Abstract
The discovery that dental pulp stem cells are capable of differentiating into endothelial cells raises the exciting possibility that these cells can be a single source of odontoblasts and vascular networks in dental tissue engineering. The purpose of this study was to begin to define signaling pathways that regulate endothelial differentiation of SHED. Stem cells from exfoliated deciduous teeth (SHED) exposed to endothelial growth medium (EGM-2MV) supplemented with vascular endothelial growth factor (VEGF) differentiated into VEGFR2-positive and CD31-positive endothelial cells in vitro. In vivo, VEGFR1-silenced SHED seeded in tooth slice/ scaffolds and transplanted into immunodeficient mice showed a reduction in human CD31-positive blood vessels as compared with controls (p = 0.02). Exposure of SHED to EGM2-MV supplemented with VEGF induced potent activation of ERK and Akt signaling, while it inhibited phosphorylation of STAT3. Notably, genetic (MEK1 silencing) or chemical (U0126) inhibition of ERK signaling restored constitutive STAT3 phosphorylation and inhibited the differentiation of SHED into endothelial cells. Collectively, analysis of these data unveiled the VEGF/MEK1/ERK signaling pathway as a key regulator of the endothelial differentiation of dental pulp stem cells.
Keywords: angiogenesis, tissue engineering, stemness, endodontics, dental pulp stem cells, blood vessels
Introduction
Dental pulp tissue engineering is emerging as a potential treatment alternative for necrotic immature permanent teeth. Engineering of dental pulp requires the generation of a complex tissue, composed of odontoblasts, sensory cells, supportive fibroblasts, and a functional vascular network. Stem cells from exfoliated deciduous teeth (SHED) are capable of differentiating into functional odontoblasts that secrete mineralizable dentin matrices (Casagrande et al., 2010; Sakai et al., 2010). Dental pulp stem cells can also differentiate into vascular endothelial cells that form functional blood vessels that anastomize with the host vasculature (d’Aquino et al., 2007; Iohara et al., 2008; Cordeiro et al., 2008; Sakai et al., 2010). Therefore, SHED are uniquely suited for dental pulp engineering, since they generate functional dental pulp while providing a vascular network that supplies the oxygen and nutrients required for the high metabolic demands of cells that are engaged in tissue regeneration (Casagrande et al., 2011). An understanding of the signaling pathways involved in the endothelial differentiation of dental pulp stem cells is critical for the optimization of methods to translate dental tissue engineering to the clinic.
Vascular endothelial growth factor (VEGF) is a potent inducer of angiogenesis (Hoeben et al., 2004). Emerging evidence demonstrates that VEGF can function as an inducer of differentiation of human adipose-derived stem cells into endothelial cells (Cao et al., 2005; Behr et al., 2011). However, the effect of VEGF on the differentiation of dental pulp stem cells is unknown. We have recently shown that SHED constitutively express the membrane-bound vascular endothelial growth factor receptor (VEGFR)-1 and its co-receptor neuropilin (NP)-1 (Sakai et al., 2010). VEGFR1 is widely expressed in normal and malignant tissues (Fan et al., 2005; Roy et al., 2006; Cao, 2009) and modulates the angiogenic potential of cells exposed to VEGF (Kearney et al., 2004; Zhang et al., 2010). Interestingly, VEGF induced VEGFR2 up-regulation in SHED, which was correlated with other evidence of endothelial differentiation of these cells. Analysis of our data, collectively, suggested that VEGFR1 is the receptor responsible for VEGF-induced endothelial differentiation, which is demonstrated by an increase in the expression of VEGFR2 along with CD31 and VE-cadherin, and an increase in capillary sprout formation in 3-D matrices (Sakai et al., 2010). Here, we will focus on the downstream signaling events triggered by VEGF signaling in SHED.
It has been shown that the MAPK pathway is critical for endothelial differentiation of bone marrow stem cells (Xu et al., 2008). Further, in adipose-derived stem cells, it is the PI3K-Akt pathway that regulates endothelial differentiation (Cao et al., 2005; Zhang et al., 2011). We know that both Akt and ERK signaling pathways are induced by VEGF in SHED (Sakai et al., 2010), but we do not know which pathways are required for this process. Therefore, the overall goal of the project is to understand the signaling pathways that regulate the differentiation of SHED into endothelial cells.
Materials & Methods
Cell Culture
Human Stem cells from Exfoliated Deciduous Teeth (SHED) were isolated from primary teeth and fully characterized, as we described previously (Miura et al., 2003). SHED were cultured in alpha-MEM medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen) and 1% penicillin/streptomycin (Invitrogen) at 37°C and 5% CO2. We induced endothelial differentiation by culturing SHED cells with endothelial cell growth medium (EGM-2MV; Lonza, Walkersville, MD, USA) supplemented with 50 ng/mL rhVEGF165 (R&D Systems, Minneapolis, MN, USA), referred to as ‘differentiation medium’ throughout this manuscript. The cell culture medium was changed every 2 days in all experiments included here. We assessed endothelial differentiation in vitro by determining the expression of 2 endothelial markers (VEGFR2 and CD31) by Western blot and RT-PCR. Human dermal microvascular endothelial cells (HDMEC; Lonza) were used as positive control. To evaluate cell morphology, we cultured SHED cells in 8-well chamber slides (Fisher, Rochester, NY, USA) in alpha-MEM or EGM2-MV medium with or without rhVEGF supplementation. Alexa Fluor 488 phalloidin (Invitrogen) was used for visualization of the cytoskeleton (F-Actin; green), and nuclei were stained with DAPI (Prolong Gold; Invitrogen).
Semi-quantitative RT-PCR
Total RNA was extracted with TRIzol Reagent (Invitrogen), and PCR reactions were performed with Superscript™ III Platinum Two-Step qRT-PCR kit (Invitrogen) according to the manufacturer’s instructions. Primers were the following: human VEGFR2 (sense 5′-gctgtctcagtgacaaacccat-3′ and anti-sense 5′-ctcccacatggattggcagagg-3′; size = 373 bp); human CD31 (sense 5’- gagtcctgctgacccttctg and anti-sense 5’-acagttgaccctcacgatcc-3’; size = 416 bp); and human GAPDH (sense 5′-gaccccttcattgacctcaact-3′ and anti-sense 5′-accaccttcttgatgt catc-3′; size = 683 bp).
Lentiviral-mediated Gene Silencing
Gene silencing was performed with lentiviral vectors encoding shRNA constructs, as described previously (Sakai et al., 2010). Briefly, 293T cells were transiently co-transfected with lentivirus packaging vector psPAX2, pMD2.G, and shRNA-C (control), shRNA-VEGFR1 or shRNA-MEK1 (Vector Core, University of Michigan) with calcium phosphate. We used supernatants containing lentiviruses to infect SHED overnight, and then cells were selected with 1 μg/mL puromycin (InVivogen, San Diego, CA, USA) for at least 1 wk. Infection efficiency was determined by fluorescence, and gene silencing by Western blot.
Tooth Slice/Scaffold Assay
To evaluate the role of VEGFR1 in the differentiation of SHED into endothelial cells in vivo, we seeded SHED stably transduced with shRNA-VEGFR1 (SHED-shRNA-VEGFR1) or control shRNA (SHED-shRNA-C) in a human tooth slice/scaffold, as described previously (Cordeiro et al., 2008). Briefly, 1-mm-thick tooth slices were obtained from healthy human third molars. The pulp chamber of the tooth slice was filled with sieved NaCl, and a PLLA/chloroform solution was gently dropped, soaking the salt. After 24 hrs, tooth slice/scaffolds were washed with Milli-Q water to remove the salt, and scaffolds were re-hydrated/ disinfected with decreasing concentrations of ethanol (100%-70%). Cells (6 x 105) were seeded in each tooth slice/scaffold that was transplanted subcutaneously into the dorsum of severe combined immunodeficient mice (CB.17 SCID; Charles River, Wilmington, MA, USA). After 28 days, specimens were retrieved and fixed with IHC Zinc Fixative (BD Pharmingen, Franklin Lakes, NJ, USA) for 24 hrs at 4oC and demineralized with Decalcifier II (Surgipath; Richmond, IL, USA) for 24 hrs at room temperature. Hematoxylin-eosin staining and immunohistochemistry with rabbit anti-human CD31 (Bethyl Laboratories, Montgomery, TX, USA) were used to evaluate the morphology and microvessel density. Vessels were counted in 5 fields per tooth slice by a calibrated evaluator (ICC = 0.95) in a blinded fashion. This work was done under a protocol reviewed and approved by the appropriate institutional committee.
Statistical Analyses
We performed a t test to compare the numbers of CD31-positive vessels in pulps generated with SHED-shRNA-VEGFR1 vs. SHED-shRNA-C cells, using the SigmaStat 2.0 software (SPSS, Chicago, IL, USA). Significance was determined at p < 0.05.
Results
Time-course of the Differentiation of SHED into Endothelial Cells
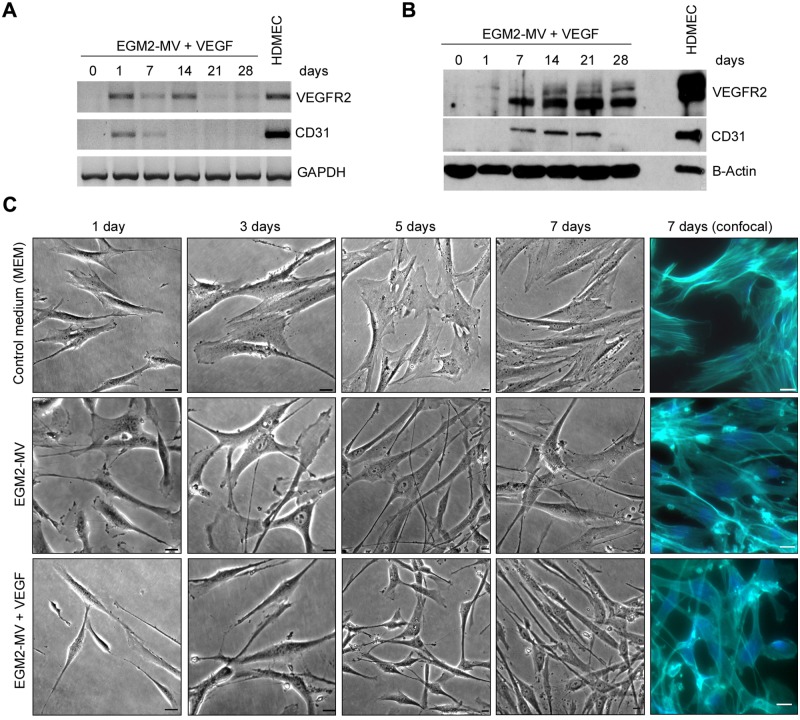
To evaluate the effect of VEGF on endothelial differentiation, we exposed SHED to EGM-2MV supplemented with rhVEGF165 and observed cells with acquired mRNA (Fig. 1A) and protein (Fig. 1B) expression of endothelial markers (VEGFR2 and CD31). In contrast, SHED cultured in α-MEM without rhVEGF165 did not express endothelial markers for up to 28 days (data not shown). VEGFR2 and CD31 mRNA expression was highest 1 day after exposure to the differentiation medium and then tapered off (Fig. 1A). Interestingly, VEGFR2 protein expression was observed after 7 days of treatment and lasted for 28 days, while CD31 expression was strong from day 7 through day 21 (Fig. 1B). Qualitative morphological evaluation revealed that SHED exposed to EGM-2MV, whether containing VEGF or not, became elongated, when compared with controls cultured in alpha-MEM (Fig. 1C). Analysis of these data, collectively, characterizes culture conditions with EGM2-MV supplemented with rhVEGF165 that induce the differentiation of dental pulp stem cells into endothelial cells.
Figure 1.
Time-course of the differentiation of SHED into endothelial cells. (A) Time-course of mRNA and (B) protein expression of endothelial cell markers VEGFR2 and CD31 upon culture of SHED cells with EGM2-MV + 50 ng/mL rhVEGF165. (C) Cell morphology over time upon culture of SHED cells with basal medium (alpha-MEM), endothelial growth medium (EGM2-MV), or EGM2-MV supplemented with 50 ng/mL rhVEGF165 (bar = 20 µm).
VEGFR1 Silencing Inhibits Endothelial Differentiation of SHED in vivo
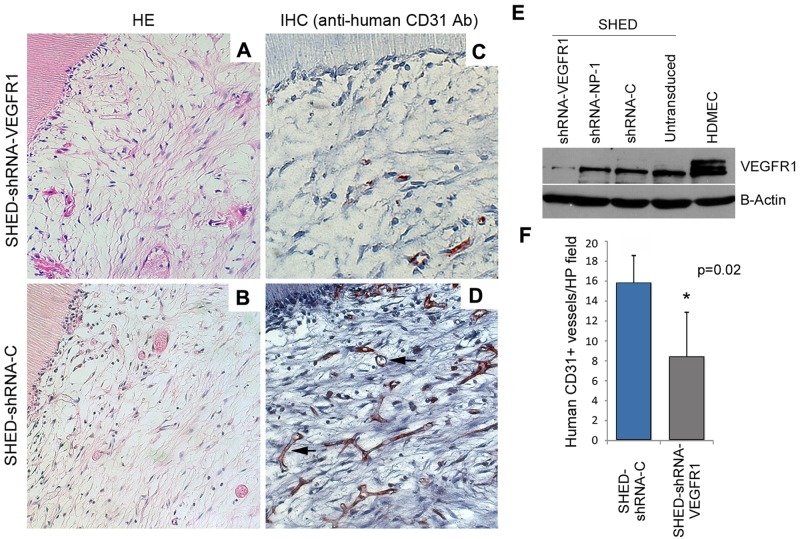
We have previously reported that VEGFR1 is constitutively expressed in SHED (Sakai et al., 2010). We have also observed that SHED-LacZ differentiate into human blood vessels when seeded in tooth slice/scaffolds and transplanted into immunodeficient mice, as determined by the consistent presence of B-galactosidase-positive blood vessels in the resulting pulp chambers (Cordeiro et al., 2008). However, the role of VEGFR1 signaling in the endothelial differentiation of SHED in vivo is unknown. Here, VEGFR1-silenced SHED or SHED transduced with control lentiviral vector (shRNA-C) (Fig. 2E) were seeded into tooth slice/scaffolds and transplanted into immunodeficient mice. After 28 days, the tooth slice/scaffolds were retrieved, and pulp-like tissues were observed in the pulp chambers (Figs. 2A, 2B). Microvessel density was evaluated with an anti-human CD31 antibody that does not cross-react with mouse blood vessels. A decrease in the density of anti-human CD31-positive cells (p = 0.02) was observed in the pulps generated with SHED-shRNA-VEGFR1 cells (Figs. 2C, 2F) as compared with pulps generated with control SHED-shRNA-C cells (Figs. 2D, 2F).
Figure 2.
VEGFR1 silencing inhibits endothelial differentiation of SHED in vivo. (A, C) Tooth slice/scaffolds seeded with VEGFR1-silenced SHED (SHED-shRNA-VEGFR1) and (B, D) vector control SHED (SHED-shRNA-C) were transplanted into the subcutaneous space of immunodeficient mice. After 28 days, tooth slice/scaffolds were retrieved, fixed, and analyzed by hematoxylin-eosin staining immunohistochemistry with anti-human CD31. Black arrows indicate CD31-positive blood vessels. (E) Western blot to evaluate the effectiveness of VEGFR1 knockdown in SHED cells. (F) Microvessel density analysis from 4 tooth slice/scaffolds and 5 microscopic fields/specimen (at 200x) per experimental condition.
MEK1/ERK Signaling is Required for Endothelial Differentiation of SHED in vitro
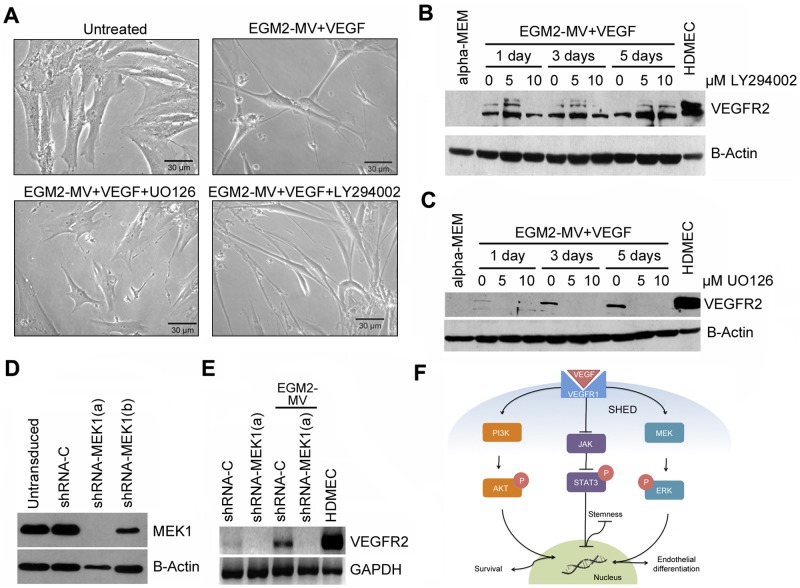
To begin to evaluate the signaling events involved in the endothelial differentiation of SHED, we exposed cells to EGM2-MV supplemented with VEGF and evaluated phosphorylation of key signaling molecules. We observed that ERK and Akt became phosphorylated within 15 min of exposure to the differentiation medium (Fig. 3A). This was followed by de-phosphorylation of STAT3, which is constitutively active in SHED (Fig. 3A). To evaluate a potential crosstalk between these pathways, we exposed SHED to the differentiation medium in the presence of well-characterized chemical inhibitors of STAT3 (Stattic V), PI3k/Akt (LY294002), or MEK/ERK (UO126). This experiment revealed that inhibition of ERK signaling with U0126 prevented differentiation medium-induced de-phosphorylation of STAT3 (Fig. 3B). Analysis of these data suggests the existence of crosstalk between ERK and STAT3 in SHED cells exposed to the differentiation medium. Notably, while inhibition of STAT3 or Pl3k/Akt pathway inhibited SHED proliferation, inhibition of MEK/ERK did not have such an effect under the experimental conditions used here (Appendix Fig.).
Figure 3.
Activation of signaling upon exposure of SHED to differentiation medium. (A) Western blot depicting effect of differentiation medium (EGM-2MV + rhVEGF165) on phosphorylated and total ERK, Akt, and STAT3. (B) Western blot showing the effects of inhibitors for ERK (U0126), Akt (LY294002), or STAT3 (Stattic V) on signaling induced by the differentiation medium.
To define a functional role for ERK signaling in the acquisition of an endothelial phenotype by SHED cells, we inhibited this pathway using 2 complementary strategies, a chemical (U0126) and a genetic (shRNA-MEK1) approach. SHED exposed to differentiation medium in the presence of U0126 no longer presented an elongated shape suggestive of endothelial differentiation (Fig. 4A). Notably, induction of the endothelial cell marker (VEGFR2) by the differentiation medium was completely inhibited by treatment with the ERK inhibitor U0126 (Fig. 4C). Similar results were observed when ERK signaling was inhibited by silencing MEK1 (Fig. 4D), the upstream kinase that phosphorylates ERK. SHED stably transduced shRNA-MEK1 and, exposed to differentiation medium, no longer expressed VEGFR2 (Fig. 4E). In contrast, inhibition of PI3k/Akt signaling with LY294002 did not affect SHED morphology (Fig. 4A), nor did it prevent expression of the endothelial marker VEGFR2 induced by the differentiation medium (Fig. 4B). Collectively, these results demonstrate that specific signaling through MEK1/ERK is required for the endothelial differentiation of SHED.
Figure 4.
MEK1/ERK signaling is required for endothelial differentiation of SHED in vitro. (A) Qualitative analysis of the morphology of SHED cells treated with differentiation medium in the presence of U0126 or LY294002. (B) Western blot showing the effect of Akt inhibition with LY294002 or (C) the effect of ERK inhibition with U0126 on endothelial cell differentiation of SHED, as determined by VEGFR2 expression. (D) Effects of 2 different shRNA-MEK1 sequences (a,b) on silencing of MEK1 in SHED cells. (E) Effect of MEK1 silencing on endothelial cell differentiation of SHED, as determined by VEGFR2 expression. (F) Schematic diagram depicting the signaling pathways studied here.
Discussion
We have recently demonstrated that human dental pulp stem cells differentiate into functional human blood vessels in immunodeficient mice, broadly recapitulating the differentiation steps observed in embryonic vasculogenesis (Sakai et al., 2010). To generate functional blood vessels, dental pulp stem cells had to undergo differentiation into endothelial cells, organize themselves into capillary structures, and anastomize with the host vasculature. While the cellular events leading to the differentiation of dental pulp stem cells into endothelial cells have been partially characterized, the molecular mechanisms and signaling pathways underlying the acquisition of the vasculogenic fate are not known. Such knowledge may accelerate the translation of dental pulp stem-cell-based therapies to the clinic.
We have shown that SHED cultured in tooth slice scaffolds in α-MEM supplemented with rhVEGF165 express endothelial markers suggesting their differentiation into endothelial cells (Sakai et al., 2010). We hypothesized that angiogenic factors residing in the dentin matrix (Roberts-Clark and Smith, 2000; Zhang et al., 2011) or stromal cells (Tran-Hung et al., 2008), together with rhVEGF165, triggered signaling pathways that were sufficient to induce endothelial differentiation of SHED. Here, we explored the possibility of creating differentiation conditions that were sufficient to induce endothelial differentiation of dental pulp stem cells without the tooth slice, which would facilitate mechanistic signaling studies. We discovered that the endothelial growth medium (EGM2-MV), which contains rhEGF, hydrocortisone, 5% fetal bovine serum, rhbFGF, R3-IGF-1, ascorbic acid, and 2 ng/mL rhVEGF supplemented with an additional 50 ng/mL rhVEGF, provides an inductive stimulus that allows for endothelial differentiation of SHED in monolayer culture. This culture condition (‘differentiation medium’) was used thereafter for the mechanistic studies included here.
VEGFR2 and CD31 are expressed primarily by vascular endothelial cells (Albelda et al., 1991; Yamaguchi et al., 1993). Both have been used as markers of endothelial differentiation of bone-marrow-derived mesenchymal stem cells (Quirici et al., 2001; Xu et al., 2008). In contrast, VEGFR1 is widely expressed in normal and malignant tissues (Fan et al., 2005; Roy et al., 2006; Cao, 2009) and modulate the angiogenic potential of cells exposed to VEGF (Kearney et al., 2004; Zhang et al., 2010). Indeed, we have observed that VEGFR1 is constitutively expressed by undifferentiated SHED cells (Sakai et al., 2010). Here, it was observed that VEGFR1-silenced SHED showed less angiogenic potential in vivo than controls, suggesting that VEGFR1 signaling plays an important role in endothelial differentiation of dental pulp stem cells. We postulate that VEGFR1 signaling allows for the differentiation of dental pulp stem cells into endothelial cells, as demonstrated by the acquisition of VEGFR2 and CD31 expression over time.
STAT3 phosphorylation is sufficient to maintain stem cells in an undifferentiated state (Matsuda et al., 1999). In contrast, unstimulated stem cells express low levels of phosphorylated ERK and AKT, while cells that are induced to undergo differentiation exhibit an increase in ERK and Akt phosphorylation (Cao et al., 2005; Xu et al., 2008; Zhang et al., 2011). Here, we observed that unstimulated SHED express high levels of phosphorylated STAT3 and that exposure of these cells to the differentiation medium quickly inhibits (within 30 min) STAT3 activity, which is in line with the observation that STAT3 activity correlates with stemness. Surprisingly, the inhibition of STAT3 phosphorylation with STATTIC V enhanced ERK, but not Akt phosphorylation, beyond what was achieved with the differentiation medium. Further, inhibition of ERK with U0126 allowed for recovery of STAT3 phosphorylation in SHED cells that were induced to differentiate. To characterize the functional relevance of ERK signaling, we inhibited ERK with U0126 or by silencing MEK1 expression and observed that SHED cells no longer differentiated into endothelial cells. Finally, we observed that inhibition of PI3K/Akt resulted in slowdown in cell proliferation and/or induction of cell death, but had no effect on the regulation of SHED stemness/differentiation. In contrast, inhibition of ERK had no effect on cell proliferation/survival, but had a profound effect on cell differentiation. These findings suggest a cause-effect relationship between ERK inhibition and maintenance of STAT3 phosphorylation, which is consistent with ERK’s role in the regulation of SHED stemness. Collectively, these results demonstrate the existence of bi-directional crosstalk between STAT3 and ERK signaling that plays a critical role in the regulation of dental pulp stem cell fate.
In conclusion, this work unveiled a pathway triggered by VEGF/MEK1 signaling that results in the inverse and reciprocal regulation of STAT3 and ERK activity that results, in turn, in the differentiation of primary tooth pulp stem cells into endothelial cells and the importance of VEGF signaling through VEGFR1 for this process. Such studies may offer clues into the mechanisms regulating cell differentiation during odontogenesis. In addition, the understanding of signaling pathways will be critical to exploit the differentiation potential of dental pulp stem cells in regenerative medicine.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
This work was funded by grant R01-DE21410 from the NIH/NIDCR (JEN) and by grant BEX 5511/09-7 from CAPES (LWB).
References
- Albelda SM, Muller WA, Buck CA, Newman PJ.(1991). Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol 114:1059-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr B, Tang C, Germann G, Longaker MT, Quarto N. (2011). Locally applied vascular endothelial growth factor A increases the osteogenic healing capacity of human adipose-derived stem cells by promoting osteogenic and endothelial differentiation. Stem Cells 29:286-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y. (2009). Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal 2(59):re1. [DOI] [PubMed] [Google Scholar]
- Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. (2005). Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun 332:370-379. [DOI] [PubMed] [Google Scholar]
- Casagrande L, Demarco FF, Zhang Z, Araujo FB, Shi S, Nör JE. (2010). Dentin-derived BMP-2 and odontoblast differentiation. J Dent Res 89:603-608. [DOI] [PubMed] [Google Scholar]
- Casagrande L, Cordeiro MM, Nör SA, Nör JE. (2011). Dental pulp stem cells in regenerative dentistry. Odontology 99:1-7. [DOI] [PubMed] [Google Scholar]
- Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, et al. (2008). Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 34:962-969. [DOI] [PubMed] [Google Scholar]
- d’Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, De Rosa A, et al. (2007). Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ 14:1162-1171. [DOI] [PubMed] [Google Scholar]
- Fan F, Wey JS, McCarty MF, Belcheva A, Liu W, Bauer TW, et al. (2005). Expression and function of vascular endothelial growth factor receptor-1 on human colorectal cancer cells. Oncogene 24:2647-2653. [DOI] [PubMed] [Google Scholar]
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. (2004). Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 56:549-580. [DOI] [PubMed] [Google Scholar]
- Iohara K, Zheng L, Wake H, Ito M, Nabekura J, Wakita H, et al. (2008). A novel stem cell source for vasculogenesis in ischemia: subfraction of side population cells from dental pulp. Stem Cells 26:2408-2418. [DOI] [PubMed] [Google Scholar]
- Kearney JB, Kappas NC, Ellerstrom C, DiPaola FW, Bautch VL. (2004). The VEGF receptor flt-1 (VEGFR-1) is a positive modulator of vascular sprout formation and branching morphogenesis. Blood 103:4527-4535. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, et al. (1999). STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J 18:4261-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. (2003). SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA 100:5807-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirici N, Soligo D, Caneva L, Servida F, Bossolasco P, Deliliers GL. (2001). Differentiation and expansion of endothelial cells from human bone marrow CD133(+) cells. Br J Haematol 115:186-194. [DOI] [PubMed] [Google Scholar]
- Roberts-Clark DJ, Smith AJ. (2000). Angiogenic growth factors in human dentine matrix. Arch Oral Biol 45:1013-1016. [DOI] [PubMed] [Google Scholar]
- Roy H, Bhardwaj S, Yla-Herttuala S. (2006). Biology of vascular endothelial growth factors. FEBS Lett 580:2879-2887. [DOI] [PubMed] [Google Scholar]
- Sakai VT, Zhang Z, Dong Z, Neiva KG, Machado MA, Shi S, et al. (2010). SHED differentiate into functional odontoblasts and endothelium. J Dent Res 89:791-796. [DOI] [PubMed] [Google Scholar]
- Tran-Hung L, Laurent P, Camps J, About I. (2008). Quantification of angiogenic growth factors released by human dental cells after injury. Arch Oral Biol 53:9-13. [DOI] [PubMed] [Google Scholar]
- Xu J, Liu X, Jiang Y, Chu L, Hao H, Liua Z, et al. (2008). MAPK/ERK signalling mediates VEGF-induced bone marrow stem cell differentiation into endothelial cell. J Cell Mol Med 12:2395-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. (1993). flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development 118:489-498. [DOI] [PubMed] [Google Scholar]
- Zhang P, Moudgill N, Hager E, Tarola N, Dimatteo C, McIlhenny S, et al. (2011). Endothelial differentiation of adipose-derived stem cells from elderly patients with cardiovascular disease. Stem Cells Dev 20:977-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Neiva KG, Lingen MW, Ellis LM, Nör JE. (2010). VEGF-dependent tumor angiogenesis requires inverse and reciprocal regulation of VEGFR1 and VEGFR2. Cell Death Differ 17:499-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.