We conclude that increased VO2 in long-lived dwarf mice reflects increased energy demand for thermogenesis under conditions imposed by housing at the standard animal room ambient temperature (approximately 22°C). It is an intriguing possibility that this increase in energy expenditure might contribute to slow aging and extended longevity of these mutants. Koizumi et al. (1996) reported that the beneficial impact of CR on cancer incidence and longevity in mice can be reduced or eliminated by housing the animals at a thermoneutral temperature. However, these authors suggested that the effects of thermoneutral temperature in their study were due to eliminating torpor which was a common (daily) occurrence under the conditions of fairly severe CR they employed (Koizumi et al., 1996). We very rarely observe torpor in our animals.
Since metabolic rate declines during aging, an increase in VO2 in long-lived mutant mice could be viewed either as a potential mechanism of extended longevity or as a “biomarker” of delayed and/or slower aging. Association of increased metabolic rate with improved life expectancy might be due to the benefits of increased uncoupling of mitochondrial electron transport from ATP production (Brand, 2000) and activation of AMPK. Reduced mTOR signaling and S6K activity in Ames dwarf and GHRKO mice (reviewed in Bartke, 2011) may provide yet another link between the regulation of aging, oxidative metabolism, and energy substrate utilization. It was recently reported that a leucine-deficient diet which suppresses hypothalamic S6KI activity produces an increase in VO2 per unit of body mass and a reduction in RQ; these are alterations similar to those we detected in long-lived dwarf mice (Xia et al., 2012). Examples of the association of increased VO2 and reduced RQ with resistance to detrimental effects of high fat diet are provided in the next section of this article.
ALTERED USAGE OF ENERGY SUBSTRATES
In addition to demonstrating an increase in VO2, indirect calorimetry studies of Ames dwarf and GHR-KO mice revealed another metabolic characteristic of these long-lived animals, namely a reduction of RQ. As was the case with VO2, these differences were detected during both dark (active) and light (resting) parts of the 24-h period, were present in both fully fed and fasted animals, and were opposite to changes measured in short-lived giant PEPCK-GH transgenics (Westbrook et al., 2009). Reduced RQ values indicate increased reliance on fat, as opposed to carbohydrate, as a metabolic fuel and thus denote an important shift in mitochondrial function. Increased “fat burning” by mitochondria is believed to be associated with improved metabolic efficiency and reduced production of potentially harmful ROS (Lopez-Lluch et al., 2006; Ukropcova et al., 2007; Anderson and Weindruch, 2010). Similar metabolic adjustments are associated with extension of longevity in animals exposed to CR (Anderson and Weindruch, 2010). Moreover, reduced RQ and enhanced VO2 were associated with protection from high fat diet-induced obesity, glucose intolerance and diabetes in mice with ablated agouti-related protein (AgRP) producing neurons and in retinaldehyde dehydrogenase 1a1 knock-out mice (Joly-Amado et al., 2012; Kiefer et al., 2012). Likely mechanisms of increased β oxidation of fatty acids in GHRKO and Ames dwarf mice include increases in adiponectin levels (Al-Regaiey et al., 2005; List et al., 2011), activation of AMPK (Al-Regaiey et al., 2005), and expression of hepatic PPARα (Masternak and Bartke, 2007).
In contrast, to findings in Ames dwarf and GHR-KO mice, extended longevity in mice with fat-specific deletion of insulin receptors, as well as improvement of the metabolic profile of obese mice after gastric bypass, are associated with increases in both VO2 and RQ (Katic et al., 2007; Nestoridi et al., 2012). From the data that are currently available, it is difficult to determine whether the association of increased VO2 and reduced RQ in long-lived GH-related mutants is in any way related to the uncommon association of increased obesity with reduced insulin and increased adiponectin levels in these animals.
SUMMARY AND RELATIONSHIP TO REGULATION OF HUMAN AGING
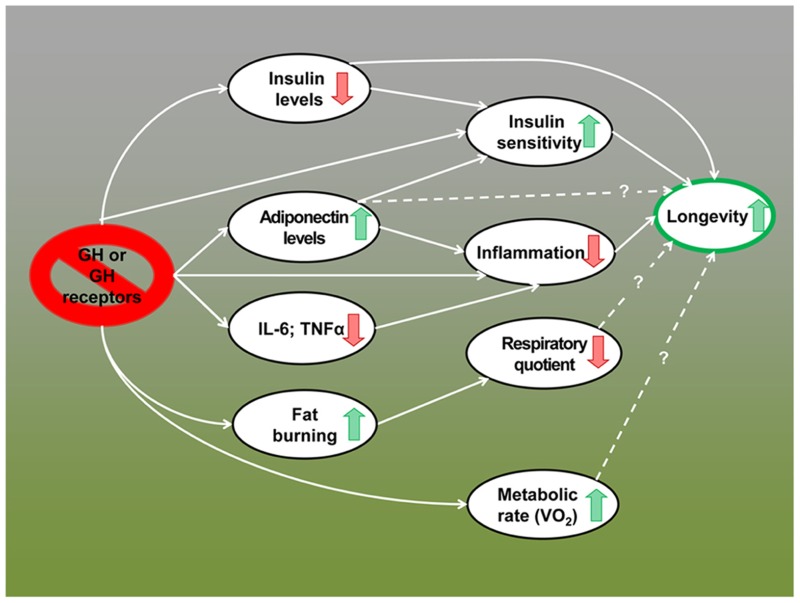
The remarkable extension of longevity in mice lacking GH or GH receptors appears to be due to multiple interacting mechanisms including reduced activation of growth-promoting pathways, greater stress resistance, reduced inflammation, increased reservoir of pluripotent stem cells, and improved genome maintenance (Flurkey et al., 2001; Coschigano et al., 2003; Murakami et al., 2003; Garcia et al., 2008; Bokov et al., 2009; Bartke, 2011; Ratajczak et al., 2011; Bartke, 2012; Brown-Borg and Bartke, 2012). Data summarized in this article indicate that alterations in energy metabolism and improved insulin control of carbohydrate homeostasis have to be added to this list. In fact, these metabolic adaptations may represent key features of the “longevous” phenotype of these animals and important mechanisms of the extension of both healthspan and lifespan in GH-related mutants (Figure 1).
FIGURE 1.
Metabolic alterations in GH-deficient and GH-resistant mice; possible mechanisms of extended longevity.
Importantly, many of the metabolic features of long-lived mutant mice described in this article have been associated with extended human longevity. Comparisons between centenarians and elderly individuals from the same population and between the offspring of exceptionally long-lived people and their partners indicate that reduced insulin, improved insulin sensitivity, increased adiponectin, and reduced pro-inflammatory markers consistently correlate with improved life expectancy (Kojima et al., 2004; Atzmon et al., 2006; Baranowska et al., 2006; Bonafè and Olivieri, 2009; Rozing et al., 2011; Wijsman et al., 2011).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Our studies and preparation of this article were supported by NIA through grants P01 AG031736, R01 AG019899, and R21 AG038850, by the Ellison Medical Foundation and by the SIU Geriatrics Research Initiative. We apologize to those whose work pertinent to this topic was not cited due to space and scope limitations or inadvertent omission.
REFERENCES
- Adochio R., Leitner J. W., Hedlund R., Draznin B. (2009). Rescuing 3t3-L1 adipocytes from insulin resistance induced by stimulation of Akt-mammalian target of rapamycin/P70 S6 kinase (S6k1) pathway and serine phosphorylation of insulin receptor substrate-1: effect of reduced expression of p85alpha subunit of phosphatidylinositol 3-kinase and S6k1 kinase. Endocrinology 150 1165–1173 [DOI] [PubMed] [Google Scholar]
- Aguirre V., Werner E. D., Giraud J., Lee Y. H., Shoelson S. E., White M. F. (2002). Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem. 277 1531–1537 [DOI] [PubMed] [Google Scholar]
- Alba M., Salvatori R. (2004). A mouse with targeted ablation of the growth hormone-releasing hormone gene: a new model of isolated growth hormone deficiency. Endocrinology 145 4134–4143 [DOI] [PubMed] [Google Scholar]
- Al-Regaiey K. A., Masternak M. M., Bonkowski M., Sun L., Bartke A. (2005). Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor I/insulin signaling and caloric restriction. Endocrinology 146 851–860 [DOI] [PubMed] [Google Scholar]
- Anderson R., Weindruch R. (2010). Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol. Metab. 21 134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R., Weindruch R. (2012). The caloric restriction paradigm: implications for healthy human aging. Am. J. Hum. Biol. 24 101–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzmon G., Rincon M., Schechter C. B., Shuldiner A. R., Lipton R. B., Bergman A., et al. (2006). Lipoprotein genotype and conserved pathway for exceptional longevity in humans. PLoS Biol. 4 e113 10.1371/journal.pbio.0040113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranowska B., Bik W., Baranowska-Bik A., Wolinska-Witort E., Szybinska A., Martynska L., et al. (2006). Neuroendocrine control of metabolic homeostasis in Polish centenarians. J. Physiol. Pharmacol. 57(Suppl. 6) 55–61 [PubMed] [Google Scholar]
- Bartke A. (2003). Can growth hormone (Gh) accelerate aging? evidence from Gh-transgenic mice. Neuroendocrinology 78 210–216 [DOI] [PubMed] [Google Scholar]
- Bartke A. (2011). Single-gene mutations and healthy ageing in mammals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366 28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A. (2012). Healthy aging: is smaller better? - a mini-review. Gerontology 58 337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A., Sun L., Longo V. (in press) Somatotropic signaling; trade-offs between growth, reproductive development and longevity. Physiol. Rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A., Wright J. C., Mattison J. A., Ingram D. K., Miller R. A., Roth G. S. (2001). Extending the lifespan of long-lived mice. Nature 414 412 [DOI] [PubMed] [Google Scholar]
- Benedict F. G., Lee R. C. (1936). La production de chaleur de la souris. etude de plusieurs races de souris. Ann. Physiol. Physicochim. Biol. 12 983–1064 [Google Scholar]
- Bokov A. F., Lindsey M. L., Khodr C., Sabia M. R., Richardson A. (2009). Long-lived Ames dwarf mice are resistant to chemical stressors. J. Gerontol. A Biol. Sci. Med. Sci. 64 819–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonafè M., Olivieri F. (2009). Genetic polymorphism in long-lived people: cues for the presence of an insulin/IGF-pathway-dependent network affecting human longevity. Mol. Cell. Endocrinol. 299 118–123 [DOI] [PubMed] [Google Scholar]
- Bonkowski M. S., Dominici F. P., Arum O., Rocha J. S., Al Regaiey K. A., Westbrook R., et al. (2009). Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS ONE 4 e4567 10.1371/journal.pone.0004567 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bonkowski M. S., Rocha J. S., Masternak M. M., Al Regaiey K. A., Bartke A. (2006). Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc. Natl. Acad. Sci. U.S.A. 103 7901–7905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M. D. (2000). Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp. Gerontol. 35 811–820 [DOI] [PubMed] [Google Scholar]
- Brown-Borg H. M. (2006). Longevity in mice: is stress resistance a common factor? Age (Dordr.) 28 145–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg H. M., Bartke A. (2012). GH and IGF1: roles in energy metabolism of long-living GH mutant mice. J. Gerontol. A Biol. Sci. Med. Sci. 67 652–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg H. M., Borg K. E., Meliska C. J., Bartke A. (1996). Dwarf mice and the ageing process. Nature 384 33 [DOI] [PubMed] [Google Scholar]
- Chen Y. F., Wu C. Y., Kao C. H., Tsai T. F. (2010). Longevity and lifespan control in mammals: lessons from the mouse. Ageing Res. Rev. 9(Suppl. 1) S28–S35 [DOI] [PubMed] [Google Scholar]
- Coschigano K., Holland A., Riders M., List E., Flyvbjerg A., Kopchick J. (2003). Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology 144 3799–3810 [DOI] [PubMed] [Google Scholar]
- Flurkey K., Papaconstantinou J., Miller R. A., Harrison D. E. (2001). Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc. Natl. Acad. Sci. U.S.A. 98 6736–6741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L., Meyer T., Klein S., Holloszy J. (2004). Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc. Natl. Acad. Sci. U.S.A. 101 6659–6663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A. M., Busuttil R. A., Calder R. B. Dollé, M. E., Diaz V., McMahan C. A., et al. (2008). Effect of Ames dwarfism and caloric restriction on spontaneous DNA mutation frequency in different mouse tissues. Mech. Ageing Dev. 129 528–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Lu Y., Houle D., Robertson K., Tang Z., Kopchick J., et al. (2005). Pancreatic islet-specific expression of an insulin-like growth factor-I transgene compensates islet cell growth in growth hormone receptor gene-deficient mice. Endocrinology 146 2602–2609 [DOI] [PubMed] [Google Scholar]
- Ishizuka T., Kajita K., Kawai Y., Kanoh Y., Miura A., Ishizawa M., et al. (2004). Protein kinase C (PKC) beta modulates serine phosphorylation of insulin receptor substrate-1 (IRS-1) – effect of overexpression of PKCbeta on insulin signal transduction. Endocr. Res. 30 287–299 [DOI] [PubMed] [Google Scholar]
- Joly-Amado A., Denis R. G., Castel J., Lacombe A., Cansell C., Rouch C., et al. (2012). Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning. EMBO J. 31 4276–4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katic M., Kennedy A. R., Leykin I., Norris A., McGettrick A., Gesta S., et al. (2007). Mitochondrial gene expression and increased oxidative metabolism: role in increased lifespan of fat-specific insulin receptor knock-out mice. Aging Cell 6 827–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer F., Orasanu G., Nallamshetty S., Brown J., Wang H., Luger P., et al. (2012). Retinaldehyde dehydrogenase 1 coordinates hepatic gluconeogenesis and lipid metabolism. Endocrinology 153 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi A., Wada Y., Tuskada M., Kayo T., Naruse M., Horiuchi K., et al. (1996). A tumor preventive effect of dietary restriction is antagonized by a high housing temperature through deprivation of torpor. Mech. Ageing Dev. 92 67–82 [DOI] [PubMed] [Google Scholar]
- Kojima T., Kamei H., Aizu T., Arai Y., Takayama M., Nakazawa S., et al. (2004). Association analysis between longevity in the Japanese population and polymorphic variants of genes involved in insulin and insulin-like growth factor 1 signaling pathways. Exp. Gerontol. 39 1595–1598 [DOI] [PubMed] [Google Scholar]
- Kurosu H., Yamamoto M., Clark J. D., Pastor J. V., Nandi A., Gurnani P., et al. (2005). Suppression of aging in mice by the hormone Klotho. Science 309 1829–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- List E. O., Sackmann-Sala L., Berryman D. E., Funk K., Kelder B., Gosney E. S., et al. (2011). Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR-/-) mouse. Endocr. Rev. 32 356–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lluch G., Hunt N., Jones B., Zhu M., Jamieson H., Hilmer S., et al. (2006). Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc. Natl. Acad. Sci. U.S.A. 103 1768–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis A., Bartke A., Masternak M. (2010). Effects of growth hormone and thyroxine replacement therapy on insulin signaling in Ames dwarf mice. J. Gerontol. A Biol. Sci. Med. Sci. 65 344–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak M. M., Bartke A. (2007). PPARs in calorie restricted and genetically long-lived mice. PPAR Res. 2007 28436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak M., Bartke A. (2012). Growth hormone, inflammation and aging. Pathobiol. Aging Age Relat. Dis. 2 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak M. M., Bartke A. Wang. F., Spong A., Gesing A., Fang Y., et al. (2012). Metabolic effects of intra-abdominal fat in GHRKO mice. Aging Cell 11 73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak M. M., Panici J. A., Bonkowski M. S., Hughes L. F., Bartke A. (2009). Insulin sensitivity as a key mediator of growth hormone actions on longevity. J. Gerontol. A Biol. Sci. Med. Sci. 64 516–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S., Salmon A., Miller R. A. (2003). Multiplex stress resistance in cells from long-lived dwarf mice. FASEB J. 17 1565–1566 [DOI] [PubMed] [Google Scholar]
- Muzumdar R., Allison D. B., Huffman D. M., Ma X., Atzmon G., Einstein F. H., et al. (2008). Visceral adipose tissue modulates mammalian longevity. Aging Cell 7 438–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestoridi E., Kvas S., Kucharczyk J., Stylopoulos N. (2012). Resting energy expenditure and energetic cost of feeding are augmented after Roux-en-Y gastric bypass in obese mice. Endocrinology 153 2234–2244 [DOI] [PubMed] [Google Scholar]
- Papaconstantinou J., Deford J. H., Gerstner A., Hsieh C. C., Boylston W. H., Guigneaux M. M., et al. (2005). Hepatic gene and protein expression of primary components of the IGF-I axis in long lived Snell dwarf mice. Mech. Ageing Dev. 126 692–704 [DOI] [PubMed] [Google Scholar]
- Parsons J. A., Bartke A., Sorenson R. L. (1995). Number and size of islets of Langerhans in pregnant, human growth hormone-expressing transgenic, and pituitary dwarf mice: effect of lactogenic hormones. Endocrinology 136 2013–2021 [DOI] [PubMed] [Google Scholar]
- Ratajczak J., Shin D. M., Wan W., Liu R., Masternak M. M., Piotrowska K., et al. (2011). Higher number of stem cells in the bone marrow of circulating low Igf-1 level Laron dwarf mice – novel view on Igf-1, stem cells and aging. Leukemia 25 729–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozing M. P., Mooijaart S. P., Beekman M., Wijsman C. A., Maier A. B., Bartke A., et al. (2011). C-reactive protein and glucose regulation in familial longevity. Age (Dordr.) 33 623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Hyttinen J. M., Kaarniranta K. (2011). AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J. Mol. Med. (Berl.) 89 667–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C., Tullet J. M., Wieser D., Irvine E., Lingard S. J., Choudhury A. I., et al. (2009). Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326 140–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Strader A. D., Woods S. C., Seeley R. J. (2007). The effect of fat removal on glucose tolerance is depot specific in male and female mice. Am. J. Physiol. Endocrinol. Metab. 293 E1012–E1020 [DOI] [PubMed] [Google Scholar]
- Taguchi A., Wartschow L. M., White M. F. (2007). Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science 317 369–372 [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Dhahbi J. M., Cui X., Mote P. L., Bartke A., Spindler S. R. (2004). Additive regulation of hepatic gene expression by dwarfism and caloric restriction. Physiol. Genomics 17 307–315 [DOI] [PubMed] [Google Scholar]
- Ukropcova B., Sereda O., de Jonge L., Bogacka I., Nguyen T., Xie H., et al. (2007). Family history of diabetes links impaired substrate switching and reduced mitochondrial content in skeletal muscle. Diabetes 56 720–727 [DOI] [PubMed] [Google Scholar]
- Wang Z., Al-Regaiey K. A., Masternak M. M., Bartke A. (2006). Adipocytokines and lipid levels in Ames dwarf and calorie-restricted mice. J. Gerontol. A Biol. Sci. Med. Sci. 61, 4 323–331 [DOI] [PubMed] [Google Scholar]
- Westbrook R. (2012). The Effects of Altered Growth Hormone Signaling on Murine Metabolism. Dissertation, Southern Illinois University Carbondale; Carbondale [Google Scholar]
- Westbrook R., Bonkowski M. S., Strader A. D., Bartke A. (2009). Alterations in oxygen consumption, respiratory quotient, and heat production in long-lived GHRKO and Ames dwarf mice, and short-lived bGH transgenic mice. J. Gerontol. A Biol. Sci. Med. Sci. 64 443–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijsman C. A., Rozing M. P., Streefland T. C., le Cessie S., Mooijaart S. P., Slagboom P. E., et al. (2011). Familial longevity is marked by enhanced insulin sensitivity. Aging Cell 10 114–121 [DOI] [PubMed] [Google Scholar]
- Xia T., Cheng Y., Zhang Q., Xiao F., Liu B., Chen S., Guo F. (2012). S6K1 in the central nervous system regulates energy expenditure via MC4R/CRH pathways in response to deprivation of an essential amino acid. Diabetes 61 2461–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R., Peters L. L., Paigen B. (2011). Mice as a mammalian model for research on the genetics of aging. ILAR J. 52 4–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Xu B. C., Maheshwari H. G., He L., Reed M., Lozykowski M., et al. (1997). A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc. Natl. Acad. Sci. U.S.A. 94 13215–13220 [DOI] [PMC free article] [PubMed] [Google Scholar]