Abstract
Sickle cell anemia is the best known hereditary blood disorder; there are serious complications associated with the condition. Diagnosis and early intervention reduce morbidity and mortality. These benefits have resulted in the widespread use of newborn screening education programs. In Brazil, the National Neonatal Screening Program established by decree 822/01 included sickle cell disease in the list of diseases tested in the so called "heel prick test". Since then, national studies of the results of this program have been periodically published. To review the literature in order to assess the prevalence of sickle cell trait and sickle cell anemia from data of national neonatal screening studies on hemoglobin S (Hb S). A bibliographic review was carried out using the key words: sickle cell anemia & hemoglobinopathies & neonatal screening & Brazil in the Bireme and SciELO databases. Original Brazilian studies presenting data on prevalence of the sickle cell trait (Hb AS) and sickle cell anemia (Hb SS) based on neonatal screening for Hb S were analysed. Twelve original national studies were identified with prevalences varying from 1.1% to 9.8% for the sickle cell trait and from 0.8 to 60 per 100,000 live births for sickle cell disease in different Brazilian regions. Conclusion: Neonatal screening for Hb S is a very useful method to assess the prevalence of sickle cell trait (Hb AS) and sickle cell anemia (Hb SS) in Brazil. There is a heterogeneous distribution of this disease with the highest prevalence in the northeastern region and the lowest prevalence in the south.
Keywords: Sickle cell anemia, Hemoglobinopathies, Neonatal screening, Brazil
Introduction
Sickle cell anemia is the best known hereditary hematological disorder in human beings. Estimates suggest that 250,000 children are born annually with sickle cell anemia worldwide and thus it is among the most important epidemiological genetic diseases in Brazil and the world.(1,2)
Originally from Africa and brought to the Americas by the forced immigration of slaves, it is more frequent where the proportion of African descendents is greater (the northeastern region and the States of São Paulo, Rio de Janeiro and Minas Gerais). In these regions, we observe new cases of sickle cell disease in every 1000 births and sickle cell trait carriers in every 27 births. It is estimated that approximately 2500 children are born every year with sickle cell disease in Brazil.(3,4)
The non-white population in Brazil was estimated at 44.66% by the 2000 Population Census and from 1% to 6% of them have the Hb S gene, thus favoring the continuation of sickle cell anemia in what is suggested by Brazilian scientific literature as a serious public health problem.(3,4)
The disease occurs due to a mutation of the beta globin gene of hemoglobin, causing a substitution of the glutamic amino acid for valine at position 6 of the beta chain, thereby producing an abnormal hemoglobin, called hemoglobin S (Hb S), instead of normal hemoglobin, hemoglobin A (Hb A).(1,2) With modified physicochemical characteristics, the molecules of hemoglobin S suffer polymerization and precipitation, leading to a change in form, a deformity of red blood cells which become sickle-shaped. In this case, the viscosity of the blood increases due to the formation of tactoids.(2,5)
The inheritance of sickle cell anemia occurs via an autosomal recessive gene with both parents, in general, asymptomatic carriers of a single affected gene (heterozygous), transmitting the defective gene to their child, who therefore is homozygous (Hb SS).(1,4)
Clinical manifestations are observed only in homozygous individuals for Hb S (Hb SS), resulting in sickle cell anemia.(5)
During fetal and early postnatal life, the lack of expression of the Hb SS phenotype is explained by the production of fetal hemoglobin (Hb F) which is sufficient to limit, by dilution, the effects of sickling. As the red cells that emerge from the bone marrow carry increasing amounts of Hb S and smaller amounts of Hb F, the results of sickling gradually appear. Therefore, newborns begin to manifest the disease from the sixth month of life, when the amount of Hb F begins to approach adult levels.(3)
The clinical manifestations of sickle cell disease are the result of two characteristic processes: severe anemia and vaso-occlusion. Anemia results from a shorter half-life of the red blood cells containing primarily Hb S. While normal red blood cells circulate for about 120 days, those containing Hb S only last for 10 to 20 days, leading patients to present moderate to severe anemia. The second process, which is physiopathologically more complicated, is vaso-occlusion. The intravascular effect from the spatial change of hemoglobin leads to the formation of helical bundles that greatly alter the membrane's permeability to ions, and the red cell/blood vessel and the red cell aggregation/red cell ratios.(4)
The effects of the phenomena of vaso-occlusion vary in intensity but include tissue ischemia, painful episodes, acute osteo-articular or abdominal crises and chronic organic injuries such as functional asplenia, cerebral-vascular disease and kidney, heart and lung failure; patients require frequent hospitalization.(2,4)
Although the sickle cell trait (Hb AS) is usually asymptomatic, there are reports of sudden death and medical complications such as hematuria, hyposthenuria, pulmonary embolism and splenic infarction, especially when carriers are exposed to extreme conditions of low oxygen tension, such as in strenuous physical exertion, depressurization of a flight cabin, and high altitude environments.(6)
In children, infections caused by encapsulated bacteria and intra-splenic vaso-occlusion (splenic sequestration) are the main causes of mortality. These begin after the first two to three months of life and affect 20-25% of children in the first 5 years of age.(2,4,7) Children who have overcome this initial barrier, face the effects of chronic vaso-occlusion. Over the years, these small strokes are the determining factor for the impairment of organs, leading to pulmonary, liver or brain infarction, kidney failure and retardation in growth and sexual maturation, with progressive impairment of multiple organs. These phenomena significantly reduce the quality of life of individuals, increase need for medical care, and diminish the capacity to work and life expectancy.(2,4)
The benefits of early diagnosis and intervention in the monitoring of sickle cell disease have led to widespread use of education programs to detect these conditions. Through neonatal screening programs, it is possible to reduce morbidity and mortality in the first five years of life. Furthermore, the prophylactic use of penicillin, the administration of pneumococcal vaccine and intensive care significantly increase the survival and quality of life of patients with sickle cell disease, reducing and extenuating the consequences of clinical complications.(5)
In 1986, one of the first Brazilian studies was published demonstrating the importance of detecting hemoglobin diseases early through a study that analyzed blood samples from the umbilical cord by means of electrophoresis in starch agar gel. The study tested 2281 samples, 78 of which had abnormal hemoglobins with preponderance among children from black mothers. In addition, it was shown that hemoglobin S represented 80.8% of abnormal samples, thereby proving the importance of neonatal screening for the detection of this alteration.(8)
In Brazil, the Newborn Screening Program began in 1976 with the pioneering project coordinated by Professor Benjamin Schmidt with the Association of Parents and Friends of the Disabled of São Paulo (APAESP), to screen for phenylketonuria (PKU). Screening for congenital hypothyroidism and PKU (Guthrie test) only became mandatory in the State of São Paulo for children born in government and maternity hospitals in 1983 (State Law 3.914 of November 14, 1983); in 1990 this requirement was extended to children born nationwide, in both government and private healthcare systems (Federal Law Nº 8069 of July 13, 1990).(9)
In 2001, the Ministry of Health included testing for hemoglobinopathies in the National Neonatal Screening Program (PNTN) through Decree Nº 822/01. Thus all children who are submitted to the Guthrie test in Brazilian states after completing implantation phases II and III of the Neonatal Screening program are also screened for hemoglobinopathies, in particular sickle cell anemia, in order to provide an early diagnosis.(10)
There are three phases of implementation: phase I includes Brazilian states that only perform neonatal screening for PKU and congenital hypothyroidism, phase II consists of states that perform screening for PKU, congenital hypothyroidism and hemoglobinopathies and phase III includes states that perform the phase II tests plus screening to identify children with cystic fibrosis. The phase in which each state is classified is determined by the structure and capacity of the state's healthcare system, the percentage of newborns screened and the regional characteristics of the population.(2)
Data released from the National Neonatal Screening Program, reported that 284 patients were identified with phenylketonuria, 2270 patients with congenital hypothyroidism, 2554 with hemoglobinopathies and 68 patients with cystic fibrosis from 2001 to 2005. Hence, hemoglobinopathies are the commonest genetic disorders in Brazilian newborns compared to other diseases diagnosed by the National Neonatal Screening Program. However, the available epidemiological data on sickle cell anemia probably do not show the true prevalence of the disease in the country because of the small number of states in phase II and III (ten states) and the low coverage (on average 58%) according to reports from the Ministry of Health.(2)
Given these data, we suppose that as more states reach Phase II of neonatal screening, information on the prevalence of the sickle cell trait and disease in Brazil will be more consistent. Therefore, the aim of this study was to review the literature on the prevalence of sickle cell trait and sickle cell disease based on national studies of neonatal screening for Hb S.
Methods
A bibliographic review was conducted using studies obtained from the SciELO and BIREME databases.
The keywords 'Sickle Cell Anemia', 'Newborn Screening' and 'Brazil' were used for an integrated search of all indices and sources of the BIREME database. Twenty papers were identified, of which only eight were selected. Additionally, a search was made using the keywords 'Neonatal' and 'Hemoglobinopathies' in BIREME, which found 113 publications but only ten were used.
In SciELO, the keywords 'Neonatal Screening' and 'Hemoglobinopathies' were used; this identified 26 papers of which 12 were selected.
Inclusion criteria were original Brazilian papers that reported on the prevalence of sickle cell trait (Hb AS) and anemia (Hb SS) from neonatal screening regardless of the sample size and coverage.
Despite having the same methods as the studies included, two studies were excluded: the first because it was a pilot project that was subsequently published in full and included in this study and the second because the statistics on a table were inaccurate. To evaluate the coverage of studies, the publication sample size was compared with the number of live births in the region in the study period using data from the Live Birth Information System (SINASC) of the Brazilian Ministry of Health.(11)
The studies were classified as being of high, medium or low relevance according to the following criteria: studies with sample sizes larger than 100,000 or coverage more than 60% of live births (higher than the national average of 58%) were considered highly relevant, studies with sample sizes lower than 100,000 but greater than 10,000 were considered medium relevance, and studies with sample sizes smaller than 10,000 were considered of low relevance.
Results
The results are shown in Tables 1 and 2, in alphabetical order of the regions analyzed.
Table 1.
Prevalence of sickle cell disease and sickle cell trait
| Author | Publication year | Location | Relevance | Period | N | Hb AS | Hb SS |
| Brandalise S(12) | 2004 | Campinas - SP | High | 1992-2000 | 281.884 | 1.98% | 10.3/100.000 |
| Diniz D(2) | 2009 | Distrito Federal | High | 2004-2006 | 116.271 | 3.23% | 90/100.000 |
| Pinheiro SL(3) | 2006 | Fortaleza - CE | Low | 2001-2002 | 389 | 3.85% | 257/100.000 |
| Holsbach DR(15) | 2008 | Mato Grosso do Sul | High | 2000-2005 | 190.809 | 1.64% | 10.5/100.000 |
| Araújo MCPE (6) | 2004 | Natal - RN | Low | 2001 | 1940 | 1.50% | 51.5/100.000 |
| WatanabeAM (10) | 2008 | Paraná | High | 2002-2004 | 548.810 | 1.52% | 2.2/100.000 |
| Sommer CK (5) | 2006 | Rio Grande do Sul | High | 2003-2004 | 117.320 | 1.14% | 0.85/100.000 |
| Magalhães PKR(9) | 2009 | Ribeirão Preto - SP | High | 2002-2005 | 103.021 | 2.60% | 14.6/100.000 |
| Lobo CLC (4) | 2003 | Rio de Janeiro - RJ | Medium | 2000-2001 | 99.260 | 3.96% | 60.5/100.000 |
| Adomo EV (16) | 2005 | Salvador - BA | Low | 2000 | 581 | 9.80% | 172/100.000 |
| Ducatti RP(14) | 2001 | S. José do Rio Preto - SP | Low | 1997-1998 | 913 | 3.72% | NR |
| Pultrini T(13) | 2004 | Western São Paulo State | Medium | 2001-2003 | 72.510 | 2.35% | 4.1/100.000 |
NR = Not reported; N = number of newborns evaluated
Table 2.
Coverage of neonatal screening in the studies
| Author | Relevance | Location | Period | Live births (Nº) | N | Coverage (%) |
| Brandalise S(12) | High | Campinas - SP | 1992-2000 | IC | 281.884 | IC |
| Diniz D(2) | High | Distrito Federal | 2004-2006 | 136.662 | 116.271 | 85.07 |
| Pinheiro SL(3) | Low | Fortaleza - CE | 2001-2002 | 40.532 | 389 | 0.95 |
| Holsbach DR(15) | High | Mato Grosso do Sul | 2000-2005 | 242.732 | 190.809 | 78.60 |
| Araújo MCPE (6) | Low | Natal - RN | 2001 | 13.818 | 1.940 | 14.03 |
| Watanabe AM (10) | High | Paraná | 2002-2004 | 482.249 | 548.810 | 100.00 |
| Sommer CK (5) | High | Rio Grande do Sul | 2003-2004 | 151.074 | 117.320 | 77.65 |
| Magalhães PKR(9) | High | Ribeirão Preto - SP | 2002-2005 | VRE | 103.021 | 94.50 |
| Lobo CLC (4) | Medium | Rio de Janeiro | 2000-2001 | 242.360 | 99.260 | 40.00 |
| Adomo EV(16) | Low | Salvador - BA | 2000 | IC | 581 | IC |
| Ducatti RP(14) | Low | S. José Rio Preto - SP | 1997-1998 | 5.158 | 913 | 17.70 |
| Pultrini T(13) | Medium | Western São Paulo State | 2001-2003 | IC | 72.510 | IC |
IC = Impossible to calculate; VRE = Value reported in the Study; N = number of newborns evaluated
The data from all studies are summarized in Table 1. Table 2 shows the estimated values of the range of neonatal screening obtained in each study according to the total live births for the period and the region according to SINASC.
Discussion
A precise calculation of the number of children born with sickle cell anemia (Hb SS) in a period of time is crucial for the organization of the healthcare system, especially if there are significant differences between regions in the country which consequently require different resources.
Therefore, a review of published studies that report the prevalence in Brazil may provide important information in scientific terms even if it is a study of secondary data from various authors with non-uniform sampling designs.
The solution found by the authors to mitigate this problem was to seek the total number of live births in the Live Births Information System (SINASC) of the Ministry of Health for the region and for the period of the studies presented. This allowed us to calculate the coverage and classify the studies according to relevance, enabling better mapping of the situation to date.
The studies conducted in the State of São Paulo produced results that varied according to their relevance. The study of Campinas was of high relevance with the sample size being higher than 100,000. With the involvement of 78 institutions in 36 cities, including 4 hospitals in the city of São Paulo, it was impossible to calculate the percentage coverage because the study did not delimit with precision the region studied. The study reported the prevalence of Hb AS in 1.98% of newborns and Hb SS in 0.01%.(12) The study of Ribeirão Preto reported Hb AS in 2.6% and Hb SS in 0.015% with high relevance, because the coverage was on average 94.5% of live births (figure indicated in the study).(9) In the west of São Paulo State there was a prevalence of 2.35% of Hb AS and 0.004% of Hb SS with medium relevance because the study was conducted in 168 cities of the state of São Paulo, the equivalent of 35% of all cities, with the sample size between 10,000 and 100,000. It was impossible to calculate coverage in the study because the statistics of some micro regions surveyed (Presidente Venceslau) are not available in SINASC. (13)
The results for São José do Rio Preto were flawed as the study was conducted in just one hospital and no cases of Hb SS were discovered probably due to a relatively low sample size. From the average figures of live births from 1997 and 1998 obtained in SINASC the coverage in São José do Rio Preto was calculated at 17.70% thus the study is considered of low relevance. As for the State of Sao Paulo, the estimated prevalence was 0.0655% of live births in the period.(14)
In the study carried out in Rio de Janeiro, prevalences of 3.96% and 0.06% were obtained for Hb AS and Hb SS, respectively. The paper presented medium relevance because the sample size was significant (between 100,000 and 10,000) and the estimate, using SINASC data, for coverage was approximately 40% of live births during the period. Taking into account that the study was conducted over 15 months and SINASC provides annual figures, the real value is perhaps less than estimated.(4)
In the Federal District, Hb AS was 3.23% and Hb SS was 0.09%. The study was considered of high relevance, as the sample size was greater than 100,000 and the coverage was 85.07% of live births during the period.(2)
The results of Rio Grande do Sul showed prevalences of 1.14% for Hb AS and 0.0009% for Hb SS, with high relevance due to the sample size and coverage. From the SINASC data, there was an average of 151,074 live births in 2003 and 2004 and so the coverage was about 77.65%.(5)
The study from the State of Paraná reported prevalences of 1.52% for Hb AS and 0.002% for Hb SS. The relevance was considered high because the coverage was 100% of the live births registered in "SRTN FEPE" (Newborn Screening Reference Service of the Ecumenical Foundation for Protection of the Handicapped) of Paraná State. This figure was confirmed by SINASC data, which show that the study size was greater than the number of live births in the period.(10)
The values for the State of Mato Grosso do Sul were 1.64% and 0.01% for Hb AS and Hb SS, respectively, with high relevance because the average coverage was 88.62% except for 2000 with coverage of 28.38% (an overall average of 78.6% of live births in the period).(15)
In Fortaleza, Ceará, a prevalence of 3.8% was reported for Hb AS and 0.2% for Hb SS with low relevance because despite of the precise technique for identifying changes in hemoglobin, the study has a small sample size and was performed in only one maternity hospital.(3) According to SINASC, there were 40,532 live births in 2001 and 2002 in Fortaleza which gives a coverage of only 0.95% for this study. On considering the state of Ceará, the coverage is approximately 0.26%.
In the study conducted in Natal, Rio Grande do Norte, the reported prevalence was 1.5% for Hb AS and 0.05% for Hb SS. The relevance was low, because although the study was carried out in three public hospitals, the sample size is small and the calculation of the coverage was 14.03% of all newborns in Natal and 3.61% of those in Rio Grande do Norte.(6)
In Salvador, Bahia, the values were 9.8% and 0.2% for Hb AS and Hb SS, respectively, with low relevance; screening was performed in only one hospital and so the sample size was small and the study period of five months was too short to calculate the coverage.(16)
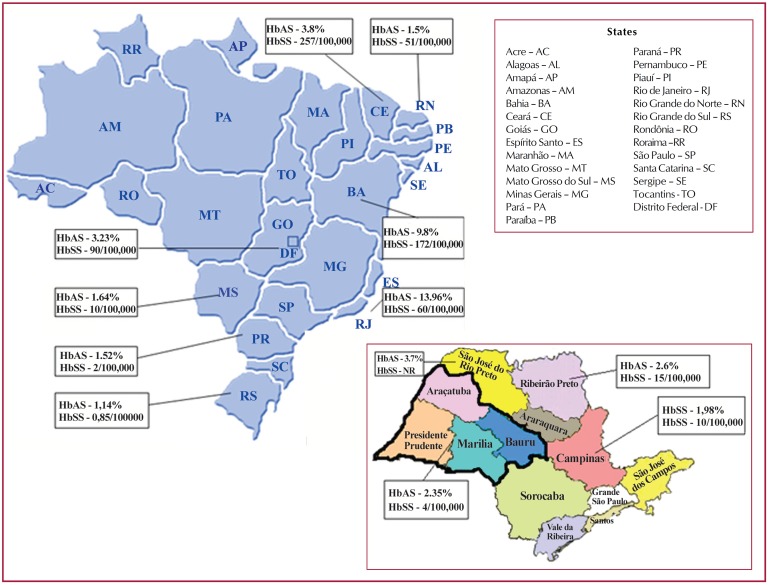
Figure 1 shows the distribution and prevalence of sickle cell anemia and sickle cell trait according to the figures found in the studies.
Figure 1.
Distribution and prevalence of sickle cell disease and trait in Brazil, according to studies. The maps are available at http://www.mz.ind.br/revendas.html and http://festivaldebesteirasnaimprensa.wordpress.com/tag/arrasando-sao-paulo/
Note that the Hb S gene is heterogeneously distributed in the Northeast with higher prevalences in the states of Bahia, Rio de Janeiro, Ceará and the Federal District. According to the Ministry of Health, sickle cell trait is present in approximately 5.3% of the population in Bahia, the state with the highest percentage, data that supports the numbers presented in our study. In addition, the Ministry of Health reported a value of 4% for sickle cell trait in the State of Rio de Janeiro, similar to the 3.9% found in our review.(17)
Like Bahia, Ceará has a high prevalence of blacks compared to other Brazilian states, such as Paraná and Rio Grande do Sul, with 3.8% of Hb AS, compared to 1.52% and 1.14%, respectively. This fact can be justified historically by the period of colonization of Brazil, where there was a great migration of black Africans to the northeast of Brazil due to slavery.
The values of Hb AS found in São Paulo, the Federal District and Rio de Janeiro (2.35%, 3.23% and 3.96%, respectively), are not as low to those of southern regions, possibly due to the great migration that took place from the northeast to these states. An example is the Federal District; the most likely explanation is the large migration that occurred during the construction of the city of Brasília.
Conclusion
The national data, despite the variability of coverage of screening for Hb S (Phase II), shows the heterogeneity of the prevalence of sickle cell trait and sickle cell disease in different regions of Brazil. This information is crucial for Public health policies.
It is observed that studies from the Northeast have the lowest coverage compared to the total live births, despite of this being the region that has the highest numbers of individuals with sickle cell anemia in Brazil. In contrast, the South has the best screening coverage and the lowest values for sickle cell trait and sickle cell disease. This fact shows not only the difference in access to health care in both regions, but the need for an extension of screening to the Northeast. There were no studies from the northern region of Brazil.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interest
References
- 1.Silva WS, Lastra A, Oliveira SF, Guimarães NK, Grisolia CK.Avaliação da cobertura do programa de triagem neonatal de hemoglobinopatias em populações do Recôncavo Baiano, Brasil Cad Saúde Pública 200622 (12): 2561-2566 [DOI] [PubMed] [Google Scholar]
- 2.Diniz D, Guedes C, Barbosa L, Tauil PL, Magalhães I.Prevalência do traço e da anemia falciforme em recém-nascidos do Distrito Federal, Brasil, 2004 a 2006 Cad Saúde Pública 200925 (1): 188-194 [DOI] [PubMed] [Google Scholar]
- 3.Pinheiro LS, Gonçalves RP, Tomé CA, Alcântara AE, Marques AR, Silva MM.Prevalência de hemoglobina S em recém-nascidos de Fortaleza: importância da investigação neonatal Rev Bras Ginecol Obstet 200628 (2): 122-125 [Google Scholar]
- 4.Lobo CL, Bueno LM, Moura P, Ogeda LL, Castilho S, Carvalho SM.Triagem neonatal para hemoglobinopatias no Rio de Janeiro, Brasil Pan Am J Public Health 200313 (2/3): 154-159 [DOI] [PubMed] [Google Scholar]
- 5.Sommer CK, Goldbeck AS, Wagner SC, Castro SM.Triagem neonatal para hemoglobinopatias: experiência de um ano na rede de saúde pública do Rio Grande do Sul, Brasil Cad Saúde Pública 200622 (8): 1709-1714 [DOI] [PubMed] [Google Scholar]
- 6.Araújo MC, Serafim ES, Castro WA, Jr, Medeiros TM.Prevalência de hemoglobinas anormais em recém-nascidos da cidade de Natal, Rio Grande do Norte, Brasil Cad Saúde Pública 200420 (1): 123-128 [DOI] [PubMed] [Google Scholar]
- 7.Ferraz MH, Murao M.Diagnóstico laboratorial da doença falciforme em neonatos e após o sexto mês de vida Rev Bras Hematol Hemoter 200729 (3): 218-222 [Google Scholar]
- 8.Ruiz MA, Guerra CC, Naoum PC.Detecção de hemoglobinas anormais em sangue de cordão de recém-nascidos na cidade de Santos, São Paulo, através da eletroforese em gel de Agar de amido Bol Soc Bras Hematol Hemot 19868 (137): 8-13 [Google Scholar]
- 9.Magalhães PK, Turcato MF, Angulo IL, Maciel LM.Programa de Triagem Neonatal do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Brasil Cad Saúde Pública 200925 (2): 445-454 [DOI] [PubMed] [Google Scholar]
- 10.Watanabe AM, Pianovski MA, Zanis J, Neto, Lichtvan LC, Maia EA, Domingos MT, et al. Prevalência da hemoglobina S no Estado do Paraná, Brasil, obtida pela triagem neonatal Cad. Saúde Pública 200824 (5): 993-1000 [DOI] [PubMed] [Google Scholar]
- 11.Banco de dados do Sistema Único de Saúde. Sistema de Informações sobre Nascidos Vivos SINASC; [ Internet] 2009[cited 2009 May 26] Avaiable from: URL: http://tabnet.datasus.gov.br/tabdata/sinasc/dados/nov_indice.htm [Google Scholar]
- 12.Brandelise S, Pinheiro V, Gabetta CS, Hambleton I, Serjeant B, Serjeant G.Newborn screening for sickle cell disease in Brazil: the Campinas experience Clin Lab Haematol 200426 (1): 15-19 [DOI] [PubMed] [Google Scholar]
- 13.Pultrini T, Pedro KP, Ferreira RR.Triagem neonatal para hemoglobinopatias em municípios da região Oeste de São Paulo Arq Ciênc Saúde 200411 (1): 20-24 [Google Scholar]
- 14.Ducatti RP, Teixeira AEA, Galão HA, Domingos CRB, Conte ACF.Investigação de hemoglobinopatias em sangue de cordão umbilical de recém-nascidos do Hospital de Base de São José do Rio Preto Rev Bras Hematol Hemoter 200123 (1): 23-29 [Google Scholar]
- 15.Holsbach DR, Ivo ML, Honer MR, Rigo L, Botelho CA.Ocorrência de hemoglobina S no estado de Mato Grosso do Sul, Brasil J Bras Patol Med Lab 200844 (4): 277-282 [Google Scholar]
- 16.Adorno EV, Couto FD, Moura JP, Neto, Menezes JF, Rêgo M, Reis MG, et al. Hemoglobinopatias em recém-nascidos de Salvador, Bahia, Nordeste do Brasil Cad Saúde Pública 200521 (1): 292-298 [DOI] [PubMed] [Google Scholar]
- 17.Cançado RD, Jesus JA.A doença falciforme no Brasil Rev Bras Hematol Hemoter 200729 (3): 204-206 [Google Scholar]