Abstract
Objectives. To quantify the risk of cancer and compare it with that for the general population in a modern cohort of UK patients with RA and to identify risk factors for cancer among this cohort.
Methods. The study population comprised biologic-naïve RA subjects receiving non-biologic disease-modifying therapy recruited to the British Society for Rheumatology Biologics Register from 2002 to 2009. Standardized incidence ratios (SIRs) for cancers were calculated using age- and gender-specific cancer rates in the English population. Poisson regression models adjusted for age and gender using England general population data were used to determine the association of other predictors with incident malignancy.
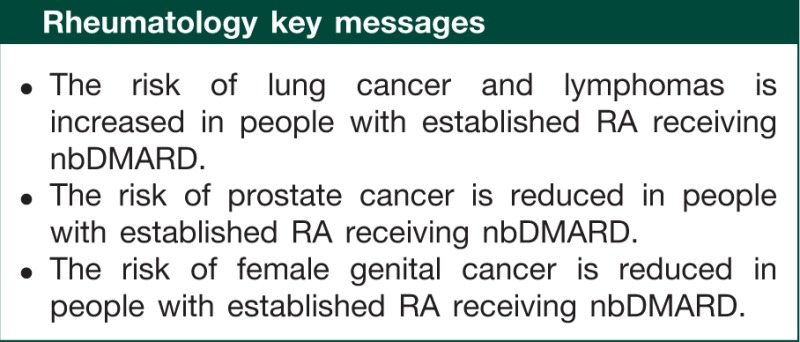
Results. The cohort comprised 3771 individuals with RA contributing 13 315 person-years of follow-up. One hundred and eighty-two cancers were reported: 156 solid and 26 myelo- or lymphoproliferative cancers. The overall SIR was 1.28 (95% CI 1.10, 1.48). Risks of lung cancer (SIR 2.39, 95% CI 1.75, 3.19), Hodgkin lymphoma (SIR 12.82, 95% CI 4.16, 29.92) and non-Hodgkin lymphoma (SIR 3.12, 95% CI 1.79, 5.07) were higher compared with the general population and risks of prostate cancer (SIR 0.35, 95% CI 0.11, 0.82) and cancers of the female genital organs (SIR 0.35, 95% CI 0.10, 0.90) were reduced. Within the cohort, cancer risk was more than 2-fold higher in current or ex-smokers than in non-smokers.
Conclusion. The overall incidence of cancer was increased in this national cohort of subjects with RA. The association of RA with certain cancers needs to be considered when studying the effects of biologic therapy, such as anti-TNF, on cancer risk.
Keywords: rheumatoid arthritis, cancer, lymphoma, standardized incidence ratio
Introduction
It has long been recognized that patients with RA have an increased risk of certain types of cancer [1]. A recent meta-analysis of observational studies of RA patients published during 1990–2007, including studies of anti-TNF-treated patients, found an overall 5% increased risk of cancer [2]. There was an increased risk of lung cancer and lymphoma but a reduced risk of colorectal and breast cancer [2]. In part, these differing directions of risk may be related to disease severity or treatment options. For example, in the case of lymphoma, increased inflammation is associated with an increased risk [3]. NSAIDs are associated with a reduced risk of colon cancer [4]. Over the past 15 years the introduction of biologic therapies to the management of RA has again raised concerns about the risk of cancer in RA, particularly with respect to anti-TNF therapies due to the role of TNF in tumour surveillance. Concurrently there have also been significant changes in the overall way that RA is managed, with an earlier and more aggressive approach to disease control [5, 6]. Therefore, in order to place the risk of cancers observed under anti-TNF therapy in context, it is important to understand further the underlying risk of cancer in patients treated with non-biologic therapies in the 21st century. The purpose of this analysis is to (i) quantify the risk of cancer and compare it with the general population in a modern cohort of UK biologic-naïve RA patients recruited and followed prospectively since 2001 and (ii) identify risk factors for cancer among this cohort.
Methods
Patients
Patients included in this study were participants in the British Society for Rheumatology Biologics Register (BSRBR), which was established primarily to assess the long-term safety of biologic therapies in RA [7]. A cohort of nearly 4000 biologic-naïve patients with RA receiving non-biologic disease-modifying therapy (nbDMARD) was recruited between 2002 and 2009. This cohort was recruited by the BSRBR Control Centre Consortium from 29 secondary or tertiary rheumatology centres from around the UK: England, 23; Northern Ireland, 2; Scotland, 2 and Wales, 2. Our purpose was to assemble a contemporaneous biologic-naïve cohort with whom patients starting biologic treatment could be compared for both efficacy and adverse events. The nbDMARD cohort was required to have active RA at recruitment (target DAS28 >4.2) despite current treatment with at least one nbDMARD.
Baseline assessment and follow-up
Baseline data including demographics, disease characteristics, current and previous nbDMARD exposure, current drug therapy, comorbidity and smoking status were reported by the clinical team at registration. Patients were asked to complete a version of the Stanford HAQ, modified for use in the UK, to indicate their level of physical disability at baseline [8]. Malignancies occurring prior to the start of the study and throughout the study were ascertained through flagging all participants with the National Health Service (NHS) Information Centre (NHS-IC) for England and Wales and the Scottish and Northern Irish Cancer Registries. Patients were not excluded from the analysis if they had a prior cancer.
Patients were followed until death, initiation of biologic therapy, 31 December 2009 or last returned follow-up form, whichever came first. Deaths were identified by flagging with NHS-IC for England, Scotland and Wales; and Health and Social Care – Northern Ireland for Northern Ireland. These agencies provided details of the date and cause of death [coded to the underlying cause of death using the International Classification of Diseases version 10 (ICD-10)]. Changes to therapy, including initiation of biologics, were reported on clinical questionnaires every 6 months for 3 years and annually thereafter. Although the analysis was censored at the end of 2009, data were collected until June 2011, which allowed 18 months for a lag in cancer reporting by the agencies to BSRBR.
Ascertainment of cancers
The BSRBR received reports of incident cancer from a number of sources: patient diaries, clinical follow-up forms, via a malignancy Event of Special Interest pro forma that can be downloaded by health care professionals from the BSRBR web site and returned at any time [9] and from the national cancer agencies. For this analysis, only cancers reported from the cancer agencies were included to reduce reporting bias and facilitate comparison with national cancer rates published by the Office for National Statistics (ONS) [10].
Cancers were reported to the BSRBR by the cancer agencies using ICD-10 and the International Classification of Diseases for Oncology morphology codes (ICD-O) [11]. Registration of cancers is mandatory in the UK and overall completeness is >99% [12]. All malignancies were included in this analysis except for non-melanoma skin cancers (ICD-10 code C44) because we have published on this previously [13].
Statistical analysis
Standardized incidence ratios
The primary outcome measure was incident cancer, defined as ICD-10 C00–C97 excluding C44 [11]. Standardized incidence ratios (SIRs) were calculated by dividing the number of observed cancers in the nbDMARD cohort by the number of cancers which would have been expected if the rate in the RA cohort was the same as in the general population and then multiplying by 100. Gender- , 5 year age- and calendar year-specific population rates for England were applied to the corresponding person-years (pyrs) of follow-up in the BSRBR (indirect standardization) to obtain the expected number of cancers. Population rates for England were derived from tables published annually by the ONS [10] and applied to the entire cohort, since the BSRBR comprises too few people in Northern Ireland, Scotland and Wales to calculate SIRs separately for each country. CIs around the SIRs were calculated assuming a Poisson distribution of cases. As well as overall cancer, an SIR was calculated separately for solid cancers (defined as ICD-10 codes C00–80 excluding C44) and myelo- and lymphoproliferative cancers (ICD-10 C81–96). Site-specific SIRs were calculated for sites where there were either at least five incident or expected malignancies.
Factors associated with incident cancer
The following baseline characteristics were analysed as possible predictors of first incident malignancy: RA disease duration, disease activity measured using DAS28 (<3.2, 3.2–5.1, >5.1), physical function measured using HAQ, prior or current exposure to ciclosporin, AZA or CYC (analysed together due to low proportion of users for each drug), current exposure to NSAID, cancer prior to registration with BSRBR and smoking status (never, prior, current) as reported by the clinical team.
Poisson regression models, adjusted for age (in 5-year age bands) and gender using England population data, were used to determine which characteristics were associated with incident malignancy during follow-up and the results are presented as relative risks (RRs) per year with 95% CIs. Models were adjusted for age and gender by multiplying England population rates of cancer stratified by gender and 5-year age bands by duration of follow-up and including the product as the exposure term. Multiple imputation was used to minimize bias due to missing baseline data. Twenty data sets were imputed using chained regression and analysed using the ICE package in Stata (StataCorp, College Station, TX, USA).
All analyses were conducted using Stata version 10.1. The subjects’ written consent was obtained according to the Declaration of Helsinki. The BSRBR study has been approved by the North West Multicentre Research Ethics Committee.
Results
The cohort comprised 3771 individuals with RA contributing 13 315 pyrs of follow-up to the analysis; median 3.7 years [interquartile range (IQR) 2.1, 4.9]. The cohort was predominantly female (72%), mean age 60 years (s.d. 12 years) (Table 1). The median disease duration was 6 years (IQR 1, 15 years) at baseline with moderate-to-severe RA disease activity [mean DAS28 5.1 (s.d. 0.8)] and moderate disability [mean HAQ 1.5 (s.d. 0.8)]. The median number of previous nbDMARDs at baseline was 2 (IQR 1, 3). The majority of the cohort was receiving monotherapy at baseline (2638, 70%), with MTX being the most frequent drug (1453, 55%). A third of the cohort were receiving combination therapy at baseline (1133, 30%), of whom 964 (85%) were taking MTX. The proportion of missing baseline data was low (Table 1).
Table 1.
Baseline characteristics of the cohort
| n = 3771 | |
|---|---|
| Gender, n (%) | |
| Male | 1039 (28) |
| Female | 2732 (72) |
| Age, mean (s.d.), years | 60 (12) |
| <55, n (%) | 1153 (31) |
| 55–64, n (%) | 1164 (31) |
| 65–74, n (%) | 1008 (29) |
| 75+, n (%) | 446 (12) |
| Ethnicity, n (%) | |
| White | 2866 (76) |
| Non-white | 74 (2) |
| Missing | 831 (22) |
| Country of residence, n (%) | |
| England | 3194 (85) |
| Northern Ireland | 372 (10) |
| Scotland | 161 (4) |
| Wales | 44 (1) |
| Smoking, n (%) | |
| Current | 892 (24) |
| Previous | 1497 (40) |
| Never | 1364 (36) |
| Missing | 18 (0) |
| Previous NHS-IC-reported cancer excluding NMSC and CIS, n (%) | 144 (4) |
| RA disease duration, median (IQR), years | 6 (1–15) |
| 0–3, n (%) | 1231 (33) |
| >3–10, n (%) | 1028 (27) |
| >10, n (%) | 1488 (39) |
| Missing, n (%) | 24 (1) |
| Disease activity, mean DAS28 (s.d.) | 5.1 (1.3) |
| Low (DAS28 <3.2), n (%) | 289 (8) |
| Moderate (DAS28 3.2–5.1), n (%) | 1551 (41) |
| High (DAS28 >5.1), n (%) | 1876 (50) |
| Missing, n (%) | 55 (1) |
| Disability, mean HAQ (s.d.) | 1.5 (0.8) |
| Low (HAQ 0–1), n (%) | 881 (23) |
| Moderate (HAQ >1–2), n (%) | 1346 (36) |
| High (HAQ >2–3), n (%) | 780 (21) |
| Missing, n (%) | 764 (20) |
| Median number of nbDMARDs ever received (IQR) | 2 (1–3) |
| Exposure to AZA, ciclosporin or CYC, n (%) | 367 (10) |
| Steroid exposure, n (%) | 853 (23) |
| NSAID exposure, n (%) | 2019 (54) |
NMSC: non-melanoma skin cancer.
Six hundred and sixty-three participants (18%) were censored prior to 31 December 2009 due to initiation of biologic therapy. In those switching to biologic therapy, median time to switching was 1.9 years (IQR 1.0, 3.3 years). Three hundred and thirty-one subjects (9%) died after a median follow-up of 2.6 years (IQR 1.3, 3.8 years).
SIRs
All cancers excluding non-melanoma skin cancers (ICD 10 C00–C97 × C44)
One hundred and eighty-two cancers were reported, equating to a crude incidence rate of 1.37/100 pyrs (95% CI 1.18, 1.58; Table 2). None of the participants had more than one incident cancer reported during follow-up. Overall, the risk of cancer was increased by 28% in the cohort compared with the general population (SIR 1.28, 95% CI 1.10, 1.48). Since population cancer rates were for England only, this analysis was then restricted to include nbDMARD subjects living in England (SIR 1.39, 95% CI 1.19, 1.62).
Table 2.
Overall and solid cancer SIRs
| Overall |
Male |
Female |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total follow-up, pyrs | 13 315 | 3732 | 9584 | |||||||||
| Cancer site and ICD-10 code | O | Rate per 100 pyrs (95% CI) | E | SIR (95% CI) | O | Rate per 100 pyrs (95% CI) | E | SIR (95% CI) | O | Rate per 100 pyrs (95% CI) | E | SIR (95% CI) |
| All sites (ICD-10 C00–C97 × C44) | 182 | 1.37 (1.18, 1.58) | 141.80 | 1.281.10, 1.48 | 58 | 1.55 (1.18, 2.01) | 51.92 | 1.11 (0.85, 1.44) | 124 | 1.29 (1.08, 1.54) | 89.88 | 1.38 (1.15, 1.64) |
| All sites—England only | 168 | 1.50 (1.28, 1.75) | 120.75 | 1.39 (1.19, 1.62) | 55 | 1.70 (1.28, 2.21) | 45.76 | 1.20 (0.91, 1.56) | 113 | 1.42 (1.17, 1.71) | 74.99 | 1.51 (1.24, 1.81) |
| Solid cancers C00–C80 × C44 | 156 | 1.17 (0.99, 1.37) | 131.09 | 1.19 (1.01, 1.39) | 49 | 1.31 (0.97, 1.74) | 47.70 | 1.03 (0.76, 1.36) | 107 | 1.12 (0.91, 1.35) | 83.39 | 1.28 (1.05, 1.55) |
| Solid cancers England only | 143 | 1.28 (1.08, 1.51) | 111.50 | 1.28 (1.08, 1.51) | 46 | 1.42 (1.04, 1.89) | 42.04 | 1.09 (0.80, 1.46) | 97 | 1.22 (0.99, 1.49) | 69.56 | 1.39 (1.13, 1.70) |
| Oesophagus C15 | 5 | 0.04 (0.01, 0.19) | 3.39 | 1.47 (0.48, 3.44) | NR | NR | NR | NR | NR | NR | NR | NR |
| Stomach C16 | 6 | 0.05 (0.02, 0.10) | 3.20 | 1.88 (0.69, 4.09) | NR | NR | NR | NR | NR | NR | NR | NR |
| Colorectal C18–C20 | 17 | 0.13 (0.07, 0.20) | 17.69 | 0.96 (0.56, 1.54) | 7 | 0.19 (0.07, 0.39) | 7.33 | 0.96 (0.38, 1.97) | 10 | 0.10 (0.05, 0.19) | 10.36 | 0.97 (0.46, 1.78) |
| Lung C34 | 46 | 0.35 (0.25, 0.46) | 19.24 | 2.39 (1.75, 3.19) | 16 | 0.43 (0.25, 0.70) | 7.96 | 2.01 (1.15, 3.26) | 30 | 0.31 (0.21, 0.45) | 11.28 | 2.66 (1.79, 3.80) |
| Melanoma C43 | 9 | 0.07 (0.03, 0.13) | 4.38 | 2.05 (0.94, 3.90) | NR | NR | NR | NR | 6 | 0.06 (0.02, 0.14) | 2.93 | 2.05 (0.75, 4.46) |
| Breast C50 | 30 | 0.31 (0.21, 0.45) | 28.16 | 1.07 (0.72, 1.52) | ||||||||
| Prostate C61 | 5 | 0.13 (0.04, 0.31) | 14.22 | 0.35 (0.11, 0.82) | ||||||||
| Female genital organs C51–C58 | 4 | 0.04 (0.01, 0.11) | 11.35 | 0.35 (0.10, 0.90) | ||||||||
O: observed; E: expected; NR: not reported (fewer than five observed or expected cancers).
Solid cancers
Solid cancer was reported in 156 subjects; crude incidence rate 1.17/100 pyrs (95% CI 0.99, 1.37) (Table 2). The risk of solid cancer was increased compared with the general population (SIR 1.19, 95% CI 1.01, 1.39). The RRs were highest for lung cancer (SIR 2.39, 95 CI 1.75, 3.19) and melanoma (SIR 2.05, 95% CI 0.94, 3.90). A reduced risk of prostate cancer and cancers of the female genital organs was observed (SIR 0.35, 95% CI 0.11, 0.82 and SIR 0.35, 95% CI 0.10, 0.90, respectively).
Myelo-and lymphoproliferative cancer
Twenty-six myelo- or lymphoproliferative cancers were reported. The incidence rate of these cancers was 0.20/100 pyrs (95% CI 0.13, 0.29) reflecting a 2.5-fold increased risk compared with the general population (SIR 2.43, 95% CI 1.58, 3.55; Table 3). When restricted to lymphomas, the risk was higher (SIR 3.81, 95% CI 2.36, 5.82). More than two-thirds of lymphomas were non-Hodgkin lymphoma (NHL). Although Hodgkin lymphoma was rare in this cohort, the risk was 13-fold higher than that in the general population (Table 3).
Table 3.
Myelo- and lymphoproliferative cancers SIRs
| Overall |
Males |
Females |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total follow-up, pyrs | 13 315 | 3732 | 9584 | |||||||||
| Cancer site and ICD-10 code | O | Rate per 100 pyrs (95% CI) | E | SIR (95% CI) | O | Rate per 100 pyrs (95% CI) | E | SIR (95% CI) | O | Rate per 100 pyrs (95% CI) | E | SIR (95% CI) |
| Myelo- and lymphoproliferative C81–C96 | 26 | 0.20 (0.13, 0.29) | 10.72 | 2.43 (1.58, 3.55) | 9 | 0.24 (0.11, 0.46) | 4.23 | 2.13 (0.97, 4.04) | 17 | 0.18 (0.10, 0.28) | 6.49 | 2.61 (1.53, 4.19) |
| Myelo- and lymphoproliferative C81–C96 England only | 25 | 0.22 (0.14, 0.33) | 9.16 | 2.73 (1.77, 4.03) | 9 | 0.28 (0.13, 0.53) | 3.73 | 2.42 (1.10, 4.59) | 16 | 0.20 (0.12, 0.33) | 5.43 | 2.95 (1.68, 4.78) |
| Lymphoma C81–85 | 21 | 0.16 (0.10, 0.24) | 5.51 | 3.81 (2.36, 5.82) | 8 | 0.21 (0.09, 0.42) | 2.02 | 3.95 (1.71, 7.79) | 13 | 0.14 (0.07, 0.23) | 3.49 | 3.73 (1.98, 6.37) |
| Hodgkin lymphoma C81 | 5 | 0.04 (0.01, 0.09) | 0.39 | 12.82 (4.16, 29.92) | NR | NR | NR | NR | NR | NR | NR | NR |
| NHL C82–85 | 16 | 0.12 (0.07, 0.20) | 5.12 | 3.12 (1.79, 5.07) | 5 | 0.13 (0.4, 0.31) | 1.88 | 2.66 (0.86, 6.21) | 11 | 0.11 (0.06, 0.21) | 3.24 | 3.39 (1.69, 6.07) |
O: observed; E: expected; NR: not reported (fewer than five observed or expected cancers).
Factors associated with incident cancer
In multivariate analysis, both current and prior smoking history were associated with incident cancer (RR 2.53, 95% CI 1.62, 3.95 and RR 2.09, 95% CI 1.40, 3.12, respectively). RA disease duration of <3 years and exposure to one or more of the nbDMARDs AZA, ciclosporin or CYC were significantly associated with incident cancer in this cohort (Table 4).
Table 4.
Factors associated with incident cancer
| RR (95% CI) | Multivariate analysis RR (95% CI) | |
|---|---|---|
| Smoking (referent never) | ||
| Prior | 2.14 (1.43, 3.19) | 2.09 (1.40, 3.12) |
| Current | 2.66 (1.71, 4.15) | 2.53 (1.62, 3.95) |
| Prior cancer (NHS-IC reported) | 1.19 (0.65, 2.19) | |
| Duration of RA (referent >10 years) | ||
| <3 years | 1.59 (1.09, 2.34) | 1.65 (1.11, 2.45) |
| 3–10 years | 1.31 (0.94, 1.83) | 1.30 (0.92, 1.82) |
| AZA, ciclosporin or CYC | 1.46 (0.96, 2.23) | 1.63 (1.05, 2.52) |
| NSAID | 0.99 (0.74, 1.32) | |
| Disease activity; DAS28 (referent >5.1) | ||
| <3.2 | 0.61 (0.32, 1.14) | |
| 3.2–5.1 | 0.79 (0.58, 1.07) | |
| Disability; HAQ (referent <1) | ||
| 1–2 | 1.32 (0.90, 1.93) | |
| >2 | 1.02 (0.65, 1.59) |
Discussion
Overall, participants in this cohort study had a 28% increase in cancer compared with the general population. The risk of lung cancer, Hodgkin lymphoma and NHL were increased. Male participants had a reduced risk of prostate cancer and females had a reduction in cancers of the genital organs.
In multivariate analysis, both current and previous smoking at baseline were associated with a more than 2-fold RR of cancer. It is well established that smoking is a risk factor for cancer in the general population and we were unable to ascertain in this analysis whether the magnitude of risk differs in people with RA. Rheumatoid disease duration of <3 years was associated with a 50% increased RR. While increasing cancer risk with RA disease duration has been described [14], the pattern of increased risk in early RA and following recruitment to a study has been observed elsewhere [15, 16] and may in part be due to unmasking of prevalent cancers (surveillance bias).
Exposure to AZA, ciclosporin or CYC was associated with a 65% increased RR for incident cancer but only 367 participants (10%) had received these less commonly used nbDMARDs. These drugs are known to be associated with increased cancer risk, and the risk appears to be related to the dose of immunosuppressant [17–21]. In the context of RA, it is noteworthy that they are usually reserved for patients with more severe disease that have failed treatment with other nbDMARDs. The observed association in the BSRBR data may actually reflect underlying disease severity, although an association between cancer risk and other markers of disease severity (HAQ, DAS28) was not observed in this study.
The observed increased risk of cancer compared with the general population may be due in part to shared genetic risk factors for RA susceptibility/severity and malignancy. For example, the HLA-DRB1 shared-epitope genotype has been shown to be associated with mortality due to malignancy in RA, particularly *0101 genotypes [22]. However, genetic risk factors were not addressed in the BSRBR data.
In this study, there was a marked reduction in risk of both prostate cancer and female genital cancers. A recent population-based study using the Californian discharge register also reported reduced risk of ovarian, uterine, cervical and prostate cancers in subjects with RA [23]. Other studies do not confirm these findings [14, 24–26] but may have lacked power to detect these less common cancers. It has been hypothesized that inflammation plays a role in the pathogenesis of prostatic and female cancers, and that NSAIDs (in particular aspirin) may be chemoprotective against them [27–29], although not all studies have reported an association [30, 31]. It is hard to attribute the reduced risk of these cancers in this study to NSAID use since we neither found a difference in risk of either colorectal or breast cancers, for which a protective role for NSAIDs has been established [4, 32] nor was NSAID use associated with cancer in regression analysis.
The finding of this study of an increased risk of both NHL and Hodgkin lymphoma has been widely reported previously and supported in meta-analysis [1, 14, 14, 23, 25, 26, 33]. Evidence for the role of chronic inflammation in this increased risk of lymphoma comes from a large case–control study from Sweden [3]. Three hundred and seventy-eight consecutive subjects with RA diagnosed with lymphoma were matched to 378 RA controls without registered cancer. Overall burden of disease activity from RA diagnosis to lymphoma date was assessed and split into deciles. Compared with the first decile (lowest cumulative disease activity), marginal increases in lymphoma risk were seen up to the seventh decile. The risk of lymphoma then rose steeply, and for those patients with highest cumulative disease activity the odds ratio for lymphoma was 61.6 (95% CI 21.0, 181.1) [3]. One might hypothesize that due to current practice of early treatment of RA and tighter control of disease activity the risk of lymphoma would be lower now than in historical RA cohorts but this was not borne out in this study. However, participants in the BSRBR were required to have active RA despite treatment with nbDMARD at registration and so they may represent a cohort with more severe RA than the general RA UK population. Furthermore, almost 40% of the cohort had >10 years of RA at baseline, and so may not have benefitted from more aggressive treatment from the outset.
This study included subjects treated with nbDMARD and did not include people exposed to biologic therapies such as anti-TNF. A degree of uncertainty remains regarding the influence (if any) of biologic therapies on site-specific cancer risk in RA. When evaluating this risk, the influence of previous exposure to nbDMARD, particularly AZA, ciclosporin or CYC, needs to be considered carefully.
A strength of this study was the flagging of all participants with the national cancer registries to identify incident cancers, which has near-complete capture of cases. However, there is a lag in the reporting of cancers from regional centres to the national cancer agencies who collate and validate events [34], and the most recently published population data are for 2009.
The limitations of cohort studies need to be borne in mind when interpreting these findings. The overall RR of cancer was higher in this study than in several other observational studies [14–16, 24–26, 33]. Recruitment to this BSRBR nbDMARD-only cohort took place in parallel with recruitment to a cohort of subjects commencing anti-TNF. It is possible that patients considered to be at high risk for developing cancer may have been preferentially recruited to the nbDMARD-only cohort if it was felt they were unsuitable for anti-TNF. In addition, patients are not screened for cancer when starting nbDMARD in routine clinical practice and so it is possible that unmasking of certain cancers, such as lymphomas, may have occurred shortly after starting nbDMARD therapy. Since this study comprised a population exposed soley to nbDAMRD, this effect may have lead to an increase in the apparent risk of cancer.
Although we found an overall increase in cancer in subjects with RA, it should not be interpreted as a causal association. For example, the association between RA and lung cancer may be partly explained by the shared risk factor of smoking [35]. Other factors, such as socio-economic status, may also be important. The cohort comprised mostly white participants and may not be generalizable to people from other ethnic groups. The BSRBR included subjects from around the UK but UK-wide rates of cancer in the general population were not available. In this analysis, all BSRBR participants were included and compared with age- and gender-adjusted rates of cancer in England only. While the majority of the cohort lived in England (85%), the SIRs for all cancers, solid cancers and myelo- and lymphoproliferative cancers were around 10% higher when those living in Northern Ireland, Scotland or Wales were excluded. This may reflect both regional differences in cancer risk and differences in promptness and completeness of registration of cancers with the national cancer agencies [36].
In conclusion, the incidence of cancer was increased by 28% in this national cohort of subjects with RA compared with the general population. While there was an increased risk of NHL, Hodgkin disease and lung cancer, the risk of prostate and female genital cancers was reduced. Smoking was the strongest independent risk factor of cancer within the RA cohort. The association of RA with certain cancers and the underlying cancer risk in patients treated with nbDMARDS needs to be considered when studying the effects of biologic therapy, such as anti-TNF, on cancer risk.
Acknowledgements
The authors acknowledge the enthusiastic collaboration of all consultant rheumatologists and their specialist nurses in the UK in providing the data. The authors would like to gratefully acknowledge the support of the National Institute for Health Research, through the Comprehensive Local Research Networks at participating centres. In addition, we acknowledge the support from Dr Ian Griffiths (Past), Professor David Isenberg (Past) and Prof. Alex MacGregor (current), Chairs of the BSRBR Management Committee, Prof. Gabriel Panayi, Prof. David G. I. Scott, Dr Andrew Bamji, Prof. Deborah Bax and Prof David L Scott, Presidents of the BSR during the period of data collection, for their active role in enabling the register to undertake its tasks and to Samantha Peters (CEO of the BSR), Mervyn Hogg, Nia Taylor, Alan Roach and members of the BSRBR Scientific Steering Committee. We also acknowledge the seminal role of the BSR Clinical Affairs Committee for establishing national biologic guidelines and recommendations for such a register. Finally, we would like to acknowledge the substantial contribution of Andy Tracey, Katie McGrother, Dr Xuejuan Fan and Dr Mark Lunt in database design and manipulation and Prof. Alan Silman in his prior role as a principal investigator of the BSRBR.
The BSR commissioned the Biologics Register (BSRBR) as a UK-wide national project to investigate the safety of biologic agents in routine medical practice. D.P.M.S. and K.L.H. are principal investigators on the BSRBR. BSR receives restricted income from UK pharmaceutical companies, presently Abbott Laboratories, Swedish Orphan Biovitrum, Merck, Pfizer, UCB and Roche. This income finances a wholly separate contract between the BSR and the University of Manchester. The principal investigators and their team have full academic freedom and are able to work independently of pharmaceutical industry influence. All decisions concerning analyses, interpretation and publication are made autonomously of any industrial contribution. Members of the Manchester team, BSR trustees, committee members and staff complete an annual declaration in relation to conflicts of interest.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Isomaki HA, Hakulinen T, Joutsenlahti U. Excess risk of lymphomas, leukemia and myeloma in patients with rheumatoid arthritis. J Chronic Dis. 1978;31:691–6. doi: 10.1016/0021-9681(78)90071-1. [DOI] [PubMed] [Google Scholar]
- 2.Smitten AL, Simon TA, Hochberg MC, et al. A meta-analysis of the incidence of malignancy in adult patients with rheumatoid arthritis. Arthritis Res Ther. 2008;10:R45. doi: 10.1186/ar2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baecklund E, Iliadou A, Askling J, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum. 2006;54:692–701. doi: 10.1002/art.21675. [DOI] [PubMed] [Google Scholar]
- 4.Flossmann E, Rothwell PM British Doctors Aspirin Trial the UK-TIA Aspirin Trial. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–13. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 5.Luqmani R, Hennell S, Estrach C, et al. British Society for Rheumatology and British Health Professionals in rheumatology guideline for the management of rheumatoid arthritis (the first two years) Rheumatology. 2006;45:1167–9. doi: 10.1093/rheumatology/kel215a. [DOI] [PubMed] [Google Scholar]
- 6.Luqmani R, Hennell S, Estrach C, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of rheumatoid arthritis (after the first 2 years) Rheumatology. 2009;48:436–9. doi: 10.1093/rheumatology/ken450a. [DOI] [PubMed] [Google Scholar]
- 7.Hyrich KL, Watson KD, Isenberg DA, et al. The British Society for Rheumatology Biologics Register: 6 years on. Rheumatology. 2008;47:1441–3. doi: 10.1093/rheumatology/ken242. [DOI] [PubMed] [Google Scholar]
- 8.Kirwan JR, Reeback JS. Stanford Health Assessment Questionnaire modified to assess disability in British patients with rheumatoid arthritis. Br J Rheumatol. 1986;25:206–9. doi: 10.1093/rheumatology/25.2.206. [DOI] [PubMed] [Google Scholar]
- 9. BSRBR. Event of Special Interest (ESI) Report; Malignancy. http://www.medicine.manchester.ac.uk/images/File/ESI_07_malig_form.pdf. (15 February 2012, date last accessed)
- 10. Office for National Statistics. Cancer statistics: registrations series MB1. http://ons.gov.uk/ons/datasets-and-tables/search/index.html?newquery=mb1 (1 January 2010, date last accessed)
- 11. World Health Organization. International statistical classification of diseases and related health problems, 10th revision. http://apps.who.int/classifications/apps/icd/icd10online/ (12 June 2010, date last accessed)
- 12. Office for National Statistics. Cancer statistics: registrations series MB1 - No. 39, 2008. http://ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77-198611 (11 July 2011, date last accessed)
- 13.Mercer LK, Green AC, Galloway JB, et al. The influence of anti-TNF therapy upon incidence of keratinocyte skin cancer in patients with rheumatoid arthritis: longitudinal results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2012;71:869–74. doi: 10.1136/annrheumdis-2011-200622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abasolo L, Judez E, Descalzo MA, et al. Cancer in rheumatoid arthritis: occurrence, mortality, and associated factors in a South European population. Semin Arthritis Rheum. 2008;37:388–97. doi: 10.1016/j.semarthrit.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Chen YJ, Chang YT, Wang CB, et al. The risk of cancer in patients with rheumatoid arthritis: a nationwide cohort study in Taiwan. Arthritis Rheum. 2011;63:352–8. doi: 10.1002/art.30134. [DOI] [PubMed] [Google Scholar]
- 16.Askling J, Fored CM, Brandt L, et al. Risks of solid cancers in patients with rheumatoid arthritis and after treatment with tumour necrosis factor antagonists. Ann Rheum Dis. 2005;64:1421–6. doi: 10.1136/ard.2004.033993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen P, Hansen S, Moller B, et al. Are renal transplant recipients on CsA-based immunosuppressive regimens more likely to develop skin cancer than those on azathioprine and prednisolone? Transplant Proc. 1999;31:1120. doi: 10.1016/s0041-1345(98)01928-9. [DOI] [PubMed] [Google Scholar]
- 18.Glover MT, Deeks JJ, Raftery MJ, et al. Immunosuppression and risk of non-melanoma skin cancer in renal transplant recipients. Lancet. 1997;349:398. doi: 10.1016/S0140-6736(97)80015-3. [DOI] [PubMed] [Google Scholar]
- 19.Dantal J, Hourmant M, Cantarovich D, et al. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet. 1998;351:623–8. doi: 10.1016/S0140-6736(97)08496-1. [DOI] [PubMed] [Google Scholar]
- 20.Fairchild WV, Spence CR, Solomon HD, et al. The incidence of bladder cancer after cyclophosphamide therapy. J Urol. 1979;122:163–4. doi: 10.1016/s0022-5347(17)56306-5. [DOI] [PubMed] [Google Scholar]
- 21.Radis CD, Kahl LE, Baker GL, et al. Effects of cyclophosphamide on the development of malignancy and on long-term survival of patients with rheumatoid arthritis. A 20-year followup study. Arthritis Rheum. 1995;38:1120–7. doi: 10.1002/art.1780380815. [DOI] [PubMed] [Google Scholar]
- 22.Mattey DL, Thomson W, Ollier WE, et al. Association of DRB1 shared epitope genotypes with early mortality in rheumatoid arthritis: results of eighteen years of followup from the early rheumatoid arthritis study. Arthritis Rheum. 2007;56:1408–16. doi: 10.1002/art.22527. [DOI] [PubMed] [Google Scholar]
- 23.Parikh-Patel A, White RH. Risk of cancer among rheumatoid arthritis patients in California. Cancer Cause Control. 2009;20:1001–10. doi: 10.1007/s10552-009-9298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cibere J, Sibley J, Haga M. Rheumatoid arthritis and the risk of malignancy. Arthritis Rheum. 1997;40:1580–6. doi: 10.1002/art.1780400906. [DOI] [PubMed] [Google Scholar]
- 25.Gridley G, McLaughlin JK, Ekbom A, et al. Incidence of cancer among patients with rheumatoid arthritis. J Natl Cancer Inst. 1993;85:307–11. doi: 10.1093/jnci/85.4.307. [DOI] [PubMed] [Google Scholar]
- 26.Mellemkjaer L, Linet MS, Gridley G, et al. Rheumatoid arthritis and cancer risk. Eur J Cancer. 1996;32A: 1753–7. doi: 10.1016/0959-8049(96)00210-9. [DOI] [PubMed] [Google Scholar]
- 27.Habel LA, Zhao W, Stanford JL. Daily aspirin use and prostate cancer risk in a large, multiracial cohort in the US. Cancer Cause Control. 2002;13:427–34. doi: 10.1023/a:1015788502099. [DOI] [PubMed] [Google Scholar]
- 28.Salinas CA, Kwon EM, FitzGerald LM, et al. Use of aspirin and other nonsteroidal antiinflammatory medications in relation to prostate cancer risk. Am J Epidemiol. 2010;172:578–90. doi: 10.1093/aje/kwq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prizment AE, Folsom AR, Anderson KE. Nonsteroidal anti-inflammatory drugs and risk for ovarian and endometrial cancers in the Iowa Women's Health Study. Cancer Epidemiol Biomarkers Prev. 2010;19:435–42. doi: 10.1158/1055-9965.EPI-09-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murad AS, Down L, Davey SG, et al. Associations of aspirin, nonsteroidal anti-inflammatory drug and paracetamol use with PSA-detected prostate cancer: findings from a large, population-based, case-control study (the ProtecT study) Int J Cancer. 2011;128:1442–8. doi: 10.1002/ijc.25465. [DOI] [PubMed] [Google Scholar]
- 31.Brasky TM, Velicer CM, Kristal AR, et al. Nonsteroidal anti-inflammatory drugs and prostate cancer risk in the VITamins And Lifestyle (VITAL) cohort. Cancer Epidemiol Biomarkers Prev. 2010;19:3185–8. doi: 10.1158/1055-9965.EPI-10-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takkouche B, Regueira-Mendez C, Etminan M. Breast cancer and use of nonsteroidal anti-inflammatory drugs: a meta-analysis. J Natl Cancer Inst. 2008;100:1439–47. doi: 10.1093/jnci/djn324. [DOI] [PubMed] [Google Scholar]
- 33.Thomas E, Brewster DH, Black RJ, et al. Risk of malignancy among patients with rheumatic conditions. Int J Cancer. 2000;88:497–502. [PubMed] [Google Scholar]
- 34. Office for National Statistics. Summary quality report for cancer registration statistics. http://www.ons.gov.uk/ons/taxonomy/index.html?nscl=Cancer (1 March 2012, date last accessed)
- 35.Symmons D, Harrison B. Early inflammatory polyarthritis: results from the Norfolk Arthritis Register with a review of the literature. I. Risk factors for the development of inflammatory polyarthritis and rheumatoid arthritis. Rheumatology. 2000;39:835–43. doi: 10.1093/rheumatology/39.8.835. [DOI] [PubMed] [Google Scholar]
- 36. Office for National Statistics. Cancer statistics: registrations series MB1 - No. 39, 2008. http://ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77-198611 (11 July 2011, date last accessed)


