Abstract
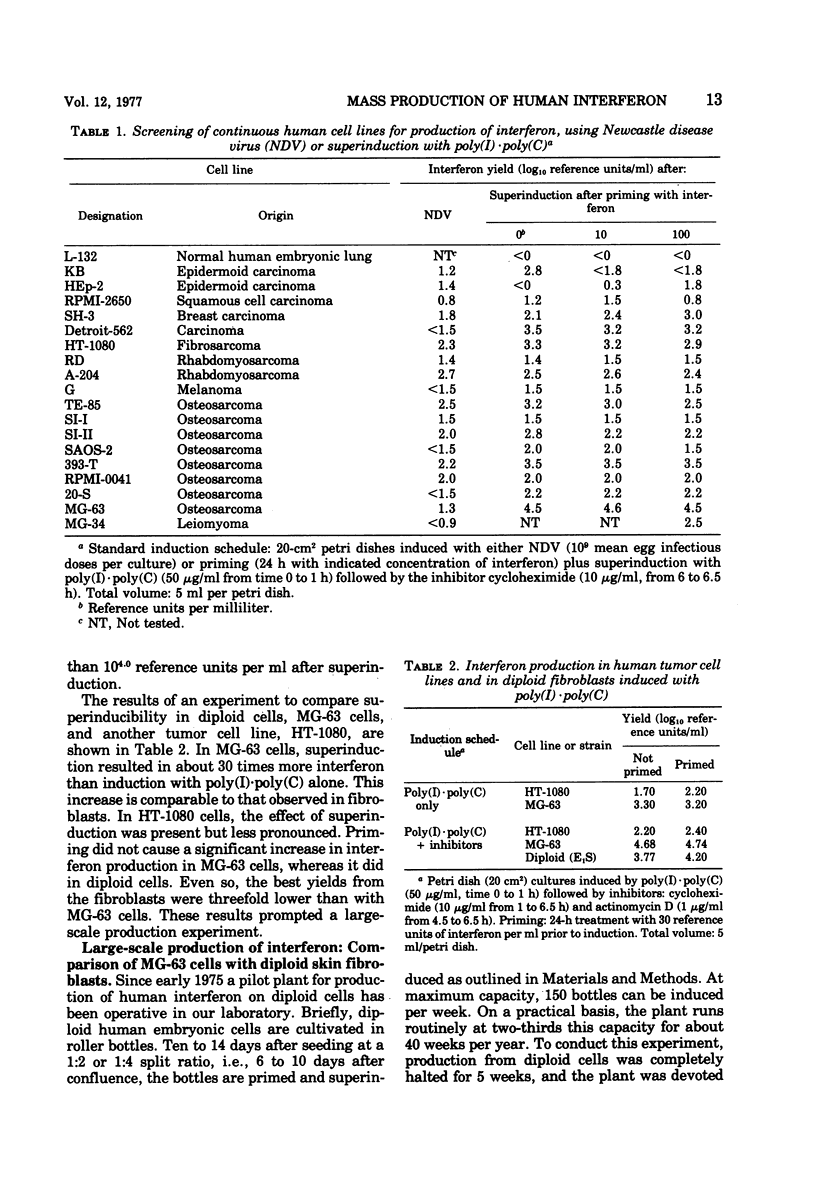
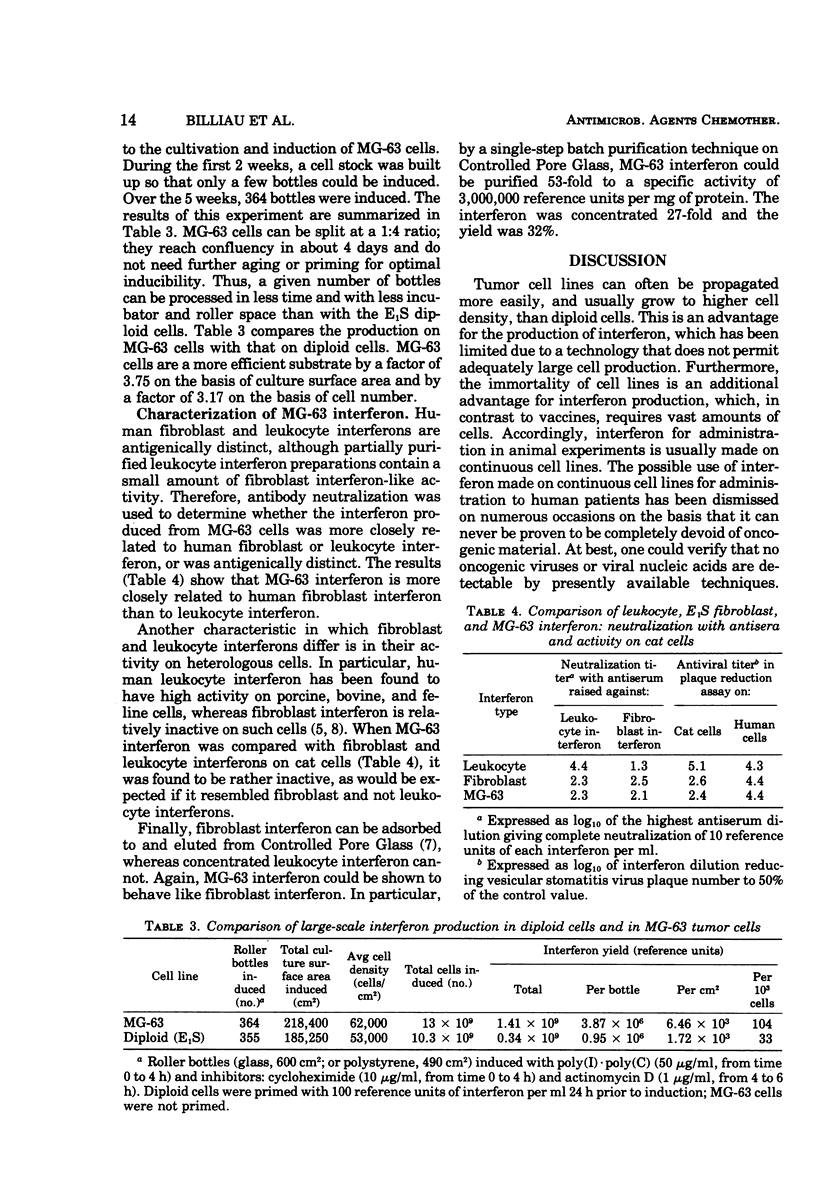
MG-63 cells, a line derived from an osteosarcoma, produced high yields of interferon after superinduction with polyinosinic acid·polycytidylic acid, cycloheximide, and actinomycin D. Advantages of MG-63 cells over diploid fibroblasts as a substrate are: no requirement for aging between confluency and induction, no requirement for priming, and 3.7-fold higher yields per square centimeter of culture surface. Physicochemically and biologically, MG-63 cell interferon resembles fibroblast rather than leukocyte interferon.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billiau A., Cassiman J. J., Willems D., Verhelst M., Heremans H. In vitro cultivation of human tumor tissues. Oncology. 1975;31(5-6):257–272. doi: 10.1159/000225032. [DOI] [PubMed] [Google Scholar]
- Billiau A., Joniau M., De Somer P. Mass production of human interferon in diploid cells stimulated by poly-I:C. J Gen Virol. 1973 Apr;19(1):1–8. doi: 10.1099/0022-1317-19-1-1. [DOI] [PubMed] [Google Scholar]
- Cantell K., Hirvonen S., Mogensen K. E., Pyhälä L. Human leukocyte interferon: production, purification, stability, and animal experiments. In Vitro Monogr. 1974;(3):35–38. [PubMed] [Google Scholar]
- De Somer P., Joniau M., Edy V. G., Billiau A. Mass production of human interferon in diploid cells. In Vitro Monogr. 1974;(3):39–46. [PubMed] [Google Scholar]
- Desmyter J., De Groote J., Desmet V. J., Billiau A., Ray M. B., Bradburne A. F., Edy V. G., De Somer P. Administration of human fibroblast interferon in chronic hepatitis-B infection. Lancet. 1976 Sep 25;2(7987):645–647. doi: 10.1016/s0140-6736(76)92460-0. [DOI] [PubMed] [Google Scholar]
- Desmyter J., Stewart W. E., 2nd Molecular modification of interferon: attainment of human interferon in a conformation active on cat cells but inactive on human cells. Virology. 1976 Apr;70(2):451–458. doi: 10.1016/0042-6822(76)90286-5. [DOI] [PubMed] [Google Scholar]
- Edy V. G., Braude I. A., De Clercq E., Billiau A., De Somer P. Purification of interferon by adsorption chromatography on controlled pore glass. J Gen Virol. 1976 Dec;33(3):517–521. doi: 10.1099/0022-1317-33-3-517. [DOI] [PubMed] [Google Scholar]
- Gresser I., Bandu M. T., Brouty-boye D., Tovey M. Pronounced antiviral activity of human interferon on bovine and porcine cells. Nature. 1974 Oct 11;251(5475):543–545. doi: 10.1038/251543a0. [DOI] [PubMed] [Google Scholar]
- Havell E. A., Berman B., Ogburn C. A., Berg K., Paucker K., Vilcek J. Two antigenically distinct species of human interferon. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2185–2187. doi: 10.1073/pnas.72.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A., Vilcek J. Production of high-titered interferon in cultures of human diploid cells. Antimicrob Agents Chemother. 1972 Dec;2(6):476–484. doi: 10.1128/aac.2.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen K. E., Pyhälä L., Cantell K. Raising antibodies to human leukocyte interferon. Acta Pathol Microbiol Scand B. 1975 Oct;83(5):443–450. doi: 10.1111/j.1699-0463.1975.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Strander H., Mogensen K. E., Cantell K. Production of human lymphoblastoid interferon. J Clin Microbiol. 1975 Jan;1(1):116–117. doi: 10.1128/jcm.1.1.116-117.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y. H., Armstrong J. A., Ke Y. H., Ho M. Regulation of cellular interferon production: enhancement by antimetabolites. Proc Natl Acad Sci U S A. 1970 Sep;67(1):464–471. doi: 10.1073/pnas.67.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y. H., Creagan R. P., Ruddle F. H. The somatic cell genetics of human interferon: assignment of human interferon loci to chromosomes 2 and 5. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2251–2255. doi: 10.1073/pnas.71.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]



