Abstract
Multiple studies demonstrate an increased cardiovascular (CV) risk associated with RA compared with the general population. While part of this risk appears to be mediated by RA-specific factors, such as long-term inflammation, traditional CV comorbidities also play an important role. We review evidence from previous studies of the relationship between RA and traditional CV comorbidities such as dyslipidaemia, obesity, insulin resistance and diabetes, hypertension, cigarette smoking and physical inactivity. We examine the prevalence and consider the effect of inflammation and RA treatments on these risk factors. Finally, we discuss three widely used CV risk estimators, the Framingham Risk Score, Reynolds Risk Score and the Systematic Coronary Risk Evaluation, and their performance in patients with RA. The traditional CV risk factors that appear to differ significantly between RA cases and controls include insulin resistance, abnormal fat distribution, cigarette smoking and lack of physical activity. Dyslipidaemia, diabetes and hypertension may also be elevated in RA; however, the evidence is conflicting. Overall, we found that the majority of information regarding CV risk factors in RA stems from data collected as covariates for studies on CV disease. A gap in knowledge exists regarding detailed information on individual risk factors in RA, their prevalence and modifications that occur as a result of inflammation or treatment. More studies are needed to develop methods for accurate CV risk estimation in RA.
Keywords: rheumatoid arthritis, traditional cardiovascular risk factors, cardiovascular disease, coronary artery disease, coronary heart disease, inflammation, C-reactive protein, Reynolds risk score/calculator, Framingham risk score/calculator, hypertension
Introduction
Illustrative case
We present a hypothetical patient, a 53-year-old man with hypertension and seropositive erosive RA diagnosed 6 years ago. His father died from sudden cardiac death at the age of 58. He has a total cholesterol (Tchol) level of 230 mg/dl (5.95 mmol/l), high density lipoprotein (HDL) level of 45 mg/dl (1.16 mmol/l) and a low density lipoprotein (LDL) level of 128 mg/dl (3.31 mmol/l). He does not smoke but has hypertension with a systolic blood pressure (BP) of 137 mmHg (on anti-hypertensive medication).
The hypothetical patient has traditional cardiovascular disease (CVD) risk factors in addition to RA. Would you recommend that he start an HMG-CoA reductase inhibitor (statin) therapy?
The inflammation associated with RA increases risk beyond what is explained by traditional CVD risk factors [1–4], but the excess risk is not accounted for by current methods of cardiovascular (CV) risk stratification. In this review, we provide an overview of the prevalence and the impact of traditional CVD risk factors among RA patients, focusing on dyslipidaemia, insulin resistance (IR) and diabetes, hypertension, obesity, smoking and physical inactivity. One of the challenges of studying the contributions of traditional CV risk factors in RA is that the factors themselves can be simultaneously affected by inflammation and treatments for RA. In areas where sufficient data are available, we will examine how inflammation and treatment modify CV risk factors. We conclude by discussing current strategies for estimating CV risk among RA patients and identifying gaps in the knowledge.
Dyslipidaemia
Cholesterol, particularly LDL, drives formation of fatty streaks in the coronary arteries that can grow over time into flow-limiting atherosclerotic plaques [5]. These plaques can rupture and cause thrombosis, leading to blockage of the coronary arteries, resulting in a myocardial infarction (MI). Despite higher risk of CVD in RA than the general population, the prevalence of dyslipidaemia (roughly defined by high levels of LDL and Tchol and low levels of HDL according to ATP III guidelines [6]) does not appear to differ significantly between RA cases and controls [3, 7]. Dyslipidaemia in one study conferred a risk for CV in RA cases similar to that in controls [3].
The differences in lipid profiles between RA cases and controls appear to occur as a result of treatment and levels of inflammation. We will focus on three types of cholesterol that have been tightly linked to CV risk [8]: Tchol, LDL and HDL. Two studies conducted on patients before their RA diagnosis, provide insight on lipid profiles before RA treatment. One cross-sectional study conducted on blood bank samples in RA patients before their diagnosis found unfavourable atherogenic profiles (Tchol/HDL), with a higher Tchol and lower HDL than their age- and gender-matched controls [9]. A second retrospective population-based RA case–control study found the opposite effect, where Tchol was lower in RA cases than in controls. In addition, the latter study found that LDL was lower in RA cases compared with controls. Furthermore, the investigators found that that LDL levels decreased significantly in the 5 years before RA diagnosis [10]. However, these lower levels of Tchol and LDL resulted in a paradoxically higher risk for CVD [11].
The majority of lipid studies in RA populations focus on changes in lipid profiles as a result of RA treatment. Of the RA treatments, tumour necrosis factor (TNF) blockade and tocilizumab appear to have the strongest association with changes in lipid levels. TNF blockers appear to elevate Tchol and HDL levels but not affect LDL levels [12]. The effect of TNF blockers on both Tchol and HDL resulted in a stable atherogenic profile (Tchol/HDL) [13–22]. Notably, changes in lipid profiles appear to occur mainly in RA treatment responders, with minimal changes in non-responders, suggesting that reduced inflammation, not a specific treatment, mediated changes in lipid levels [19, 23]. Tocilizumab, a humanized antibody that targets the IL-6 receptor, elevates LDL and Tchol [24]. However, the impact of these changes on CVD risk is unclear and a subject of current studies [25, 26]. The effects of DMARDs other than TNF and IL-6 R blockers on lipid profiles in RA are less studied. MTX does not appear to alter lipid profiles independent of inflammation [27], whereas there is some evidence that hydroxycholoroquine improves atherogenic profiles in RA [28, 29].
Inflammation alters lipid levels and lipid function. RA treatment studies that monitor changes in cholesterol levels provide data on how inflammation may modify levels. In RA, disease activity levels appear to be inversely related to HDL levels [16, 17, 21]. One study of untreated RA patients >60 years compared with a non-RA control population found that RA patients had had significantly lower HDL levels than controls [30]. HDL in inflammatory states has been shown to undergo modification to a pro-inflammatory particle, which can lead to accelerated atherosclerosis [31, 32]. No clear association has been observed between levels of inflammation in RA and LDL levels [16, 17, 21, 30].
Conventional therapy with statins is effective for targeting LDL in RA patients. A randomized placebo-controlled trial of 6 months of statin therapy in RA demonstrated significantly reduced Tchol and LDL levels [33]. This study also demonstrated that subjects in the statin-treated group experienced significant decreases in disease activity and inflammatory markers (CRP and ESR). These findings suggest that statins when used in RA improve CV risk through both lipid lowering and a modest anti-inflammatory effect.
Insulin resistance and diabetes
There are strong epidemiological data supporting the association between RA and IR. We will first review the literature about RA and IR and then RA and diabetes mellitus (DM). In many studies, the homeostatic model assessment (HOMA) calculation is used to quantify insulin resistance (HOMA-IR) [34]. Among 124 subjects with RA, one study found that 54% had evidence of IR as measured by HOMA-IR [35], whereas general population estimates for IR are 40–45% [36]. In a study of HOMA-IR in RA versus controls, all had co-existing thyroid disease, RA subjects had a HOMA-IR of 1.65 compared with 1.16 in controls (P = 0.031) [37].
Two cross-sectional studies have examined the relationship between IR and inflammation in RA. A comparison of RA patients with low- and high-grade inflammation found that the HOMA-IR was significantly higher in patients with high-grade inflammation [38]. Strong predictors of HOMA-IR included CRP, ESR and waist circumference but not current steroid dose. Another cross-sectional study found that CRP, IL-6 and TNFα were significantly associated with HOMA-IR in subjects with RA [35]; there was also a trend towards higher HOMA-IR associated with cumulative steroid dosage.
Previous studies of the association between RA and DM are not as clear. At least five studies have found a positive association. In one study using a large administrative database from Canada, subjects with RA were found to have a 50% [95% confidence interval (CI) 1.4, 1.5] increase in the risk of DM, even after controlling for glucocorticoid use [39]. These findings replicate an earlier administrative database study that found a 40% (95% CI 1.3, 1.4) increase in DM associated with RA [40]. Several other studies found an increased prevalence of DM in RA [41–43]. Finally, a population-based study found an increase in the risk of DM associated with RA [odds ratio (OR) 1.3], but the number of events was small and the CI spanned 1 [44]. However, several studies found no association. One study examined DM in RA and found a strong relationship with DM overall, but this was only significant for type 1 DM (OR 4.9, 95% CI 1.8, 13) and not type 2 DM (OR 1.1, 95% CI 0.7, 1.6) [45]. At least two other studies not directly examining DM risk, but instead focused on CVD endpoints, found no difference in the prevalence of DM among RA compared with the non-RA subjects [3, 46].
The data regarding immunosuppressives’ effect on IR in RA is conflicting. Three research groups investigated the effect of TNF blockade on IR. A study of 45 patients starting infliximab found no reduction in IR for 6 months, but did find a reduction in the group with the highest IR at baseline [47]. A study investigating adalimumab found no reduction in IR among the nine patients with RA [48]. A third study enrolled 19 patients with RA and treated them with 14 weeks of infliximab, and found that IR improved after infliximab treatment [20]. Another recent study of 11 patients with rheumatoid diseases found that tocilizumab produced a significant drop in HOMA-IR; however, many details are omitted in this article [49]. In a small study of 22 RA subjects, 14 patients who started MTX had a decrease in HOMA-IR during the first 8 weeks [43].
RA treatments have also been associated with reduced risk of diabetes in RA patients. In a large administrative database study, TNFα blockers and HCQ reduced the risk of future DM compared with other non-biologic DMARDs by 30–50% [50]. This work confirmed an earlier study demonstrating a reduced risk of DM in an RA cohort using HCQ [51].
Obesity
According to the Centers for Disease Control in the United States, the prevalence of obesity is estimated to be 54% higher in individuals with arthritis than those without [52]. According to the World Health Organization, individuals with a BMI of ≥30 kg/m2 are considered obese, with a lower cutpoint of 25 kg/m2 for Asians [53]. Whether individuals with RA have a higher BMI compared with the general population varies between studies [1, 3, 54]. A study that compared the magnitude of effect for individual CV risk factors in RA cases compared with controls, found that obesity had a similar effect on CV risk in RA as in controls[3].
BMI was developed as a clinically measurable proxy for body fat percentage [55]. Bioelectrical impedence studies, which allow for the characterization of actual body fat composition demonstrate that RA patients have more body fat for a given BMI than healthy controls [56]. A study using abdominal CT scans revealed that male RA patients had more visceral fat compared with controls, and female RA patients had more s.c. fat than controls with similar BMI and waist circumference [57]. Together these findings suggest that using BMI may underestimate body fat composition in RA versus controls, leading to an underestimation of CVD risk in RA.
Obesity itself is believed to contribute to low-grade inflammation, as adipose tissue has been found to release pro-inflammatory cytokines such as IL-6 and adiponectin [58–60]. In the general population, a higher BMI is associated with elevated CRP levels [61], which in turn are associated with increased CVD risk [62]. In RA, abnormal body fat composition is also associated with higher CRP levels and more severe RA [63, 64].
Hypertension
Previous studies that have assessed the prevalence of hypertension in RA in the context of studies of CVD do not suggest a clear increase in risk compared with non-RA controls. One meta-analysis that included 7 RA case-control studies found no clear increase in the prevalence of hypertension in RA cases compared with controls [7]. However, a number of other studies that included hypertension as a baseline covariate have found an increased prevalence of hypertension in RA or higher BPs [65]. To date, there has been no longitudinal study of the relative risk of hypertension in RA compared with controls. Hypertension as a risk factor was found to have a relatively similar contribution to CV risk in RA as in the general population [3].
Several studies have examined arterial wall pliability in RA compared with controls, and all have found increased stiffness associated with RA [66]. These studies have generally matched on BP. So, while we cannot determine the risk of hypertension in RA versus controls from these studies, it appears that the ability of the arterial system to respond to changes in blood volume is diminished in RA.
Currently available disease modifying anti-rheumatic drugs do not seem to have substantial effects on BP; however, several agents being tested appear to raise BP [67]. Also, NSAID (selective and non-selective) and glucocorticoids are known to raise BP [68–71]. In some patients, these agents cause clinically significant hypertension requiring treatment.
Cigarette smoking
Smoking is the strongest known environmental risk factor for RA [72, 73]. Despite decreasing prevalence of smoking in the general population, it remains one of the largest modifiable risk factors for CVD [74]. The prevalence of cigarette smoking appears to be higher in RA cases as was found in a recent meta-analysis (OR 1.56, 95% CI 1.34, 1.80) [7]. Three studies not included in the meta-analysis also found a higher prevalence of current and past smokers in RA cases compared with controls [3, 4, 75].
A study assessing the relative contribution of cigarette smoking to risk of CVD in a population-based RA case-control study found that current smoking status contributed to CV risk, but its contribution to overall CV risk was significantly less in RA cases than controls [3]. Cigarette smoking is also associated with increased severity of RA particularly in men with seropositive disease [76, 77].
Physical activity
Physical activity is an integral component of lifestyle modification to decrease CV risk. A small questionnaire-based study found that RA patients were significantly less physically active than population-based controls [78]. Another small prospective study found that RA patients expend similar levels of energy than non-RA controls [79]. However, when actual activities were compared in the study, RA cases walked significantly less than controls. As in the general population, RA patients who are physically inactive have a worse CV risk profile, i.e. higher BP, higher Tchol and LDL than those who are active [80]. Exercise interventions have been proven to help maintain and even improve functional status in RA patients [81] and would likely be of great benefit for CV risk as well.
Potential strategies for estimating CV risk
The accurate estimation of CV risk among RA patients remains an area of active investigation. Currently, for the general population, the two methods for estimating CV risk are the Framingham Risk Score (FRS) [82] and the Reynolds Risk Score (RRS) [62] (Table 1). The FRS incorporates traditional risk factors above such as age, gender and Tchol levels to estimate the 10-year risk for a coronary event. The RRS takes into account the added risk of inflammation as measured by high-sensitivity CRP (hsCRP) and estimates 10-year risk for a coronary event or stroke. In Europe, the Systematic Coronary Risk Evaluation (SCORE) is widely used to determine 10-year risk of fatal CVD [83] (Table 1).
Table 1.
Comparison of clinical variables and predicted outcomes for the FRS, RRS and the SCORE
| Clinical variable | FRS | RRS | SCORE |
|---|---|---|---|
| Age | ✓ | ✓ | ✓ |
| Gender | ✓ | ✓ | ✓ |
| Tchol | ✓ | ✓ | ✓ |
| HDL | ✓ | ✓ | |
| Current smoker | ✓ | ✓ | ✓ |
| Systolic BP | ✓ | ✓ | |
| On medication for hypertension | ✓ | ||
| hsCRP | ✓ | ||
| Mother or father with heart attack or stroke before age 60 | ✓ | ||
| High-/low-risk European country | ✓ | ||
| Predicted 10-year risk | MI or coronary death | MI or stroke | Fatal CVD |
Can these risk scores be applied to patients with RA? Although there is evidence that a higher FRS is associated with higher CV risk in RA [84], both FRS and SCORE include only traditional CV risk factors (not inflammatory markers); thus, they would underestimate CV risk in RA. Data from several studies demonstrate that traditional risk factors account for only a portion of the overall risk for CVD in RA, with the additional risk attributed to inflammation [1–3]. A recent population-based cohort study quantified the degree of underestimation by FRS and found that observed CV rates in female RA patients were roughly 50% higher than predicted by FRS [85].
The RRS, which incorporates inflammation into the score, may also be a poor estimator for CV risk because it was calibrated on a population where the mean hsCRP was 2.0 mg/l, well below that of the average RA patient. As an example, the mean hsCRP was 9.7 mg/l in the Brigham Rheumatoid Arthritis Sequential Study [86], a prospective cohort of ∼1100 treated RA patients, which is consistent with other established RA cohorts. As CRP can change dramatically in RA due to disease flares and treatment, it is also unclear how well CRP at one time point would inform CV risk. In addition, significant interactions between CV risk factors and level of inflammation would not be accounted for by the RRS. In a study that used carotid intima media thickness (cIMT) as a surrogate for CV risk, patients with the highest number of CV risk factors and highest levels of the ESR had higher cIMT than would be expected by adding cIMT measures from each factor alone [87]. Indeed, one study found that RRS underestimated CV risk, similar to FRS, despite inclusion of CRP into the risk calculator [85].
The European League Against Rheumatism (EULAR) convened an expert panel to provide recommendations on how to improve CV risk estimation in RA. Based on relatively low quality evidence, EULAR recommends a multiplier of 1.5 for FRS or SCORE if a patient has 2 or more of the following: RA disease duration >10 years, RF or antibodies to citrullinated peptide antibodies, or extra-articular manifestations [88]. This multiplier has not undergone validation.
Conclusion
Returning to our hypothetical patient, who has a Tchol of 230 mg/dl, HDL 45 mg/dl, LDL 128 mg/dl, is a non-smoker and has a systolic BP of 137 mmHg (on anti-hypertensive medication), according to FRS he has a 10% chance of MI or death due to coronary disease in the next 10 years (Table 2). His CRP at his last visit was 15 mg/dl. Thus, according to the RRS, his 10-year risk for CVD was 13%. Assuming he is in a high-risk European country (i.e. England), his risk of 10-year CVD mortality based on the SCORE is 3% (Table 2).
Table 2.
Comparison of predicted 10-year risk of CVD disease for hypothetical patient based on the FRS, RRS and the SCORE
| Variables | FRS | RRS | SCORE |
|---|---|---|---|
| Age, years | 53 | 53 | 53 |
| Gender | Male | Male | Male |
| Tchol | 230 mg/dl | 230 mg/dl | 5.95 mmol/l |
| HDL, mg/dl | 45 | 45 | – |
| Current smoker | No | No | No |
| Systolic BP, mmHg | 137 | – | 137 |
| On medication for hypertension | Yes | – | – |
| hsCRP, mg/l | – | 15 | – |
| Mother or father with heart attack or stroke before age 60 | – | Yes | – |
| High-/low-risk European country | – | – | High |
| Predicted 10-year risk | 10% MI or coronary death | 13% MI or stroke | 3% fatal CVD |
Applying the EULAR-recommended multiplier to FRS would increase his risk to 15%. If we applied the Adult Treatment Panel III guidelines [6] (which provide LDL targets for statin therapy) to FRS (with or without the multiplier) and RRS, the patient would not require additional treatment intervention for his LDL level. However, given his strong family history of cardiac disease and longstanding RA, many might consider adding a statin to his regimen. However, evidence supporting this consideration is lacking. This hypothetical case illustrates the need for the development of a data-driven method to estimate CV risk validated for patients with RA.
In summary, the CV risk factors IR, fat mass distribution, physical activity and smoking very likely have a higher prevalence in RA cases than controls. There may be elevations in the prevalence of dyslipidaemia, diabetes and hypertension but the evidence around these CV comorbidities is conflicting. Studies have demonstrated that the inflammation and treatment associated with RA can modify the levels and change the nature of how CVD risk is conferred compared with the general population. Thus, we suggest reframing the typical conceptual model of CV risk in RA. Rather than thinking of traditional risk factors and inflammation separately (Fig. 1A), to accurately describe CV risk, we must consider traditional risk factors and how they may be modified by inflammation without clear separation between the two groups (Fig. 1B). Finally, accurately estimating CV risk remains a challenge in RA, and more work is needed with focused study on each CV risk factor and improved CV risk estimation to help inform our decisions for primary and secondary prevention of CVD in our RA patients.
Fig. 1.
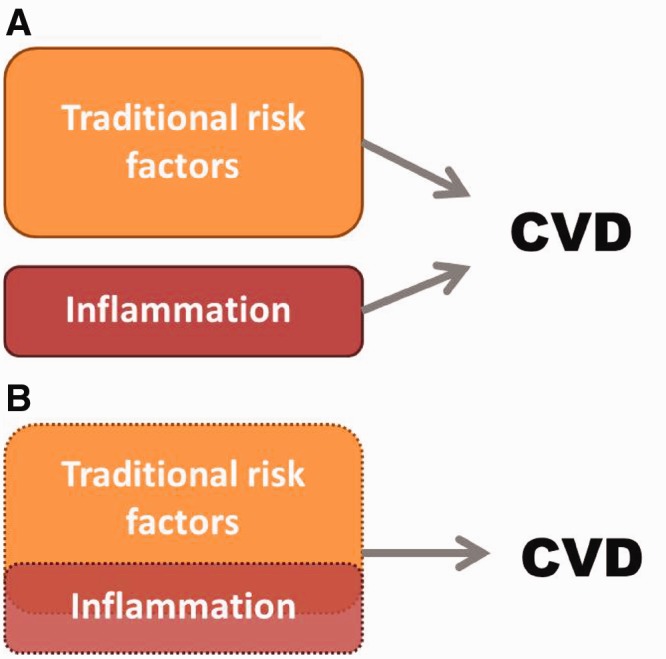
Conceptual model of factors contributing to CV risk in RA.
(A) A conceptual model of CV risk in RA where traditional risk factors and inflammation are considered separately. (B) Proposed conceptual model where traditional risk factors, inflammation and the overlap between the two, requires study to accurately understand CV risk in RA.

Acknowledgements
K.P.L. is supported by NIH K08 AR060257 and the Katherine Swan Ginsburg Fund.
D.H.S. is supported by NIH K24 AR055989.
Disclosure statement: D.H.S. receives research support from Lilly and Amgen and serves, in an unpaid role, on two Pfizer-sponsored trials. He also serves as an epidemiologic consultant to the Consortium of Rheumatology Researchers of North America (CORRONA). The other author has declared no conflicts of interest.
References
- 1.del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–45. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 2.Solomon DH, Kremer J, Curtis JR, et al. Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann Rheum Dis. 2010;69:1920–5. doi: 10.1136/ard.2009.122226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez A, Maradit Kremers H, Crowson CS, et al. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis. 2008;67:64–9. doi: 10.1136/ard.2006.059980. [DOI] [PubMed] [Google Scholar]
- 4.Brady SR, de Courten B, Reid CM, et al. The role of traditional cardiovascular risk factors among patients with rheumatoid arthritis. J Rheumatol. 2009;36:34–40. doi: 10.3899/jrheum.080404. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–24. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 6.NCEP. Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III Final Report): National Heart, Blood, Lung Institute, 2002 http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3_rpt.htm (29 August 2012, date last accessed) [Google Scholar]
- 7.Boyer JF, Gourraud PA, Cantagrel A, Davignon JL, Constantin A. Traditional cardiovascular risk factors in rheumatoid arthritis: a meta-analysis. Joint Bone Spine. 2012;78:179–83. doi: 10.1016/j.jbspin.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 9.van Halm VP, Nielen MM, Nurmohamed MT, et al. Lipids and inflammation: serial measurements of the lipid profile of blood donors who later developed rheumatoid arthritis. Ann Rheum Dis. 2007;66:184–8. doi: 10.1136/ard.2006.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myasoedova E, Crowson CS, Kremers HM, et al. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010;69:1310–4. doi: 10.1136/ard.2009.122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70:482–7. doi: 10.1136/ard.2010.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollono EN, Lopez-Olivo MA, Lopez JA, Suarez-Almazor ME. A systematic review of the effect of TNF-alpha antagonists on lipid profiles in patients with rheumatoid arthritis. Clin Rheumatol. 2010;29:947–55. doi: 10.1007/s10067-010-1405-7. [DOI] [PubMed] [Google Scholar]
- 13.Dahlqvist SR, Engstrand S, Berglin E, Johnson O. Conversion towards an atherogenic lipid profile in rheumatoid arthritis patients during long-term infliximab therapy. Scand J Rheumatol. 2006;35:107–11. doi: 10.1080/03009740500474578. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Gay MA, De Matias JM, Gonzalez-Juanatey C, et al. Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006;24:83–6. [PubMed] [Google Scholar]
- 15.Peters MJ, Vis M, van Halm VP, Wolbink GJ, Voskuyl AE, Lems WF, et al. Changes in lipid profile during infliximab and corticosteroid treatment in rheumatoid arthritis. Ann Rheum Dis. 2007;66:958–61. doi: 10.1136/ard.2006.059691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popa C, van den Hoogen FH, Radstake TR, et al. Modulation of lipoprotein plasma concentrations during long-term anti-TNF therapy in patients with active rheumatoid arthritis. Ann Rheum Dis. 2007;66:1503–7. doi: 10.1136/ard.2006.066191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seriolo B, Paolino S, Sulli A, Fasciolo D, Cutolo M. Effects of anti-TNF-alpha treatment on lipid profile in patients with active rheumatoid arthritis. Ann N Y Acad Sci. 2006;1069:414–9. doi: 10.1196/annals.1351.039. [DOI] [PubMed] [Google Scholar]
- 18.Soubrier M, Jouanel P, Mathieu S, et al. Effects of anti-tumor necrosis factor therapy on lipid profile in patients with rheumatoid arthritis. Joint Bone Spine. 2008;75:22–4. doi: 10.1016/j.jbspin.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Spanakis E, Sidiropoulos P, Papadakis J, et al. Modest but sustained increase of serum high density lipoprotein cholesterol levels in patients with inflammatory arthritides treated with infliximab. J Rheumatol. 2006;33:2440–6. [PubMed] [Google Scholar]
- 20.Tam LS, Tomlinson B, Chu TT, Li TK, Li EK. Impact of TNF inhibition on insulin resistance and lipids levels in patients with rheumatoid arthritis. Clin Rheumatol. 2007;26:1495–8. doi: 10.1007/s10067-007-0539-8. [DOI] [PubMed] [Google Scholar]
- 21.Vis M, Nurmohamed MT, Wolbink G, et al. Short term effects of infliximab on the lipid profile in patients with rheumatoid arthritis. J Rheumatol. 2005;32:252–5. [PubMed] [Google Scholar]
- 22.Kiortsis DN, Mavridis AK, Filippatos TD, et al. Effects of infliximab treatment on lipoprotein profile in patients with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol. 2006;33:921–3. [PubMed] [Google Scholar]
- 23.Jamnitski A, Visman IM, Peters MJ, et al. Beneficial effect of 1-year etanercept treatment on the lipid profile in responding patients with rheumatoid arthritis: the ETRA study. Ann Rheum Dis. 2010;69:1929–33. doi: 10.1136/ard.2009.127597. [DOI] [PubMed] [Google Scholar]
- 24.Singh JA, Beg S, Lopez-Olivo MA. Tocilizumab for rheumatoid arthritis: a Cochrane systematic review. J Rheumatol. 2011;38:10–20. doi: 10.3899/jrheum.100717. [DOI] [PubMed] [Google Scholar]
- 25.Hospital OU. Effect of the interleukin-6 receptor antagonist tocilizumab in non-ST elevation myocardial infarction. ClinicalTrials.gov. [cited 26 April 2012]; http://clinicaltrials.gov/show/NCT01491074 (12 December 2012, date last accessed)
- 26.Roche H-L. A study of the effect of tocilizumab on markers of atherogenic risk in patients with moderate to severe rheumatoid arthritis. ClinicalTrials.gov. [cited 26 April 2012] http://clinicaltrials.gov/show/NCT00535782 (7 December 2012, date last accessed)
- 27.Westlake SL, Colebatch AN, Baird J, et al. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology. 2010;49:295–307. doi: 10.1093/rheumatology/kep366. [DOI] [PubMed] [Google Scholar]
- 28.Morris SJ, Wasko MC, Antohe JL, et al. Hydroxychloroquine use associated with improvement in lipid profiles in rheumatoid arthritis patients. Arthritis Care Res. 2011;63:530–4. doi: 10.1002/acr.20393. [DOI] [PubMed] [Google Scholar]
- 29.Wallace DJ, Metzger AL, Stecher VJ, Turnbull BA, Kern PA. Cholesterol-lowering effect of hydroxychloroquine in patients with rheumatic disease: reversal of deleterious effects of steroids on lipids. Am J Med. 1990;89:322–6. doi: 10.1016/0002-9343(90)90345-e. [DOI] [PubMed] [Google Scholar]
- 30.Choi HK, Seeger JD. Lipid profiles among US elderly with untreated rheumatoid arthritis—the Third National Health and Nutrition Examination Survey. J Rheumatol. 2005;32:2311–6. [PubMed] [Google Scholar]
- 31.Van Lenten BJ, Hama SY, de Beer FC, et al. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest. 1995;96:2758–67. doi: 10.1172/JCI118345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hahn BH, Grossman J, Ansell BJ, Skaggs BJ, McMahon M. Altered lipoprotein metabolism in chronic inflammatory states: proinflammatory high-density lipoprotein and accelerated atherosclerosis in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res Ther. 2008;10:213. doi: 10.1186/ar2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCarey DW, McInnes IB, Madhok R, et al. Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet. 2004;363:2015–21. doi: 10.1016/S0140-6736(04)16449-0. [DOI] [PubMed] [Google Scholar]
- 34.Reilly MP, Wolfe ML, Rhodes T, et al. Measures of insulin resistance add incremental value to the clinical diagnosis of metabolic syndrome in association with coronary atherosclerosis. Circulation. 2004;110:803–9. doi: 10.1161/01.CIR.0000138740.84883.9C. [DOI] [PubMed] [Google Scholar]
- 35.Chung CP, Oeser A, Solus JF, et al. Inflammation-associated insulin resistance: differential effects in rheumatoid arthritis and systemic lupus erythematosus define potential mechanisms. Arthritis Rheum. 2008;58:2105–12. doi: 10.1002/art.23600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheal KL, Abbasi F, Lamendola C, et al. Relationship to insulin resistance of the adult treatment panel III diagnostic criteria for identification of the metabolic syndrome. Diabetes. 2004;53:1195–200. doi: 10.2337/diabetes.53.5.1195. [DOI] [PubMed] [Google Scholar]
- 37.Dessein PH, Joffe BI, Stanwix AE. Subclinical hypothyroidism is associated with insulin resistance in rheumatoid arthritis. Thyroid. 2004;14:443–6. doi: 10.1089/105072504323150750. [DOI] [PubMed] [Google Scholar]
- 38.Dessein PH, Joffe BI. Insulin resistance and impaired beta cell function in rheumatoid arthritis. Arthritis Rheum. 2006;54:2765–75. doi: 10.1002/art.22053. [DOI] [PubMed] [Google Scholar]
- 39.Solomon DH, Love TJ, Canning C, Schneeweiss S. Risk of diabetes among patients with rheumatoid arthritis, psoriatic arthritis and psoriasis. Ann Rheum Dis. 2010;69:2114–7. doi: 10.1136/ard.2009.125476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han C, Robinson DW Jr, Hackett MV, et al. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006;33:2167–72. [PubMed] [Google Scholar]
- 41.del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–45. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 42.Chung CP, Oeser A, Solus JF, et al. Prevalence of the metabolic syndrome is increased in rheumatoid arthritis and is associated with coronary atherosclerosis. Atherosclerosis. 2008;196:756–63. doi: 10.1016/j.atherosclerosis.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Dessein PH, Joffe BI, Stanwix AE. Effects of disease modifying agents and dietary intervention on insulin resistance and dyslipidemia in inflammatory arthritis: a pilot study. Arthritis Res. 2002;4:R12. doi: 10.1186/ar597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simard JF, Mittleman MA. Prevalent rheumatoid arthritis and diabetes among NHANES III participants aged 60 and older. J Rheumatol. 2007;34:469–73. [PubMed] [Google Scholar]
- 45.Liao KP, Gunnarsson M, Kallberg H, et al. Specific association of type 1 diabetes mellitus with anti-cyclic citrullinated peptide-positive rheumatoid arthritis. Arthritis Rheum. 2009;60:653–60. doi: 10.1002/art.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solomon DH, Karlson EW, Rimm EB, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–7. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 47.Kiortsis DN, Mavridis AK, Vasakos S, Nikas SN, Drosos AA. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis. 2005;64:765–6. doi: 10.1136/ard.2004.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenvinge A, Krogh-Madsen R, Baslund B, Pedersen BK. Insulin resistance in patients with rheumatoid arthritis: effect of anti-TNFalpha therapy. Scand J Rheumatol. 2007;36:91–6. doi: 10.1080/03009740601179605. [DOI] [PubMed] [Google Scholar]
- 49.Schultz O, Oberhauser F, Saech J, et al. Effects of inhibition of interleukin-6 signalling on insulin sensitivity and lipoprotein (a) levels in human subjects with rheumatoid diseases. PloS one. 2010;5:e14328. doi: 10.1371/journal.pone.0014328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;22;305:2525–31. doi: 10.1001/jama.2011.878. [DOI] [PubMed] [Google Scholar]
- 51.Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298:187–93. doi: 10.1001/jama.298.2.187. [DOI] [PubMed] [Google Scholar]
- 52.CDC. Prevalence of obesity among adults with arthritis—United States, 2003–2009. MMWR Morb Mortal Wkly Rep. 60:509–13. [PubMed] [Google Scholar]
- 53.WHO. BMI classification Global Database on Body Mass Index. 2006. [cited 13 April 2012]; http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
- 54.Stavropoulos-Kalinoglou A, Metsios GS, Koutedakis Y, Kitas GD. Obesity in rheumatoid arthritis. Rheumatology. 2011;50:450–62. doi: 10.1093/rheumatology/keq266. [DOI] [PubMed] [Google Scholar]
- 55.Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. J Chronic Dis. 1972;25:329–43. doi: 10.1016/0021-9681(72)90027-6. [DOI] [PubMed] [Google Scholar]
- 56.Stavropoulos-Kalinoglou A, Metsios GS, Koutedakis Y, et al. Redefining overweight and obesity in rheumatoid arthritis patients. Ann Rheum Dis. 2007;66:1316–21. doi: 10.1136/ard.2006.060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giles JT, Allison M, Blumenthal RS, et al. Abdominal adiposity in rheumatoid arthritis: association with cardiometabolic risk factors and disease characteristics. Arthritis Rheum. 62:3173–82. doi: 10.1002/art.27629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Panagiotakos DB, Pitsavos C, Yannakoulia M, Chrysohoou C, Stefanadis C. The implication of obesity and central fat on markers of chronic inflammation: The ATTICA study. Atherosclerosis. 2005;183:308–15. doi: 10.1016/j.atherosclerosis.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 59.McGuire TR, Brusnahan SK, Bilek LD, et al. Inflammation associated with obesity: relationship with blood and bone marrow endothelial cells. Obesity. 2011;19:2130–6. doi: 10.1038/oby.2011.246. [DOI] [PubMed] [Google Scholar]
- 60.Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004;15:2792–800. doi: 10.1097/01.ASN.0000141966.69934.21. [DOI] [PubMed] [Google Scholar]
- 61.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–5. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 62.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–9. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 63.Giles JT, Bartlett SJ, Andersen R, et al. Association of body fat with C-reactive protein in rheumatoid arthritis. Arthritis Rheum. 2008;58:2632–41. doi: 10.1002/art.23766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giles JT, Allison M, Blumenthal RS, et al. Abdominal adiposity in rheumatoid arthritis: association with cardiometabolic risk factors and disease characteristics. Arthritis Rheum. 2010;62:3173–82. doi: 10.1002/art.27629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McEntegart A, Capell HA, Creran D, et al. Cardiovascular risk factors, including thrombotic variables, in a population with rheumatoid arthritis. Rheumatology. 2001;40:640–4. doi: 10.1093/rheumatology/40.6.640. [DOI] [PubMed] [Google Scholar]
- 66.Maki-Petaja KM, Hall FC, Booth AD, et al. Rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by anti-tumor necrosis factor-alpha therapy. Circulation. 2006;114:1185–92. doi: 10.1161/CIRCULATIONAHA.105.601641. [DOI] [PubMed] [Google Scholar]
- 67.Kremer JM, Bloom BJ, Breedveld FC, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60:1895–905. doi: 10.1002/art.24567. [DOI] [PubMed] [Google Scholar]
- 68.Aw TJ, Haas SJ, Liew D, Krum H. Meta-analysis of cyclooxygenase-2 inhibitors and their effects on blood pressure. Arch Intern Med. 2005;165:490–6. doi: 10.1001/archinte.165.5.IOI50013. [DOI] [PubMed] [Google Scholar]
- 69.Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Intern Med. 1994;121:289–300. doi: 10.7326/0003-4819-121-4-199408150-00011. [DOI] [PubMed] [Google Scholar]
- 70.Pope JE, Anderson JJ, Felson DT. A meta-analysis of the effects of nonsteroidal anti-inflammatory drugs on blood pressure. Arch Intern Med. 1993;153:477–84. [PubMed] [Google Scholar]
- 71.Mazzantini M, Talarico R, Doveri M, et al. Incident comorbidity among patients with rheumatoid arthritis treated or not with low-dose glucocorticoids: a retrospective study. J Rheumatol. 2010;37:2232–6. doi: 10.3899/jrheum.100461. [DOI] [PubMed] [Google Scholar]
- 72.Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119:503 e1–9. doi: 10.1016/j.amjmed.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 73.Heliovaara M, Aho K, Aromaa A, Knekt P, Reunanen A. Smoking and risk of rheumatoid arthritis. J Rheumatol. 1993;20:1830–5. [PubMed] [Google Scholar]
- 74.Yang Q, Cogswell ME, Flanders WD, et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012;307:1273–83. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Solomon DH, Karlson EW, Rimm EB, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–7. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 76.Albano SA, Santana-Sahagun E, Weisman MH. Cigarette smoking and rheumatoid arthritis. Semin Arthritis Rheum. 2001;31:146–59. doi: 10.1053/sarh.2001.27719. [DOI] [PubMed] [Google Scholar]
- 77.Wolfe F. The effect of smoking on clinical, laboratory, and radiographic status in rheumatoid arthritis. J Rheumatol. 2000;27:630–7. [PubMed] [Google Scholar]
- 78.van den Berg MH, de Boer IG, le Cessie S, Breedveld FC, Vliet Vlieland TP. Are patients with rheumatoid arthritis less physically active than the general population? J Clin Rheumatol. 2007;13:181–6. doi: 10.1097/RHU.0b013e318124a8c4. [DOI] [PubMed] [Google Scholar]
- 79.Mancuso CA, Rincon M, Sayles W, Paget SA. Comparison of energy expenditure from lifestyle physical activities between patients with rheumatoid arthritis and healthy controls. Arthritis Rheum. 2007;57:672–8. doi: 10.1002/art.22689. [DOI] [PubMed] [Google Scholar]
- 80.Metsios GS, Stavropoulos-Kalinoglou A, Panoulas VF, et al. Association of physical inactivity with increased cardiovascular risk in patients with rheumatoid arthritis. Eur J Cardiovasc Prev Rehabil. 2009;16:188–94. doi: 10.1097/HJR.0b013e3283271ceb. [DOI] [PubMed] [Google Scholar]
- 81.Metsios GS, Stavropoulos-Kalinoglou A, Veldhuijzen van Zanten JJ, et al. Rheumatoid arthritis, cardiovascular disease and physical exercise: a systematic review. Rheumatology. 2008;47:239–48. doi: 10.1093/rheumatology/kem260. [DOI] [PubMed] [Google Scholar]
- 82.D'Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–7. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 83.Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 84.Chung CP, Oeser A, Avalos I, et al. Utility of the Framingham risk score to predict the presence of coronary atherosclerosis in patients with rheumatoid arthritis. Arthritis Res Ther. 2006;8:R186. doi: 10.1186/ar2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Crowson CS, Matteson EL, Roger VL, Therneau TM, Gabriel SE. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110:420–4. doi: 10.1016/j.amjcard.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iannaccone CK, Lee YC, Cui J, et al. Using genetic and clinical data to understand response to disease-modifying anti-rheumatic drug therapy: data from the Brigham and Women's Hospital Rheumatoid Arthritis Sequential Study. Rheumatology. 2011;50:40–6. doi: 10.1093/rheumatology/keq263. [DOI] [PubMed] [Google Scholar]
- 87.Del Rincon I, Williams K, Stern MP, et al. Association between carotid atherosclerosis and markers of inflammation in rheumatoid arthritis patients and healthy subjects. Arthritis Rheum. 2003;48:1833–40. doi: 10.1002/art.11078. [DOI] [PubMed] [Google Scholar]
- 88.Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325–31. doi: 10.1136/ard.2009.113696. [DOI] [PubMed] [Google Scholar]


