Abstract
Auto-degradation of collagen matrices occurs within hybrid layers created by contemporary dentin bonding systems, by the slow action of host-derived matrix metalloproteinases (MMPs). This study tested the null hypothesis that there are no differences in the activities of MMP-2 and -9 after treatment with different etch-and-rinse or self-etch adhesives. Tested adhesives were: Adper Scotchbond 1XT (3M ESPE), PQ1 (Ultradent), Peak LC (Ultradent), Optibond Solo Plus (Kerr), Prime&Bond NT (Dentsply) (all 2-step etch-and-rinse adhesives), and Adper Easy Bond (3M ESPE), Tri-S (Kuraray), and Xeno-V (Dentsply) (1-step self-etch adhesives). MMP-2 and -9 activities were quantified in adhesive-treated dentin powder by means of an activity assay and gelatin zymography. MMP-2 and MMP-9 activities were found after treatment with all of the simplified etch-and-rinse and self-etch adhesives; however, the activation was adhesive-dependent. It is concluded that all two-step etch-and-rinse and the one-step self-etch adhesives tested can activate endogenous MMP-2 and MMP-9 in human dentin. These results support the role of endogenous MMPs in the degradation of hybrid layers created by these adhesives.
Keywords: dentin bonding systems, collagen, non-collageneous proteins, hybrid layer, auto-degradation, biochemical assays
Introduction
Hybrid layers created by contemporary adhesives are unstable in aqueous environments because of hydrolytic degradation of both adhesive resins and collagen fibrils (Carrilho et al., 2007a,b; Breschi et al., 2008). It has been shown that endogenous protease enzymes bound to the dentin organic matrix can degrade the exposed collagen fibrils within the hybrid layers, if unprotected by adhesive monomers (Pashley et al., 2004; Mazzoni et al., 2006; Nishitani et al., 2006). Because of their unequivocal presence in dentin (Mazzoni et al., 2007; Sulkala et al., 2007) and their well-known ability to degrade almost any component of extracellular matrices, including native and denatured collagen (Visse and Nagase, 2003), matrix metalloproteinases (MMPs) have been considered as having a major role on degradation of poorly resin-infiltrated hybrid layers (Pashley et al., 2011).
MMPs are a family of Zn- and Ca-dependent enzymes that regulate the physiological and pathological metabolism of collagen-based tissues (Visse and Nagase, 2003). As in other collagen-based tissues, dentin contains different MMPs: collagenase MMP-8 (Sulkala et al., 2007), gelatinases MMP-2 and -9 (Martin-De Las Heras et al., 2000; Mazzoni et al., 2006, 2007, 2009, 2011a), stromelysin MMP-3 (Mazzoni et al., 2011b), and enamelysin MMP-20 (Sulkala et al., 2002). These MMPs play active roles during tooth development (Tjäderhane et al., 2002). However, when the dentin matrix mineralizes, MMPs become covered with apatitic nanocrystals, making them immobile and non-functional. As long as dentin is mineralized, its proteases remain structurally stable (Nishitani et al., 2006).
The presence of MMP-2 and -9 in human sound and carious dentin powder was assayed by zymography, Western-blot analysis, and different immunohistochemical approaches that revealed both activated and latent isoforms of these MMPs in partially demineralized dentin (Mazzoni et al., 2007, 2009). Evidence of collagenolytic and gelatinolytic activities in partially demineralized dentin treated with either etch-and-rinse or self-etch adhesives further confirmed the potential involvement of these endoproteases in the disruption of incompletely resin-infiltrated collagen fibrils within hybrid layers (Mazzoni et al., 2006; Nishitani et al., 2006). These studies were based on a fluorescent enzyme assay kit that allows for screening of the relative proteolytic activity in adhesive-treated dentin. However, although the existence of intrinsic proteolytic activity was clearly revealed, such activity could not be related to a specific enzyme. The purpose of this study was to investigate the effects of different bonding agents on MMP-2 and -9 activities, by means of a correlative analysis based on ELISA activity assay and gelatin zymography. The tested null hypothesis was that the exposures and activities of host-derived MMP-2 and -9 are not different regardless of whether dentin is exposed to etch-and-rinse or self-etch adhesive systems.
Materials & Methods
Reagents were purchased from Sigma Chemical Co. (St. Louis, MO, USA) unless otherwise specified.
Preparation of Mineralized Dentin Powder
Fifty-two extracted human third molars were obtained from anonymous individuals (average age, 29 yrs) following their signed consent under a protocol approved by the University of Trieste (Italy). Teeth, ground free of enamel, pulpal soft tissue, and cementum, were reduced to fine powder when the dentin was frozen in liquid nitrogen and triturated by means of a steel mortar/pestle (Reimiller, Reggio Emilia, Italy). The fine mineralized dentin powder was pooled, dried, and kept frozen until use.
Treatment of Dentin Powder with Adhesive Systems
Preliminary tests allowed for definition of the appropriate concentration for acid-etching and adhesive incubation time with the dentin powder. One lot of untreated mineralized dentin powder served as control for all treatments (G1). Six one-gram lots of dentin powder were treated with 1 mL of 1% phosphoric acid for 10 min to simulate the etching procedure as the first step of the etch-and-rinse bonding technique. One of these lots was not treated with adhesives and was simply left partially demineralized (G2); the other 5 lots of phosphoric-acid-etched dentin powder were mixed for 30 min with 1 mL of one of the following etch-and-rinse adhesives: G3 = Adper Scotchbond 1XT (3M ESPE, St. Paul, MN, USA); G4 = PQ1 (Ultradent, Salt Lake City, UT, USA); G5 = Peak LC (Ultradent); G6 = Optibond Solo Plus (Kerr, Orange, CA, USA); or G7 = Prime&Bond NT (Dentsply, Konstanz, Germany).
The 3 remaining lots of mineralized dentin powder were mixed for 30 min with 1 mL of self-etch adhesives: G8 = Adper Easy Bond (3M ESPE); G9 = Tri-S (Kuraray, Tokyo, Japan); or G10 = Xeno-V (Dentsply).
The adhesive was extracted from each dentin-treated powder with 1 mL of acetone, centrifuged (20,800 g for 20 min), re-suspended in acetone, and re-centrifuged twice more for proper removal of the resin (Mazzoni et al., 2006).
Assay for the Activities of MMP-2 and -9
The enzymatic activities of MMP-2 and -9 were determined with the Biotrak™ activity assay system (GE Healthcare, Buckinghamshire, UK). Protein extraction from etched and treated dentin powder was performed in 50 mM Tris-HCl buffer, pH 7.4. Standard curves were prepared, and samples were incubated in the supplier-provided assay buffer for 2 and 6 hrs, for MMP-9 and MMP-2, respectively, at 4°C. After extensive rinses, the detection reagent was added, and absorbance was read at 405 nm (Bio-Rad, Segrate Milano, Italy). Assays were performed in duplicate and completed according to the manufacturer’s instructions.
The statistical analysis was performed with all adhesives, and since values were normally distributed (Kolmogorov-Smirnov test), data were analyzed with a one-way analysis of variance (ANOVA) and Tukey’s post hoc test (p < 0.05).
Zymographic Analysis
Dentin powder aliquots were treated as described above, and specimens were re-suspended in extraction buffer (50 mM Tris-HCl, pH 6, containing 5 mM CaCl2, 100 mM NaCl, 0.1% Triton X-100, 0.1% non-ionic detergent P-40, 0.1 mM ZnCl2, 0.02% NaN3) for 24 hrs at 4°C as previously described (Breschi et al., 2010). The specimens were then sonicated for 10 min (at ≈ 30 pulses) and centrifuged for 20 min at 4°C (20,800 g); the supernatant was removed and re-centrifuged. The protein content was further concentrated by means of a Vivaspin centrifugal concentrator (10,000-kDa cut-off) for 30 min at 4ºC (15,000 g, 3 times). Total protein concentrations of dentin extracts were determined by the Bradford assay. Dentin protein aliquots were diluted in Laemmli sample buffer at a 4:1 ratio and subjected to electrophoresis under non-reducing conditions in 10% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) containing 1 mg/mL gelatin which had been fluorescently labeled with MDPF by the method of O’Grady et al. (1984). Pre-stained low-range molecular-weight SDS-PAGE standards (Bio-Rad) were used as molecular-weight markers. After electrophoresis, the gels were washed for 1 hr in 2% Triton X-100 and were then incubated in activation solution (50 mmol/L Tris-HCl, 5 mmol/L CaCl2, pH 7.4) for 48 hrs. After that, the gels were photographed under UV illumination with long-wavelength UV (Gel Doc XR System, Bio-Rad). Gelatinases (MMP-2 and -9) in the samples were analyzed in duplicate by gelatin zymography.
Results
Assay for the Activities of MMP-2 and -9
The data were shown to have a normal distribution and had equal variances. The MMP-2 and -9 activities (ng/mL) recorded in untreated mineralized, partially demineralized, and adhesive-treated dentin powder are summarized in Fig. 1. The differences among the 10 groups were highly significant (p < 0.01). With mineralized dentin powder as the control, the enzyme activity increased approximately. 9 to 83% for MMP-2 and 24 to 74% for MMP-9 in etch-and-rinse adhesives (Fig. 1A) and up to approximately 13 to 20% for MMP-2 and 23 to 48% for MMP-9 in self-etch adhesives (Fig. 1B).
Figure 1.
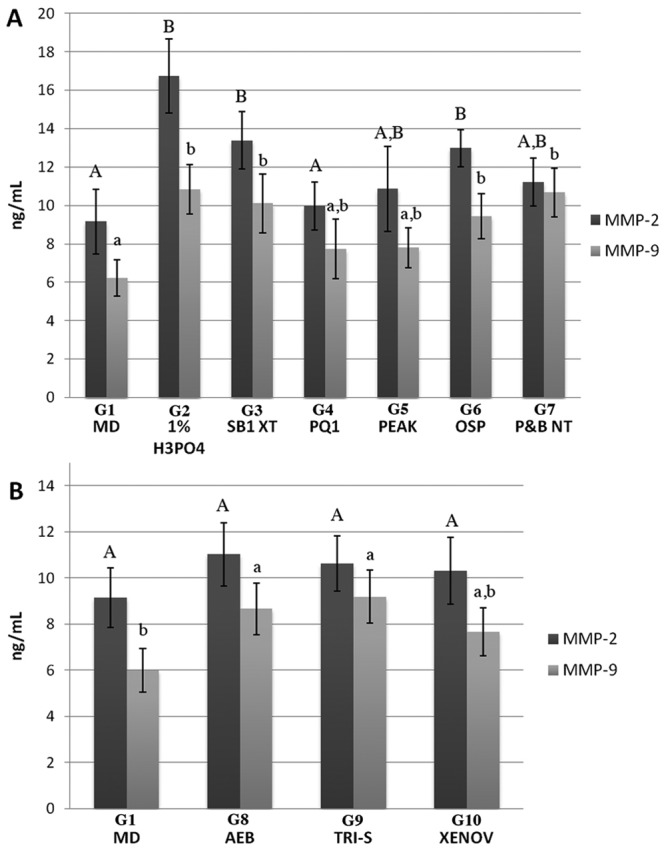
Expression of MMP-2 and -9 activities (ng/mL) obtained with the Biotrak™ activity assay system. (A) Comparison of enzyme activity in the etch-and-rinse group. Bar 1, control mineralized dentin powder (G1 - MD); Bar 2, dentin powder etched with 1% phosphoric acid solution (G2 - 1% H3PO4); Bar 3, etched-dentin plus treatment with ScotchBond 1XT (G3 - SB1XT); Bar 4, etched-dentin plus PQ-1 (G4 – PQ1); Bar 5, etched-dentin plus Peak LC (G5 - Peak); Bar 6, etched-dentin plus OptiBond Solo Plus (G6 - OSP); and Bar 7, etched-dentin plus Prime&Bond NT (G7 - P&B NT). (B) Comparison of the MMP-2 and -9 enzyme activities in the self-etch group. Bar 1, control mineralized dentin powder (G1 - MD); Bar 2, mineralized dentin treated with Adper Easy Bond (G8 - AEB); Bar 3, mineralized dentin treated with Tri-S (G9 - TRI-S); and Bar 4, mineralized dentin treated with Xeno-V (G10 - XENOV).
Zymographic Analysis
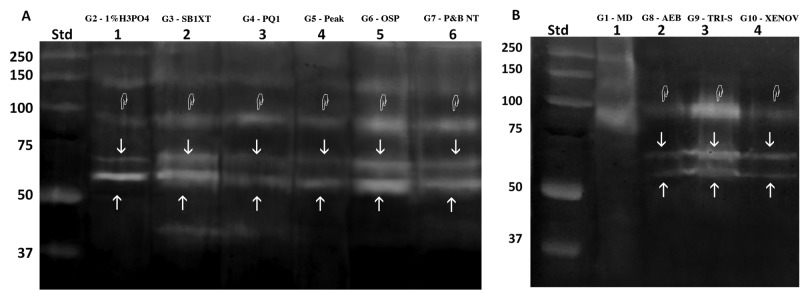
Zymograms of gelatinolytic activity are shown in Fig. 2. Zymograms of demineralized dentin (G2) showed MMP-2 pro- and active-form (72- and 66-kDa, respectively) and pro-MMP-9 (95 kDa) (Fig. 2A, Lane 1). Increased gelatinolytic activity of MMP-9 was found after treatment with all tested etch-and-rinse adhesives (G3-G7) compared with demineralized dentin. Treatment with Scotchbond 1XT (G3, Fig. 1A, lane 2), Optibond Solo Plus (G6, Fig. 2A, lane 5), and Prime&Bond NT (G7, Fig. 1A, lane 6) produced an increase of MMP-2 pro- and active-form, while the other etch-and-rinse adhesives showed decreased MMP-2 activity (G4-G5, Fig. 1A, lanes 3, 4). Treatment of mineralized dentin powder with the self-etch adhesives Adper Easy Bond and Xeno-V (G8 and G10, Fig. 2B, lanes 2, 4) increased gelatinolytic activity of MMP-2 while simultaneously decreasing pro-MMP-9. In contrast, treatment with Tri-S (G9, Fig. 2B, lane 3) produced a massive activation of both MMP-2 pro- and active-form and pro-MMP-9.
Figure 2.
Zymographic analysis of dentin powder treated with etch-and-rinse and self-etch adhesives. MMP-2 pro- and active-form (72- and 66-kDa respectively) are labeled with arrows while MMP-9 pro- and active-form (92- and 86-kDa respectively) are labeled with pointers. (A) Gelatin zymography of the etch-and-rinse group. Molecular masses, expressed in kDa, are reported in the standard lane (std). Lane 1: Proteins extracted from dentin powder demineralized with 1% phosphoric acid (G2 - 1% H3PO4), showing the presence of MMP-2 pro- and active-form (72- and 66-kDa, respectively) and pro-MMP-9 (95 kDa). Lane 2: Demineralized dentin powder after incubation with Adper Scotchbond 1XT (G3 - SB1XT) produced an increase of MMP-2 pro- and active-form and of MMP-9 compared with the demineralized dentin; in addition, a lower molecular band of approximately 40 kDa was detected. Lanes 3, 4: Incubation of demineralized dentin powder with PQ1 (G4 – PQ1) and Peak LC (G5 - Peak), respectively, produced a fainter increase in the activity of MMP-2 and similar activity in MMP-9 compared with the demineralized dentin. Lanes 5, 6: Incubation of demineralized dentin powder with Optibond Solo Plus (G6 - OSP) and Prime&Bond NT (G7 - P&B NT), respectively, produced an increase of both MMP-2 and -9 activities. (B) Zymographic analysis of mineralized dentin powder treated with self-etch adhesive systems. Relative molecular masses, expressed in kDa, are reported in the standard lane (std). Lane 1: Mineralized dentin (G1 - MD) in which faint activity was recorded. Lane 2: Incubation of mineralized dentin powder with Adper Easy Bond (G8 - AEB) produced an increase of MMP-2 activity and a fainter band corresponding to slight activity of the MMP-9 active form. Lane 3: Incubation of mineralized dentin powder with Tri-S (G9 - TRI-S) resulted in a massive activation of MMP-2 pro- and active-form and MMP-9 activity. Lane 4: Incubation of mineralized dentin powder with Xeno-V (G10 - XENOV) produced an increased gelatinolytic activity of MMP-2 with a simultaneous decrease of MMP-9.
Control zymograms incubated with 5 mM EDTA and 2 mM 1,10-phenanthroline showed no enzymatic activity (data not shown).
Discussion
The present study showed, for the first time, the differential changes in dentinal MMP-2 and -9 enzyme activities after the application of etch-and-rinse or self-etch adhesives. These findings require rejection of the null hypothesis that the activities of host-derived dentinal MMP-2 and -9 are not different regardless of the dentin bonding agents used (i.e., etch-and-rinse or self-etch).
The ability of etch-and-rinse and self-etch adhesives to increase the gelatinolytic activities of host-derived dentinal MMPs is consistent with our previous findings (Mazzoni et al., 2006; Nishitani et al., 2006). However, the innovative approach of this study includes the ability to relate the activities of the tested specific dentinal MMPs (i.e., MMP-2 and -9) selectively to a specific adhesive application by a specific assay for the activities of MMP-2 and -9 compared with non-specific gelatin zymography. In fact, in this study, the assay for the activities of MMP-2 and -9 was innovatively adopted as a suitable correlative method for determination of selective MMP activities in adhesive-treated human dentin substrates. The MMP-2 and MMP-9 activities detected with this assay first increased with acid-etching compared with the mineralized dentin control. This finding is in conflict with results of previous studies, where acid-etching resulted in significantly lower MMP activities than mineralized dentin powder (Mazzoni et al., 2006, 2011a; Nishitani et al., 2006). However, previous methods may have underestimated the presence of MMPs in demineralized dentin, since the Biotrak™ assay did not interfere with specific tissue inhibitors of MMPs (TIMPs) or non-specific MMP inhibitors. Analysis of these data provides, for the first time, direct evidence of the gelatinase activities in acid-etched dentin, since previous studies have demonstrated activity only after exposure to adhesives (Mazzoni et al., 2006, 2011a; Nishitani et al., 2006).
The activities of MMP-2 and -9 after treatment with either etch-and-rinse or self-etch adhesives were adhesive-dependent (Figs. 1A, 1B, respectively). Similarly, with self-etch adhesives, the exposure of matrix-bound MMPs was accompanied with increased activity, but sometimes showed reduced extent of activation (Figs. 1B, 2B).
Gelatin zymography is also an extremely sensitive technique mainly used for the detection of MMP-2 and -9 activities (Snoek-van Beurden and Von den Hoff, 2005), including gelatinase activities in adhesive-treated dentin (De Munck et al., 2009; Breschi et al., 2010). Our findings further support evidence of endogenous dentin MMP activation after the application of etch-and-rinse adhesives and self-etch adhesive systems. These results are in contrast to other studies that used gelatin zymography to investigate the effects of mild self-etch adhesive systems on dentinal MMP-2 and -9 activities (De Munck et al., 2009, 2010). These contrasting results may be explained by the fact that we performed the protein extraction and concentration after treatment with adhesives, enriching the concentrations of gelatinolytic enzymes. In addition, since the zymography band intensity is time-related, and incubation time may have an effect on the observed results, we incubated in activation buffer for 48 hrs, to confirm the gelatinolytic activities, while De Munck and co-workers (2009, 2010) did not report the incubation time.
It is known that MMPs can be activated in low-pH environments (Davis, 1991; Tjäderhane et al., 1998) by inducing the cysteine switch (Chaussain-Miller et al., 2006), and that etch-and-rinse and self-etch adhesives probably contribute to the activation process due to their acidity. These acidic resin monomers may, in fact, activate latent forms of MMPs (pro-MMPs) via the cysteine-switch mechanism that exposes the catalytic domains of these enzymes that were blocked by pro-peptides (Tallant et al., 2010). Relatively acidic environments also provide excellent conditions for the activation of cysteine cathepsins, another class of proteases recently described for dentin (Tersariol et al., 2010). Positive correlation between the proteolytic activities of MMPs and cysteine cathepsins in dentin were also recently demonstrated (Tersariol et al., 2010). Cathepsin B, one of the most prevalent cathepsins observed in cultured odontoblasts and pulp tissue (Tersariol et al., 2010), has been shown to be involved in the activation of MMP-1 (Cox et al., 2006). Conversely, procathepsin B can be activated by active MMPs (Hara et al., 1988). Thus, it is quite likely that, during the application of acidic monomers, not only MMPs but also cysteine cathepsins are activated, which in turn could represent the potential MMP-cathepsin interactions in the degradation of the hybrid layer collagen. The 38- to 40-kDa zymographic bands in lanes 2 and 3 of Fig. 2A were probably a cathepsin.
In addition to the acidic activation induced by etch-and- rinse and self-etching adhesives, an adjunctive contribution to the increase in MMP-2 and -9 protein levels and activity in adhesive-treated dentin could be related to the loss of inhibiting activity of TIMPs. MMP activity can be regulated at multiple levels, and TIMPs are specific inhibitors of matrixins that participate in controlling the local activities of MMPs in tissues (Brew et al., 2000). Most TIMPs inhibit active MMPs, and some TIMPs also prevent proMMP activation; for instance, TIMP-1 binds to proMMP-9, and TIMP-2 and -4 bind to pro-MMP-2, and TIMP-related inhibition of MMPs is a reversible mechanism (Visse and Nagase, 2003). TIMP-1 has been identified in root dentin (Ishiguro et al., 1994). Thus, it is possible that acid-etchants and acidic monomers in the dentin-bonding agents may play a role in MMP-2 and -9 activation by removing TIMP-1 and -2 binding to these MMPs.
In conclusion, the results of this study showed direct evidence of increased MMP-2 and -9 activities following adhesive application, regardless of the use of etch-and-rinse or self-etch adhesive systems. Since the present study design allowed for identification of the exact MMP isoforms investigated, this evidence confirms that application of these adhesives substantially increases the dentin-degrading activity exerted by MMP-2 and -9, indicating that these proteases may play a direct role in hybrid layer degradation and loss of bond strength over time. The generally higher level of activity seen in etch-and-rinse adhesives compared with self-etching adhesives seems to correlate with the more rapid destruction of hybrid layers seen in etch-and-rinse bonds, relative to self-etch adhesives. However, that difference may be due to the fact that pre-etched dentin treated with etch-and-rinse adhesives simply exposes more dentin matrix than occurs with self-etching adhesives.
Acknowledgments
The authors thank Mr. Aurelio Valmori for photographic assistance.
Footnotes
The study was funded by grants from MIUR (Italy) [FIRB RBAP1095CR to Breschi L (P.I.), PRIN 2009SAN9K5 to Breschi L (P.I.), and PRIN 2009FXT3WL to Di Lenarda R (P.I.)], grants R21 DE 091213 (P.I. FRT) and R01 DE 015306 from the NIH/NIDCR (P.I. DHP), and grants from FAPESP [07/54618-4 (P.I. MC, Brazil) and 2009/14005-9 (P.I. PS, Brazil)].
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. (2008). Dental adhesion review: aging and stability of the bonded interface. Dent Mater 24:90-101. [DOI] [PubMed] [Google Scholar]
- Breschi L, Mazzoni A, Nato F, Carrilho M, Visintini E, Tjäderhane L, et al. (2010). Chlorhexidine stabilizes the adhesive interface: a 2-year in vitro study. Dent Mater 26:320-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K, Dinakarpandian D, Nagase H. (2000). Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta 1477:267-283. [DOI] [PubMed] [Google Scholar]
- Carrilho MR, Carvalho RM, de Goes MF, di Hipólito V, Geraldeli S, Tay FR, et al. (2007a). Chlorhexidine preserves dentin bond in vitro. J Dent Res 86:90-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrilho MR, Geraldeli S, Tay F, de Goes MF, Carvalho RM, Tjäderhane L, et al. (2007b). In vivo preservation of hybrid layer by chlorhexidine. J Dent Res 86:529-533. [DOI] [PubMed] [Google Scholar]
- Chaussain-Miller C, Fioretti F, Goldberg M, Menashi S. (2006). The role of matrix metalloproteinases (MMPs) in human caries. J Dent Res 85:22-32. [DOI] [PubMed] [Google Scholar]
- Cox SW, Eley BM, Kiili M, Asikainen A, Tervahartiala T, Sorsa T. (2006). Collagen degradation by interleukin-1beta-stimulated gingival fibroblasts is accompanied by release and activation of multiple matrix metalloproteinases and cysteine proteinases. Oral Dis 12:34-40. [DOI] [PubMed] [Google Scholar]
- Davis GE. (1991). Identification of an abundant latent 94 kDa gelatin-degrading metalloproteinase in human saliva which is activated by acid-exposure: implications for a role in digestion of collagenous proteins. Arch Biochem Biophys 286:551-554. [DOI] [PubMed] [Google Scholar]
- De Munck J, Van den Steen PE, Mine A, Van Landuyt KL, Poitevin A, Opdenakker G, et al. (2009). Inhibition of enzymatic degradation of adhesive-dentin interfaces. J Dent Res 88:1101-1106. [DOI] [PubMed] [Google Scholar]
- De Munck J, Mine A, Van den Steen PE, Van Landuyt KL, Poitevin A, Opdenakker G, et al. (2010). Enzymatic degradation of adhesive-dentin interfaces produced by mild self-etch adhesives. Eur J Oral Sci 118:494-501. [DOI] [PubMed] [Google Scholar]
- Hara K, Kominami E, Katunuma N. (1988). The effect of proteinase inhibitors on intracellular processing of cathepsin B, H and L in rat macrophages. FEBS Lett 231:229-231. [DOI] [PubMed] [Google Scholar]
- Ishiguro K, Yamashita K, Nakagaki H, Iwata K, Hayakawa T. (1994). Identification of tissue inhibitor of metalloproteinases-1 (TIMP-1) in human teeth and its distribution in cementum and dentine. Arch Oral Biol 39:345-349. [DOI] [PubMed] [Google Scholar]
- Martin-De Las Heras S, Valenzuela A, Overall CM. (2000). The matrix metalloproteinase gelatinase A in human dentine. Arch Oral Biol 45:757-765. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Tjäderhane L, Toledano M, et al. (2006). Reactivation of inactivated endogenous proteolytic activities of phosphoric acid-etched dentin by etch-and-rinse adhesives. Biomaterials 27:4470-4476. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Mannello F, Tay FR, Tonti GA, Papa S, Mazzotti G, et al. (2007). Zymographic analysis and characterization of MMP-2 and -9 isoforms in human sound dentin. J Dent Res 86:436-440. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Pashley DH, Tay FR, Gobbi P, Orsini G, Ruggeri A, et al. (2009). Immunohistochemical identification of MMP-2 and MMP-9 in human dentin: correlative FEI-SEM/TEM analysis. J Biomed Mater Res A 88:697-703. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Papa V, Nato F, Carrilho M, Tjäderhane L, Ruggeri A, et al. (2011a). Immunohistochemical and biochemical assay of MMP-3 in human dentine. J Dent 39:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni A, Carrilho M, Papa V, Tjäderhane L, Gobbi P, Di Lenarda R, et al. (2011b). MMP-2 assay within the hybrid layer created by a two-step etch-and-rinse adhesive: biochemical and immunohistochemical analysis. J Dent 39:470-477. [DOI] [PubMed] [Google Scholar]
- Nishitani Y, Yoshiyama M, Wadgaonkar B, Elrod D, Breschi L, Mannello F, et al. (2006). Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur J Oral Sci 114:160-166. [DOI] [PubMed] [Google Scholar]
- O’Grady RL, Nethery A, Hunter N. (1984). A fluorescent screening assay for collagenase using collagen labeled with 2- methoxy-2,4-diphenyl-3(2H)-furanone. Anal Biochem 140:490-494. [DOI] [PubMed] [Google Scholar]
- Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. (2004). Collagen degradation by host-derived enzymes during aging. J Dent Res 83:216-221. [DOI] [PubMed] [Google Scholar]
- Pashley DH, Tay FR, Breschi L, Tjäderhane L, Carvalho RM, Carrilho M, et al. (2011). State of the art etch-and-rinse adhesives. Dent Mater 27:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoek-van Beurden PA, Von den Hoff JW. (2005). Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. Biotechniques 38:73-83. [DOI] [PubMed] [Google Scholar]
- Sulkala M, Larmas M, Sorsa T, Salo T, Tjäderhane L. (2002). The localization of matrix metalloproteinase-20 (MMP-20, enamelysin) in mature human teeth. J Dent Res 81:603-607. [DOI] [PubMed] [Google Scholar]
- Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjäderhane L. (2007). Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch Oral Biol 52:121-127. [DOI] [PubMed] [Google Scholar]
- Tallant C, Marrero A, Gomis-Rüth FX. (2010). Matrix metalloproteinases: fold and function of their catalytic domains. Biochim Biophys Acta 1803:20-28. [DOI] [PubMed] [Google Scholar]
- Tersariol IL, Geraldeli S, Minciotti CL, Nascimento FD, Pääkkönen V, Martins MT, et al. (2010). Cysteine cathepsins in human dentin-pulp complex. J Endod 36:475-481. [DOI] [PubMed] [Google Scholar]
- Tjäderhane L, Larjava H, Sorsa T, Uitto VJ, Larmas M, Salo T. (1998). The activation and function of host matrix metalloproteinase in dentin matrix during breakdown in caries lesions. J Dent Res 77:1622-1629. [DOI] [PubMed] [Google Scholar]
- Tjäderhane L, Palosaari H, Sulkala M, Wahlgren J, Salo T. (2002). The expression of matrix metalloproteinases (MMPs) in human odontoblasts. Proceedings of the International Conference on the Dental/Pulp Complex Ishikawa T, Takahashi K, Maeda T, Suda H, Shimono M, Inoue T, editors. Tokyo, Japan: Quintessence Publishing Co. Ltd., pp. 45-51. [Google Scholar]
- Visse R, Nagase H. (2003). Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92:827-839. [DOI] [PubMed] [Google Scholar]