Abstract
Background
Estimated glomerular filtration rate (eGFR) is very important in clinical practice, although it is not adequately tested in different populations. We aimed at establishing the best eGFR formulas for a Brazilian population with emphasis on the need for race correction.
Methods
We evaluated 202 individuals with chronic kidney disease (CKD) and 42 without previously known renal lesions that were additionally screened by urinalysis. Serum creatinine and plasma clearance of iohexol were measured in all cases. GFR was estimated by the Mayo Clinic, abbreviated Modification of Diet in Renal Disease (MDRD) and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formulas, and creatinine clearance was estimated by the Cockcroft-Gault (CG) formula. Plasma clearance of iohexol was used as the gold standard for GFR determination and for the development of a Brazilian formula (BreGFR).
Results
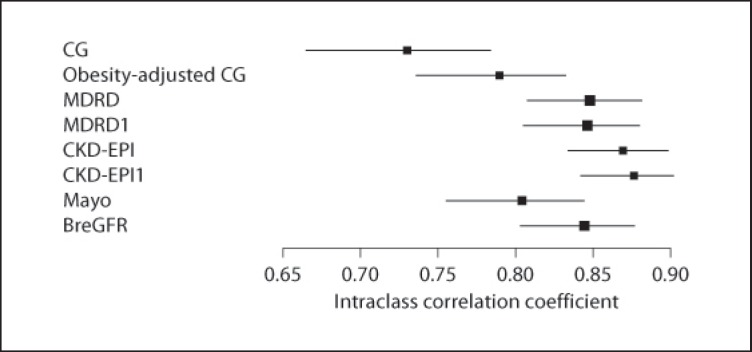
Measured and estimated GFR were compared in 244 individuals, 57% female, with a mean age of 41 years (range 18–82). Estimates of intraclass correlation coefficients among the plasma clearance of iohexol and eGFR formulas were all significant (p < 0.001) and corresponded to the following scores: CG 0.730; obesity-adjusted CG 0.789; Mayo Clinic 0.804; MDRD 0.848; MDRD1 (without race adjustment) 0.846; CKD-EPI 0.869; CKD-EPI1 (without race adjustment) 0.876, and BreGFR 0.844.
Conclusions
All cited eGFR formulas showed a good correlation with the plasma clearance of iohexol in the healthy and diseased conditions. The formulas that best detected reduced eGFR were the BreGFR, CKD-EPI, and CKD-EPI1 formulas. Notably, the race correction included in the MDRD and CKD-EPI formulas was not necessary for this population, as it did not contribute to more accurate results.
Key Words: Biomarkers, Creatinine clearance, Glomerular filtration rate, Glomerulonephritis
Introduction
Renal function is mainly measured by glomerular filtration rate (GFR). Presently, serum creatinine is the endogenous marker most commonly used in clinical practice to monitor renal function, although its concentration presents considerable intra- and inter-individual variations, and there are many interfering factors that make its determination imprecise in certain circumstances [1]. To increase the accuracy of this test, which is an easily available and simple laboratory determination, formulas for estimating GFR were developed. The following formulas are used, among others: Cockcroft-Gault (CG) [2], CG with correction for obese subjects [3], 4- and 6-parameter Modification of Diet in Renal Disease (MDRD), Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [4], and Mayo Clinic [5]. Most of these formulas are used in adults, but some were developed for application in children, such as those proposed by Schwartz and Work [6]. The formulas cited use serum creatinine (eventually associated to other laboratory analytes) as the main marker, others use serum cystatin C [7] or both.
GFR estimates are an approximation of true GFR that attempt to overcome the limitations of serum creatinine and creatinine clearance without increasing costs and time loss. Variability in GFR estimation is partially determined by interassay serum creatinine differences in different laboratories [8].
It is already known that values estimated by the CG formula tend to be higher than true GFR estimates by other GFR formulas; this overestimation may be due to the fact that the CG formula predicts creatinine clearance, which involves both true GFR and creatinine excretion via tubular secretion. In the case of large adiposity or edema, the inclusion of a weight coefficient in the formula causes a GFR overestimation. It is noteworthy that the formula of the MDRD study proved to be inadequate to predict normal GFR, especially for upper normal levels of GFR.
In addition, some eGFR formulas involve race adjustment factors to provide better performance in multiracial populations. Such correction factors may not be adequate in populations that have a widely different ethnic composition compared to those for which the original formula was developed. To study the effects of racial differences, we evaluated different eGFR formulas in a Brazilian population that has a multiethnic composition.
Materials and Methods
Study Participants
We evaluated 244 individuals, of whom 138 (57%) were female. The mean age of males was 40.6 ± 15.5 years (range 18–82) and of females 42.6 ± 14.4 years (range 18–81). Race was evaluated for 230 participants: 3 (1.3%) were of Asian descent, 119 (51.7%) of Caucasian, and 18 (7.8%) of African descent, and 90 (39.1%) were biracial (mixed Caucasian and African descent). Of the subjects, 202 had chronic kidney disease (CKD); most of these had glomerulopathies and were followed in the Glomerulopathy Outpatient Clinic of the Federal University of São Paulo (Brazil). As a control group, 42 subjects without previously known renal disease were screened by clinical evaluation and urine dipstick test.
This study was approved by the local ethical committee and all participants gave informed consent.
Methods
Proteinuria and Serum Albumin
Proteinuria was measured by the pyrogallol red technique (Olympus AU400), and serum albumin was determined by the bromocresol green technique (Olympus AU400).
Creatinine
Serum creatinine was determined by an automated method based on the alkaline picrate reaction in a Hitachi 912-Roche isotope dilution mass spectrometry (IDMS)-calibrated solution, and the results were expressed as mg/dl.
The estimated GFR (eGFR) was determined by the following formulas: traditional and obesity-adjusted CG, Mayo Clinic [5], MDRD [2] and CKD-EPI [4], as well as MDRD1 and CKD-EPI1 (i.e. the MDRD and CKD-EPI formulas without race correction, respectively). The eGFR results were adjusted for body surface area in each formula.
Iohexol Clearance
Each patient was given a 5-milliliter iohexol solution (Omnipaque, Sanofi) intravenously. Blood samples were obtained via an intravenous cannula in the contralateral arm at 120, 180, and 240 min after injection. Serum iohexol levels were determined by capillary electrophoresis, as described by Shihabi and Constantinescu [9]. After deproteinization of the samples by mixing with acetonitrile, the supernatants were introduced into the capillary and electrophoresed using a capillary electrophoresis system (Beckman, model 5010). GFR was calculated from the plasma clearance of iohexol using the slope intercept method (one-compartment model) and approximated to a two-compartment model using the Brochner-Mortensen equation.
Statistical Analysis
Quantitative variables were presented as median, minimum and maximum, mean and standard deviation values, and the categorical variables were presented as absolute and relative (percentage) frequencies. The reproducibility of the eGFR formulas versus iohexol clearance was estimated by the intraclass correlation coefficient. A Brazilian formula of GFR (BreGFR) was created using a multiple linear regression model.
The statistical significance corresponded to a p value <5% (p < 0.05). The statistical analysis was performed with the SPSS 15.0 software for Windows.
Results
The CG, CG with correction for obesity, MDRD, MDRD1 (without race adjustment), CKD-EPI, CKD-EPI1 (without race adjustment), and Mayo Clinic formulas for estimating GFR were applied to the group of individuals involved in the present study concerning iohexol clearance. These results are presented in table 1 and figure 1a–f. Using the available laboratory and demographic data, an additional formula intended to perform better in a Brazilian population was developed. This equation (table 1; fig. 1f) is as follows: BreGFR for females = 2.464 − 0.285(age, years) + 0.216(weight, kg) + 61.073(1/creatinine, mg/dl), and BreGFR for males = 9.734 − 0.285(age, years) + 0.216(weight, kg) + 61.073(1/creatinine, mg/dl).
Table 1.
Results of eGFR formulas in the populations of healthy and CKD individuals
| GFR measures (ml/min/1.73 m2) |
||||||
|---|---|---|---|---|---|---|
| n | mean | median | min. | max. | SD | |
| Iohexol clearance | 244 | 61.31 | 57.00 | 8.00 | 135.00 | 33.58 |
| CGa | 244 | 74.39 | 67.35 | 4.80 | 231.30 | 44.15 |
| Obesity adjusted CGa | 244 | 69.57 | 64.49 | 4.27 | 204.56 | 40.34 |
| Mayo Clinic | 244 | 74.61 | 79.05 | 5.30 | 150.10 | 41.43 |
| MDRD (Afro-descendant-adjusted) | 230 | 60.40 | 53.50 | 2.00 | 201.00 | 37.09 |
| MDRD1 (without race adjustment) | 230 | 64.87 | 59.00 | 3.00 | 244.00 | 40.41 |
| CKD-EPI | 230 | 64.64 | 58.50 | 2.00 | 149.00 | 37.55 |
| CKD-EPI1 | 230 | 68.08 | 63.00 | 3.00 | 172.00 | 39.53 |
| BreGFR | 244 | 61.54 | 58.05 | 7.33 | 160.07 | 28.67 |
a CG formula estimates creatinine clearance (not GFR).
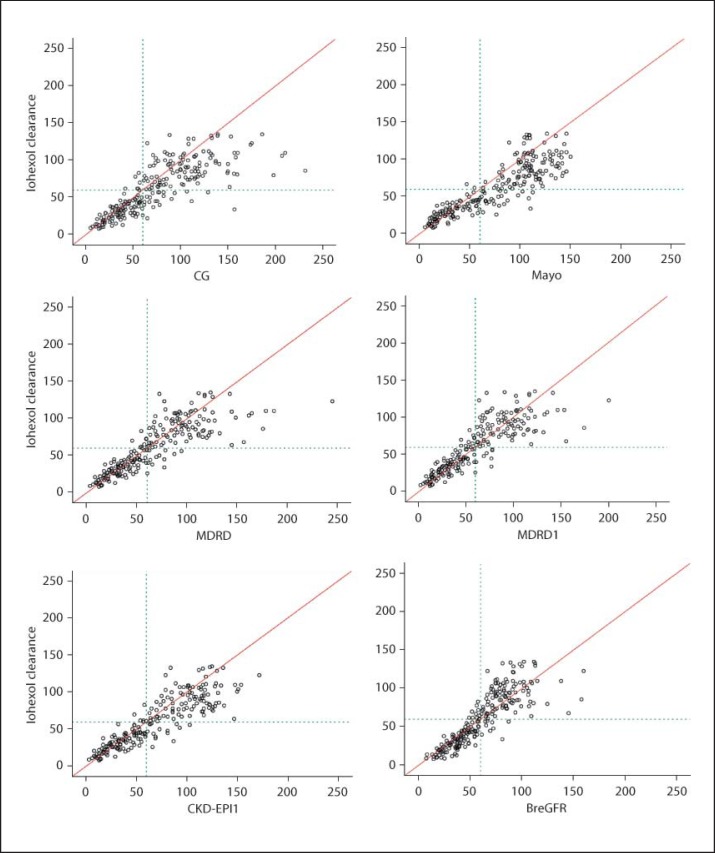
Fig. 1.
Distribution of data according to plasma clearance of iohexol and eGFR formulas (ml/min/1.73 m2).
The concordance between iohexol clearance and the tests for estimating GFR was then evaluated, and we observed a good correlation among iohexol and the CG, obesity-adjusted CG, Mayo Clinic, MDRD1, MDRD, CKD-EPI, and CKD-EPI1 formulas, respectively. In figure 1, the circles next to the 45° line indicate a good correlation (reproducibility of measures); the circles above the line indicate values that are underestimated, and those below are overestimated based on iohexol clearance as the gold standard.
The CG, obesity-adjusted CG, MDRD, MDRD1, and Mayo Clinic formulas showed a tendency to overestimate iohexol clearance. Notably, the CKD-EPI, CKD-EPI1, and BreGFR formulas provided a more precise estimate of iohexol clearance levels <60 ml/min as opposed to more elevated levels. This result can be observed in figure 1, in which the horizontal line represents the value of 60 ml/min for each of the methods used to estimate GFR.
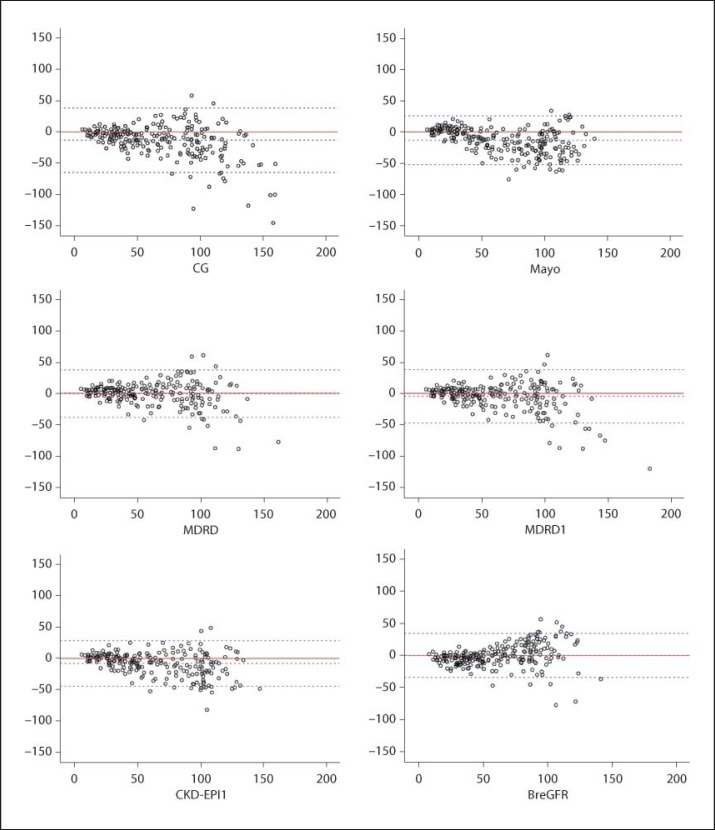
In the Bland-Altman plot presented in figure 2, the central (continuous) line represents total concordance, and the dashed lines represent the acceptable limits of discordance between these methods. The limits were not completely respected by any of the methods; however, discordance was certainly more marked for higher mean values (>100 ml/min) in each pair of measures that were compared.
Fig. 2.
Distribution of differences and mean plasma clearance of iohexol and each of the eGFR formulas.
Intraclass correlation coefficients were estimated between the plasma clearance of iohexol and the eGFR markers, and these data are presented in table 2 and figure 3. As shown, the concordance of the CG formula with iohexol clearance is statistically lower when compared to the MDRD, MDRD1, CKD-EPI, CKD-EPI1, and BreGFR formulas.
Table 2.
Estimates of intraclass correlation coefficients among iohexol clearance and eGFR formulas (CG, obesity-adjusted CG, Mayo Clinic, MDRD, MDRD1, CKD-EPI, CKD-EPI1, and BreGFR)
| Iohexol clearance vs. evaluated formulas | ICC | 95% CI (ICC) | p |
|---|---|---|---|
| CG | 0.730 | 0.665–0.783 | <0.001 |
| Obesity-adjusted CG | 0.789 | 0.736–0.832 | <0.001 |
| Mayo Clinic | 0.804 | 0.755–0.844 | <0.001 |
| MDRD | 0.848 | 0.808–0.881 | <0.001 |
| MDRD1 | 0.817 | 0.769–0.856 | <0.001 |
| CKD-EPI | 0.869 | 0.834–0.898 | <0.001 |
| CKD-EPI1 | 0.845 | 0.804–0.878 | <0.001 |
| BreGFR | 0.844 | 0.803–0.876 | <0.001 |
ICC = Intraclass correlation coefficient; CI = confidence interval.
Fig. 3.
Graphic comparison of estimates of intraclass correlation coefficients.
We considered a patient with a low GFR to have iohexol clearance ≤60 ml/min, and the cutoff points for the eGFR were established by using this parameter for comparison. These cutoffs and the sensitivity and specificity for each eGFR formula are shown in table 3.
Table 3.
Estimates of the best cutoff point, sensitivity and specificity for the CG, obesity-adjusted CG, MDRD, MDRD1, CKD-EPI, CKD-EPI1, Mayo Clinic, and BreGFR formulas, considering iohexol clearance ≤60 ml/min as the gold standard for reduced GFR
| Cutoff point | Sensitivity | 95% CI (sensitivity) | Specificity | 95% CI (specificity) | AUC | 95% CI (AUC) | |
|---|---|---|---|---|---|---|---|
| CG | 61.9 | 0.846 | 0.773–0.899 | 0.933 | 0.873–0.965 | 0.945 | 0.917–0.972 |
| Obesity-adjusted CG | 65.4 | 0.887 | 0.819–0.931 | 0.883 | 0.813–0.929 | 0.951 | 0.926–0.976 |
| Mayo Clinic | 81.7 | 0.935 | 0.877–0.966 | 0.925 | 0.863–0.960 | 0.971 | 0.953–0.990 |
| MDRD | 54 | 0.901 | 0.835–0.942 | 0.953 | 0.896–0.980 | 0.975 | 0.963–0.987 |
| MDRD1 | 61 | 0.909 | 0.845–0.948 | 0.916 | 0.849–0.955 | 0.973 | 0.958–0.988 |
| CKD-EPI | 61 | 0.909 | 0.845–0.948 | 0.935 | 0.872–0.968 | 0.974 | 0.960–0.989 |
| CKD-EPI1 | 70 | 0.942 | 0.886–0.971 | 0.898 | 0.826–0.942 | 0.974 | 0.960–0.988 |
| BreGFR | 58.48 | 0.924 | 0.868–0.961 | 0.933 | 0.874–0.966 | 0.976 | 0.960–0.992 |
CI = Confidence interval; AUC = area under the curve (ROC).
Notably, the respective cutoff points for all formulas evaluated here are adequate measures to classify the individuals as having or not having CKD or GFR deficits, as they have high sensitivity and specificity.
Discussion
In a world where the frequency of CKD is increasing, adequately measuring GFR has become a matter of concern, and estimating GFR has been widely recommended to improve the detection, evaluation, and management of CKD.
Several precise methods for the determination of GFR using inulin, the gold standard method, and isotopic markers have been developed; however, they are usually cumbersome, expensive and, consequently, not applicable in daily practice. Thus, to evaluate GFR easily and quickly, formulas have been developed to estimate GFR as accurately as possible.
Such formulas present specific limitations that are at least in part inherent to the demographic profile of the original population as the populations in which the formulas are applied are often different from those for which they were developed. Thus, researchers are still looking for an ideal formula to estimate GFR.
In this study, we used plasma iohexol clearance as the main measure of renal function (glomerular filtration); this measurement is a widely recognized and applied technique and is a gold standard for estimating GFR. In the evaluation of normal subjects and patients with CKD, most of whom have glomerular diseases and various GFR levels, iohexol clearance showed a good correlation with all estimates of GFR tested here. This result confirms the usefulness of these estimations as measures of GFR. However, the best correlations were observed between iohexol clearance and the BreGFR, CKD-EPI, and MDRD formulas.
Recently, it has been demonstrated that the CKD-EPI formula performs better in healthy people than others [8], which is easily understandable when we analyze the populations involved in the development of each formula. For example, the MDRD was created in a study involving only patients with CKD, and this aspect could explain its more reliable estimation in patients with GFR ≤60 ml/min. Nevertheless, the accuracy of the CKD-EPI formula is close to that of the MDRD [10].
Next, the limitations frequently observed in the application of eGFR formulas could have many explanations (for example, the type of population studied to develop a formula, as mentioned above). Moreover, patients with CKD may have lower muscle mass and dietary protein intake than healthy people, leading to increased errors when estimation formulas derived from diseased populations are applied to healthy people [11]. Differences in the creatinine assays can also contribute, but this error source has been minimized by assay standardization and correction for ethnic population characteristics, among other measures.
It is also necessary to emphasize that differences between the estimated and measured GFR could reflect, in part, measurement errors in the GFR and normal biologic variations of GFR, both of which are greater at higher GFR levels. Thus, reported differences would tend to overestimate the magnitude of the differences between the estimated and true GFR, especially at higher GFR levels when reported on the raw scale rather than as a percentage. Such differences represent a limitation of the GFR measurement rather than of the estimation formulas themselves [11].
We have used the correction of the CG formula created by Saracino et al. [3] for overweight and obese patients. However, in a randomly selected population such as ours, this correction did not improve the performance of the formula. Nevertheless, it is an interesting tool in studies directed specifically toward an obese population.
As all existing formulas have limitations, there has been a tendency in recent years to develop new formulas to estimate GFR. For example, the 4 variable MDRD was not adequate when applied to hospitalized Japanese patients with an eGFR <60 ml/min/1.73 m2. Therefore, the Japanese Society of Nephrology-Chronic Kidney Disease Initiatives (JSN-CKDI) coefficient was created, and this formula had good performance in an initial study [12].
In fact, according to several authors [13, 14, 15, 16], the MDRD study formula does not adequately reflect measured GFR in the Japanese population or any other Asian population, suggesting that it is necessary to develop a new formula or an ethnic correction coefficient for the MDRD study formula.
Certainly, this phenomenon is not restricted to Asians. Similar concerns exist in several countries [13, 14, 15, 16], and the same problem may occur in all populations whose ethnic compositions and levels of renal involvement, age ranges or other relevant characteristics are markedly different from those populations for whom the formulas were developed.
The Brazilian population has a peculiar ethnical composition that was recently evaluated in a comprehensive genetics-based study. It has been shown that, irrespective of their ‘color’ or ‘race’ self-identification, most Brazilians share European and African genetic ancestries and many also have a significant proportion of Amerindian ancestry [17]. In fact, the heterogeneity of our population cannot be adequately represented by arbitrary ‘race/color’ categories, and this finding represents another reason why it is not advisable to add such an imprecise parameter to formulas proposed to increase the accuracy of a laboratory test.
Nevertheless, we have tested the contribution of ‘race/color’ parameters in well-established eGFR formulas. It was shown that the correction suggested in previous studies for race (initially directed to African-Americans in the USA) has not added useful information for Brazilians, particularly when a practical and simple test is the goal of physicians and laboratories, as is the case when the intention is to estimate GFR in the general population for screening purposes.
Notably, the CG formula is widely used by students, residents, and physicians, but not the MDRD and CKD-EPI formulas; the latter two are more adequate for clinical laboratories.
Although estimating GRF is recognized as a tool for prevention and early diagnosis of CKD [18], imprecise results can be an obstacle to its use by highly respected clinical laboratories whenever the serum creatinine level is used. Thus, establishing more accurate formulas or adequate correction factors for specific populations could be a tool to generalize the use of eGFR and to contribute to a more precise evaluation of GFR based only on simple laboratory tests and formulas.
Considering the increase of CKD worldwide, estimating GFR has been advocated as a tool for early diagnosis of CKD in the general population. Although widely used, the adequacy of the available formulas for various target populations has been increasingly questioned. Awareness of population differences and the need of a more rigorous selection of formulas must be addressed. For instance, it is possible that formulas that are adequate for CKD screening are not similarly appropriate for drug dosage adjustments in special groups of patients, such as the elderly.
It is necessary to clarify that the Brazilian formula developed here is not necessarily a preferential or alternative equation to be used in our population because its performance was not markedly superior to most other equations tested within this study.
Ultimately, in this studied Brazilian population, all formulas were similarly successful, and the race adjustment was not associated with better performance, demonstrating that it is not necessary, especially as part of an automatic eGFR laboratory reporting system for CKD screening purposes, when serum creatinine is measured.
Disclosure Statement
The authors declare no conflicts of interest.
Acknowledgements
G.M.K., M.T.P., and A.R.P. are partially supported by grants from the National Council for Scientific and Technological Development (CNPq, Brazil).
References
- 1.Shemesh O, Golbetz H, Kriss JP, Myers BD. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985;28:830–838. doi: 10.1038/ki.1985.205. [DOI] [PubMed] [Google Scholar]
- 2.Bostom AG, Kronenberg F, Ritz E. Predictive performance of renal function equations for patients with chronic kidney disease and normal serum creatinine levels. J Am Soc Nephrol. 2002;13:2140–2144. doi: 10.1097/01.asn.0000022011.35035.f3. [DOI] [PubMed] [Google Scholar]
- 3.Saracino A, Morrone LF, Suriano V, Niccoli-Asabella A, Ramunni A, Fanelli M, Rubini G, Coratelli P. A simple method for correcting overestimated glomerular filtration rate in obese subjects evaluated by the Cockcroft and Gault formula: a comparison with 51Cr EDTA clearance. Clin Nephrol. 2004;62:97–103. doi: 10.5414/cnp62097. [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration), A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rule AD, Larson TS, Bergstralh EJ, et al. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009;4:1832–1843. doi: 10.2215/CJN.01640309. [DOI] [PubMed] [Google Scholar]
- 7.Eriksen BO, Mathisen UD, Melsom T, Ingebretsen OC, Jenssen TG, Njølstad I, Solbu MD, Toft I. Cystatin C is not a better estimator of GFR than plasma creatinine in the general population. Kidney Int. 2010;78:1305–1311. doi: 10.1038/ki.2010.321. [DOI] [PubMed] [Google Scholar]
- 8.Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, Hostetter T, Levey AS, Panteghini M, Welch M, Eckfeldt JH. National Kidney Disease Education Program Laboratory Working Group, Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52:5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 9.Shihabi ZK, Constantinescu MS. Iohexol in serum determined by capillary electrophoresis. Clin Chem. 1992;38:2117–2120. [PubMed] [Google Scholar]
- 10.Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol. 2010;5:1003–1009. doi: 10.2215/CJN.06870909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function – measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2478. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 12.Imai E, Horio M, Nitta K, Yamagata K, Iseki K, Tsukamoto Y, Ito S, Makino H, Hishida A, Matsuo S. Modification of the Modification of Diet in Renal Disease (MDRD) Study equation for Japan. Am J Kidney Dis. 2007;50:927–937. doi: 10.1053/j.ajkd.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Jafer TH, Schmid CH, Levey AS. Serum creatinine as marker of kidney function in South Asians: a study of reduced GFR in adults in Pakistan. J Am Soc Nephrol. 2005;16:1413–1419. doi: 10.1681/ASN.2004121100. [DOI] [PubMed] [Google Scholar]
- 14.Kang YS, Han KH, Han SY, Kim HK, Cha DR. Characteristics of population with normal serum creatinine impaired renal function and: the validation of an MDRD formula in a healthy general population. Clin Nephrol. 2005;63:258–266. doi: 10.5414/cnp63258. [DOI] [PubMed] [Google Scholar]
- 15.Mahajan S, Mukhiya GK, Singh R, Tiwari SC, Kalra V, Bhowmik DM, Gupta S, Agarwal SK, Dash SC. Assessing glomerular filtration rate in healthy Indian adults: a comparison of various prediction equations. J Nephrol. 2005;18:257–261. [PubMed] [Google Scholar]
- 16.Zuo L, Ma YC, Zhou YH, Wang M, Xu GB, Wang HY. Application of GFR-estimating equations in Chinese patients with chronic kidney disease. Am J Kidney Dis. 2005;45:463–472. doi: 10.1053/j.ajkd.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Pena SD, Di Pietro G, Fuchshuber-Moraes M, Genro JP, Hutz MH, Kehdy Fde S, Kohlrausch F, Magno LA, Montenegro RC, Moraes MO, de Moraes ME, de Moraes MR, Ojopi EB, Perini JA, Racciopi C, Ribeiro-Dos-Santos AK, Rios-Santos F, Romano-Silva MA, Sortica VA, Suarez-Kurtz G. The genomic ancestry of individuals from different geographical regions of Brazil is more uniform than expected. PLoS One. 2011;16:e17063. doi: 10.1371/journal.pone.0017063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mastroianni Kirsztajn G. Renal function markers as prevention tools. J Bras Nefrol. 2006;28:48–52. [Google Scholar]