Abstract
The ameloblast cell layer of the enamel organ is in contact with the forming enamel as it develops into the hardest substance in the body. Ameloblasts move in groups that slide by one another as the enamel layer thickens. Each ameloblast is responsible for the formation of one enamel rod, and the rods are the mineralized trail that moving ameloblasts leave behind. Matrix metalloproteinases (MMPs) facilitate cell movement in various tissues during development, and in this review we suggest that the tooth-specific MMP, enamelysin (MMP20), facilitates ameloblast movements during enamel development. Mmp20 null mice have thin brittle enamel with disrupted rod patterns that easily abrades from the underlying dentin. Strikingly, the Mmp20 null mouse enamel organ morphology is noticeably dysplastic during late-stage development, when MMP20 is no longer expressed. We suggest that in addition to its role of cleaving enamel matrix proteins, MMP20 also cleaves junctional complexes present on ameloblasts to foster the cell movement necessary for formation of the decussating enamel rod pattern. Therefore, inactivation of MMP20 would result in tight ameloblast cell-cell attachments that may cause maturation-stage enamel organ dysplasia. The tight ameloblast attachments would also preclude the ameloblast movement necessary to form decussating enamel rod patterns.
Keywords: enamel, mineralized tissue/development, matrix metalloproteinases (MMPs), desomsomes, tight junctions, adherens junctions
Introduction
The enamel organ guides dental enamel development, and its innermost cell layer, composed of ameloblasts, is in direct contact with the forming enamel as it transforms into the hardest mineralized substance in the body. Each ameloblast is responsible for creating one enamel rod (prism), all of which collectively form a highly ordered structure. The enamel rod is the mineralized trail that the moving ameloblast leaves behind. Ameloblasts move in groups that slide by one another as the enamel layer thickens (secretory stage of development), and this movement culminates in the characteristic decussating enamel prism pattern observed in rodent incisors (Reith and Ross, 1973) or the entwined gnarled prism pattern seen in human molars (Boyde, 1989). However, little is known about how the ameloblasts move, detach and re-attach themselves, and communicate with one another.
Dental enamel malformation, termed Amelogenesis Imperfecta (AI), results from the non-syndromic inheritance of gene mutations that affect only the dental enamel. To date, 4 different mutations have been identified that cause AI, resulting from homozygous mutation of human matrix metalloproteinase-20 (Mmp20, Enamelysin) alleles (Kim et al., 2005; Ozdemir et al., 2005; Papagerakis et al., 2008; Lee et al., 2010). In addition (Fig. 1), Mmp20 null mice have thin brittle enamel with a dysplastic rod pattern (Caterina et al., 2002). MMPs facilitate cell movement in various tissues during development, and in this review we provide evidence to suggest that the tooth-specific MMP (MMP20) facilitates the movement of ameloblasts during dental enamel development.
Figure 1.
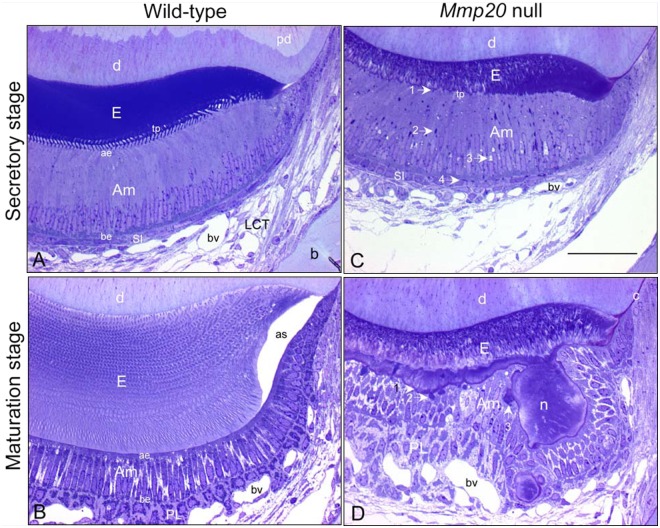
Semi-thin (0.5 µm) sections from glutaraldehyde-fixed, decalcified, and plastic-embedded mandibular incisors of wild-type (A,B) and Mmp20 null (C,D) mice stained with toluidine blue to illustrate the appearance of enamel organ cells at mid-secretory stage (A,C) and near-mid-maturation stage (B, D) of enamel development; magnification bar in C equals 50 µm and applies to all panels. The absence of MMP20 does not alter the basic configuration of the enamel organ in terms of size, number, types and arrangement of cell layers (compare C with A, D with B), the tall columnar appearance of ameloblasts as they form enamel (compare C with A, Am), and their shorter and wider appearance as they participate in enamel maturation (compare D with B, Am). However, there are several location-specific alterations in the enamel organ of Mmp20 null mice, including: (a) thinner, highly disorganized, and poorly formed inner and outer enamel layers (E) (compare C with A, D with B); (b) abnormalities in secretory-stage ameloblasts, such as disruption of row organization of Tomes’ processes (tp) (compare C with A), irregular and ragged apical surface applied to forming enamel (C, arrow 1), presence of large dense-staining bodies (C, arrow 2) and vacuoles (C, arrow 3) in supranuclear region of ameloblasts and dense-staining bodies at their bases near stratum intermedium cells (SI) (C, arrow 4); and (c) abnormalities in maturation-stage ameloblasts, including undulating and metachromatic (mauve)-staining at the enamel surface (D, arrow 1), presence of large dense-staining bodies in apical region of modulating ameloblasts (D, arrow 2), some appearing in continuity to the outer edges of matrix nodules (n) projecting from the enamel surface (D, arrow 3) that are covered by unevenly distributed and distorted ameloblasts (Am) and papillary layer cells (PL, D). Other abbreviations: pd, predentin; D, dentin; ae, apical end; be, basal end; bv, blood vessel; as, artifact space; b, bone; c, cementum.
Dental Enamel Development Overview
Dental enamel development progresses through defined stages. The enamel organ is composed of an outer enamel epithelium that, during the secretory stage of development, encompasses the perimeter of the maturing tooth crown. The cells in the center of the enamel organ secrete hydrophilic glycosaminoglycans into the extracellular compartment. This causes water to diffuse into the enamel organ, which, in turn, forces these cells apart. Since these cells are all interconnected by desmosomes, they are stretched into a star shape and are therefore termed the stellate reticulum (Nanci, 2003). The next layer of cells is the stratum intermedium, which forms a boundary between the stellate reticulum and the inner enamel epithelium. During development, the inner enamel epithelium becomes the ameloblast layer, which is attached at its basal (proximal) end to the stratum intermedium, while its apical (distal) end is in contact, initially, with dentin and, later, with the forming enamel (Fig. 1). The ameloblasts are responsible for secreting enamel matrix proteins and proteinases, inducing mineral ribbons to form, and organizing them into rod and interrod patterns typical for each vertebrate species.
When ameloblasts progress to the secretory stage, they elongate and eventually start secreting large amounts of enamel matrix proteins, including amelogenin, ameloblastin, enamelin, and MMP20, as they move away from the dentin surface. In association with newly secreted proteins, long, thin mineral ribbons form rapidly normal to the secretory surfaces of the ameloblasts. Within a short time, ameloblasts develop apical Tomes’ processes, thereby establishing a two-compartmental system where proteins destined to occupy spaces between rods (interrod) tend to exit from the “base” of the process, whereas those involved in rod formation tend to exit from the “tip” of the process. Mineral crystallites forming within the rod will grow progressively in c-axis length parallel to one another as ameloblasts move away from the dentin surface. Mineral crystallites developing between the rods (interrod) may have more limited lengths, but they are always positioned spatially to be at angles relative to rod crystallites (Nanci, 2003). In rodents, groups of ameloblasts move in different directions to form complex decussating enamel rod patterns (Reith and Ross, 1973). Just prior to when the enamel layer reaches its full thickness, the ameloblasts no longer move relative to each other. They retract their Tomes’ processes, smooth the enamel surface with a final coating of interrod material, and transition (transition stage) into shorter and fatter maturation-stage cells (Smith, 1998). It is during the maturation stage that ameloblasts actively secrete Kallikrein-4 (KLK4) to help remove the mass of previously secreted matrix proteins from the enamel layer so that the crystallites can expand in width and thickness. Therefore, ameloblasts progress through defined developmental stages that require contact, detachment, movement, re-attachment to each other, and intercommunication.
Epithelial Junctional Complexes Overview
Epithelial intercellular junctional complexes include tight junctions (TJs), adherens junctions (AJs), and desmosomes (Terry et al., 2010). TJs and AJs are linked to the actin filament system, while desmosomes are linked to intermediate filaments. Ameloblasts have TJs at both their proximal and distal ends (terminal webs), and they are also interconnected to one another by AJs located near the TJs. Desmosomes are transmembrane macromolecular complexes that provide strong cell-cell adhesion at focal locations. Desmosomes are present in a variety of epithelial tissues and are prominent in stratified squamous epithelial tissues such as the skin and oral mucosa. Desmogleins and desmocollins are transmembrane desmosomal cadherins that mediate extracellular cell-cell adhesion. The cytoplasmic domains of these proteins interact with desmosomal plaque proteins, such as plakoglobin and desmoplakin, which recruit intermediate filaments to the sites of cell-cell contact (Roberts et al., 2011). TJs function as dynamic barriers to selectively regulate diffusion of water, ions and other small molecules through the paracellular space between neighboring cells. They are therefore typically located at the apical ends of polarized epithelial cells, where they form an apical ring. In contrast, AJs are typically distributed along the basolateral membrane (Terry et al., 2010). AJs perform multiple functions, including cell-cell adhesion, regulation of the actin cytoskeleton, intracellular signaling, and transcription regulation. The AJ is composed of several classes of protein, including classic cadherins, catenins, and nectins. Cadherins are transmembrane proteins with extracellular domains that provide important calcium-dependent adhesive contacts between neighboring cells. The catenins link the intracellular cadherin domains to the actin cytoskeleton (Hartsock and Nelson, 2008). Nectins are a subfamily of calcium-independent immunoglobulin-like adhesion molecules that are also transmembrane proteins linked to the actin cytoskeleton through the adaptor protein afadin (Barron et al., 2008). In addition, pre-secretory and maturation-stage ameloblasts also have integrin-based hemi-desmosomal junctions present at their apical surfaces to provide strong contact/adhesion with the extracellular environment. Gap junction channels are often present between adjacent cells. However, gap junctions are not tethering junctional complexes and are therefore beyond the scope of this review.
Therefore, epithelial junctional complexes restrict the flow of ions and small molecules between cells (TJs), form tight bonds between cells undergoing physical stress (desmosomes), and are cell-signaling mediators via their extracellular attachments and simultaneous intracellular linkage to the actin cytoskeleton (AJs).
Matrix Metalloproteinases (MMPs) Overview
MMPs are a family of proteinases capable of cleaving virtually all extracellular matrix proteins. MMPs play critical roles in reproduction, development, morphogenesis, wound healing, tissue repair, regeneration, remodeling, and cell movement (reviewed in Roycik et al., 2009). MMP20 is required for healthy dental enamel development. People and mice with homozygous MMP20 mutations have soft discolored enamel that may be hypoplastic and easily abrades from the dentin surface (Caterina et al., 2002; Kim et al., 2005; Ozdemir et al., 2005; Papagerakis et al., 2008; Lee et al., 2010). Analyses of Mmp20 null mouse enamel revealed that the most abundant enamel matrix protein, amelogenin, is not processed properly, the enamel has altered or non-existent rod patterns, and the enamel organ has a deteriorating morphology as enamel development progresses (Caterina et al., 2002; Bartlett et al., 2011a). Strikingly, the Mmp20 null mouse enamel organ morphology becomes most noticeably dysplastic during the maturation stage of development, when MMP20 is no longer expressed (Fig. 1D). We suggest that, in addition to cleaving enamel matrix proteins, MMP20 may also cleave junctional complexes present on ameloblasts to foster the cell movement in 3D necessary to form spatially complex enamel rod patterns (decussating and gnarling). Therefore, in the absence of MMP20 activity, this inability to cleave secretory-stage ameloblast cadherins may result in tight ameloblast cell-cell attachments that may lead to maturation-stage enamel organ dysplasia.
The Role of Junctional Complexes in Enamel Formation
Junctional complexes are essential for enamel formation. Therefore, cell-cell adhesion, intercellular communication, and maintenance of epithelial barriers are required as the enamel develops from a soft cheese-like substance into a highly mineralized elastic substance with a hardness between that of iron and carbon steel (Cole and Eastoe, 1988).
Desmosomes and Enamel Development
The desmosomal proteins, desmoglein and desmoplakin (Fig. 2), are most prominent at the proximal ends of ameloblasts, where they attach to the stratum intermedium (Sasaki et al., 1984; Fausser et al., 1998; Jheon et al., 2011). But, desmosomes also locate to secretory ameloblasts near the apical and basal terminal webs (Nishikawa et al., 1988). Desmosomes are present predominantly in tissues that undergo strong physical stress. So, the attachment of ameloblasts to the stratum intermedium by desmosomes suggests that considerable physical stress occurs in this region during enamel development. Three reports demonstrated that desmosomes are essential for enamel development. The first showed that loss of the desmoplakin tail causes lethal acantholytic epidermolysis bullosa, and when one examines the displayed teeth closely, a severe loss of enamel is observed (Jonkman et al., 2005). The second showed that compound heterozygous desmoplakin mutations caused horizontal lines of enamel dysplasia in a female patient (Mahoney et al., 2010). This could possibly be due to secondary systemic disturbances. The third publication demonstrated that PERP, a tetraspan protein essential for stable desomosome assembly, was essential for proper enamel formation in mice (Jheon et al., 2011). In aggregate, desmosomes appear essential for proper enamel development.
Figure 2.
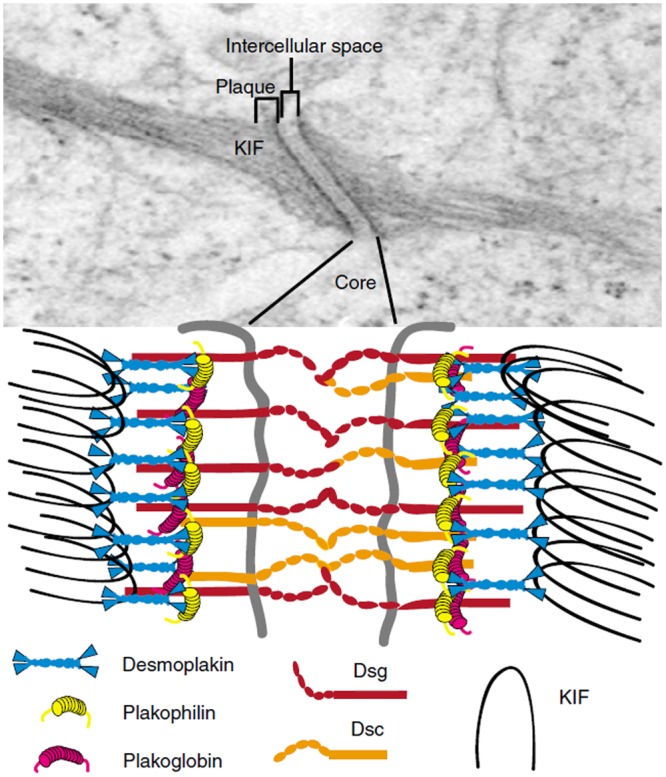
Electron micrograph and schematic representation of the desmosome. The desmosome is an electron-dense complex (upper panel) found in tissues subjected to mechanical stress, such as stratified squamous epithelia cells and the myocardium. This intercellular junction is composed of a core region, which mediates tight cell-cell adhesion, and a plaque region, which mediates attachment to the intermediate filament cytoskeleton. The core region contains the extracellular domains of the desmosomal cadherins, the desmocollins (Dsc) and desmogleins (Dsg). The cytoplasmic plaque region includes the C-terminal tails of the desmosomal cadherins, which associate directly and indirectly with various cytoplasmic proteins. The armadillo family proteins in the desmosome include plakoglobin and plakophilins. These proteins mediate interactions between the desmosomal cadherin tails and desmoplakin, a plakin family protein that binds directly to keratin intermediate filaments (KIF). These components of the desmosome allow tethering of the intermediate filaments to the plasma membrane, thereby acting as a scaffold to provide structural integrity to cells and tissues. KIF, keratin intermediate filaments. [Reproduced with permission from: Kottke MD, Delva E, Kowalczyk AP (2006). The desmosome: cell science lessons from human disease. J Cell Sci119:797-806. doi:10.1242/jcs.02888. http://jcs.biologists.org/content/119/5/797.long]
Nectins are a subfamily of 4 immunoglobulin-like transmembrane cell adhesion molecules that engage in homophilic and heterophilic adhesion at cell-cell junctions. Interestingly, 2 studies showed that nectins are required for normal ameloblast function, and that ablation of either nectin-1 or nectin-3 resulted in smaller and less numerous desmosomal contacts between ameloblasts and the stratum intermedium (Barron et al., 2008; Yoshida et al., 2010). This is interesting because nectins are usually associated with regulating the formation of AJs and TJs. Only one other study has implicated nectins in the regulation of desmosome formation (Inagaki et al., 2005). Even so, the nectin-1- or nectin-3-ablated enamel phenotype was mild, much like the Perp null phenotype. Perhaps nectins are master regulators of junctional complex formation. Conditional ablation of all 4 nectins may someday shed light on this area.
Tight Junctions and Enamel Development
TJs form an apical ring around the cell that binds adjacent cells such that the intercellular space is virtually eliminated (Fig. 3). They function as dynamic barriers to regulate ion diffusion and may act as signaling complexes (Terry et al., 2010). TJs are heteromeric protein complexes, and the first identified protein associated within TJs was zonula occludens-1 (ZO-1). ZO-1 lacks a transmembrane domain, but interacts with the tetraspanning membrane proteins occludin and the claudins to link them to the actin cytoskeleton. Occludin has adhesive properties. However, occludin-deficient cells still possess TJs. So, a search for additional TJ components led to the discovery of the claudins. Claudins are a family of at least 24 proteins that are also tetraspanning integral membrane proteins. This family regulates ion selectivity in TJs, and individual family members form cation- or anion-selective pores (reviewed in Shen et al., 2011). Several other proteins are present within the TJ, but the best characterized in the enamel organ are ZO proteins, occludin, and the claudins.
Figure 3.

Structure of tight junctions between 2 adjacent cells. (top) View of a tight junction, by transmission electron microscopy, of an ultrathin section of mouse epididymis reveals focal attachments of adjacent cells, known as ‘kissing points’ (arrows in the image on the right), where the intercellular space is obliterated. Scale bars, 200 nm. (bottom) Schematic representation of adjacent cells where integral membrane proteins of apposing cells obliterate the intercellular space at kissing points. Note that the TJ forms an apical ring around each joined cell. [Adapted with permission from: Mariano C, Sasaki H, Brites D, Brito DM (2011). A look at tricellulin and its role in tight junction formation and maintenance. Eur J Cell Biol 90:787-796. doi:10.1016/j.ejcb.2011.06.005. http://www.sciencedirect.com/science/article/pii/S0171933511001270].
ZO-1 and ZO-2, but not ZO-3, are expressed by ameloblasts. ZO-1 accumulates at the basal and apical ends of pre-ameloblasts and ameloblasts in late-bell-stage molars (Unda et al., 2003). However, in incisors, pre-ameloblasts expressed ZO-1 at their basal end and little to no ZO-1 at their apical end. Once the ameloblasts reached the secretory stage, strong ZO-1 expression was observed at both the apical and basal ends (Unda et al., 2003; Inai et al., 2008). ZO-2, in contrast, was observed along lateral surfaces of pre-ameloblasts and across ameloblast cell membranes. It was postulated that ZO-2 binds AJ proteins to promote ameloblast lateral cell-cell contacts (Unda et al., 2003). Thus, for ameloblasts, it appears that ZO-1 plays the major role in linking occludins and claudins to the actin cytoskeleton.
Although agreement exists that occludin is expressed by ameloblasts, no consensus exists on where it localizes in the ameloblast cell layer during enamel development. One group has reported that, in mouse incisors, occludin was detected at the apical ends of the ameloblasts (Unda et al., 2003). Another group showed that occludin was present at the apical and basal ends in rat molars during early development (pre-ameloblasts), but was present only at the basal end in secretory-stage ameloblasts (Joao and Arana-Chavez, 2004). Yet another group demonstrated that, in transition and maturation-stage rat incisor ameloblasts, occludin was present at both the apical and basal ends. However, during the maturation stage, staining for occludin was strongest at the apical ends of the ameloblasts. But, when these ameloblasts became smooth-ended, occludin was absent at the apical ends (Inai et al., 2008). The final group to assess occludin expression in ameloblasts did so in mouse incisors and found that maturation-stage ameloblasts expressed occludin at their basal and apical ends regardless of whether the ameloblasts were smooth- or ruffle-ended (Hata et al., 2010). So what do we make of all this? The results presented by Inai et al. (2008) logically make the most sense. Ameloblasts remove their apical TJs when they become smooth-ended (Nanci, 2003), presumably as part of an effort to neutralize the acidic extracellular pH caused by hydroxyapatite precipitation during the maturation stage. Thus, since the smooth-ended apical TJ is removed, it is reasonable to conclude that occludin is also removed from the cell surface, and confocal microscopy results presented in the Inai paper demonstrate this definitively.
The ameloblast claudin story is much the same as that of occludin. Agreement exists that ameloblasts express at least one claudin, but which ones and where they are located is an open question. Claudin-1 (Joao and Arana-Chavez, 2004; Bello et al., 2007; Inai et al., 2008; Hata et al., 2010; Nishikawa and Abe, 2010), claudin-2 (Ohazama and Sharpe, 2007), claudin-4 (Inai et al., 2008), claudin-7 (Bello et al., 2007), and claudins -6, -8, -9, and -10 (Hata et al., 2010) were all shown to be expressed in ameloblasts. The most convincing data were generated from rat incisors and demonstrated that both claudin-1 (Nishikawa and Abe, 2010) and claudins-1 and -4 (Inai et al., 2008) were expressed in maturation-stage ameloblasts. Both studies showed that apical claudin-1 was removed from smooth-ended ameloblasts and was returned when the ameloblasts once again became ruffle-ended. Intriguingly, the study by Nishikawa and Abe used an antibody confirmed for claudin-1 specificity to demonstrate that claudin-1 was not present at the ameloblast basal end, but instead was present in the ameloblast supranuclear region. Co-localization studies showed that claudin-1 was likely present in the Golgi apparatus. Therefore, since most of the occludin and claudin studies did not have the resolution or nucleus-specific staining procedures to identify the ameloblast nucleus, it is possible that, in certain cases, supranuclear protein staining was misinterpreted as protein present at the basal terminal web. Future studies will be necessary to clarify the disparate occludin and claudin expression pattern results in ameloblasts during enamel development.
Adherens Junctions and Enamel Development
AJs are composed of cadherins and several other protein components that function in calcium-dependent cell-cell attachment (Fig. 4). The cadherin extracellular domain connects through homotypic trans-pairing between cadherins on adjacent cells (reviewed in Hartsock and Nelson, 2008). p120-catenin binds to an intracellular cadherin domain near the cell membrane termed the “juxtamembrane domain” (JMD). Binding of p120 to the JMD prevents cadherins from becoming internalized and degraded (Davis et al., 2003; Xiao et al., 2003). α- and β-catenin are also part of the AJ. They link the actin cytoskeleton to cadherins. A major pathway for signal transduction by AJs involves regulation of β-catenin, which can act as either a structural protein at cell-cell junctions or a transcription factor in the cell nucleus. Disruption of AJs releases β-catenin for subsequent degradation or translocation to the nucleus (reviewed in Munshi and Stack, 2006).
Figure 4.
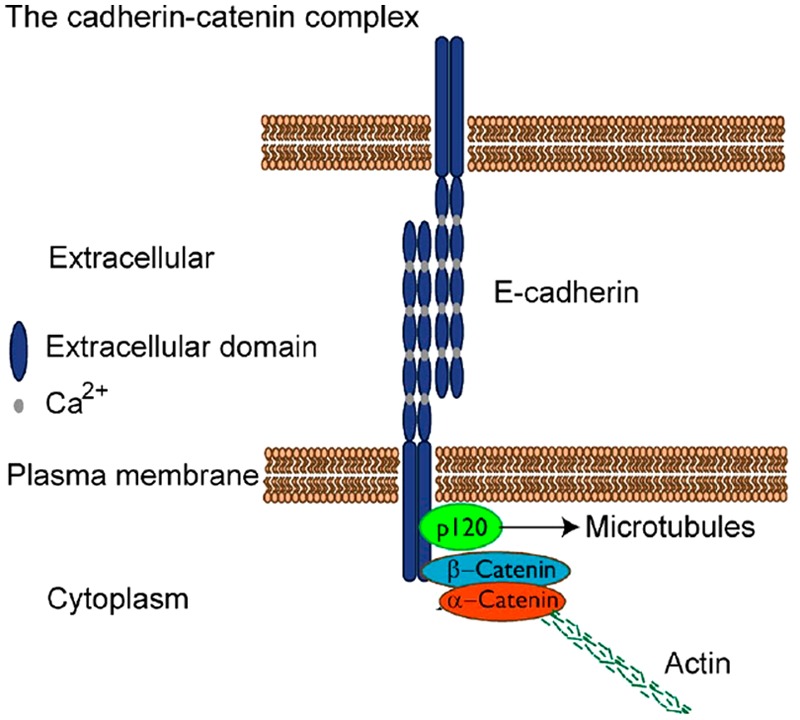
Selected adherens junction components. E-cadherin can dimerize to form trans-homophilic interactions that may result in cadherin clusters. Ca2+ ions are required to stiffen the extracellular domain and are essential to form homophilic interactions. The E-cadherin intracellular domain contains binding sites for the catenins p120 and β-catenin, thereby forming the cadherin-catenin complex. p120-catenin links cadherins to microtubules and is also important to prevent cadherin endocytosis and degradation. β-Catenin binds α-catenin, which in turn binds actin and several actin-associated proteins. The cadherin-catenin complex also binds many other proteins, including signaling proteins and cell-surface receptors, and forms a hub for protein-protein interactions. [Adapted with permission from Baum B and Georgiou M (2011). Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J Cell Biol 192(6):907–917. www.jcb.org/cgi/doi/10.1083/jcb.201009141. http://jcb.rupress.org/content/192/6/907.long].
Adherens junction proteins are present on ameloblasts. Ameloblasts have been shown to express E-cadherin (Palacios et al., 1995), N-cadherin (Heymann et al., 2002), P-cadherin (Palacios et al., 1995), cadherin-related neuronal receptors (Fukumoto et al., 2003), β-catenin and γ-catenin (Fausser et al., 1998), plus α-catenin and p120-catenin (Sorkin et al., 2000). Reports vary, but it appears that both E- and P-cadherin are expressed in pre-ameloblasts and that these cadherins are down-regulated and N-cadherin is predominantly expressed once the ameloblasts reach the secretory stage of enamel development. This is intriguing because although the mechanism remains unknown, expression of N-cadherin in epithelial cells is associated with cell motility (reviewed in Wheelock et al., 2008). Thus, N-cadherin is expressed by ameloblasts at the point when they initiate coordinated cell movement to form the characteristic decussating and gnarling enamel rod patterns.
Two studies have genetically manipulated AJ protein expression to determine its effect on tooth development. The first conditionally ablated p120-catenin from epithelial tissues, which includes the enamel organ. Tooth development in these mice proceeded somewhat normally (the molars were slightly misshapen) until the secretory stage of enamel development, when the ameloblasts lost polarity, detached from each other and surrounding tissues (Fig. 5), and flattened, and cadherin expression became undetectable by immunostaining (Bartlett et al., 2010). These results are consistent, but not exclusive, with p120-catenin playing a key role in ameloblast attachment and detachment during the secretory stage.
Figure 5.
The p120-catenin null ameloblasts detach from the incisor dentin surface, the stratum intermedium, and from each other. Secretory ameloblasts (Am) are identified by positive immunostaining for amelogenin. Panels A-D represent sequential sections of an incisor from a p120-catenin epithelial tissue conditional knock-out mouse. Pre-secretory ameloblasts do not express amelogenin (A), whereas secretory-stage ameloblasts do express amelogenin (B-D). Note the vacuoles above the ameloblasts in the stratum intermedium (SI) that grow progressively larger as development progresses. The right side of panel D shows ameloblasts that have completely lost contact with the stratum intermedium. However, the ameloblasts also lost contact with the dentin surface and with each other (E, F). The ameloblasts in panels E and F are disorganized and appear to have lost most but not all cell adhesion properties, so they appear stretched between the stratum intermedium and dentin. Od, odontoblast; SR, stellate reticulum. (Reproduced with permission from: PLoS One 5(9)e12703, 2010. doi:10.1371/journal.pone.0012703.g006; http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0012703)
The second study conditionally inactivated E-cadherin, where the stem cells locate in the cervical loop region of the continuously erupting mouse incisor. This caused an abnormal morphology of the labial cervical loop, a decrease in label-retaining stem cells, increased cell proliferation, and decreased cell migration of the stem cell progeny along the proximal-distal axis of the incisor. The study concluded that E-cadherin is an important regulator of stem cells and their progeny during mouse incisor growth (Li et al., 2012).
Interestingly, both of these studies demonstrate the essential role of cadherins in tooth development. During the secretory stage, the ameloblasts have TJs at their apical and basal ends and are connected to each other and the stratum intermedium by desmosomes. Yet when the cadherin-stabilizing protein p120-catenin is ablated, the secretory-stage ameloblasts fall apart (Fig. 5). Perhaps the extensive remodeling necessary for ameloblast cell movement requires all these various junctional complexes to be functional so that the ameloblasts can maintain proper grouping for coordinated cell movement.
Matrix Metalloproteinases and Cell Movement
It has been demonstrated previously that when matrix metalloproteinases (MMPs) cleave an extracellular classic cadherin domain, β-catenin is removed from its position near the cell membrane and in many cases will translocate to the cell nucleus (Lochter et al., 1997; Sanceau et al., 2003; Ichikawa et al., 2006; Cowden Dahl et al., 2008; Dwivedi et al., 2009; Zheng et al., 2009; Lynch et al., 2010). This translocation is associated with cell movement (reviewed in Tanaka et al., 2011).
MMP20 and Cell Movement during Enamel Development
MMPs can initiate cell movement through cadherin hydrolysis. MMPs-3, -7, and -9 cleave the extracellular domain of E-cadherin, thereby promoting cell movement, cell invasion, and/or cell proliferation (Lochter et al., 1997; McGuire et al., 2003; Sanceau et al., 2003; Cowden Dahl et al., 2008; Lynch et al., 2010). Additionally, MMPs-2, -9, -12, and -28 also cleave the extracellular domains of various classic cadherins (Illman et al., 2006; Dwivedi et al., 2009; Hartland et al., 2009). Significantly, adherens junctions were identified along the possible sliding interface of adjacent secretory-stage ameloblast migrating rows (Nishikawa et al., 1990). Therefore, MMPs cleave cadherins to promote cell movement, and we previously demonstrated in vitro that MMP20 cleaves the extracellular domain of E-cadherin (Bartlett et al., 2011b) and N-cadherin (unpublished observations). Since the enamel rod is the mineralized trail of the migrating ameloblast that formed it, the possibility exists that the malformed rod pattern in Mmp20 null mice results from the ameloblasts that do not migrate properly. Also, the ameloblast dysplasia observed in the Mmp20 null mouse maturation-stage enamel organ is consistent with a failure of ameloblasts to detach from one another, as might normally occur if MMP20 had cleaved cadherins during the secretory stage. Alternative possibilities would include disturbances in matrix-cell interactions that are necessary to form a proper Tomes’ process, or problems forming or sustaining the mineralization front. Therefore, it is possible, among several possibilities, that the Mmp20 null mouse phenotype is caused by an inability to cleave the extracellular junctional domains that may be required for ameloblasts to move in rows to form decussating enamel rod patterns.
However, if MMP20 does cleave cadherin extracellular domains along the lateral surface of the ameloblast, MMP20 must either be secreted from the lateral membrane or it must degrade the apical tight junction proteins. Both of these alternatives are possibilities. Many polarized epithelial cells secrete proteins from their basal and lateral surfaces, in addition to predominant secretion from their apical surfaces. Ameloblasts are among these cells. Ameloblasts may secrete as much as 10% of their exported proteins to their lateral and basal surfaces (reviewed in Nanci and Smith, 1992). Alternatively, it has been demonstrated that MMP2, MMP3, and MMP9 (reviewed in Rosenberg and Yang, 2007) and MMP8 (Schubert-Unkmeir et al., 2010) can cleave various components of the TJ complex. Additionally, general MMP inhibition blocked the shedding of the desmosomal cadherin desmoglein-2 (Klessner et al., 2009), and shedding of desmoglein-3 was abolished when both MMP2 and MMP9 were inhibited (Cirillo et al., 2007). So, the possibility exists that MMP20 can make its way to the ameloblast lateral membrane by boring through the apical TJ. Perhaps both routes to the lateral membrane are necessary, since AJs, TJs, and desmosomes would require remodeling to allow ameloblasts to slide by one another in rows. The mechanism that decides which groups of ameloblasts will remain attached to one another during group cell movement remains a mystery.
Concluding Remarks
Evidence is accumulating demonstrating that extensive remodeling occurs among junctional complexes (Shen et al., 2011), and this remodeling would be enhanced in a dynamic environment where cohorts of cells are moving in different directions relative to one another. Logically, this remodeling would require proteolytic activity. Although the role of proteinases in degrading junctional complexes has been extensively studied in the pathogenesis of tumor progression and metastasis (Munshi and Stack, 2006), relatively little research has examined how junctional complexes are remodeled during normal developmental processes. We believe this is a fertile area for future research studies, and we are excited about defining the role of MMP20 and ameloblast cell-cell contacts during dental enamel development.
Acknowledgments
We thank James P. Simmer and the University of Michigan Dental Research Laboratory for the histological material used to make Fig. 1.
Footnotes
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Portions of the research reported here were supported by NIDCR research grant DE016276 (JDB).
References
- Barron MJ, Brookes SJ, Draper CE, Garrod D, Kirkham J, Shore RC, et al. (2008). The cell adhesion molecule nectin-1 is critical for normal enamel formation in mice. Hum Mol Genet 17:3509-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JD, Dobeck JM, Tye CE, Perez-Moreno M, Stokes N, Reynolds AB, et al. (2010). Targeted p120-catenin ablation disrupts dental enamel development. PLoS One 5:e12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JD, Skobe Z, Nanci A, Smith CE. (2011a). Matrix metalloproteinase 20 promotes a smooth enamel surface, a strong dentino-enamel junction, and a decussating enamel rod pattern. Eur J Oral Sci 119(Suppl 1):199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JD, Yamakoshi Y, Simmer JP, Nanci A, Smith CE. (2011b). MMP20 cleaves E-cadherin and influences ameloblast development. Cells Tissues Organs 194:222-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello IO, Soini Y, Slootweg PJ, Salo T. (2007). Claudins 1, 4, 5, 7 and occludin in ameloblastomas and developing human teeth. J Oral Pathol Med 36:48-54. [DOI] [PubMed] [Google Scholar]
- Boyde A. (1989). Enamel. In: Handbook of microscopic anatomy. Oksche A, Vollrath L, editors. Berlin: Springer-Verlag, pp. 309-473. [Google Scholar]
- Caterina JJ, Skobe Z, Shi J, Ding Y, Simmer JP, Birkedal-Hansen H, et al. (2002). Enamelysin (matrix metalloproteinase 20)-deficient mice display an amelogenesis imperfecta phenotype. J Biol Chem 277:49598-49604. [DOI] [PubMed] [Google Scholar]
- Cirillo N, Femiano F, Gombos F, Lanza A. (2007). Metalloproteinase 9 is the outer executioner of desmoglein 3 in apoptotic keratinocytes. Oral Dis 13:341-345. [DOI] [PubMed] [Google Scholar]
- Cole AS, Eastoe JE, editors (1988). Biochemistry and oral biology. London: Butterworth & Co Ltd. [Google Scholar]
- Cowden Dahl KD, Symowicz J, Ning Y, Gutierrez E, Fishman DA, Adley BP, et al. (2008). Matrix metalloproteinase 9 is a mediator of epidermal growth factor-dependent e-cadherin loss in ovarian carcinoma cells. Cancer Res 68:4606-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Ireton RC, Reynolds AB. (2003). A core function for p120-catenin in cadherin turnover. J Cell Biol 163:525-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi A, Slater SC, George SJ. (2009). MMP-9 and -12 cause N-cadherin shedding and thereby beta-catenin signalling and vascular smooth muscle cell proliferation. Cardiovasc Res 81:178-186. [DOI] [PubMed] [Google Scholar]
- Fausser JL, Schlepp O, Aberdam D, Meneguzzi G, Ruch JV, Lesot H. (1998). Localization of antigens associated with adherens junctions, desmosomes, and hemidesmosomes during murine molar morphogenesis. Differentiation 63:1-11. [DOI] [PubMed] [Google Scholar]
- Fukumoto E, Sakai H, Fukumoto S, Yagi T, Takagi O, Kato Y. (2003). Cadherin-related neuronal receptors in incisor development. J Dent Res 82:17-22. [DOI] [PubMed] [Google Scholar]
- Hartland SN, Murphy F, Aucott RL, Abergel A, Zhou X, Waung J, et al. (2009). Active matrix metalloproteinase-2 promotes apoptosis of hepatic stellate cells via the cleavage of cellular N-cadherin. Liver Int 29:966-978. [DOI] [PubMed] [Google Scholar]
- Hartsock A, Nelson WJ. (2008). Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta 1778:660-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata M, Kawamoto T, Kawai M, Yamamoto T. (2010). Differential expression patterns of the tight junction-associated proteins occludin and claudins in secretory and mature ameloblasts in mouse incisor. Med Mol Morphol 43:102-106. [DOI] [PubMed] [Google Scholar]
- Heymann R, About I, Lendahl U, Franquin JC, Obrink B, Mitsiadis TA. (2002). E- and N-cadherin distribution in developing and functional human teeth under normal and pathological conditions. Am J Pathol 160:2123-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa Y, Ishikawa T, Momiyama N, Kamiyama M, Sakurada H, Matsuyama R, et al. (2006). Matrilysin (MMP-7) degrades VE-cadherin and accelerates accumulation of beta-catenin in the nucleus of human umbilical vein endothelial cells. Oncol Rep 15:311-315. [PubMed] [Google Scholar]
- Illman SA, Lehti K, Keski-Oja J, Lohi J. (2006). Epilysin (MMP-28) induces TGF-beta mediated epithelial to mesenchymal transition in lung carcinoma cells. J Cell Sci 119(Pt 18):3856-3865. [DOI] [PubMed] [Google Scholar]
- Inagaki M, Irie K, Ishizaki H, Tanaka-Okamoto M, Morimoto K, Inoue E, et al. (2005). Roles of cell-adhesion molecules nectin 1 and nectin 3 in ciliary body development. Development 132:1525-1537. [DOI] [PubMed] [Google Scholar]
- Inai T, Sengoku A, Hirose E, Iida H, Shibata Y. (2008). Differential expression of the tight junction proteins, claudin-1, claudin-4, occludin, ZO-1, and PAR3, in the ameloblasts of rat upper incisors. Anat Rec (Hoboken) 291:577-585. [DOI] [PubMed] [Google Scholar]
- Jheon AH, Mostowfi P, Snead ML, Ihrie RA, Sone E, Pramparo T, et al. (2011). PERP regulates enamel formation via effects on cell-cell adhesion and gene expression. J Cell Sci 124(Pt 5):745-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joao SM, Arana-Chavez VE. (2004). Tight junctions in differentiating ameloblasts and odontoblasts differentially express ZO-1, occludin, and claudin-1 in early odontogenesis of rat molars. Anat Rec A Discov Mol Cell Evol Biol 277:338-343. [DOI] [PubMed] [Google Scholar]
- Jonkman MF, Pasmooij AM, Pasmans SG, van den Berg MP, Ter Horst HJ, Timmer A, et al. (2005). Loss of desmoplakin tail causes lethal acantholytic epidermolysis bullosa. Am J Hum Genet 77:653-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Simmer JP, Hart TC, Hart PS, Ramaswami MD, Bartlett JD, et al. (2005). MMP-20 mutation in autosomal recessive pigmented hypomaturation amelogenesis imperfecta. J Med Genet 42:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessner JL, Desai BV, Amargo EV, Getsios S, Green KJ. (2009). EGFR and ADAMs cooperate to regulate shedding and endocytic trafficking of the desmosomal cadherin desmoglein 2. Mol Biol Cell 20:328-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Seymen F, Kang HY, Lee KE, Gencay K, Tuna B, et al. (2010). MMP20 hemopexin domain mutation in amelogenesis imperfecta. J Dent Res 89:46-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Cha W, Luder HU, Charles RP, McMahon M, Mitsiadis TA, et al. (2012). E-cadherin regulates the behavior and fate of epithelial stem cells and their progeny in the mouse incisor. Dev Biol 366:357-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. (1997). Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol 139:1861-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CC, Vargo-Gogola T, Matrisian LM, Fingleton B. (2010). Cleavage of E-cadherin by matrix metalloproteinase-7 promotes cellular proliferation in nontransformed cell lines via activation of RhoA. J Oncol 2010:530745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney MG, Sadowski S, Brennan D, Pikander P, Saukko P, Wahl J, et al. (2010). Compound heterozygous desmoplakin mutations result in a phenotype with a combination of myocardial, skin, hair, and enamel abnormalities. J Invest Dermatol 130:968-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JK, Li Q, Parks WC. (2003). Matrilysin (matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. Am J Pathol 162:1831-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi HG, Stack MS. (2006). Reciprocal interactions between adhesion receptor signaling and MMP regulation. Cancer Metastasis Rev 25:45-56. [DOI] [PubMed] [Google Scholar]
- Nanci A. (2003). Ten Cate's oral histology, development, structure, and function. St Louis, MO: Mosby. [Google Scholar]
- Nanci A, Smith CE. (1992). Development and calcification of enamel. In: Calcification in biological systems. Boca Raton, FL: CRC Press, pp. 313-343. [Google Scholar]
- Nishikawa S, Abe M. (2010). Immunocytochemical localization of claudin-1 in the maturation ameloblasts of rat incisors. Front Physiol 1:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa S, Fujiwara K, Kitamura H. (1988). Formation of the tooth enamel rod pattern and the cytoskeletal organization in secretory ameloblasts of the rat incisor. Eur J Cell Biol 47:222-232. [PubMed] [Google Scholar]
- Nishikawa S, Tsukita S, Tsukita S, Sasa S. (1990). Localization of adherens junction proteins along the possible sliding interface between secretory ameloblasts of the rat incisor. Cell Struct Funct 15:245-249. [DOI] [PubMed] [Google Scholar]
- Ohazama A, Sharpe PT. (2007). Expression of claudins in murine tooth development. Dev Dyn 236:290-294. [DOI] [PubMed] [Google Scholar]
- Ozdemir D, Hart PS, Ryu OH, Choi SJ, Ozdemir-Karatas M, Firatli E, et al. (2005). MMP20 active-site mutation in hypomaturation amelogenesis imperfecta. J Dent Res 84:1031-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios J, Benito N, Berraquero R, Pizarro A, Cano A, Gamallo C. (1995). Differential spatiotemporal expression of E- and P-cadherin during mouse tooth development. Int J Dev Biol 39:663-666. [PubMed] [Google Scholar]
- Papagerakis P, Lin HK, Lee KY, Hu Y, Simmer JP, Bartlett JD, et al. (2008). Premature stop codon in MMP20 causing amelogenesis imperfecta. J Dent Res 87:56-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith EJ, Ross MH. (1973). Morphological evidence for the presence of contractile elements in secretory ameloblasts of the rat. Arch Oral Biol 18:445-448. [DOI] [PubMed] [Google Scholar]
- Roberts BJ, Pashaj A, Johnson KR, Wahl JK. (2011). Desmosome dynamics in migrating epithelial cells requires the actin cytoskeleton. Exp Cell Res 317:2814-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg GA, Yang Y. (2007). Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg Focus 22:E4. [DOI] [PubMed] [Google Scholar]
- Roycik MD, Fang X, Sang QX. (2009). A fresh prospect of extracellular matrix hydrolytic enzymes and their substrates. Curr Pharm Des 15:1295-1308. [DOI] [PubMed] [Google Scholar]
- Sanceau J, Truchet S, Bauvois B. (2003). Matrix metalloproteinase-9 silencing by RNA interference triggers the migratory-adhesive switch in Ewing's sarcoma cells. J Biol Chem 278:36537-36546. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Segawa K, Takiguchi R, Higashi S. (1984). Intercellular junctions in the cells of the human enamel organ as revealed by freeze-fracture. Arch Oral Biol 29:275-286. [DOI] [PubMed] [Google Scholar]
- Schubert-Unkmeir A, Konrad C, Slanina H, Czapek F, Hebling S, Frosch M. (2010). Neisseria meningitidis induces brain microvascular endothelial cell detachment from the matrix and cleavage of occludin: a role for MMP-8. PLoS Pathog 6:e1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. (2011). Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol 73:283-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CE. (1998). Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med 9:128-161. [DOI] [PubMed] [Google Scholar]
- Sorkin BC, Wang MY, Dobeck JM, Albergo KL, Skobe Z. (2000). The cadherin-catenin complex is expressed alternately with the adenomatous polyposis coli protein during rat incisor amelogenesis. J Histochem Cytochem 48:397-406. [DOI] [PubMed] [Google Scholar]
- Tanaka SS, Kojima Y, Yamaguchi YL, Nishinakamura R, Tam PP. (2011). Impact of WNT signaling on tissue lineage differentiation in the early mouse embryo. Dev Growth Differ 53:843-856. [DOI] [PubMed] [Google Scholar]
- Terry S, Nie M, Matter K, Balda MS. (2010). Rho signaling and tight junction functions. Physiology (Bethesda) 25:16-26. [DOI] [PubMed] [Google Scholar]
- Unda FJ, Perez-Nanclares G, Le Morvan V, Hernandez C, Vilaxa A, De-la-Fuente M, et al. (2003). Dynamic assembly of tight junction-associated proteins ZO-1, ZO-2, ZO-3 and occludin during mouse tooth development. Histol Histopathol 18:27-38. [DOI] [PubMed] [Google Scholar]
- Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. (2008). Cadherin switching. J Cell Sci 121(Pt 6):727-735. [DOI] [PubMed] [Google Scholar]
- Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, et al. (2003). Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol 163:535-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Miyoshi J, Takai Y, Thesleff I. (2010). Cooperation of nectin-1 and nectin-3 is required for normal ameloblast function and crown shape development in mouse teeth. Dev Dyn 239:2558-2569. [DOI] [PubMed] [Google Scholar]
- Zheng G, Lyons JG, Tan TK, Wang Y, Hsu TT, Min D, et al. (2009). Disruption of E-cadherin by matrix metalloproteinase directly mediates epithelial-mesenchymal transition downstream of transforming growth factor-beta1 in renal tubular epithelial cells. Am J Pathol 175:580-591. [DOI] [PMC free article] [PubMed] [Google Scholar]