Abstract
This study determined if dentin proteases are denatured by phosphoric acid (PA) used in etch-and-rinse dentin adhesives. Dentin beams were completely demineralized with EDTA for 30 days. We “acid-etched” experimental groups by exposing the demineralized dentin beams to 1, 10, or 37 mass% PA for 15 sec or 15 min. Control beams were not exposed to PA but were incubated in simulated body fluid for 3 days to assay their total endogenous telopeptidase activity, by their ability to solubilize C-terminal crosslinked telopeptides ICTP and CTX from insoluble dentin collagen. Control beams released 6.1 ± 0.8 ng ICTP and 0.6 ± 0.1 ng CTX/mg dry-wt/3 days. Positive control beams pre-incubated in p-aminophenylmercuric acetate, a compound known to activate proMMPs, released about the same amount of ICTP peptides, but released significantly less CTX. Beams immersed in 1, 10, or 37 mass% PA for 15 sec or 15 min released amounts of ICTP and CTX similar to that released by the controls (p > 0.05). Beams incubated in galardin, an MMP inhibitor, or E-64, a cathepsin inhibitor, blocked most of the release of ICTP and CTX, respectively. It is concluded that PA does not denature endogenous MMP and cathepsin activities of dentin matrices.
Keywords: demineralized, MMPs, cathepsins, collagen, bonding
Introduction
Matrix metalloproteinases (MMPs) have been implicated in various physiological and pathological processes of the pulpodentin complex (Tjäderhane et al., 2001; Bourd-Boittin et al., 2005; Lehmann et al., 2009). Detection of MMPs in mineralized dentin (Martin-De Las Heras et al., 2000; Sulkala et al., 2004, 2007; Mazzoni et al., 2007, 2011) and carious dentin (Tjäderhane et al., 1998; Chaussain-Miller et al., 2006) indicates that MMPs trapped within peripheral dentin may be activated once they are exposed by acid demineralization. The recent discovery of cysteine cathepsins in normal and carious dentin (Nascimento et al., 2011; Tersariol et al., 2010; Scaffa et al., 2012) increases the list of potential endogenous proteases in dentin matrices. Demineralization of the sound, unaffected collagen matrix exposes endogenous dentin proteases, although most of them remain bound to the collagen (Martin-De Las Heras et al., 2000).
Dentin demineralization is also involved in dentin bonding. In etch-and-rinse bonding procedures, 32 to 37 mass% phosphoric acid (PA) is used to expose the collagen fibril meshwork for micromechanical retention of adhesive resins (Nakabayashi and Pashley, 1998). Although a small fraction of these proteases may be extracted by acids (Mazzoni et al., 2007; DeMunck et al., 2009), most remain bound to the matrix in their active forms, where they can slowly hydrolyze the collagen matrix (Pashley et al., 2004).
Traditionally, investigators have extracted proteases from the dentin matrix for identification by Western blotting and evaluation of their functional activity by zymography (Tjäderhane et al., 1998; Martin-De Las Heras et al., 2000; Sulkala et al., 2004, 2007; Mazzoni et al., 2007; Breschi et al., 2010). In those studies, it was assumed that soluble proteases functioned similarly to those that were matrix-bound, and that all proteases were equally extractable. An alternative approach is to study the functional activity of matrix-bound MMPs in situ. This approach measures the end results of protease activity by quantifying the rate of solubilization of hydroxyproline-containing peptide fragments (Garnero et al., 1998), or the rate of release of collagen C-terminal telopeptides by cysteine cathepsins and MMPs (CTX and ICTP, respectively), as these proteases slowly degrade the dentin matrix (Garnero et al., 2003; Fedarko et al., 2004; Osorio et al., 2011). The advantage of this indirect approach is that one may assay the total protease activities of the dentin matrix in their natural bound state, and their responses to activators and inhibitors.
Clinical acid-etching of dentin with 37% PA has been reported to decrease MMP activity (Pashley et al., 2004; Mazzoni et al., 2006; Nishitani et al., 2006). It is not clear if this is due to denaturation of MMPs or the creation of CaHPO4 precipitates over the enzymes. To differentiate between these 2 possibilities, we demineralized dentin beams completely in EDTA to remove all mineral. This prevented any calcium precipitates from forming when such dentin was “acid-etched” with PA. Thus, the conditions used in this experiment were far removed from clinical conditions.
If one completely demineralizes dentin with ethylenediaminetetra-acetic acid (EDTA), most of the matrix-bound proteases remain on the dentin matrix (Martin-De Las Heras et al., 2000). One may then expose the matrix-bound MMPs and cathepsins to various concentrations of PA without the buffering effects of apatite mineral. If matrix-bound MMPs and cathepsins of EDTA-demineralized dentin are already active, and if they are susceptible to denaturation by PA, then acid-etching EDTA-demineralized dentin matrix with PA should denature MMPs and cathepsins and reduce their activities.
Thus, the purpose of this work was to measure the total functional MMP and cathepsin enzyme activities of EDTA-demineralized dentin matrices before and after exposure to various concentrations of PA. The null hypotheses were that EDTA-demineralization of human dentin does not activate protease enzymes, and that further exposure of EDTA-demineralized dentin to PA does not inactivate dentin MMP or cathepsin activity.
Materials & Methods
Measurement of Matrix-bound Endogenous Proteases in Demineralized Dentin
Seventy extracted human third molars were obtained from 18- to 21-year-old patients under a protocol approved by the Georgia Health Sciences University. The teeth were stored frozen until required. After they were thawed, the enamel and superficial dentin were removed by means of an Isomet saw (Buehler Ltd., Lake Bluff, IL, USA) under water cooling. Dentin beams with dimensions 6 x 2 x 1 mm were sectioned from the mid-coronal dentin (70 beams, 1 per tooth).
The beams were completely demineralized in 0.5 M EDTA (pH 7.4) for 30 days at 4°C with constant stirring. Demineralization was followed by serial measurements of beam stiffness by 3-point loading. Mineralized dentin has a modulus of elasticity between 16,000 and 19,000 MPa. Dentin beams completely demineralized in EDTA have a modulus of elasticity of 4 to 6 MPa (Carrilho et al., 2009). Ten beams were assigned to each of 7 groups. Group 1 was the negative control, which was not treated with any acid. Group 2 medium contained the same medium as group 1 plus 2 mM p-aminophenylmercuric acetate (APMA), an agent known to activate pre-forms of MMPs. After 2 hrs of incubation in APMA, the beams were transferred to APMA-free incubation buffer. Groups 3, 4, and 6 were treated with 1, 10, or 37% PA, respectively, for 15 min to determine if increasingly higher PA concentrations would cause more denaturation. Group 5 specimens were treated with 37% PA for 15 sec to simulate normal clinical etching to serve as controls for the prolonged etching for 15 min used in groups 3, 4, and 6. Group 7 beams were incubated in the same buffer as controls but contained 200 µM galardin, an MMP inhibitor, plus 50 µM E-64, a cathepsin inhibitor. All beams exposed to PA or APMA were then dropped into 50 mL of buffered medium (pH 7) for 5 min to dilute the reagents and buffer the specimens. The pH values of 1, 10, and 37% PA were 1.42, 0.51, and -0.37, respectively. Each beam was then immersed in 0.5 mL of a buffered medium composed of 5 mM HEPES, 2.5 mM CaCl2 .H2O, 0.05 mM ZnCl2, and 120 mM NaCl adjusted to pH 7.4. The sealed tubes were incubated in a shaker-water bath at 37°C for 3 days. The entire 0.5 mL of medium was removed after 3 days. From 10- to 20-µL aliquots of the incubation medium were used to measure solubilized ICTP and CTX collagen fragments.
Solubilized Telopeptides
We determined matrix degradation by MMPs by measuring the quantity of solubilized type I collagen C-terminal cross-linked telopeptides (ICTP) (Garnero et al., 2003; Osorio et al., 2011) over the 3-day incubation periods, using the ICTP ELISA kit (TSZ ELISA, Framingham, MA, USA; Cat. #HU9655). The only source of ICTP telopeptide fragments in mineralized matrices is attributed to the telopeptidase activity of MMPs (Garnero et al., 1998, 2003; Osorio et al., 2011). We determined matrix degradation by cysteine cathepsins by measuring the amount of solubilized C-terminal peptide, CTX, in the incubation medium using the Serum CrossLaps ELISA (Immunodiagnostic System, Scottsdale, AZ, USA).
Statistical Analyses
The ICTP and CTX release rates (in ng telopeptide/mg dry dentin per 3 days) from all groups were compared for normality (Kolmogorov-Smirnov test) and homoscedasticity (modified Levine test). Since the normality and equality variance assumptions of the data were valid, they were analyzed by 2 different one-way analyses of variance (ANOVAs) (one for ICTP and the other for CTX), with dentin treatment (APMA PA, etc.) as the single factor. Post hoc multiple comparisons were performed with the Tukey test using SigmaStat 3.11 (Systat Software, San Jose, CA, USA). Statistical significance was pre-set at α = 0.05.
Results
For the negative control group that was never exposed to PA (incubated in SBF only), the EDTA-demineralized dentin beams released 6.1 ± 0.8 ng ICTP and 0.6 ± 0.1 ng CTX per mg dentin dry-weight/3 days (Fig.). For the positive control group that was incubated in 2 mM APMA to activate MMP proforms, the ICTP release rate remain unchanged compared with controls, but APMA caused a large, significant (p < 0.05) decrease in CTX release (Fig.). The rate of release of ICTP from EDTA-demineralized beams did not change significantly as the PA concentration or exposure time increased. Group 7 specimens were incubated in a buffered medium containing 200 µM galardin, an MMP inhibitor, and E-64, a cathepsin inhibitor. The presence of both inhibitors significantly reduced (p < 0.05) the rate of release of ICTP and CTX to near zero, indicating that ICTP and CTX are released from dentin collagen by MMPs and cathepsins. Other experiments in which demineralized dentin beams were incubated in only galardin or only E-64 revealed that their action was limited to MMPs or cathepsins, respectively (data not shown). The rate of release of CTX from EDTA-demineralized dentin was unchanged as a function of PA concentration or exposure time. These values were not significantly different from those of the corresponding negative controls (Fig.). Treatment of dentin with APMA or galardin/E-64 was the only variable that significantly (p < 0.05) lowered CTX values below those of the control.
Figure.
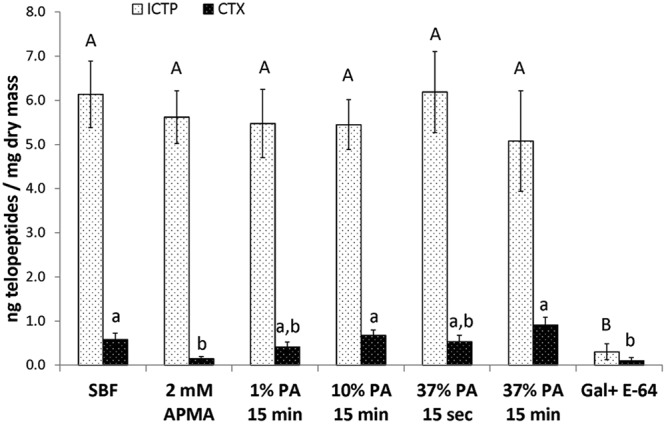
Bar chart of C-terminal telopeptide (ICTP and CTX) release from EDTA-demineralized dentin beams after various treatments with increasing concentrations of phosphoric acid. Values are ng telopeptide/mg dentin dry-wt/3 days. Bar heights are mean values (n = 10); brackets indicate ± SD. Bars identified by different letters are significantly different. APMA = 2 mM p-aminophenylmercuric acetate pre-treatment for 2 hrs at 37°C. PA = phosphoric acid. SBF = simulated body fluid. Bars identified with similar upper-case letters are not significantly different (p > 0.05) by one-way ANOVA for effects of treatments on ICTP release rate. Bars identified by different lower-case letters are significantly different (p < 0.05) by one-way ANOVA followed by Tukey’s test for effects of treatments on CTX release rate. Gal/E-64 refers to group 7, where the incubation medium contained 200 µM galardin, a potent MMP inhibitor, and E-64, a cathepsin inhibitor.
Discussion
Osorio et al. (2011) were the first to demonstrate that MMP-2 has telopeptidase activity in dentin, although they did not use that term. Using an ICTP-specific ELISA assay, they incubated completely demineralized dentin beams in a medium for 1, 7, or 21 days. The control media contained 0.87 ± 0.11 µg/L ICTP per 24 hrs. When they added 10 µg of exogenous active recombinant human MMP-2, the ICTP concentration increased to 210 ± 11 µg/L in the media. This indicated that MMP-2 attacked the C-terminal telopeptides in demineralized dentin, to release cross-linked ICTP fragments. The results of the present study confirm that endogenous dentin MMPs-2, -8, and -9, known to exist in the dentin matrix (Mazzoni et al., 2007; Sulkala et al., 2007), do indeed release ICTP from dentin matrix. In addition, endogenous cathepsins also attack C-terminal telopeptides in dentin matrix to release the smaller CTX peptide fragments.
The total MMP activity of EDTA-demineralized dentin, measured as the release of ICTP telopeptide fragments, is an order of magnitude larger than the total cysteine cathepsin activity of demineralized dentin, measured as the release of CTX telopeptide fragments. By measuring the relative amounts of ICTP and CTX telopeptide fragments in the incubation medium, one can follow the relative amounts of MMPs vs. cathepsin protease activity. Thus, the dentin matrix contains 2 classes of endogenous proteases that can act as telopeptidases for demineralized collage matrix degradation. Both ICTP and CTX release were inhibited by galardin and E-64, respectively. Future work will identify their relative collagenolytic activity.
The results of this work require rejection of the first null hypothesis, that EDTA-demineralization of human dentin does not activate protease enzymes. That is, EDTA-demineralized control dentin exhibited significant MMP and cathepsin activity without the use of acids or APMA to activate proforms of dentin MMPs, confirming previous findings (Carrilho et al., 2009).
Treatment of the demineralized dentin with 2 mM APMA for 1 hr at 37°C did not activate matrix-bound MMPs. Rather, APMA treatment significantly lowered the total cathepsin activity of dentin because it reduced the release of CTX peptide fragments, indicating that some cathepsins were already active. Organic mercurials are known to oxidize with sulfuryl groups in proteins, causing their inactivation (Means and Feeney, 1971; Pasternak et al., 1975).
It is possible that the MMP proforms are activated by members of the small integrin-binding ligand N-linked glycoproteins (SIBLINGS) in dentin. Bone sialoprotein activates proMMP-2; dentin matrix protein-1 activates MMP-9 (Fedarko et al., 2004). MMP-2 is known to activate other proforms of MMPs (Nagase, 1997). Cathepsins are also known to activate MMPs (Tersariol et al., 2010).
Our previous work indicated that acid-etching mineralized dentin powder with 37% PA for 15 sec caused a large (65%) reduction in the collagenolytic activity of that powder (Pashley et al., 2004). Similar results were obtained by Nishitani et al. (2006). Mazzoni et al. (2006) acid-etched dentin powder with 10% PA for 15 sec, resulting in 98.1% reduction in collagenolytic activity compared with mineralized dentin powder control. These results were interpreted as being due to very rapid exposure of the collagen matrix and its matrix-bound MMPs by PA-demineralization, which was followed, almost immediately, by denaturation of the MMPs by the very low pH of these solutions. However, a recent experiment on the effects of self-etching adhesives provides an alternative explanation. Iwasa et al. (2011) examined the buffering capacity of dentin powder exposed to single-step, self-etching adhesives. The baseline pHs of the self-etching adhesives before etching ranged from 0.97 to 2.83. After the adhesives were mixed with dentin powder for 3 min, the pH values rose to 6.3-7.11. Scanning electron microscopy examination of the etched dentin powder revealed that the surfaces were covered with a “dense, insoluble precipitate” that obscured the fibrillar nature of the underlying collagen matrix. We believe that such precipitates are reaction products of the interaction of acids with dentin matrix apatite that mask the collagen fibrils from matrix proteases.
The present paper investigated the question from a different perspective. By completely demineralizing dentin powder with 0.5 M EDTA, and then exposing the demineralized powder to PA, there is no possibility of a layer of CaHPO4 forming over the demineralized collagen fibrils, because all of the calcium was removed by EDTA for 30 days. “Acid-etching” of the EDTA-demineralized beams with all concentrations of PA tested for up to 15 min had no effect on the total MMP activity of dentin (i.e., on the release of ICTP fragments). The prolonged exposure of EDTA-demineralized dentin was used to show that even under extreme conditions that are far from clinical practice, PA does not denature matrix-bound proteases. That is, there was no large decrease in MMP or cathepsin activity following exposure to PA. These results argue against the idea that 37% PA can chemically denature matrix-bound proteases. Thus, these results require acceptance of the second test null hypothesis, that exposure of EDTA-demineralized dentin to PA does not inactivate dentin MMP activity.
Within the limits of the present study, it may be concluded that treatment of dentin with 37% PA does not denature the endogenous proteases of the dentin matrix. We speculate that matrix-bound endogenous proteases are far more stable to thermal or chemical denaturation than are those same proteases in soluble form (Fernandez et al., 2002; Berberich et al., 2005; Roessl et al., 2010). The closeness of the active site of MMPs and cathepsins to their collagen-binding sites (Nagase et al., 2006; Brömme and Wilson, 2011) may stabilize collagen-bound MMPs compared with soluble forms.
Acknowledgments
The authors are grateful to Michelle Barnes for outstanding secretarial support.
Footnotes
This work was supported by grant R01 DE015306 from the National Institute of Dental and Craniofacial Research (P.I. DHP) and by grant #8126372 from the Academy of Finland (P.I. AM).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Berberich JA, Yang LW, Madura J, Bahar I, Russell AJ. (2005). A stable three-enzyme creatinine biosensor. I. Impact of structure, function and environment on PEGylated and immobilized sarcosine oxidase. Acta Biomater 1:173-181. [DOI] [PubMed] [Google Scholar]
- Bourd-Boittin K, Fridman R, Fanchon S, Septier D, Goldberg M, Menashi S. (2005). Matrix metalloproteinases inhibition impairs the processing, formation and mineralization of dental tissues during mouse molar development. Exp Cell Res 304:493-505. [DOI] [PubMed] [Google Scholar]
- Breschi L, Martin-P, Mazzoni A, Nato F, Carrilho M, Tjäderhane L, et al. (2010). Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dent Mater 26:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brömme D, Wilson S. (2011). Role of cysteine cathepsins in extracellular proteolysis. In: Extracellular matrix degradation. Parks WC, Mecham RP, editors. Berlin: Springer-Verlag, p. 26. [Google Scholar]
- Carrilho MR, Tay FR, Donnelly AM, Agee KA, Tjäderhane L, Mazzoni A, et al. (2009). Host-derived loss of dentin matrix stiffness associated with solubilization of collagen. J Biomed Mater Res B Appl Biomater 90:373-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaussain-Miller S, Fioretti F, Goldberg M, Menashi S. (2006). The role of matrix metalloproteinase (MMPs) in human caries. J Dent Res 85:22-32. [DOI] [PubMed] [Google Scholar]
- De Munck J, Van den, Steen PE, Mine A, Van Landuyt KL, Poitevin A, Opdenakker G, et al. (2009). Inhibition of enzymatic degradation of adhesive-dentin interfaces. J Dent Res 88:1101-1106. [DOI] [PubMed] [Google Scholar]
- Fedarko NS, Jain A, Karadag A, Fisher LW. (2004). Three small integrin-binding ligand N-linked glycoproteins (SIBLINGS) bind and activate specific matrix metalloproteinases. FASEB J 18:734-736. [DOI] [PubMed] [Google Scholar]
- Fernandez S, Geueke B, Delgado O, Coleman J, Hatti-Kaul R. (2002). β-Galactosidase from cold-adapted bacterium: purification, characterization, and application for lactose hydrolysis. Appl Microbiol Biotechnol 58:313-321. [DOI] [PubMed] [Google Scholar]
- Garnero P, Borrel O, Byrialsen I, Ferreras M, Drake FH, McQueney MS, et al. (1998). The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J Biol Chem 273:32347-32352. [DOI] [PubMed] [Google Scholar]
- Garnero P, Ferreras M, Karsdal MA, Nicamhlaoibh R, Risteli J, Borel O, et al. (2003). The type I collagen fragments ICTP and CTX reveal distinct enzymatic pathways of bone collagen degradation. J Bone Miner Res 18:859-867. [DOI] [PubMed] [Google Scholar]
- Iwasa M, Tsubota K, Shimamura Y, Ando S, Miyazaki M, Platt JA. (2011). pH changes upon mixing single-step self-etching adhesives with powdered dentin. J Adhes Dent 13:207-212. [DOI] [PubMed] [Google Scholar]
- Lehmann N, Debret R, Roméas A, Magloire H, Degrange M, Bleicher F, et al. (2009). Self-etching increases matrix metalloproteinase expression in the dentin-pulp complex. J Dent Res 88:77-82. [DOI] [PubMed] [Google Scholar]
- Martin-De Las Heras S, Valenzuela A, Overall CM. (2000). The matrix metalloproteinase gelatinase A in human dentin. Arch Oral Biol 45:757-765. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Mannello F, Tjäderhane L, et al. (2006). Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials 27:4470-4476. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Mannello F, Tay FR, Toni GA, Papa S, Mazotti G, et al. (2007). Zymographic analysis and characteristics of MMP-2 and -9 forms in human sound dentin. J Dent Res 86:436-440; erratum in J Dent Res 86:792, 2007. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Papa V, Nato F, Carrilho M, Tjäderhane L, Ruggeri A, et al. (2011). Immunohistochemical and biochemical assay of MMP-3 in human dentin. J Dent 39:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means GE, Feeney RE. (1971). Chemical modification of proteins. San Francisco, CA: Holden-Day Inc., pp. 217-220. [Google Scholar]
- Nagase H. (1997). Activation mechanisms of matrix metalloproteinases. Biol Chem 378:151-160. [PubMed] [Google Scholar]
- Nagase H, Visse R, Murphy G. (2006). Structure and function of metalloproteinases and TIMPs. Cardivasc Res 69:562-573. [DOI] [PubMed] [Google Scholar]
- Nakabayashi N, Pashley DH. (1998). Hybridization of dental hard tissues. Chicago, IL: Quintessence Publishing Co. [Google Scholar]
- Nascimento FD, Minciotti CL, Geraldeli S, Carrilho MR, Pashley DH, Tay FR, et al. (2011). Cysteine cathepsins in human carious dentin. J Dent Res 90:506-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani Y, Yoshiyama M, Wadgaonkar B, Elrod D, Breschi L, Mannello F, et al. (2006). Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur J Oral Sci 114:160-166. [DOI] [PubMed] [Google Scholar]
- Osorio R, Yamauti M, Osorio E, Ruiz-Requena ME, Pashley D, Tay F, et al. (2011). Effect of dentin etching and chlorhexidine application on metalloproteinase-mediated collagen degradation. Eur J Oral Sci 119:79-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. (2004). Collagen degradation by host-derived enzymes during aging. J Dent Res 83:216-221. [DOI] [PubMed] [Google Scholar]
- Pasternak GW, Wilson HA, Snyder SH. (1975). Differential effects of protein modifying reagents on receptor binding of opiate agonists and antagonists. Mol Pharmacol 11:340-351. [PubMed] [Google Scholar]
- Roessl U, Nahálka J, Nidetzky B. (2010). Carrier-free immobilized enzymes for biocatalysis. Biotechnol Lett 32:341-350. [DOI] [PubMed] [Google Scholar]
- Scaffa PM, Vidal CM, Barros N, Gesteira TF, Carmona AK, Breschi L, et al. (2012). Chlorhexidine inhibits the activity of dentin cysteine cathepsins. J Dent Res 91:420-425. [DOI] [PubMed] [Google Scholar]
- Sulkala M, Pääkkönen V, Larmas M, Salo T, Tjäderhane L. (2004). Matrix metalloproteinase-13 (MMP-13, collagenase 3) is highly expressed in human tooth pulp. Connect Tissue Res 45:231-237. [DOI] [PubMed] [Google Scholar]
- Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjäderhane L. (2007). Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch Oral Biol 52:121-127. [DOI] [PubMed] [Google Scholar]
- Tersariol IL, Geraldeli S, Minciotti CL, Nascimento FD, Pääkkönen V, Martins MT, et al. (2010). Cysteine cathepsins in human dentin-pulp complex. J Endod 36:475-481. [DOI] [PubMed] [Google Scholar]
- Tjäderhane L, Larjava H, Sorsa T, Uitto VJ, Larmas M, Salo T. (1998). The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J Dent Res 77:1622-1629. [DOI] [PubMed] [Google Scholar]
- Tjäderhane L, Palosaari H, Sulkala M, Wahlgren J, Salo T. (2001). The expression of matrix metalloproteinases (MMPs) in human odontoblasts. In: Proceedings of the International Conference on the Dentin/Pulp Complex, June 28-30, Kazusa, Japan Ishikawa T, Takahashi K, Maeda T, Suda H, Shimono M, Inoue T, editors. Tokyo, Japan: Quintessence Publishing Co., pp. 45-51. [Google Scholar]


