Abstract
Mammalian spermatogenesis and sperm maturation are susceptible to the effects of internal and external factors. However, how male germ cells interact with and respond to these elements including those potentially toxic substances is poorly understood. Here, we show that many bitter-taste receptors (T2rs), which are believed to function as gatekeepers in the oral cavity to detect and innately prevent the ingestion of poisonous bitter-tasting compounds, are expressed in mouse seminiferous tubules. Our in situ hybridization results indicate that Tas2r transcripts are expressed postmeiotically. Functional analysis showed that mouse spermatids and spermatozoa responded to both naturally occurring and synthetic bitter-tasting compounds by increasing intracellular free calcium concentrations, and individual male germ cells exhibited different ligand-activation profiles, indicating that each cell may express a unique subset of T2r receptors. These calcium responses could be suppressed by a specific bitter-tastant blocker or abolished by the knockout of the gene for the G protein subunit α-gustducin. Taken together, our data strongly suggest that male germ cells, like taste bud cells in the oral cavity and solitary chemosensory cells in the airway, utilize T2r receptors to sense chemicals in the milieu that may affect sperm behavior and fertilization.
Keywords: signal transduction, gene expression, germ cells, membrane receptors
Introduction
Mammalian spermatogenesis and sperm maturation include at least three phases (Eddy, 2002): (i) mitotic: stem cells in the testis differentiate into spermatogonia, which undergo a limited number of mitoses; (ii) meiotic: diploid spermatogonia enter meiosis, each of them giving rise to four haploid spermatocytes; (iii) post-meiotic: haploid spermatocytes undergo the most dramatic morphological changes and transform into immature spermatozoa, which are released into the lumen of seminiferous tubules of the testis. Sperm then further mature in the epididymis and fuse with prostasomes derived from the prostate glands before being ejaculated (Ronquist and Brody, 1985; Park et al., 2011). In the female reproductive tract, the sperm are capacitated and hyperactivated, and finally one of them initiates the acrosome reaction and fuses with the oocyte (Suarez, 2008).
Each phase of mammalian spermatogenesis consists of many critical cellular and molecular steps, many of which are susceptible to the influence of intrinsic and extrinsic factors. For example, intrinsically, the testis expresses tissue-specific or cell-differentiation stage-specific genes or splicing variants that are found only in spermatogonia and spermatocytes and stored in spermatids (Schultz et al., 2003; Almstrup et al., 2004; Schlecht et al., 2004; Shima et al., 2004; Wu et al., 2004; Hong et al., 2005; Iguchi et al., 2006). Mutations in at least 200 genes can adversely affect mammalian sperm production and function (Matzuk and Lamb, 2002, 2008; de Rooij and de Boer, 2003). Extrinsically, environmental agents such as pesticides, phytoestrogen, heavy metals and other toxic compounds can significantly contribute to the development of infertility and subfertility, and possibly also to epigenetic changes (Hew et al., 1993; Feist et al., 2005; Cederroth et al., 2010; Howard et al., 2011). Further studies are needed to comprehensively identify all potential extrinsic factors and to understand the molecular and cellular mechanisms by which male germ cells detect and respond to these environmental stimuli.
Bitter-taste receptors (T2rs) were initially identified from taste bud cells in the oral cavity (Adler et al., 2000; Chandrashekar et al., 2000; Matsunami et al., 2000). Activation of these receptors triggers an innate aversion response (Glendinning, 1994). Since many bitter-tasting compounds are potentially toxic, these receptors seem to provide warning signals against ingestion of these poisons (Dubois et al., 2008). However, some of these bitter compounds appear to have health benefits and are possibly of hedonic valence as well (Hofmann, 2009; Maehashi and Huang, 2009). Bitter-tasting food and drinks such as certain vegetables, chocolate, coffee, beer and tea are consumed and even liked by adult humans. Interestingly, T2r receptors are also expressed in the gastrointestinal tract, and thus the ingested bitter-tasting compounds are continuously monitored along the intestines, although the exact functions of these receptors in these tissues are yet unknown (Wu et al., 2002; Rozengurt et al., 2006).
T2r receptors are also found in the solitary chemosensory cells in the nasal cavity and lung, and possibly in other cells of the respiratory system, suggesting that these receptors may play a role in removing potentially harmful substances and initiating protective responses (Finger et al., 2003; Sbarbati et al., 2004; Shah et al., 2009; Deshpande et al., 2010; Tizzano et al., 2010, 2011). Moreover, T2rs are reported to be present in the central nervous system, suggesting a possible role of these receptors in detecting internal toxic compounds (Singh et al., 2011).
In this study, we have identified the expression of all the 35 T2rs in mouse testis, particularly in the post-meiotic germ cells. Thus, this finding may have identified an important molecular mechanism underlying the interactions between male germ cells and their microenvironment, which can affect sperm behavior and fertilization process.
Materials and Methods
Reagents and animals
Bitter-tasting compounds: caffeine, N-phenylthiourea (PTC), 6-propyl-2 thiouracil (PROP), picrotin, salicin, denatonium, procainamide and cycloheximide and the bitter blocker probenecid (Greene et al., 2011) were purchased from Sigma-Aldrich (St. Louis, MO). All studies involving animals were performed according to protocols approved by the Institutional Animal Care and Use Committee of the Monell Chemical Senses Center. C57BL/6J wild-type and α-gustducin-knockout (Gnat3−/−) (Wong et al., 1996) mice were housed in a climate-controlled environment at the Animal Care Facility of the Monell Chemical Senses Center.
Reverse transcription-PCR analysis
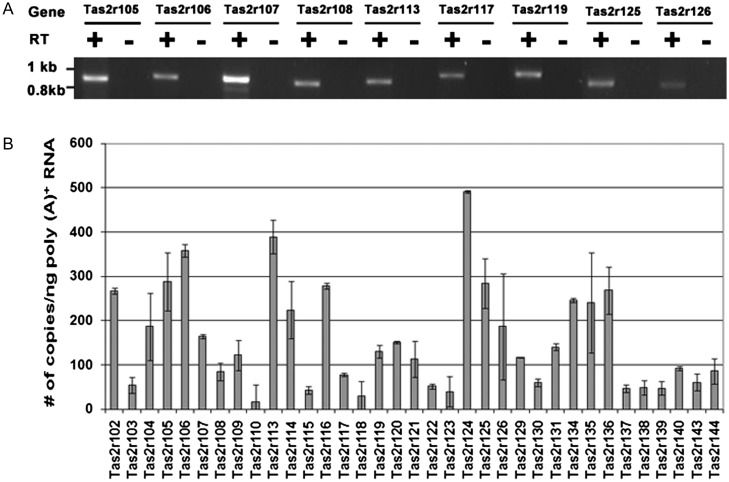
To determine the expression of mouse Tas2r genes in the testis, total RNA was extracted from mouse testes using TRIzol reagent (Life Technologies, Grand Island, NY). To enrich the target transcripts, poly(A)+ RNA was isolated from total RNA using oligo(dT)25 Dynabeads (Life Technologies). One microgram of poly(A)+ RNA was used as template to synthesize first-strand cDNA with oligo(dT)15 primers and avian myeloblastosis virus DNA polymerase. A negative control was prepared with the omission of the DNA polymerase. The reaction mixtures for both cDNA synthesis and negative control were diluted to 100 μl, of which 1 μl was used for each PCR with the PCR primers covering nearly the entire Tas2r coding region (Supplementary data, Table SI). PCRs were set up using the FailSafe PCR System (Epicentre, Madison, WI), and PCR products were fractionated by agarose gel electrophoresis (Fig. 1) and confirmed by sequencing.
Figure 1.
Tas2r gene expression in testis. (A) Reverse-transcription PCR covering nearly full coding sequences was performed for 9 Tas2r genes with cDNA templates reverse transcribed from mouse testis poly(A)+ RNA in the presence (+) or absence (−) of reverse transcriptases. The identities of the amplified products were confirmed by sequence analysis. (B) Quantitative real-time PCR was conducted with cDNA prepared from mouse testis poly(A)+ RNA. Transcripts for all the 35 known Tas2rs were detected. An average transcript copy number per nanogram of poly(A)+ RNA was obtained from three independent experiments with three mice. Data are means ± SEM.
Quantitative real-time PCR analysis
Real-time PCR primers were designed and synthesized for all the 35 predicted mouse Tas2rs (Supplementary data, Table SII). The PCR was set up with FastStart TaqMan Probe Master (Roche Applied Science, Indianapolis, IN) and 1 μl of the diluted cDNA described above. The PCR parameters were 95°C for 10 min, followed by 45 cycles of 95°C for 10 s, 50°C for 15 s and 72°C for 20 s. PCR products were fractionated by agarose gel electrophoresis and confirmed by sequencing. The transcript copy number per nanogram of input poly(A)+ RNA was calculated on the basis of the Ct (threshold cycle number) value against the standard curve. The standard curves for these intronless Tas2r genes were plotted using data from a series of PCRs with the diluted mouse genomic DNA: 860, 86, 8.6, 0.86 and 0.086 ng that contains 300 000, 30 000, 3000, 300 and 30 copies of a single-copy gene, using a previously described procedure (Yun et al., 2006). The results from three independent experiments with three adult male mice were averaged (Fig. 1).
In situ hybridization
Linearized plasmid DNAs containing 860 and 1013 bp of Tas2r105 and Tas2r108 receptor genes in pGEM-T Easy (Promega, Madison, WI) and pCR4-TOPO (Life Technologies) vector, respectively, were used to prepare ribonucleotide probes. Sense and antisense RNA probes were synthesized and digoxigenin-labeled from SP6, T3 and T7 promoters with the DIG RNA labeling Kit (Roche Applied Science). Tissue processing and probe hybridization were performed following previously reported procedures with some modifications (Schaeren-Wiemers and Gerfin-Moser, 1993; Braissant and Wahli, 1998). Briefly, mouse testes were fresh frozen and sliced into 10-μm-thick sections, which were then fixed in 4% formaldehyde in 0.1 M phosphate-buffered saline (PBS; pH 7.4) for 10 min. The sections were treated twice in 0.1% diethylpyrocarbonate in PBS for 15 min, followed by washing three times in 5 × saline-sodium citrate (SSC) for 5 min each. The sections were pre-hybridized in 5 × SSC, 50% formamide, 50 µg/ml denatured sonicated salmon sperm DNA, 250 µg/ml yeast RNA and 1 × Denhardt's solution for 2 h at 60°C. Adjacent sections were hybridized with 0.5–1 µg/ml DIG-labeled antisense probes or sense control probes. After overnight hybridization, the sections were washed at 68°C in 0.1 × SSC for 1 h. Signals were detected using alkaline phosphatase-conjugated anti-digoxigenin antibodies and standard chromogenic substrates of 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium salt (Roche Applied Science).
Calcium imaging with testicular cells and epididymal sperm
Eight adult male mice of each genotype: C57BL/6J and Gnat−/−, were sacrificed, and the testis and cauda epididymis were immediately removed and transferred into HS medium containing (mM): 135 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 30 HEPES, 10 glucose, 10 lactic acid and 1 pyruvic acid (pH adjusted to 7.4 with NaOH; Xia et al., 2007). For isolation of spermatids, seminiferous tubules from mouse testicle were dissected out and placed in a 15-mm dish containing 3 ml of HS medium. The tissue was finely minced and gently triturated with a fire-polished pipette, and the mixture was filtered with a 100-µm nylon cell strainer (BD Falcon, Bedford, MA). The dissociated cells were collected in a 1.5-ml plastic tube, washed once in HS medium by centrifugation at 300g for 4 min, and resuspended in HS medium.
For isolation of epididymal spermatozoa, three incisions were made to the cauda epididymis, which was incubated in a 1.5-ml tube containing 1 ml of HS medium and 5 mg/ml bovine serum albumin in a 5% CO2 incubator at 37°C for 20 min, as described previously (Xia et al., 2007). Sperm cells released into the medium were collected from the top 0.1 ml and adjusted to 1 × 107 cells/ml with HS medium.
Mouse spermatogenic cells were loaded with 5 µM Fura-2-acetoxymethyl ester (Fura-2/AM) and 80 μg/ml pluronic F127 (Molecular Probes, Eugene, Oregon) and transferred onto coverslips (22 × 60 mm; No. 0, Thomas Scientific Co., Swedesboro, NJ) for at least 1 h at room temperature. The coverslips with spermatogenic cells were mounted in a recording chamber and superfused with HS medium or HS containing tasting compounds or the bitter blocker via a valve controller (VC-8, Warner Instruments, Hamden, CT).
Imaging of calcium responses was conducted as previously described (Gomez et al., 2005). Stimulation duration was 30 s, and perfusion rate was 0.8 ml/min. Cells were excited at 340 and 380 nm, and signals at 510 nm were captured by a cooled CCD camera. The change in fluorescence ratio (F= F340/F380) was recorded for regions of interest drawn on the cells.
Data analysis
To analyze the dose-dependent calcium responses, the response intensity (FN) was normalized and expressed as ΔF/F0= (F–F0)/F0, where F0 is the fluorescence ratio at the beginning of stimulation (i.e. time 0) and F is the peak fluorescence ratio evoked by the stimulation. Dose–response curves and EC50 values were generated on the basis of the normalized responses using the software Origin (OriginLab Corp., Northampton, MA, USA) by nonlinear regression.
Results
Mouse testis expresses the T2r transcripts
To understand whether a mammalian male germ cell is capable of detecting and responding to potentially toxic, naturally occurring or synthetic compounds, we set out to examine the expression of T2rs that are known to sense poisonous substances in the oral cavity and other organs. Reverse transcription-PCR with poly(A)+ RNA prepared from mouse testis and gene-specific primers for 9 Tas2rs (Supplementary data, Table SI) produced the expected PCR products that were absent in the control samples with the omission of the reverse transcriptases (Fig. 1A). Sequencing analysis of the amplified products confirmed their complete matches with mouse Tas2r105, 106, 107, 108, 113, 117, 119, 125 and 126 genes.
To determine the expression levels of all the 35 identified mouse Tas2r genes, quantitative real-time PCR was carried out with cDNA templates prepared from poly(A)+ RNA. Multiple pairs of PCR primers were designed and tested for each gene. Those that engendered specific and efficient amplification were used in the subsequent quantification experiments (Supplementary data, Table SII). Since Tas2r genes are known to be intronless, a series of diluted mouse genomic DNA was used to obtain standard curves of Ct (threshold cycle number) values versus template copy numbers for all the optimized pairs of PCR primers, which were used to determine the transcript copy number of the target gene in the testis poly(A)+ RNA samples. The averaged copy number of transcripts per nanogram of poly (A)+ RNA from three mice varied from gene to gene (Fig. 1). On the basis of these numbers, these genes can be largely classified into three categories: (i) abundant: 11 genes with their copy numbers of 200 or more (Tas2r102, 105, 106, 113, 114, 116, 124, 125, 134, 135 and 136); (ii) medium abundant: 9 genes with transcript copy numbers ranging from 100 to 200 (Tas2r104, 107, 109, 119, 120, 121, 126, 129 and 131) and (iii) rare: the remaining 15 genes with a transcript copy number below 100.
Tas2r transcripts are localized to post-meiotic spermatids
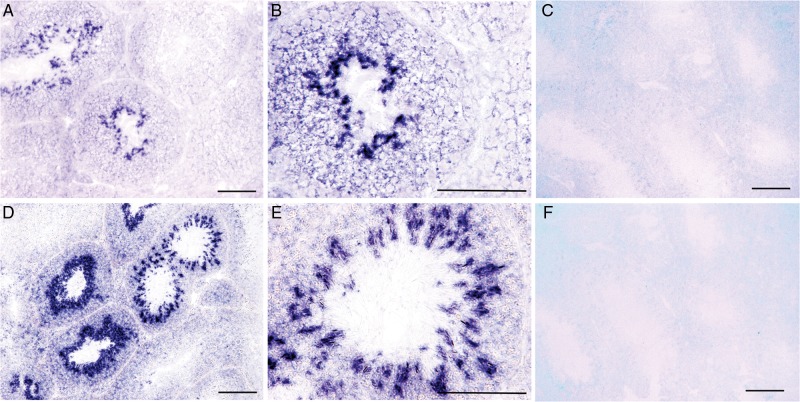
To localize Tas2r transcripts to expressing cells in the testis, in situ hybridization was performed on mouse testicular tubule sections with sense and antisense riboprobes for the two T2r receptors with the known bitter-tasting ligands and different expression levels in the testis: the abundantly expressed Tas2r105 and the rarely expressed Tas2r108, activated by cycloheximide and denatonium, respectively. The antisense probes hybridized to some cells of adjacent tubule sections, whereas cells in other sections were not hybridized (Fig. 2). Further examination of these seminiferous sections indicated that cells expressing Tas2rs appeared to be the spermatocytes undergoing meiosis as well as those in the later stages of spermatogenesis. The negative controls of sense probes did not produce any specific or non-specific hybridization on the sections.
Figure 2.
In situ hybridization of mouse testicular sections with Tas2r probes. (A and D) Antisense probes of Tas2r105 (A) and Tas2r108 (D) hybridized to subsets of cells in some seminiferous sections (blue staining). (B and E) High-magnification images of Tas2r105 (B) and Tas2r108 (E) show that the stained cells were post-meiotic cells. (C and F) No signals with the sense probes Tas2r105 (C) and Tas2r108 (F) were detected. Scale bars: 100 μm.
Bitter-taste compounds elicit calcium responses from spermatids and spermatozoa in a dose-dependent manner
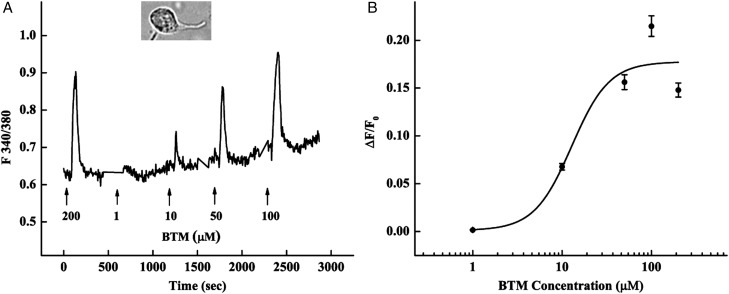
Among the 35 mouse T2rs, only T2r105 and 108 are known to be activated by the bitter compounds cycloheximide and denatonium, respectively (Chandrashekar et al., 2000). To evoke responses from additional T2r receptors expressed in mouse testis, we used a mixture of six bitter tastants [bitter-taste mixture (BTM)]: caffeine, PTC, PROP, picrotin, salicin and denatonium (200 μM each). Calcium responses were elicited from a number of freshly dissociated spermatids that were identified by their emerging tail and immature head structure (Fig. 3). The responses were concentration-dependent within the range 0.1–200 μM of each BTM compound and a calculated EC50 of 13 ± 11 μM (Fig. 3).
Figure 3.
Activation of spermatids by bitter tastants increases intracellular calcium concentrations. (A) A typical calcium response trace from the head area of a male germ cell. Spermatids were loaded with the calcium-sensitive dye Fura-2/AM (inset: image of a representative spermatid). The BTM (200 μM each of caffeine, PTC, PROP, picrotin, salicin and denatonium) was first used to identify responsive spermatids, followed by a series of concentrations of the mixture. (B) A dose–response curve from 13 responsive cells with an EC50 of 13 ± 11 μM.
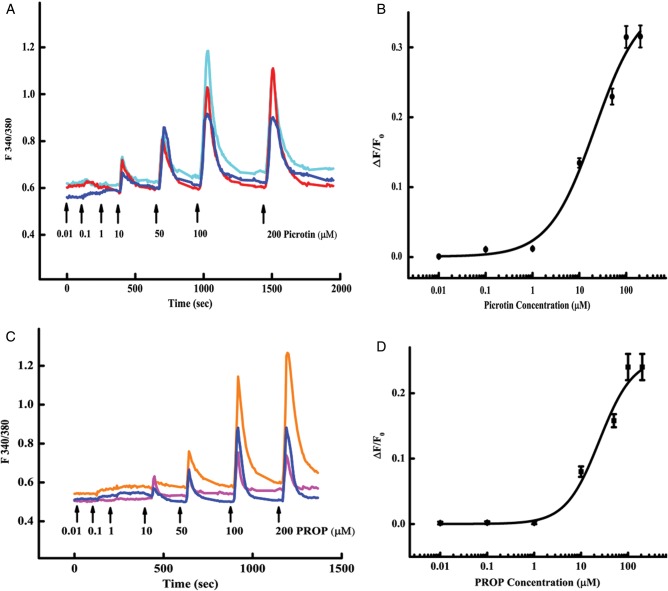
To test whether mouse spermatogenic cells can be activated by single bitter compounds, picrotin and PROP were used in the calcium imaging assays. The results showed that out of 200 BTM-responsive mouse spermatids, 33 and 45 responded to picrotin and PROP in a dose-dependent fashion with calculated EC50 values of 20 ± 10 and 24 ± 12 μM, respectively (Fig. 4).
Figure 4.
Single bitter-tasting compounds picrotin and PROP activate spermatids. (A and C) Shown are traces from three representative cells in response to a series of picrotin (A) or PROP (C) concentrations. No responses were detected to 0.01, 0.1 and 1 μM of either compound. Thus, the shorter intervals were given between these stimuli than between higher concentrations. (B and D) Dose–response curves were plotted with the data from 20 to 44 responsive cells to picrotin (B) and PROP (D), with calculated EC50 values of 20 ± 10 and 24 ± 12 µM, respectively.
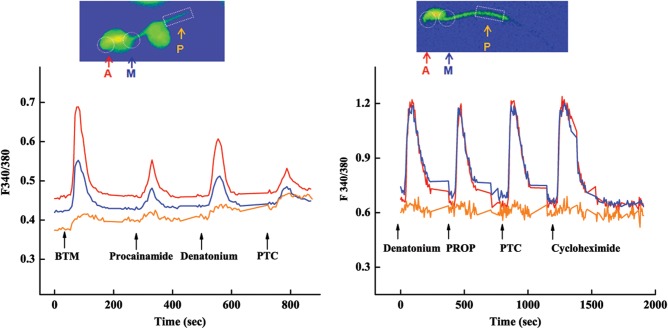
To examine subcellular differences in calcium responses, areas of acrosome, midpiece and principal piece were monitored separately (Fig. 5). The increases in intracellular free calcium concentrations in responses to 200 μM BTM or individual compounds procainamide, denatonium and PTC were much larger in the acrosome than in the midpiece of mouse spermatids, whereas the responses in the principal piece were detectable but much smaller than those in the other two areas (Fig. 5, left). The initiation of the calcium response in these three areas, however, seemed to be simultaneous.
Figure 5.
Spatiotemporal characterization of bitter-tastant-evoked calcium responses in mouse male germ cells: traces of calcium responses from acrosome (A), midpiece (M) and principal piece (P) of a spermatid (left) and epididymal sperm (right) to 200 µM bitter-tastant mixture (BTM) or individual bitter tastants: procainamide, denatonium, PTC, PROP and cycloheximide. Insets: Fura-2/AM-loaded spermatid (left) and epididymal sperm (right).
To reveal whether the subcellular response pattern is altered over the sperm maturation, calcium imaging was performed with more mature sperm cells isolated from mouse cauda epididymis (Fig. 5, right). The results showed that the amplitudes of the responses to 200 μM denatonium, PROP, PTC or cycloheximide from the acrosome and midpiece were equally strong, whereas the responses from the principal pieces were mostly undetectable, suggesting that there was a shift in the distribution of the corresponding receptors and their downstream signaling components during this maturation period.
Individual germ cells exhibit different ligand-activation profiles
In taste bud cells, one receptor cell can be activated by multiple bitter compounds, and the activation profiles differ among cells (Caicedo and Roper, 2001). To characterize the response profiles of male germ cells, we selected five bitter compounds: denatonium, PROP, PTC, cycloheximide and salicin, to stimulate mouse spermatids and epididymal sperm cells. Individual cells displayed different response profiles (Fig. 6), with at least 10 and 13 different response profiles among testicular spermatids and epididymal sperm cells, respectively (Tables I and II). Some cells responded to different compounds with similar intensity, whereas others exhibited different response intensities to different bitter compounds (Fig. 6).
Figure 6.
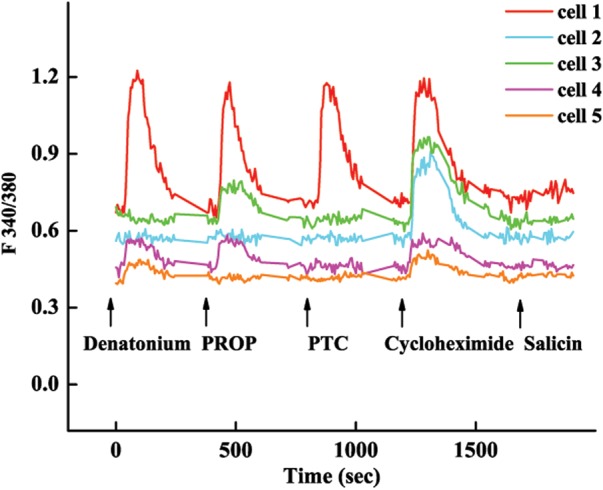
Individual male germ cell exhibits different ligand-activation profiles: traces of five epididymal sperm cells in response to denatonium, PROP, PTC, cycloheximide and salicin. Cell 1 responded to the first four compounds with similar intensity; cell 2 responded only to cycloheximide; cell 3 responded to cycloheximide and weakly to PROP; cell 4 responded weakly to denatonium, PROP and cycloheximide and cell 5 responded weakly to denatonium and cycloheximide. Cells responsive to salicin were rare and are not shown here.
Table I.
Response profiles of individual spermatids to bitter compounds.
| Profile | Compounds to which cells responded |
# of cells responded | ||||
|---|---|---|---|---|---|---|
| Profile 1 | Cycloheximide | Denatonium | PROP | PTC | 3 | |
| Profile 2 | Cycloheximide | Denatonium | PTC | 1 | ||
| Profile 3 | Cycloheximide | PROP | PTC | 1 | ||
| Profile 4 | Cycloheximide | Denatonium | 7 | |||
| Profile 5 | Cycloheximide | PROP | 1 | |||
| Profile 6 | Denatonium | PTC | 1 | |||
| Profile 7 | Cycloheximide | 5 | ||||
| Profile 8 | Denatonium | 4 | ||||
| Profile 9 | PROP | 3 | ||||
| Profile 10 | PTC | 3 | ||||
| Total | 29 | |||||
Table II.
Response profiles of individual epididymal sperm cells to bitter compounds.
| Profile | Compounds to which cells responded |
# of cells responded | ||||
|---|---|---|---|---|---|---|
| Profile 1 | Cycloheximide | Denatonium | PROP | PTC | Salicin | 4 |
| Profile 2 | Cycloheximide | Denatonium | PROP | PTC | 13 | |
| Profile 3 | Cycloheximide | Denatonium | PROP | Salicin | 1 | |
| Profile 4 | Cycloheximide | PROP | PTC | Salicin | 1 | |
| Profile 5 | Cycloheximide | Denatonium | PROP | 4 | ||
| Profile 6 | Cycloheximide | Denatonium | PTC | 1 | ||
| Profile 7 | Cycloheximide | PROP | PTC | 1 | ||
| Profile 8 | Denatonium | PROP | PTC | 1 | ||
| Profile 9 | Cycloheximide | Denatonium | 1 | |||
| Profile 10 | Cycloheximide | PROP | 5 | |||
| Profile 11 | Denatonium | PTC | 1 | |||
| Profile 12 | Cycloheximide | 15 | ||||
| Profile 13 | Salicin | 1 | ||||
| Total | 49 | |||||
Ligand-based analysis indicated that, of the five bitter compounds tested on the spermatids, T2r105 receptor's ligand cycloheximide was the most effective, inducing 18 of 29 (62.1%) cells to increase intracellular calcium concentrations; the second and third most effective compounds were T2r108 receptor's ligand denatonium and PROP, respectively, stimulating 55.2 and 27.6% cells to respond (Table I). Some spermatids responded to both of the N-C = S moiety-containing compounds PROP and PTC, but others responded to either or neither of the two. About half (51.7%) of the cells responded to a single compound only. No spermatids responded to salicin (Table I).
Among the epididymal sperm cells, cycloheximide again was the most effective compound and evoked the calcium responses from 46 to 49 (93.9%) of cells; 61.2 and 53.1% cells responded to the second and third most effective compounds, PROP and denatonium, respectively (Table II). As with the spermatids, some epididymal sperm cells responded to both PROP and PTC, whereas others responded to either or neither of the two. Compared with spermatids, fewer epididymal sperm cells (34.7%) responded to a single compound only. Further, ∼14.3% of these cells were responsive to salicin.
Germ cells’ responses to bitter tastants can be suppressed by a bitter blocker and abolished by the α-gustducin gene knockout
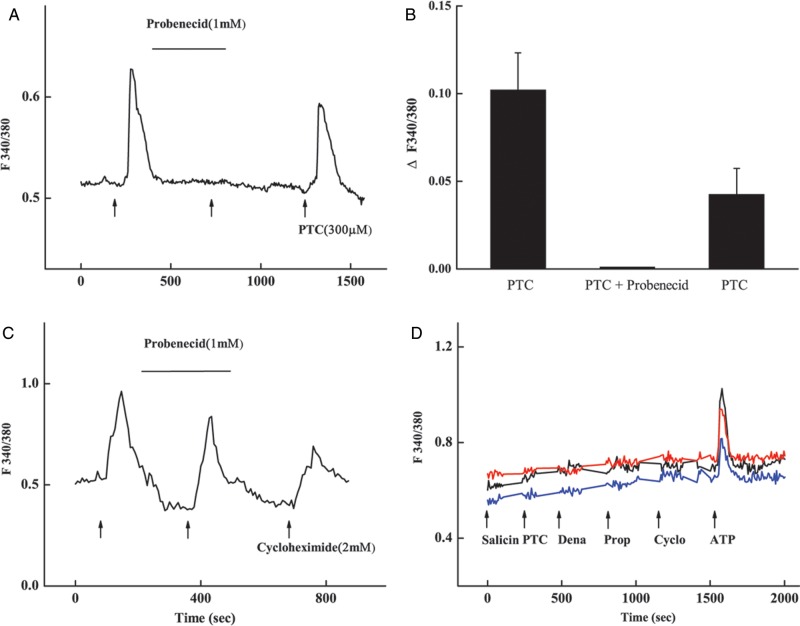
To confirm whether the germ cells’ responses to bitter tastants were mediated by the T2rs, we examined the effect of the T2r blocker probenecid and nullification of the α-gustducin gene Gnat3 (Fig. 7) (Wong et al., 1996; Greene et al., 2011). The results showed that preincubation of the C57B/6L wild-type sperm with 1 mM prebenecid for 5 min nearly completely suppressed the responses to 0.3 mM PTC (Fig. 7A and B). The suppression was reversible; and after the wash-off of the blocker, the cells’ responses to PTC were restored although the amplitude was somewhat smaller than the pre-blocking ones. In contrast, probenecid did not block the responses to another bitter tastant, cycloheximide (Fig. 7C).
Figure 7.
Calcium responses of mouse sperm were suppressed by the bitter blocker and abolished by the Gnat3 gene knockout. (A) A response trace from a representative sperm: the increase in the intracellular calcium concentration was significant in response to 300 μM PTC alone, nearly undetectable after the incubation with 1 mM probenecid and partially recovered after the wash-off of probenecid (arrows). (B) Quantification of the peak responses from 26 sperm at the three different time points described in (A). Values are mean ± SE. (C) Probenecid has no inhibitory effect on the response to cycloheximide. (D) Sperm isolated from the Gnat3−/− mutant mice did not respond to 2 mM salicin, PTC, denatonium (Dena), 6 propyl-2-thiouracil (PROP), cycloheximide (Cyclo) but did respond to 2 mM ATP (177 sperm imaged and only 3 cells' traces shown).
Calcium imaging of sperm isolated from the Gnat3−/− mutant mice showed that these cells were unresponsive to the bitter tastants tested: salicin, PTC, denatonium, PROP and cyclohexmide at 2 mM while the responses to 2 mM ATP seemed to be normal (Rodriguez-Miranda et al., 2008) (Fig. 7D).
Discussion
Previous studies have shown that many molecular and cellular processes of spermatogenesis are susceptible to the influences of a number of internal and external elements, including environmental agents, medications, dietary selection and lifestyle factors (Huynh et al., 2000; Amory, 2007; Sharpe, 2010). However, little is known about how these factors change production and function of male germ cells, or in some cases, cause transgenerational epigenetic alterations (Anway et al., 2005; Carone et al., 2010; Howard et al., 2011). Our discovery of Tas2r expression in haploid male germ cells may provide a novel mechanism underlying the interaction of mammalian male germ cells with environmental activators and toxicants.
Our reverse-transcription PCR and quantitative real-time PCR results revealed the expression in the testis of all the 35 known genes in mouse genome that encode T2rs (Wu et al., 2005; Bachmanov and Beauchamp, 2007). On the basis of expression levels, these genes can be roughly classified into three groups: abundant, medium abundant and rare (Fig. 1). In situ hybridization results localized the transcripts of two receptors with the known ligands, T2r105 and 108, in post-meiotic cells (Fig. 2). These results, together with the Tas2r expression patterns in taste buds and solitary chemosensory cells, lead to the prediction that the other 33 Tas2rs are likely expressed approximately at the same stage (i.e. the meiotic phase), although some Tas2rs may be expressed in the spermatogonia. However, these Tas2r genes may not be transcribed in spermatozoa since RNA synthesis ends before spermatids are released into the lumen (Kierszenbaum and Tres, 1978).
To functionally characterize T2r receptors expressed in the male germ cells, we performed calcium imaging of the responses of acutely dissociated mouse testicular cells to bitter-tasting compounds. Currently, only T2r105 and 108 receptors have been deorphanized, whereas the ligands for the remaining 33 mouse T2r receptors are yet to be determined (Chandrashekar et al., 2000). To help identify responsive cells from a heterogeneous pool of dissociated testicular cells, we initially applied a mixture of six bitter compounds: caffeine, PTC, PROP, picrotin, salicin and denatonium. The response amplitude from the spermatids was concentration-dependent, with an EC50 of 13 ± 11 μM (Fig. 3). Part of the variation may be attributed to the heterogeneity in the developmental stages of these germ cells dissociated from whole testes. Spermatids isolated from the same seminiferous location, with germ cells more likely at the same maturation stage and possibly expressing the same subset of Tas2rs, may exhibit a more homogeneous response.
The responsive cells identified with the bitter-tasting mixture were tested with individual bitter-tasting compounds picrotin and PROP. Some of these cells displayed a concentration-dependent response, with calculated EC50 values of 20 ± 10 μM to picrotin and 24 ± 12 μM to PROP (Fig. 4). In humans, picrotin can activate five heterologously expressed human T2Rs, the most sensitive of which is T2R14, with an EC50 value of 18 μM (Behrens et al., 2004; Meyerhof et al., 2010). The similarity in picrotin EC50 values between human T2R14 and mouse sperm responses suggests that a mouse receptor orthologous to human T2R14 may exist. Unlike picrotin, however, PROP can activate only one human bitter receptor: T2R38. Six variants of this receptor have been found: two unresponsive to PROP and four responding with EC50 values of 2–4 μM (Kim et al., 2003; Bufe et al., 2005). Our data suggest that one or more less-sensitive receptors with EC50 values of 24 µM may occur in rodent testicular spermatids. Interestingly, while PROP and PTC act on the same T2R38 in humans, our calcium imaging data showed that cells responding to these two compounds can be segregated (Fig. 6, Tables I and II), suggesting that there are two different murine receptors for PTC and PROP, respectively, which is consistent with previous genetic observations (Nelson et al., 2003). Identification of these two receptors can provide additional information into the species differences in bitter taste.
Calcium imaging results of multiple subcellular areas of the spermatids indicated that the increase in intracellular calcium concentrations seemed to be simultaneous, although the amplitudes of the calcium responses differed remarkably, with the acrosome, midpiece and principal piece having highest, medium and least response, respectively (Fig. 5). These results suggest that T2rs and their signaling components are distributed across the spermatid, with apparent concentrations in the head region. The response pattern in epididymal sperm differed: the responses from the acrosome and midpiece appeared equally large, while that from the principal piece became barely detectable (Fig. 5). This change may have resulted from the redistribution of the proteins within the sperm cell during maturation: the receptor proteins and their downstream signal transduction components are more concentrated in the cytosol-rich midpiece. Restricted subcellular localization for some functionally specialized proteins has been reported. For example, a sperm-specific, calcium-ion-permeable channel, CatSper, is located exclusively in the sperm tail (Ren and Xia, 2010). More studies are needed to reveal any further changes in the T2r distribution during the fusion with prostasome, capacitation and hyperactivation processes in the female reproductive tract (Suarez, 2008; Visconti, 2009; Park et al., 2011) to elucidate the physiological role of these T2r receptors at different stages.
Comparison of bitter-tastant response profiles indicated that individual male germ cells may have different response profiles: some cells responded to more tastants than did others, and cells varied in response intensity (Fig. 6). On the basis of the limited number of tastants used in this study, responsive cells can be grouped by response profile (Tables I and II). Similar observations of different response profiles among T2r cells from the oral cavity have also been reported (Caicedo and Roper, 2001). And a recent molecular study has revealed that each taste receptor cell expresses a different subset of receptor genes and varies in gene expression level (Behrens et al., 2007). Similarly, differential response profiles of haploid male germ cells can be explained by the heterogeneity of Tas2r expression patterns. The previous transgenic studies (Li and Zhou, 2012) show that nearly all spermatogenic cells express Tas2r105, which is consistent with the functional data that indicate the responsiveness of 93.9% epididymal sperm to the T2r105 receptor's ligand cycloheximide. These results also support the conclusion of heterogeneous expression patterns of Tas2rs in male germ cells. The variations in response intensity to the same stimuli may be partially due to the activation of multiple receptors by the same compound. Cells expressing the cognate receptors may have stronger responses than cells carrying non-cognate receptors.
Comparative analysis of response profiles of testicular spermatids versus epididymal sperm cells identifies contrasting changes in the profiles (Tables I and II). Although the molecular and cellular events for this shift in responsiveness are yet to be defined, we hypothesize that after Tas2r genes are transcribed during the meiotic phase, only a few receptor proteins along with downstream signaling components are sorted to the cell surface. During the maturation process from testicular spermatids to epididymal sperm, additional receptors are localized to the cell surface, and each cell becomes responsive to environmental ligands.
Probenecid is known to inhibit the responses of human T2R16, 38 and 43 to salicin, PTC and PROP and aloin, but not that of T2R31 to saccharin (Greene et al., 2011). We found that probenecid can also inhibit the mouse sperm's response to PTC, but not to cycloheximide (Fig. 7A–C), suggesting that the mouse sperm's responses to bitter tastants were mediated by T2rs.
We believe that Tas2rs expressed in mammalian male germ cells may have functions similar to those of Tas2rs in the digestive and respiratory systems: to detect bitter-tasting compounds. A large number of naturally occurring and synthetic bitter-tasting substances have been identified, and many of them are present in foodstuffs and medicine (Belitz and Weiser, 1985; Maehashi and Huang, 2009). These compounds display an enormous diversity of chemical structures. Many of these bitter tastants are hydrophobic and can readily cross cell membranes (Kumazawa et al., 1988; Peri et al., 2000), suggesting that they may be able to enter male and female reproductive tracts and activate these receptors on sperm. Calcium-signaling pathways play critical roles from spermatogenesis to fertilization (Publicover et al., 2007). T2r-mediated increases in intracellular calcium concentrations may affect many molecular and cellular steps, including those that may lead to altered DNA or histone modifications, chromatin packaging, sperm motility and fertilization. In the oral cavity, taste bud cells utilize a heterotrimeric G-protein consisting of α-gustducin, Gβ3 and γ13 subunits, and the effector enzyme phospholipase C β2 (PLCβ2) and inositol trisphosphate (IP3) receptor IP3R3, to release calcium ions from the intracellular stores, which are sequestered back into the endoplasmic reticulum by the sarco/endoplasmic reticulum Ca2+-ATPase Serca 3 (Wong et al., 1996; Rossler et al., 1998; Huang et al., 1999; Clapp et al., 2001, Hisatsune et al., 2007, Iguchi et al., 2011). Gustducin has been reported to be expressed in spermatids (Fehr et al., 2007). Our results showed that the knockout of the α-gustducin gene abolished the sperm's calcium responses to several bitter tastants (Fig. 7D), indicating that testicular T2rs utilize a similar signal transduction pathway. Further studies are needed to delineate the T2r-mediated calcium signaling pathways and their regulatory mechanisms in the testis.
Chemotaxis is also known to play an important role in fertilization (Garbers, 1989). Olfactory receptors, in addition to T2rs, have been found in mammalian germ cells, and activation of these receptors by some volatile odorants attracts sperm (Parmentier et al., 1992; Vanderhaeghen et al., 1993; Spehr et al., 2003; Fukuda et al., 2004; Fukuda and Touhara, 2006; Veitinger et al., 2011). Bitter compounds like caffeine can induce sperm hyperactivation and improve artificial insemination success rates and this effect of caffeine is mediated by calcium ions instead of cAMP or inhibition of phosphodiesterases (Colas et al., 2009; Yamaguchi et al., 2009). Our data suggest that this calcium release is induced by caffeine-induced T2r receptor activation and that other bitter-tasting compounds may be able to trigger the hyperactivation and facilitate IVF as well.
In summary, we found that many T2rs are expressed post-meiotically in haploid germ cells in mouse seminiferous tubules. Wild-type spermatids and spermatozoa responded to bitter-tasting compounds by increasing intracellular free calcium concentrations; this response is probably mediated by α-gustducin and can be inhibited by the specific bitter blocker. Many mouse sperm respond to the bitter-tastant cyclohexmimide although individual male germ cells may have a slightly different subset of T2r receptors. Male germ cells, like taste bud cells in the oral cavity and solitary chemosensory cells in the airway, may use T2r receptors to detect and respond to bitter-tasting compounds. Further studies are needed to determine the specific roles of these receptors in mammalian reproductive system.
Supplementary data
Supplementary data are available at http://molehr.oxfordjournals.org/.
Authors’ roles
L.H. designed the study, analyzed the data and prepared the manuscript. J.X. designed and carried out the calcium imaging experiments and helped write part of the manuscript. J.C. carried out qPCR and in situ hybridization. N.I. helped interpret the data and participated in the manuscript preparation. D.R. helped design the experiments, prepare and revise the manuscript.
Funding
This work was supported by National Institutes of Health grant R01 DC007487 to L.H., by NIH-NIDCD Core grant P30 DC011735 to R. Margolskee in support of Monell Core Facilities and by National Science Foundation Equipment grant DBI-0216310 to N. Rawson in support of Monell's Confocal Microscopy.
Conflict of interest
None declared.
Supplementary Material
Acknowledgments
We thank Dr Karen Yee for helpful discussions and Dr Robert Margolskee for the α-gustducin-knockout mice.
References
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Almstrup K, Nielsen JE, Hansen MA, Tanaka M, Skakkebaek NE, Leffers H. Analysis of cell-type-specific gene expression during mouse spermatogenesis. Biol Reprod. 2004;70:1751–1761. doi: 10.1095/biolreprod.103.026575. [DOI] [PubMed] [Google Scholar]
- Amory JK. Drug effects on spermatogenesis. Drugs Today (Barc) 2007;43:717–724. doi: 10.1358/dot.2007.43.10.1131829. [DOI] [PubMed] [Google Scholar]
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Beauchamp GK. Taste receptor genes. Annu Rev Nutr. 2007;27:389–414. doi: 10.1146/annurev.nutr.26.061505.111329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens M, Brockhoff A, Kuhn C, Bufe B, Winnig M, Meyerhof W. The human taste receptor hTAS2R14 responds to a variety of different bitter compounds. Biochem Biophys Res Commun. 2004;319:479–485. doi: 10.1016/j.bbrc.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Behrens M, Foerster S, Staehler F, Raguse JD, Meyerhof W. Gustatory expression pattern of the human TAS2R bitter receptor gene family reveals a heterogenous population of bitter responsive taste receptor cells. J Neurosci. 2007;27:12630–12640. doi: 10.1523/JNEUROSCI.1168-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belitz HD, Weiser H. Bitter compounds: occurrence and structure-activity relationships. Food Rev Int. 1985;1:271–354. [Google Scholar]
- Braissant O, Wahli W. Differential expression of peroxisome proliferator-activated receptor-alpha, -beta, and -gamma during rat embryonic development. Endocrinology. 1998;139:2748–2754. doi: 10.1210/endo.139.6.6049. [DOI] [PubMed] [Google Scholar]
- Bufe B, Breslin PA, Kuhn C, Reed DR, Tharp CD, Slack JP, Kim UK, Drayna D, Meyerhof W. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol. 2005;15:322–327. doi: 10.1016/j.cub.2005.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, Roper SD. Taste receptor cells that discriminate between bitter stimuli. Science. 2001;291:1557–1560. doi: 10.1126/science.291.5508.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, Li R, Bock C, Li C, Gu H, Zamore PD, et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143:1084–1096. doi: 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederroth CR, Auger J, Zimmermann C, Eustache F, Nef S. Soy, phyto-oestrogens and male reproductive function: a review. Int J Androl. 2010;33:304–316. doi: 10.1111/j.1365-2605.2009.01011.x. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- Clapp TR, Stone LM, Margolskee RF, Kinnamon SC. Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction. BMC Neurosci. 2001;2:6. doi: 10.1186/1471-2202-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas C, Cebrian-Perez JA, Muino-Blanco T. Caffeine induces ram sperm hyperactivation independent of cAMP-dependent protein kinase. Int J Androl. 2009;33:e187–e197. doi: 10.1111/j.1365-2605.2009.00991.x. [DOI] [PubMed] [Google Scholar]
- de Rooij DG, de Boer P. Specific arrests of spermatogenesis in genetically modified and mutant mice. Cytogenet Genome Res. 2003;103:267–276. doi: 10.1159/000076812. [DOI] [PubMed] [Google Scholar]
- Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16:1299–1304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois GE, Desimone J, Lyall V. Chemistry of gustatory stimuli. In: Firestein S, Beauchamp GK, editors. Olfaction and Taste. Amsterdam: Elsivier; 2008. p. 48. [Google Scholar]
- Eddy EM. Male germ cell gene expression. Recent Prog Horm Res. 2002;57:103–128. doi: 10.1210/rp.57.1.103. [DOI] [PubMed] [Google Scholar]
- Fehr J, Meyer D, Widmayer P, Borth HC, Ackermann F, Wilhelm B, Gudermann T, Boekhoff I. Expression of the G-protein alpha-subunit gustducin in mammalian spermatozoa. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2007;193:21–34. doi: 10.1007/s00359-006-0168-8. [DOI] [PubMed] [Google Scholar]
- Feist GW, Webb MA, Gundersen DT, Foster EP, Schreck CB, Maule AG, Fitzpatrick MS. Evidence of detrimental effects of environmental contaminants on growth and reproductive physiology of white sturgeon in impounded areas of the Columbia River. Environ Health Perspect. 2005;113:1675–1682. doi: 10.1289/ehp.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE, Bottger B, Hansen A, Anderson KT, Alimohammadi H, Silver WL. Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci USA. 2003;100:8981–8986. doi: 10.1073/pnas.1531172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda N, Touhara K. Developmental expression patterns of testicular olfactory receptor genes during mouse spermatogenesis. Genes Cells. 2006;11:71–81. doi: 10.1111/j.1365-2443.2005.00915.x. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Yomogida K, Okabe M, Touhara K. Functional characterization of a mouse testicular olfactory receptor and its role in chemosensing and in regulation of sperm motility. J Cell Sci. 2004;117:5835–5845. doi: 10.1242/jcs.01507. [DOI] [PubMed] [Google Scholar]
- Garbers DL. Molecular basis of fertilization. Annu Rev Biochem. 1989;58:719–742. doi: 10.1146/annurev.bi.58.070189.003443. [DOI] [PubMed] [Google Scholar]
- Glendinning JI. Is the bitter rejection response always adaptive? Physiol Behav. 1994;56:1217–1227. doi: 10.1016/0031-9384(94)90369-7. [DOI] [PubMed] [Google Scholar]
- Gomez G, Lischka FW, Haskins ME, Rawson NE. Evidence for multiple calcium response mechanisms in mammalian olfactory receptor neurons. Chem Senses. 2005;30:317–326. doi: 10.1093/chemse/bji026. [DOI] [PubMed] [Google Scholar]
- Greene TA, Alarcon S, Thomas A, Berdougo E, Doranz BJ, Breslin PA, Rucker JB. Probenecid inhibits the human bitter taste receptor TAS2R16 and suppresses bitter perception of salicin. PLoS ONE. 2011;6:e20123. doi: 10.1371/journal.pone.0020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hew KW, Ericson WA, Welsh MJ. A single low cadmium dose causes failure of spermiation in the rat. Toxicol Appl Pharmacol. 1993;121:15–21. doi: 10.1006/taap.1993.1123. [DOI] [PubMed] [Google Scholar]
- Hisatsune C, Yasumatsu K, Takahashi-Iwanaga H, Ogawa N, Kuroda Y, Yoshida R, Ninomiya Y, Mikoshiba K. Abnormal taste perception in mice lacking the type 3 inositol 1,4,5-trisphosphate receptor. J Biol Chem. 2007;282:37225–37231. doi: 10.1074/jbc.M705641200. [DOI] [PubMed] [Google Scholar]
- Hofmann T. Identification of the key bitter compounds in our daily diet is a prerequisite for the understanding of the hTAS2R gene polymorphisms affecting food choice. Ann N Y Acad Sci. 2009;1170:116–125. doi: 10.1111/j.1749-6632.2009.03914.x. [DOI] [PubMed] [Google Scholar]
- Hong S, Choi I, Woo JM, Oh J, Kim T, Choi E, Kim TW, Jung YK, Kim DH, Sun CH, et al. Identification and integrative analysis of 28 novel genes specifically expressed and developmentally regulated in murine spermatogenic cells. J Biol Chem. 2005;280:7685–7693. doi: 10.1074/jbc.M412444200. [DOI] [PubMed] [Google Scholar]
- Howard TD, Ho SM, Zhang L, Chen J, Cui W, Slager R, Gray S, Hawkins GA, Medvedovic M, Wagner JD. Epigenetic changes with dietary soy in cynomolgus monkeys. PLoS ONE. 2011;6:e26791. doi: 10.1371/journal.pone.0026791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF. Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci. 1999;2:1055–1062. doi: 10.1038/15981. [DOI] [PubMed] [Google Scholar]
- Huynh PN, Hikim AP, Wang C, Stefonovic K, Lue YH, Leung A, Atienza V, Baravarian S, Reutrakul V, Swerdloff RS. Long-term effects of triptolide on spermatogenesis, epididymal sperm function, and fertility in male rats. J Androl. 2000;21:689–699. [PubMed] [Google Scholar]
- Iguchi N, Tobias JW, Hecht NB. Expression profiling reveals meiotic male germ cell mRNAs that are translationally up- and down-regulated. Proc Natl Acad Sci USA. 2006;103:7712–7717. doi: 10.1073/pnas.0510999103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi N, Ohkuri T, Slack JP, Zhong P, Huang L. Sarco/Endoplasmic reticulum Ca2+-ATPases (SERCA) contribute to GPCR-mediated taste perception. PLoS ONE. 2011;6:e23165. doi: 10.1371/journal.pone.0023165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum AL, Tres LL. RNA transcription and chromatin structure during meiotic and postmeiotic stages of spermatogenesis. Fed Proc. 1978;37:2512–2516. [PubMed] [Google Scholar]
- Kim UK, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299:1221–1225. doi: 10.1126/science.1080190. [DOI] [PubMed] [Google Scholar]
- Kumazawa T, Nomura T, Kurihara K. Liposomes as model for taste cells: receptor sites for bitter substances including N-C=S substances and mechanism of membrane potential changes. Biochemistry. 1988;27:1239–1244. doi: 10.1021/bi00404a025. [DOI] [PubMed] [Google Scholar]
- Li F, Zhou M. Depletion of bitter taste transduction leads to massive spermatid loss in transgenic mice. Mol Hum Reprod. 2012;18:289–297. doi: 10.1093/molehr/gas005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehashi K, Huang L. Bitter peptides and bitter taste receptors. Cell Mol Life Sci. 2009;66:1661–1671. doi: 10.1007/s00018-009-8755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunami H, Montmayeur JP, Buck LB. A family of candidate taste receptors in human and mouse. Nature. 2000;404:601–604. doi: 10.1038/35007072. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lamb DJ. Genetic dissection of mammalian fertility pathways. Nat Cell Biol. 2002;4(Suppl):s41–s49. doi: 10.1038/ncb-nm-fertilityS41. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14:1197–1213. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses. 2010;35:157–170. doi: 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]
- Nelson TM, Munger SD, Boughter JD,, Jr Taste sensitivities to PROP and PTC vary independently in mice. Chem Senses. 2003;28:695–704. doi: 10.1093/chemse/bjg062. [DOI] [PubMed] [Google Scholar]
- Park KH, Kim BJ, Kang J, Nam TS, Lim JM, Kim HT, Park JK, Kim YG, Chae SW, Kim UH. Ca2+ signaling tools acquired from prostasomes are required for progesterone-induced sperm motility. Sci Signal. 2011;4:ra31. doi: 10.1126/scisignal.2001595. [DOI] [PubMed] [Google Scholar]
- Parmentier M, Libert F, Schurmans S, Schiffmann S, Lefort A, Eggerickx D, Ledent C, Mollereau C, Gerard C, Perret J, et al. Expression of members of the putative olfactory receptor gene family in mammalian germ cells. Nature. 1992;355:453–455. doi: 10.1038/355453a0. [DOI] [PubMed] [Google Scholar]
- Peri I, Mamrud-Brains H, Rodin S, Krizhanovsky V, Shai Y, Nir S, Naim M. Rapid entry of bitter and sweet tastants into liposomes and taste cells: implications for signal transduction. Am J Physiol Cell Physiol. 2000;278:C17–C25. doi: 10.1152/ajpcell.2000.278.1.C17. [DOI] [PubMed] [Google Scholar]
- Publicover S, Harper CV, Barratt C. [Ca2+]i signalling in sperm—making the most of what you've got. Nat Cell Biol. 2007;9:235–242. doi: 10.1038/ncb0307-235. [DOI] [PubMed] [Google Scholar]
- Ren D, Xia J. Calcium signaling through CatSper channels in mammalian fertilization. Physiology (Bethesda) 2010;25:165–175. doi: 10.1152/physiol.00049.2009. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Miranda E, Buffone MG, Edwards SE, Ord TS, Lin K, Sammel MD, Gerton GL, Moss SB, Williams CJ. Extracellular adenosine 5′-triphosphate alters motility and improves the fertilizing capability of mouse sperm. Biol Reprod. 2008;79:164–171. doi: 10.1095/biolreprod.107.065565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist G, Brody I. The prostasome: its secretion and function in man. Biochim Biophys Acta. 1985;822:203–218. doi: 10.1016/0304-4157(85)90008-5. [DOI] [PubMed] [Google Scholar]
- Rossler P, Kroner C, Freitag J, Noe J, Breer H. Identification of a phospholipase C beta subtype in rat taste cells. Eur J Cell Biol. 1998;77:253–261. doi: 10.1016/s0171-9335(98)80114-3. [DOI] [PubMed] [Google Scholar]
- Rozengurt N, Wu SV, Chen MC, Huang C, Sternini C, Rozengurt E. Colocalization of the alpha-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G792–G802. doi: 10.1152/ajpgi.00074.2006. [DOI] [PubMed] [Google Scholar]
- Sbarbati A, Merigo F, Benati D, Tizzano M, Bernardi P, Crescimanno C, Osculati F. Identification and characterization of a specific sensory epithelium in the rat larynx. J Comp Neurol. 2004;475:188–201. doi: 10.1002/cne.20172. [DOI] [PubMed] [Google Scholar]
- Schaeren-Wiemers N, Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry. 1993;100:431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- Schlecht U, Demougin P, Koch R, Hermida L, Wiederkehr C, Descombes P, Pineau C, Jegou B, Primig M. Expression profiling of mammalian male meiosis and gametogenesis identifies novel candidate genes for roles in the regulation of fertility. Mol Biol Cell. 2004;15:1031–1043. doi: 10.1091/mbc.E03-10-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz N, Hamra FK, Garbers DL. A multitude of genes expressed solely in meiotic or postmeiotic spermatogenic cells offers a myriad of contraceptive targets. Proc Natl Acad Sci USA. 2003;100:12201–6. doi: 10.1073/pnas.1635054100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM. Environmental/lifestyle effects on spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1697–1712. doi: 10.1098/rstb.2009.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima JE, McLean DJ, McCarrey JR, Griswold MD. The murine testicular transcriptome: characterizing gene expression in the testis during the progression of spermatogenesis. Biol Reprod. 2004;71:319–330. doi: 10.1095/biolreprod.103.026880. [DOI] [PubMed] [Google Scholar]
- Singh N, Vrontakis M, Parkinson F, Chelikani P. Functional bitter taste receptors are expressed in brain cells. Biochem Biophys Res Commun. 2011;406:146–151. doi: 10.1016/j.bbrc.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Spehr M, Gisselmann G, Poplawski A, Riffell JA, Wetzel CH, Zimmer RK, Hatt H. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science. 2003;299:2054–2058. doi: 10.1126/science.1080376. [DOI] [PubMed] [Google Scholar]
- Suarez SS. Control of hyperactivation in sperm. Hum Reprod Update. 2008;14:647–657. doi: 10.1093/humupd/dmn029. [DOI] [PubMed] [Google Scholar]
- Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, Churchill ME, Silver WL, Kinnamon SC, Finger TE. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci USA. 2010;107:3210–3215. doi: 10.1073/pnas.0911934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizzano M, Cristofoletti M, Sbarbati A, Finger TE. Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm Med. 2011;11:3. doi: 10.1186/1471-2466-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhaeghen P, Schurmans S, Vassart G, Parmentier M. Olfactory receptors are displayed on dog mature sperm cells. J Cell Biol. 1993;123:1441–1452. doi: 10.1083/jcb.123.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veitinger T, Riffell JR, Veitinger S, Nascimento JM, Triller A, Chandsawangbhuwana C, Schwane K, Geerts A, Wunder F, Berns MW, et al. Chemosensory Ca2+ dynamics correlate with diverse behavioral phenotypes in human sperm. J Biol Chem. 2011;286:17311–17325. doi: 10.1074/jbc.M110.211524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti PE. Understanding the molecular basis of sperm capacitation through kinase design. Proc Natl Acad Sci USA. 2009;106:667–668. doi: 10.1073/pnas.0811895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GT, Gannon KS, Margolskee RF. Transduction of bitter and sweet taste by gustducin. Nature. 1996;381:796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]
- Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci USA. 2002;99:2392–2397. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SM, Baxendale V, Chen Y, Pang AL, Stitely T, Munson PJ, Leung MY, Ravindranath N, Dym M, Rennert OM, et al. Analysis of mouse germ-cell transcriptome at different stages of spermatogenesis by SAGE: biological significance. Genomics. 2004;84:971–981. doi: 10.1016/j.ygeno.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Wu SV, Chen MC, Rozengurt E. Genomic organization, expression and function of bitter taste receptors (T2R) in mouse and rat. Physiol Genomics. 2005;22:139–149. doi: 10.1152/physiolgenomics.00030.2005. [DOI] [PubMed] [Google Scholar]
- Xia J, Reigada D, Mitchell CH, Ren D. CATSPER channel-mediated Ca2+ entry into mouse sperm triggers a tail-to-head propagation. Biol Reprod. 2007;77:551–559. doi: 10.1095/biolreprod.107.061358. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Funahashi H, Murakami T. Improved fertility in gilts and sows after artificial insemination of frozen-thawed boar semen by supplementation of semen extender with caffeine and CaCl2. J Reprod Dev. 2009;55:645–649. doi: 10.1262/jrd.20238. [DOI] [PubMed] [Google Scholar]
- Yun JJ, Heisler LE, Hwang II, Wilkins O, Lau SK, Hyrcza M, Jayabalasingham B, Jin J, McLaurin J, Tsao MS, et al. Genomic DNA functions as a universal external standard in quantitative real-time PCR. Nucleic Acids Res. 2006;34:e85. doi: 10.1093/nar/gkl400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.