Abstract
The importance of susceptibility genes in the risk for dental caries has been clearly established. While many candidate caries genes have been proposed, to date, few of them have been rigorously validated through observational and experimental studies. Moreover, most genetic epidemiological studies have analyzed global caries phenotypes that ignore the possibility that genes may exert differential effects across tooth surfaces of the dentition. Therefore, we performed genome-wide association studies (GWAS) of 5 novel dental caries phenotypes (developed by clustering the permanent dentition into categories of tooth surfaces based on co-occurrence of caries) to nominate new candidate caries genes. GWAS was performed in 920 self-reported white participants, aged 18 to 75 years, with genotype data on 518,997 genetic variants. We identified a significant genetic association between dental caries of the anterior mandibular teeth and LYZL2 (p value = 9e-9), which codes a bacteriolytic agent thought to be involved in host defense. We also identified a significant genetic association between caries of the mid- dentition tooth surfaces and AJAP1 (p value = 2e-8), a gene possibly involved in tooth development. Suggestive genetic associations were also observed for ABCG2, PKD2, the dentin/bone SCPP sub-family, EDNRA, TJFBR1, NKX2-3, IFT88, TWSG1, IL17D, and SMAD7 (p values < 7e-6). We nominate these novel genes for future study.
Keywords: genome-wide association study, genetic association, genomics, hierarchical clustering, tooth decay, cluster analysis
Introduction
Dental caries is the most common chronic disease worldwide and affects approximately 90% of adults in the US (Beltran-Aguilar et al., 2005). Many factors contribute to disease, including environmental, endogenous, and behavioral factors such as diet, bacterial flora, fluoride exposures, oral hygiene, salivary flow and composition, morphological and positional characteristics of the dentition, host immune response, and preventive interventions. Genes and gene-by-environmental interactions are widely acknowledged to contribute to caries susceptibility through mechanisms affecting these and other unknown risk factors. The heritability of dental caries is high (30-55%) (Boraas et al., 1988; Shaffer et al., 2012a), and previous studies, including a genome-wide association study (GWAS) (Shaffer et al., 2011) have sought to identify the specific genetic factors involved. However, to date, the causal roles of caries genes have not been rigorously established. The vast majority of genetic variants affecting disease remain unknown.
To date, a potential limitation of most genetic epidemiological studies of dental caries is the global phenotype definition. Most genetics studies have used a single measure of decay, such as a binary (yes/no) affection status or the DMFT/S index (calculated as the sum of decayed, missing due to decay, or filled/restored teeth/surfaces). As global measures of decay, phenotypes such as these ignore the fact that tooth surfaces across the dentition exhibit differences in susceptibility to dental caries and are differentially affected by risk factors. By ignoring the patterns of decay, global caries measures may be poor phenotypes for identifying genetic variants affecting dental caries, which are likely to exert individually weak and differential effects across the various surfaces of the permanent dentition. In a related study presented in this issue of the Journal, we describe the use of hierarchical clustering to generate novel caries phenotypes that capture the non-uniform risk of caries across tooth surfaces of the dentition (Shaffer et al., 2013). In this study, we performed GWAS for these novel dental caries phenotypes to nominate candidate caries genes for future observational and experimental studies.
Methods
Sample Recruitment
The sample was recruited as part of an initiative by the Center for Oral Health Research in Appalachia (COHRA) to study the factors affecting oral health in a rural, underserved population. Details regarding recruitment and data collection for this sample have been described previously (Polk et al., 2008) and are summarized in a separate article (Shaffer et al., 2013). Nine hundred twenty self-reported whites, aged 18 to 75 yrs, with dental caries assessments and genome-wide genotype data were included in this study.
Novel Caries Phenotypes
To study the genetic factors influencing dental caries, which we hypothesized may exert differential effects across tooth surfaces of the permanent dentition, we first developed novel caries phenotypes that capture the patterns of decay. Development of these novel phenotypes is fully described in a separate article (Shaffer et al., 2013). In summary, we used hierarchical clustering to group tooth surfaces based on co-occurrence of dental caries. We hypothesized that tooth surfaces that co-vary with respect to dental caries may be similarly influenced by genetic risk factors. Cluster analysis yielded 5 stable clusters (Table 1), one of which, the mid-dentition surfaces, could be further partitioned into sub-clusters comprised of maxillary and mandibular tooth surfaces. For each cluster (or sub-cluster), we generated the “partial DMFS index”, which is the count of carious surfaces (i.e., non-cavitated or cavitated decay, missing due to decay, or restored) for a given cluster. We denote these novel phenotypes as DMFS1, DMFS2, DMFS3, DMFS4, and DMFS5 (which is sub-divided into DMFS5max and DMFS5mand).
Table 1.
Novel Dental Caries Phenotypes Generated via Cluster Analysis on Tooth-surface-level Caries Data
| Partial DMFS |
Heritability |
GWAS Results (# loci) |
||||||
|---|---|---|---|---|---|---|---|---|
| Phenotype | Tooth Surfaces Included | Mean | SD | h2 | p value | λ | Significanta | Suggestiveb |
| DMFS1 | pit and fissure molar | 6.37 | 3.75 | 0.27 | 0.057 | 1.022 | - | - |
| DMFS2 | mandibular anterior (incisors, canines, first pre-molar) | 0.66 | 2.07 | 0.54 | 0.003 | 1.030 | 1 | 8 |
| DMFS3 | posterior (molars and maxillary pre-molars excluding molar pit and fissure) | 7.78 | 8.84 | 0.43 | 0.004 | 1.053 | 0 | 5 |
| DMFS4 | maxillary anterior (incisors) | 2.42 | 3.98 | 0.00 | 0.500 | 0.997 | - | - |
| DMFS5 | mid-dentition (pre-molars and canines) | 3.70 | 6.01 | 0.40 | 0.008 | 1.047 | 0 | 10 |
| DMFS5max | maxillary mid-dentition | 1.86 | 3.73 | 0.34 | 0.041 | 1.041 | 1 | 6 |
| DMFS5mand | mandibular mid-dentition | 1.84 | 2.97 | 0.31 | 0.016 | 1.038 | 0 | 10 |
p value threshold for genome-wide significant associations = 10−7.3λ.
p value threshold for suggestive associations = 10−5λ.
Mean = mean number of carious surfaces within a cluster.
SD = standard deviation.
h2 = heritability estimate.
p value = significance of test that heritability estimate differs from zero; bold indicates p value < 0.05.
λ = genomic inflation factor.
Genotyping
Genotyping was performed at the Center of Inherited Disease Research (CIDR) of Johns Hopkins University. Data cleaning and quality assurance procedures were conducted in conjunction with the CIDR data cleaning center at the University of Washington. The Illumina Human610-Quadv1_B BeadChip (Illumina, Inc., San Diego, CA, USA) and Illumina Infinium II assay protocol were used. In general, genotyping quality was excellent; 518,997 genetic markers (single-nucleotide polymorphisms; SNPs) passing quality control and analysis filters (participant call rates > 90%; SNP call rates > 99%; Hardy-Weinberg p values > 0.0001; minor allele frequency > 0.02) were analyzed in this study. Details regarding genotyping and data cleaning are publicly available (www.genevastudy.org).
Statistical Analysis
GWAS were performed for each heritable dental caries phenotype (i.e., DMFS2, DMFS3, DMFS5, DMFS5max, DMFS5mand). Genetic association for each of the 518,997 SNPs was tested by linear regression in PLINK (Purcell et al., 2007), adjusted for the effects of sex, age, and age2. The genomic inflation factor, λ, and Manhattan plots were generated in R (R Foundation for Statistical Computing, Vienna, AU). Given the issue of multiple comparisons, conservative p value thresholds for statistical significance were set equal to 5e-8 after adjustment for genomic inflation (i.e., p ≤ 10−7.3λ). The p value thresholds for suggestive significance were set equal to 1e-5 after adjustment for genomic inflation (i.e., p ≤ 10−5λ). Under the GWAS approach, associated markers are not expected to be causal, but are assumed to be physically proximal and in linkage disequilibrium (i.e., correlated due to ancestry) with unobserved causal variants. Moreover, where a causal variant may be situated with respect to the gene it influences is currently unknown, but, typically, is thought to be also physically proximal. Therefore, we explored and report the known biological functions of genes near our significant and suggestive hits for plausible roles in dental caries.
Results
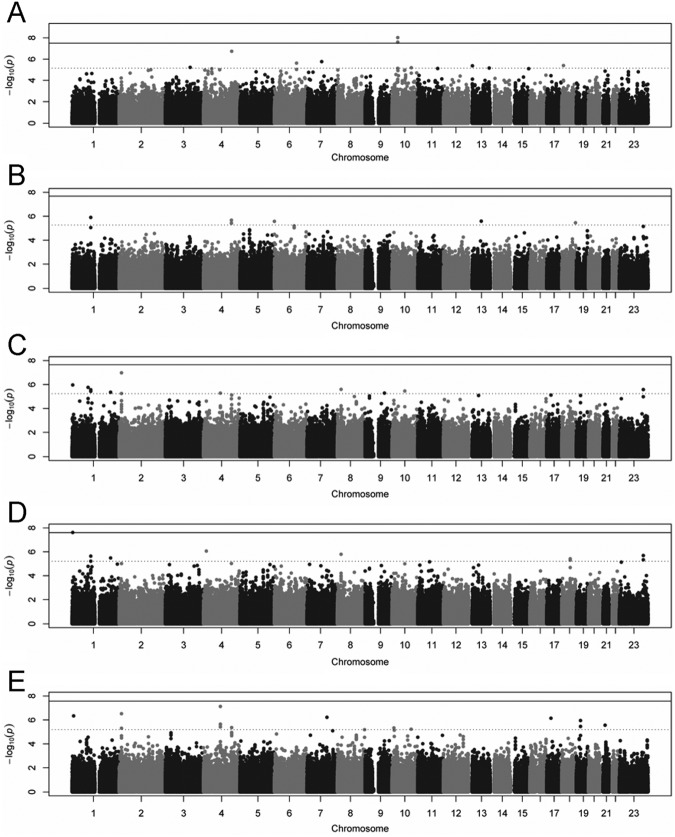
The study sample included 920 self-reported whites, aged 18 to 75 yrs, with available data for caries phenotypes, covariates (i.e., age, age2, and sex), and genetic markers. Novel dental caries phenotypes reflecting decay in different categories of tooth surfaces were generated by cluster analysis. A separate article describes the development and heritability estimation of the phenotypes used herein (Shaffer et al., 2013). Descriptive statistics for novel caries phenotypes are shown in Table 1. The genomic inflation factor (λ) ranged from 1.00 to 1.05, indicating minimal to minor inflation of p values compared with that expected by chance alone. Thresholds for genome-wide and suggestive statistical significance were adjusted for the genomic inflation factor observed for each GWAS. Manhattan plots illustrating the GWAS results for the 5 heritable dental caries phenotypes (i.e., DMFS2, DMFS3, DMFS5, DMFS5max, and DMFS5mand) are shown in the Fig.
Figure.
Manhattan plots showing GWAS results for (A) DMFS2, (B) DMFS3, (C) DMFS5, (D) DMFS5max, and (E) DMFS5mand. Solid lines represent thresholds for genome-wide significance (p value < 10−7.3λ). Dotted lines represent thresholds for suggestive significance (p value < 10−5λ).
Two loci exceeded the threshold for genome-wide significance (Table 2). The most significant association that we observed was for DMFS2 (mandibular anterior tooth surfaces) and LYZL2 on chromosome 10p11.23 (rs399593; p value = 9.4e-9). Though very little is known about LYZL2 specifically, it belongs to the family of c-type lysosomes, which are well-recognized bacteriolytic factors of host defense (Zhang et al., 2005). It is currently unknown whether LYZL2 affects dental caries, although its putative antibacterial function suggests a biologically plausible mechanism through which LYZL2 may influence cariogenesis. The second most significant association that we observed was for DMFS5max and the upstream un-translated region of AJAP1 on chromosome 1p (rs3896439; p value = 2.4e-8). The protein product of AJAP1, SHREW1, interacts with basigin, a mediator of matrix metalloprotease (MMP) activity (Schreiner et al., 2007) involved in tooth development in rat (Schwab et al., 2007) and mouse models (Kumamoto and Ooya, 2006). This association was also observed for DMFS5 at the suggestive level of significance (rs3896439; p value = 1.1e-6). Likewise, associated genetic variants upstream of AJAP1 were among the top hits in an independent GWAS of dental caries in COHRA children (unpublished observations). These lines of evidence suggest that AJAP1 may influence dental caries, potentially through the regulation tooth development.
Table 2.
Significant (p value and gene symbol in bold) and Suggestive Associated Loci
| Chr. | Phenotype(s) | Top SNP | BP | p value | Gene(s) | Corroborative Evidence/Plausible Role in Dental Caries |
|---|---|---|---|---|---|---|
| 1p36 | DMFS5max, DMFS5 | rs3896439 | 4568530 | 2E-8 | AJAP1 | Interacts with basagin (Schreiner et al., 2007), a mediator of MMP activity during tooth development (Kumamoto and Ooya, 2006; Schwab et al., 2007) |
| 1p36 | DMFS5mand | rs9308447 | 9354977 | 5E-7 | SPSB1 | Unknown |
| 1p22 | DMFS5 | rs1750491 | 84998887 | 2E-6 | LPAR3 | Unknown |
| 1p21 | DMFS3, DMFS5, DMFS5max | rs11166135 | 99121424 | 1E-6 | LPPR5 | Unknown |
| 1q32 | DMFS5, DMFS5max | rs7552806 | 203050641 | 3E-6 | NFASC | Unknown |
| 2p24 | DMFS5, DMFS5mand | rs10180496 | 12886351 | 1E-7 | TRIB2 | Unknown |
| 3q21 | DMFS2 | rs9810890 | 130135243 | 6E-6 | ACAD9 | Unknown |
| 4p15 | DMFS5max | rs2531154 | 15627422 | 9E-7 | PROM1 | Unknown |
| 4q22 | DMFS5mand, DMFS5 | rs3114018 | 89283605 | 7E-8 | ABCG2 | Transporter protein and stem-cell marker expressed in human dental pulp (Honda et al., 2007) and in developing mouse incisor (Li et al., 2011); increased expression in ameloblastic tumors suggests that ABCG2 may regulate the maintenance of odontogenic tissues (Kumamoto and Ohki, 2010) |
| PKD2 | Pkd2 mutations in mice caused dental loss, root fractures (unpublished observations presented at IADR 2012 by Khonsari, http://iadr.confex.com/iadr/2012rio/webprogram/Paper160464.html) | |||||
| dentin/bone sub-family SCPP (SPP1, MEPE, IBSP, DMP1, and DSPP) | SCPP sub-family consisting of paralogous genes coding extracellular matrix proteins of dentin/bone; shares homology with enamel SCPP sub-family (Kawasaki and Weiss, 2008) | |||||
| 4q31 | DMFS3 | rs11100904 | 147123754 | 2E-6 | ZNF827 | Unknown |
| 4q31 | DMFS2, DMFS5mand | rs1429138 | 148501792 | 2E-7 | EDNRA | Signaling gene expressed during early craniofacial development; mice knock-outs exhibit severe craniofacial defects (Ruest et al., 2004) |
| 6p25 | DMFS3 | rs2476842 | 511741 | 3E-6 | EXOC2 | Unknown |
| 6q22 | DMFS2 | rs1204798 | 116650540 | 2E-6 | NT5DC1 (6q22.1) | Unknown; modest genetic association (p value = 0.02) in a candidate gene study following up linkage signal (Vieira et al., 2008) |
| 7q11 | DMFS2 | rs848452 | 77434748 | 2E-6 | PHTF2 | Unknown |
| 7q22 | DMFS5mand | rs10242311 | 105060956 | 6E-7 | ATXN7L1 | Unknown |
| 8p22 | DMFS5max, DMFS5 | rs10111661 | 20380853 | 2E-6 | LZTS1 | Unknown |
| 9q22 | DMFS5 | rs649057 | 101274144 | 5E-6 | 300 Kb from TGFBR1 | Strongly expressed in ameloblasts; promotes MMP20 expression during amelogenesis (Gao et al., 2009) |
| 300 Kb from NR4A3 | Transcription factor up-regulated in dental follicle cells during osteogenic differentiation (Morsczeck et al., 2009) | |||||
| 10p14 | DMFS5mand | rs11256676 | 10641866 | 5E-6 | SFTA1P | Unknown |
| 10p11 | DMFS2 | rs399593 | 30952036 | 9E-9 | LYZL2 | Bacteriolytic factor thought to be involved in host defense (Zhang et al., 2005) |
| 10q22 | DMFS5 | rs2441755 | 67835273 | 4E-6 | CTNNA3 | Unknown |
| 10q24 | DMFS5mand, DMFS2 | rs7078219 | 101264355 | 6E-6 | NKX2-3 | Involved in salivary gland and tooth morphogenesis (Biben et al., 2002) |
| 13q12 | DMFS2 | rs735539 | 20178034 | 4E-6 | IFT88 | Mutation in IFT88 leads to increased SHH signaling during development, resulting in ectopic extra molars (Ohazama et al., 2009) |
| IL17D | Cytokine that enhances pro-inflammatory response during bacterial infection via up-regulation of TLR4 (Guzzo et al., 2012) | |||||
| 13q21 | DMFS3 | rs2875517 | 66594146 | 3E-6 | PCDH9 | Unknown |
| 13q33 | DMFS2 | rs17485138 | 108300850 | 7E-6 | MYO16 | Unknown |
| 17q11 | DMFS5mand | rs12602978 | 22591207 | 7E-7 | WSB1 | Unknown |
| 18p11 | DMFS2 | rs2864527 | 9278456 | 4E-6 | TWSG1 | Regulates BMP signaling in the mandibular arch; mouse knock-outs exhibit craniofacial defects and salivary gland dysmorphogenesis (Melnick et al., 2006; MacKenzie et al., 2009) |
| 18q21 | DMFS5max | rs357894 | 44833968 | 4E-6 | SMAD7 | Regulator of TGF-beta mediated tooth development (Xu et al., 2003); expressed in human tooth bud (Bao et al., 2003); regulator of enamel deposition (Klopcic et al., 2007) |
| 18q23 | DMFS3 | rs13381277 | 72447598 | 4E-6 | ZNF516 | Unknown |
| 19p12 | DMFS5mand | rs931608 | 22405962 | 1E-6 | ZNF98 | Unknown |
| 21q21 | DMFS5mand | rs2829459 | 25201222 | 3E-6 | (gene desert) | Unknown |
| Xq26 | DMFS5, DMFS5max | rs3788848 | 129027493 | 3E-6 | BCORL1 | Shares homology with BCOR, a gene affecting tooth development (Fan et al., 2009); association observed in GWAS of related dental caries phenotype in this cohort |
In addition to the 2 genome-wide significant associations described above, we also observed several suggestive association signals. The strongest of these was observed for DMFS5mand and a locus on chromosome 4q22.1 harboring several tooth-related genes. The top SNP in this region (rs3114018; p value = 7.478e-8) was in ABCG2, a transporter protein and stem-cell marker expressed in human dental pulp (Honda et al., 2007) and in developing mouse incisors (Li et al., 2011). Increased expression in ameloblastic tumors suggests that ABCG2 may regulate the maintenance of odontogenic tissues (Kumamoto and Ohki, 2010). The adjacent gene, PKD2, has also been implicated in craniofacial development: PKD2 mutations in mice cause dental loss, root fractures, and other craniofacial anomalies, and are associated with facial asymmetry in humans (Khonsari; unpublished observations presented at IADR 2012, http://iadr.confex.com/iadr/2012rio/webprogram/Paper160464). The associated locus also includes the 5 paralogous genes of the dentin/bone SCPP sub-family (i.e., SPP1, MEPE, IBSP, DMP1, and DSPP), which are extracellular matrix proteins involved in tooth (and bone) formation (Kawasaki and Weiss, 2008). Genetic variation at this locus may affect one or more of these genes related to tooth development, which, in turn, may affect susceptibility to dental caries.
Many additional suggestive associations were observed, including several in or near genes with biologically plausible roles in caries susceptibility, as summarized in Table 2. Although none of these genes has previously been implicated in dental caries, their putative roles in tooth, salivary gland, and craniofacial development, and in response to bacterial infection, suggest plausible mechanisms through which they may influence cariogenesis. Other suggestive associations were observed for genetic loci that have no obvious biological functions related to dental caries.
Discussion
Dental caries is an extremely multi-factorial disease, whereby genes operating through numerous biological avenues may influence susceptibility. In this study, we performed genome-wide association scans for novel dental caries phenotypes developed under the hypothesis that genetic variants (as is the case with environmental risk factors [Shaffer et al., 2012b]) may differentially affect caries risk across tooth surfaces of the permanent dentition. Two genome-wide significant loci were observed: LYZL2 for mandibular anterior surfaces (i.e., surfaces of the incisors, canines, and premolars), and AJAP1 for mid-dentition surfaces (i.e., surfaces of the premolars and maxillary canines). Numerous suggestive loci were also observed.
A theme among the associated loci was genes thought to be involved in craniofacial, salivary gland, and tooth development, including AJAP1, ABCG2, PKD2, the dentin/bone SCPP sub-family, EDNRA, TJFBR1, NKX2-3, IFT88, TWSG1, and SMAD7. Developmental genes such as these add to the growing list of host genes, including taste preference, enamel-related, and bactericidal genes, that have been chosen for investigation. In light of the observational study design, we cautiously interpret our results to nominate these loci as putative caries genes, pending confirmation in additional epidemiological and experimental studies. More work, including functional studies, will be required to determine whether associated loci may be useful for disease prediction, diagnosis, or treatment. Either way, the novel associations identified here may lead to better understanding of the biological mechanisms influencing cariogenesis.
For the most part, the significant and suggestive associations identified in this study did not overlap with loci identified via GWAS of global caries phenotypes of the permanent dentition in this sample (unpublished observations) or global phenotypes of the primary dentition in a related sample of children from the same population (Shaffer et al., 2011). This observation suggests that data-driven caries phenotypes that attempt to capture biologically informative patterns of tooth decay, such as those generated for this study, may be useful for identifying and understanding the genetic contributions to dental caries. Indeed, the X-linked association between DMFS5 and BCORL1 was also observed in a GWAS of smooth-surface caries, a similar caries phenotype defined based on a priori tooth-surface classifications (unpublished observations).
In the interpretation of our results, a few design aspects of the GWAS approach warrant discussion. First, we view GWAS as a discovery-driven approach, with the goal of generating new hypotheses (as opposed to the paradigm of testing hypotheses). Therefore, in addition to scrutinizing our genome-wide significant hits (which met a very strict significance threshold, even considering the multiple testing), we also examined and report suggestive associations. While the genome-wide significant associations are extremely unlikely to occur by chance alone, associations meeting suggestive significance may occur by chance (though likely not as many as we observed). Therefore, we expect that some of the suggestive associations observed in this study may be spurious, whereas others are likely true reflections of genetic risk. Ideally, these associations should be replicated in an independent sample; however, we analyzed novel caries phenotypes developed specifically for this GWAS from tooth-surface-level caries data not currently available in any other adult sample. Therefore, replication in an independent sample is not currently possible. This limitation is partly countered by the corroborating evidence of gene functions connecting many of our associated loci to tooth-related biological processes. In short, many of our associations make sense, and therefore, we propose prioritizing them among the growing list of caries candidate genes. Additional work is needed to explore the independent or interacting effects of these candidate genes with known environmental risk factors, such as fluoride exposures, dietary and oral hygiene behaviors, and demographics.
A major strength and innovation of this study was the use of novel caries phenotypes, which were developed by hierarchical clustering analysis on tooth-surface-level data. We have previously shown that grouping surfaces based on co-occurrence of caries resulted in sensible categories, which were reproducible in NHANES 1999-2000, an independent national cohort (Shaffer et al., 2013). Some of the novel caries phenotypes were heritable and/or exhibited significant correlations with putative environmental predictors such as age, sex, educational attainment, and toothbrushing behaviors (Shaffer et al., 2013). Here, we showed that these novel caries outcomes were also useful for identifying genetic loci, which may exert differential effects across categories of tooth surfaces. For example, bacteriolytic LYZL2 and pro-inflammatory IL17D, both genes involved in host response to bacterial infection, were implicated in DMFS2, which reflects caries of the mandibular anterior tooth surfaces (i.e., incisors, canines, and first premolars). These surfaces are similar in their positions in the mouth and their comparatively low prevalence of caries. In contrast, several tooth development genes were implicated for DMFS5 (and sub-clusters DMFS5max and DMFS5mand), reflecting caries of the mid-dentition surfaces (i.e., pre-molars and canines, which exhibit moderate caries prevalence). DMFS3, which reflects posterior surfaces excluding the molar pit and fissure surfaces, showed associations with a number of genetic loci, though no obvious candidate genes were identified. In contrast, DMFS1 (reflecting molar pit and fissure surfaces, which exhibit the greatest caries prevalence) and DMFS4 (reflecting maxillary incisors, which exhibit moderate caries prevalence) were not significantly heritable, and therefore, GWAS for these outcomes were not considered. These two categories of tooth surfaces are likely strongly influenced by important environmental factors (e.g., sealants and dietary choices) that may render them less informative for discovering genetic risk factors. Overall, we take these results to support our premise that susceptibility genes exert their effects differentially across the permanent dentition and that modeling tooth-surface-level caries data can benefit gene-mapping efforts.
In conclusion, we identified many novel genetic associations for dental caries and nominated several new caries genes. Moreover, we observed that different genetic variants were associated with caries across the different categories of tooth surfaces, which supports our central hypothesis. This study demonstrated the utility of novel dental caries phenotypes for identifying genetic risk factors, which we believe may prove useful for understanding the biological mechanisms predisposing to dental caries, and, ultimately, may lead to improvements in disease prevention, detection, and treatment.
Footnotes
This work was funded through NIH grants U01-DE018903, R01-DE014899, and R03-DE021425. Genotyping was performed as part of the GENEVA consortium (www.genevastudy .org) by the Center for Inherited Disease Research (www.cidr.jhmi.edu) through an NIH contract (HHSN268200782096C). Phenotype harmonization and genotype data cleaning and quality assurance were conducted in conjunction with the GENEVA coordinating center (funded through U01-HG004446).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Bao L, Niu Z, Shi J. (2003). [The expression and function of Smad7 during human tooth germ development]. Hua Xi Kou Qiang Yi Xue Za Zhi 21:438-440. [PubMed] [Google Scholar]
- Beltran-Aguilar ED, Barker LK, Canto MT, Dye BA, Gooch BF, Griffin SO, et al. (2005). Surveillance for dental caries, dental sealants, tooth retention, edentulism, and enamel fluorosis—United States, 1988-1994 and 1999-2002. MMWR Surveill Summ 54:1-43. [PubMed] [Google Scholar]
- Biben C, Wang CC, Harvey RP. (2002). NK-2 class homeobox genes and pharyngeal/oral patterning: Nkx2-3 is required for salivary gland and tooth morphogenesis. Int J Dev Biol 46:415-422. [PubMed] [Google Scholar]
- Boraas JC, Messer LB, Till MJ. (1988). A genetic contribution to dental caries, occlusion, and morphology as demonstrated by twins reared apart. J Dent Res 67:1150-1155. [DOI] [PubMed] [Google Scholar]
- Fan Z, Yamaza T, Lee JS, Yu J, Wang S, Fan G, et al. (2009). BCOR regulates mesenchymal stem cell function by epigenetic mechanisms. Nat Cell Biol 11:1002-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Li D, Han T, Sun Y, Zhang J. (2009). TGF-beta1 and TGFBR1 are expressed in ameloblasts and promote MMP20 expression. Anat Rec (Hoboken) 292:885-890. [DOI] [PubMed] [Google Scholar]
- Guzzo C, Ayer A, Basta S, Banfield BW, Gee K. (2012). IL-27 enhances LPS-induced proinflammatory cytokine production via upregulation of TLR4 expression and signaling in human monocytes. J Immunol 188:864-873. [DOI] [PubMed] [Google Scholar]
- Honda MJ, Nakashima F, Satomura K, Shinohara Y, Tsuchiya S, Watanabe N, et al. (2007). Side population cells expressing ABCG2 in human adult dental pulp tissue. Int Endod J 40:949-958. [DOI] [PubMed] [Google Scholar]
- Kawasaki K, Weiss KM. (2008). SCPP gene evolution and the dental mineralization continuum. J Dent Res 87:520-531. [DOI] [PubMed] [Google Scholar]
- Klopcic B, Maass T, Meyer E, Lehr HA, Metzger D, Chambon P, et al. (2007). TGF-beta superfamily signaling is essential for tooth and hair morphogenesis and differentiation. Eur J Cell Biol 86:781-799. [DOI] [PubMed] [Google Scholar]
- Kumamoto H, Ohki K. (2010). Detection of CD133, Bmi-1, and ABCG2 in ameloblastic tumors. J Oral Pathol Med 39:87-93. [DOI] [PubMed] [Google Scholar]
- Kumamoto H, Ooya K. (2006). Immunohistochemical detection of MT1-MMP, RECK, and EMMPRIN in ameloblastic tumors. J Oral Pathol Med 35:345-351. [DOI] [PubMed] [Google Scholar]
- Li L, Kwon HJ, Harada H, Ohshima H, Cho SW, Jung HS. (2011). Expression patterns of ABCG2, Bmi-1, Oct-3/4, and Yap in the developing mouse incisor. Gene Expr Patterns 11:163-170. [DOI] [PubMed] [Google Scholar]
- MacKenzie B, Wolff R, Lowe N, Billington CJ, Peterson A, Schmidt B, et al. (2009). Twisted gastrulation limits apoptosis in the distal region of the mandibular arch in mice. Dev Biol 328:13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick M, Petryk A, Abichaker G, Witcher D, Person AD, Jaskoll T. (2006). Embryonic salivary gland dysmorphogenesis in Twisted gastrulation deficient mice. Arch Oral Biol 51:433-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsczeck C, Schmalz G, Reichert TE, Vollner F, Saugspier M, Viale-Bouroncle S, et al. (2009). Gene expression profiles of dental follicle cells before and after osteogenic differentiation in vitro. Clin Oral Investig 13:383-391. [DOI] [PubMed] [Google Scholar]
- Ohazama A, Haycraft CJ, Seppala M, Blackburn J, Ghafoor S, Cobourne M, et al. (2009). Primary cilia regulate Shh activity in the control of molar tooth number. Development 136:897-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk DE, Weyant RJ, Crout RJ, McNeil DW, Tarter RE, Thomas JG, et al. (2008). Study protocol of the Center for Oral Health Research in Appalachia (COHRA) etiology study. BMC Oral Health 8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. (2007). PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruest LB, Xiang X, Lim KC, Levi G, Clouthier DE. (2004). Endothelin-A receptor-dependent and -independent signaling pathways in establishing mandibular identity. Development 131:4413-4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner A, Ruonala M, Jakob V, Suthaus J, Boles E, Wouters F, et al. (2007). Junction protein shrew-1 influences cell invasion and interacts with invasion-promoting protein CD147. Mol Biol Cell 18:1272-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab W, Harada H, Goetz W, Nowicki M, Witt M, Kasper M, et al. (2007). Immunocytochemical and biochemical detection of EMMPRIN in the rat tooth germ: differentiation-dependent co-expression with MMPs and co-localization with caveolin-1 in membrane rafts of dental epithelial cells. Histochem Cell Biol 128:195-203. [DOI] [PubMed] [Google Scholar]
- Shaffer JR, Wang X, Feingold E, Lee M, Begum F, Weeks DE, et al. (2011). Genome-wide association scan for childhood caries implicates novel genes. J Dent Res 90:1457-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer JR, Feingold E, Wang X, Tcuenco K, Weeks DE, Desensi RS, et al. (2012a). Heritable patterns of tooth decay in the permanent dentition: principal components and factor analyses. BMC Oral Health 12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer JR, Polk DE, Feingold E, Wang X, T.Cuenco K, Weeks DE, et al. (2012b). Demographic, socioeconomic, and behavioral factors affecting patterns of tooth decay in the permanent dentition: principal components and factor analyses. Community Dent Oral Epidemiol (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer JR, Feingold E, Wang X, Weeks DE, Weyant RJ, Crout R, et al. (2013). Clustering tooth surfaces into biologically informative caries outcomes. J Dent Res 92:32-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira AR, McHenry TG, Daack-Hirsch S, Murray JC, Marazita ML. (2008). Candidate gene/loci studies in cleft lip/palate and dental anomalies finds novel susceptibility genes for clefts. Genet Med 10:668-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Jeong L, Han J, Ito Y, Bringas P, Chai Y. (2003). Developmental expression of Smad1-7 suggests critical function of TGF-beta/BMP signaling in regulating epithelial-mesenchymal interaction during tooth morphogenesis. Int J Dev Biol 47:31-39. [DOI] [PubMed] [Google Scholar]
- Zhang K, Gao R, Zhang H, Cai X, Shen C, Wu C, et al. (2005). Molecular cloning and characterization of three novel lysozyme-like genes, predominantly expressed in the male reproductive system of humans, belonging to the c-type lysozyme/alpha-lactalbumin family. Biol Reprod 73:1064-1071. [DOI] [PubMed] [Google Scholar]