Abstract
Although the association between maternal smoking and low birthweight infants has been well established, the mechanisms behind reduced fetal growth are still being elucidated. While many infants are exposed to tobacco smoke in utero, not all are born growth restricted or small for gestational age. Many hypotheses have emerged to explain the differential response to in utero maternal tobacco smoke exposure (MTSE). Studies have shown that both maternal and fetal genotypes may contribute to the discrepant outcomes. However, the contribution of epigenetic changes cannot be ignored. In this review we address two important questions regarding the effect of MTSE on the fetal epigenome. First, does exposure to maternal tobacco smoke in utero alter the fetal epigenome? Secondly, could these alterations be associated with the reduced fetal growth observed with MTSE?
Keywords: epigenetics, DNA methylation, pregnancy, tobacco smoke
Tobacco use modifies pregnancy outcomes
Despite the myriad public health warnings describing the harmful effects of tobacco smoke exposure, as many as 20% of all women smoke during pregnancy (Gergen et al., 1998). Smoking while pregnant results in a higher risk of perinatal and obstetric complications such as preterm birth and impaired lung function of the developing fetus (Hofhuis et al., 2003; Suter et al., 2010a, b). The association between MTSE and restricted fetal growth has been well studied since Simpson's seminal report on smoking and prematurity in 1957 (Simpson, 1957). Birthweight is significantly less and the prevalence of SGA infants is significantly greater in tobacco-exposed infants (Aagaard-Tillery et al., 2008a, b). The effect of smoke exposure appears to be dose dependent, as there is an observed reduction in infant birthweight in women who smoke >10 cigarettes per day compared with all smokers (Voigt et al., 2009a, b). Furthermore, the outcome is more favorable in women who quit smoking while pregnant compared with those who continue to smoke throughout gestation (Nordstrom and Cnattingius, 1994).
Many hypothesized mechanisms have been proposed to contribute to reduced fetal growth with MTSE (Suter et al., 2010a, b). Chronic fetal hypoxia is potentially a main contributor to IUGR with MTSE. Carbon monoxide is a component of tobacco smoke which forms carboxyhemoglobin in the fetus (Rogers, 2009). Carbon monoxide exposure during pregnancy has been repeatedly associated with adverse pregnancy outcomes including reduced birthweight (Wilhelm and Ritz, 2005; Liu et al., 2007). Another hypothesis is that nicotine, a principle metabolite found in tobacco smoke, is associated with the constriction of placental blood vessels and increased apoptosis of placental syncytiotrophoblasts (Voigt et al., 2009a, b).
While many fetuses experience MTSE in utero, not all are born growth restricted or SGA. Mechanisms behind this differing susceptibility to MTSE and IUGR are beginning to be explored. It has been known for decades that specific genetic polymorphisms are associated with a differing susceptibility to cancer. However, more recent research implicates epigenetic modifications with cancer susceptibility. Similarly, the potential for genetic polymorphisms to contribute to IUGR has become the focus of many studies. However, if genetic polymorphisms contribute to IUGR susceptibility, the contribution of epigenetic modifications may be similarly important. Such modifications can alter the expression patterns of genes which ultimately work in concert to influence fetal growth.
Genetic polymorphisms associated with restricted fetal growth
Over 4000 compounds have been reported in tobacco smoke (Brunnemann and Hoffmann, 1991) and many of these compounds are water soluble with a small molecular weight, allowing them to easily cross the placenta (Jauniaux and Burton, 2007). The genes which respond to and metabolize xenobiotic compounds help the body process these carcinogenic and teratogenic compounds found in tobacco smoke. Polymorphisms within the genes which metabolize the polycyclic aromatic hydrocarbons (PAHs) and nicotine alter an individuals' susceptibility to cancer (Bartsch et al., 2000; Roos and Bolt, 2005). PAHs are metabolized in a two-step process (Shimada, 2006). Phase I enzymes recognize the PAH and transform it into a reactive intermediate. The intermediate is processed by the Phase II enzymes to produce an excretable metabolite.
When a xenobiotic enters the cell it is recognized by the aryl hydrocarbon receptor (AhR). Upon binding the xenobiotic, AhR couples with the aryl hydrocarbon receptor nuclear translocator protein (ARNT), binds to genes with a xenobiotic response element (XRE) within the promoter and initiates expression of the Phase I and II genes necessary for processing the compound (Kohle and Bock, 2007). A wild-type (WT) maternal AhR genotype, compared with a polymorphism in AhR (an arginine (Arg) to lysine (Lys) substitution at codon 554 (G/A) in exon 10), is associated with a reduced birthweight and length (Sasaki et al., 2006).
The most extensively studied gene polymorphisms associated with maternal tobacco smoke exposure and fetal growth are the Phase I enzyme, CYP1A1 and the Phase II enzyme GSTT1. The CYP1A1 gene has a well-characterized polymorphism in the 3′ non-coding region, which gives rise to an MspI restriction site (Kawajiri et al., 1990). This polymorphism has been highly studied for its association with lung cancer risk (Hayashi et al., 1991; Anttila et al., 1994; Sugimura et al., 1994). Interestingly, it is also implicated in growth restriction with maternal smoking. If the maternal genotype is homozygous for the MspI polymorphism (aa), the average reduction in birthweight is 520 g with MTSE, compared with mothers who are homozygous for the WT allele (AA), which have a 252 g reduction in birthweight (Wang et al., 2002). Further studies of the maternal CYP1A1 and GSTT1 genotypes have concluded that these polymorphisms influence infant birthweight with MTSE (Nukui et al., 2004; Tsai et al., 2008; Delpisheh et al., 2009). While most studies have focused on the maternal genotype, we have shown that fetal genotype is also associated with reduced fetal weight with MTSE (Aagaard-Tillery et al., 2010). Offspring with a homozygous deletion at the GSTT1 locus (null phenotype) showed a reduced mean birthweight of 262 g compared with fetuses that had the GSTT1 allele in MTSE newborns.
Epigenetic changes with environmental influence
Because polymorphisms are associated with reduced birthweight, it stands to reason that epigenetic changes may also contribute to a growth restricted phenotype. Unlike our genome, the ‘epigenome’ is modifiable by the environment. These epigenetic changes, such as DNA methylation, or alterations in the histone code, can alter the patterns of gene expression (Jenuwein and Allis, 2001; Choudhuri et al., 2010). The study of epigenetics and perinatal health is becoming increasingly important. In animal models, it is demonstrated that various changes within the in utero milleu can lead to adverse outcomes in the offspring. Many of these in utero exposure models have also shown that these adverse experiences are associated epigenetic changes as well. For example, in utero exposure to a high fat diet is associated with fetal non-alcoholic fatty liver in a non-human primate model (McCurdy et al., 2009). In these same animals, the fetal hepatic epigenome is altered (Aagaard-Tillery et al., 2008a, b; Suter et al., 2011a, b). Because it is known that the fetal epigenome can respond to and be altered by different environmental exposures, the questions must be asked, how does exposure to maternal tobacco smoke in utero alter the fetal epigenome and what impact does this have on susceptibility to fetal growth restriction?
Exposure to tobacco smoke in adults has been associated with changes in DNA methylation. Gene specific changes in promoter methylation were reported in induced sputum from smokers with either chronic obstructive pulmonary disease or lung cancer compared with non-smoking healthy controls (Guzman et al., 2012). Studies have shown gene-specific altered methylation in adenocarcinoma (Kim et al., 2004; Liu et al., 2006), (Tessema et al., 2009). Furthermore, tobacco smoke exposure is associated with aberrant DNA methylation patterns in prostate, gastric and colorectal tumors (Cortessis et al., 2012).
Reduced birthweight in the absence of MTSE has been associated with changes in DNA methylation. Methylation status of both coding and non-coding regions of the genome from cord blood is associated with low birthweight (Fryer et al., 2009; Fryer et al., 2011). Gene specific changes in methylation have also been reported in human placenta from SGA infants (Ferreira et al., 2011; Filiberto et al., 2011). Factors which contribute to reduced birthweight, such as maternal stress and depression, have also been associated with gene-specific promoter methylation patterns (Liu et al., 2012; Mulligan et al., 2012). If both tobacco smoke exposure and reduced birthweight are associated with DNA methylation changes, it is necessary to study how MTSE can contribute to an altered fetal epigenome.
Epigenetics and MTSE
‘Does MTSE alter the fetal epigenome?’ While respiratory epithelia exposed directly to tobacco smoke show epigenomic alterations (Liu et al., 2010), the question remains if indirect tobacco smoke exposure (i.e. through maternal bloodstream and through the placenta) could alter the epigenome. Because the epigenome varies between different cell types and tissues, it is important to consider which tissue is being studied when comparing environmental exposures and epigenetic changes (Suter and Aagaard, 2012). Studies from the placenta, cord blood, buccal cells and peripheral blood indicate that in utero MTSE is associated with alterations in DNA methylation (summarized in Table I).
Table I.
DNA methylation changes associated with in utero MTSE
| Observed change | Sample studied | Methodology | Reference |
|---|---|---|---|
| Hypomethylation of the CYP1A1 promoter | Placenta | Bisulfite sequencing | Suter et al. (2010a, b) |
| Global, site-specific, CpG methylation changes | Placenta | Illumina Infinium Array | Suter et al. (2011a, b) |
| Decreased AluYb8 methylation | Placenta | Bisulfite pyrosequencing | Wilhelm-Benartzi et al. (2012) |
| Global DNA methylation inversely correlates with cotinine levels | Cord blood | ELISA | Guerrero-Preston et al. (2010) |
| AluYb8 methylation decreases | Buccal cells | Bisulfite pyrosequencing | Breton et al. (2009) |
| AXL promoter methylation increases | Buccal cells | Bisulfite pyrosequencing | Breton et al. (2011) |
| Decrease in Sat2 methylation | Peripheral blood granulocytes | MethyLight Assay | Flom et al. (2011) |
| Increased IGF2 DMR methylation | Cord blood | Bisulfite pyrosequencing | Soubry et al. (2011); Murphy et al. (2012) |
| Altered methylation of 26 individual CpGs associated with six genes | Cord blood | 450 K array | Joubert et al. (2012) |
Tobacco smoke exposure was shown to be associated with changes in the methylation of the CYP1A1 promoter in the lungs of smokers compared with non-smokers (Anttila et al., 2003). Furthermore, methylation within this promoter was dose dependent, increasing days after quitting smoking. Because of this data, we interrogated the methylation status of the CYP1A1 promoter in the placentas of smokers and non-smokers and found that this promoter is significantly hypomethylated in smokers (Suter et al., 2010a, b). Interestingly, methylation in this promoter surrounding a critical xenobiotic response element is inversely correlated with the expression of the CYP1A1 gene.
We wanted to further interrogate the correlation between methylation and gene expression on a genome-wide scale to see if other genes were similarly altered in the placentas of smokers. Utilizing the genome-wide Illumina bead arrays, we analyzed the CpG methylation and gene expression of placentas from smokers and non-smokers (Suter et al., 2011a, b). We found significant methylation changes at 1024 individual CpGs. When correlating the methylation of specific CpG sites and expression of their associated genes, only 13 genes showed a correlation when analyzing the total cohort together. When only non-smokers were included in the analysis, 25 genes showed a significant correlation. However, 438 genes revealed a significant correlation between methylation and gene expression when only smokers were included in the analysis. Using Ingenuity Pathway Analysis to investigate the potential functions and mechanisms of the 438 genes from the smoking cohort, we found that this gene list is enriched for genes involved in oxidative phosphorylation, mitochondrial dysfunction and the cells' response to hypoxia (Fig. 1).
Figure 1.
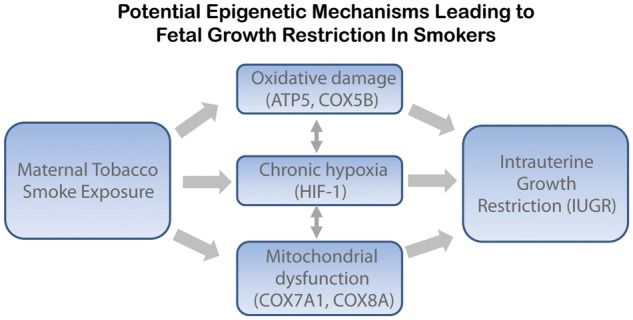
Potential epigenetic mechanisms leading to fetal growth restriction in smokers. We have found that in placenta the oxidative damage, hypoxia and mitochondrial dysfunction pathways are altered with maternal smoking. Representative genes which were modified are listed underneath each pathway. These pathways interact and could potentially influence fetal growth leading to IUGR.
Another study using the placenta interrogated the methylation status of the repeat regions of the genome (Wilhelm-Benartzi et al., 2012). They found that both tobacco smoke and alcohol exposure were associated with differential methylation of repeat regions. MTSE is associated with a decrease in methylation levels at the AluYb8 repeats. A similar decrease was not seen with alcohol exposure. However, alcohol exposure was associated with changes in LINE-1 methylation, a region which did not change with MTSE. It is possible that specific regions of the genome are more susceptible to various exposures than others.
Another readily available sample used to test DNA methylation and that can give good insight into the in utero experience is cord blood. Guerrero-Preston et al. (2010) asked the question if DNA methylation levels in fetal cord blood could be used as a biomarker for in utero exposure to tobacco smoke. They set out to correlate genome-wide methylation levels using an ELISA kit, and correlate the data with cord blood cotinine levels, a metabolite of nicotine and an indicator of tobacco smoke exposure. They reported that global DNA methylation inversely correlates with cotinine levels; global DNA methylation was lowest in the cord blood of mothers who smoked (higher cotinine) and methylation was highest in the non-smoking mothers.
Using cord blood to decipher site-specific changes in CpG methylation, Joubert et al. (2012) subjected DNA isolated from cord blood to the Infinium HumanMethylation450 BeadChip. This array measures the methylation status of over 450 000 individual CpG sites throughout the genome. Methylation levels were correlated with maternal cotinine levels. They determined that 26 individual CpGs associated with six genes, including CYP1A1 are altered by virtue of maternal smoking. While studies of the placenta and cord blood give an indication of epigenetic changes experienced in utero, the question remains if this in utero exposure persists into childhood and adulthood. Three recent studies have shown differences in DNA methylation in children and adults associated with MTSE. Using buccal cells from children in kindergarten and first grade, it has been reported that DNA methylation of the AluYb8 repeat is decreased, while methylation within the site-specific AXL gene is slightly increased (Breton et al., 2009; Breton et al., 2011). In a study of DNA methylation in peripheral blood granulocytes from adult women (mean age was 43 years), there was a difference in the DNA methylation of Sat2 repeats between those exposed to MTS and those who were not (Flom et al., 2011).
Not only are there apparent changes in the DNA methylation in the placenta and cord blood from MTSE, it appears that an epigenetic memory of this exposure can still be identified in childhood and adulthood. The importance of this ‘epigenetic memory’ remains to be discovered.
‘Could epigenetic changes associated with MTSE influence fetal growth?’ The idea that genetic polymorphisms can influence infant phenotype with respect to MTSE is not novel. Potential epigenetic changes in response to MTSE may further increase the susceptibility to growth restriction; however, such research itself is still in its infancy. While others have reported that polymorphisms in the CYP1A1 gene may influence fetal growth, we have shown that this gene is amenable to epigenetic alterations with MTSE. This could be a potential mechanism behind growth restriction without a subsequent change in the genome.
Other studies have focused on the differentially methylated region (DMR) of the insulin-like growth factor 2 (IGF2) gene. IGF2 is an imprinted gene, expressed from the paternal allele and repressed from the maternal allele (Biliya and Bulla, 2010). Adult individuals who were exposed in utero to the Dutch famine have decreased methylation in this region in peripheral blood cells 60 years after exposure (Heijmans et al., 2008; Tobi et al., 2012). This region of the genome is a model for an epigenetically regulated genomic region whose methylation is sensitive to environmental exposures. Studies of this region with MTSE have not been conclusive. Two studies have reported that MTSE is associated with an increase in methylation in cord blood of the DMR which regulates IGF2 expression (Soubry et al., 2011; Murphy et al., 2012). However, another group reported no significant changes in methylation in this region (Tobi et al., 2011).
While current studies are far from determining the exact molecular mechanisms behind fetal growth restriction with MTSE, determining the contribution of epigenetics to this phenotype is an exciting new field, and likely an important yet understudied piece of the puzzle. While it is likely that tobacco smoke exposure can alter the fetal epigenome, we still do not know how the epigenome is influencing the phenotype of growth restriction. Current data suggest that there are many different regions of the genome, which are susceptible to environmental exposures, and these regions provide a good reference point as we start to map these fetal epigenomic changes. Only then can we discern the contribution of MTSE to the fetal epigenome and the differing susceptibility to growth restriction.
Authors’ roles
M.A.S. and K.M.A. wrote the manuscript. A.M.A. made the figure and revised the manuscript.
Funding
This work was supported by the U.S. National Institutes of Health (NIH) Director New Innovator Award (DP2120OD001500-01; to K.M.A.); the NIH Research Education and Career Horizon Institutional Research and Academic Career Development Award (REACH IRACDA) grant K12 GM084897 (to M.A.S.) and NIH NIGMS grant GM069234 (to A.M.A.).
Conflict of interest
None declared.
References
- Aagaard-Tillery KM, Grove K, Bishop J, Ke X, Fu Q, McKnight R, Lane RH. Developmental origins of disease and determinants of chromatin structure: maternal diet modifies the primate fetal epigenome. J Mol Endocrinol. 2008a;41:91–102. doi: 10.1677/JME-08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aagaard-Tillery KM, Porter TF, Lane RH, Varner MW, Lacoursiere DY. In utero tobacco exposure is associated with modified effects of maternal factors on fetal growth. Am J Obstet Gynecol. 2008b;198:66e1–66e6. doi: 10.1016/j.ajog.2007.06.078. [DOI] [PubMed] [Google Scholar]
- Aagaard-Tillery K, Spong CY, Thom E, Sibai B, Wendel G, Jr, Wenstrom K, Samuels P, Simhan H, Sorokin Y, Miodovnik M, et al. Pharmacogenomics of maternal tobacco use: metabolic gene polymorphisms and risk of adverse pregnancy outcomes. Obstet Gynecol. 2010;115:568–577. doi: 10.1097/AOG.0b013e3181d06faf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttila S, Hirvonen A, Husgafvel-Pursiainen K, Karjalainen A, Nurminen T, Vainio H. Combined effect of CYP1A1 inducibility and GSTM1 polymorphism on histological type of lung cancer. Carcinogenesis. 1994;15:1133–1135. doi: 10.1093/carcin/15.6.1133. [DOI] [PubMed] [Google Scholar]
- Anttila S, Hakkola J, Tuominen P, Elovaara E, Husgafvel-Pursiainen K, Karjalainen A, Hirvonen A, Nurminen T. Methylation of cytochrome P4501A1 promoter in the lung is associated with tobacco smoking. Cancer Res. 2003;63:8623–8628. [PubMed] [Google Scholar]
- Bartsch H, Nair U, Risch A, Rojas M, Wikman H, Alexandrov K. Genetic polymorphism of CYP genes, alone or in combination, as a risk modifier of tobacco-related cancers. Cancer Epidemiol Biomarkers Prev. 2000;9:3–28. [PubMed] [Google Scholar]
- Biliya S, Bulla LA., Jr Genomic imprinting: the influence of differential methylation in the two sexes. Exp Biol Med (Maywood) 2010;235:139–147. doi: 10.1258/ebm.2009.009251. [DOI] [PubMed] [Google Scholar]
- Breton CV, Byun HM, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med. 2009;180:462–467. doi: 10.1164/rccm.200901-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton CV, Salam MT, Gilliland FD. Heritability and role for the environment in DNA methylation in AXL receptor tyrosine kinase. Epigenetics. 2011;6:895–898. doi: 10.4161/epi.6.7.15768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunnemann KD, Hoffmann D. Analytical studies on tobacco-specific N-nitrosamines in tobacco and tobacco smoke. Crit Rev Toxicol. 1991;21:235–240. doi: 10.3109/10408449109017910. [DOI] [PubMed] [Google Scholar]
- Choudhuri S, Cui Y, Klaassen CD. Molecular targets of epigenetic regulation and effectors of environmental influences. Toxicol Appl Pharmacol. 2010;245:378–393. doi: 10.1016/j.taap.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortessis VK, Thomas DC, Levine AJ, Breton CV, Mack TM, Siegmund KD, Haile RW, Laird PW. Environmental epigenetics: prospects for studying epigenetic mediation of exposure-response relationships. Hum Genet. 2012 doi: 10.1007/s00439-012-1189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpisheh A, Brabin L, Topping J, Reyad M, Tang AW, Brabin BJ. A case-control study of CYP1A1, GSTT1 and GSTM1 gene polymorphisms, pregnancy smoking and fetal growth restriction. Eur J Obstet Gynecol Reprod Biol. 2009;143:38–42. doi: 10.1016/j.ejogrb.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Ferreira JC, Choufani S, Grafodatskaya D, Butcher DT, Zhao C, Chitayat D, Shuman C, Kingdom J, Keating S, Weksberg R. WNT2 promoter methylation in human placenta is associated with low birthweight percentile in the neonate. Epigenetics. 2011;6:440–449. doi: 10.4161/epi.6.4.14554. [DOI] [PubMed] [Google Scholar]
- Filiberto AC, Maccani MA, Koestler D, Wilhelm-Benartzi C, Avissar-Whiting M, Banister CE, Gagne LA, Marsit CJ. Birthweight is associated with DNA promoter methylation of the glucocorticoid receptor in human placenta. Epigenetics. 2011;6:566–572. doi: 10.4161/epi.6.5.15236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flom JD, Ferris JS, Liao Y, Tehranifar P, Richards CB, Cho YH, Gonzalez K, Santella RM, Terry MB. Prenatal smoke exposure and genomic DNA methylation in a multiethnic birth cohort. Cancer Epidemiol Biomarkers Prev. 2011;20:2518–2523. doi: 10.1158/1055-9965.EPI-11-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer AA, Nafee TM, Ismail KM, Carroll WD, Emes RD, Farrell WE. LINE-1 DNA methylation is inversely correlated with cord plasma homocysteine in man: a preliminary study. Epigenetics. 2009;4:394–398. doi: 10.4161/epi.4.6.9766. [DOI] [PubMed] [Google Scholar]
- Fryer AA, Emes RD, Ismail KM, Haworth KE, Mein C, Carroll WD, Farrell WE. Quantitative, high-resolution epigenetic profiling of CpG loci identifies associations with cord blood plasma homocysteine and birth weight in humans. Epigenetics. 2011;6:86–94. doi: 10.4161/epi.6.1.13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergen PJ, Fowler JA, Maurer KR, Davis WW, Overpeck MD. The burden of environmental tobacco smoke exposure on the respiratory health of children 2 months through 5 years of age in the United States: Third National Health and Nutrition Examination Survey, 1988 to 1994. Pediatrics. 1998;101:E8. doi: 10.1542/peds.101.2.e8. [DOI] [PubMed] [Google Scholar]
- Guerrero-Preston R, Goldman LR, Brebi-Mieville P, Ili-Gangas C, Lebron C, Witter FR, Apelberg BJ, Hernandez-Roystacher M, Jaffe A, Halden RU, et al. Global DNA hypomethylation is associated with in utero exposure to cotinine and perfluorinated alkyl compounds. Epigenetics. 2010;5:539–546. doi: 10.4161/epi.5.6.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman L, Depix MS, Salinas AM, Roldan R, Aguayo F, Silva A, Vinet R. Analysis of aberrant methylation on promoter sequences of tumor suppressor genes and total DNA in sputum samples: a promising tool for early detection of COPD and lung cancer in smokers. Diagn Pathol. 2012;7:87. doi: 10.1186/1746-1596-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, Watanabe J, Nakachi K, Kawajiri K. Genetic linkage of lung cancer-associated MspI polymorphisms with amino acid replacement in the heme binding region of the human cytochrome P450IA1 gene. J Biochem. 1991;110:407–411. doi: 10.1093/oxfordjournals.jbchem.a123594. [DOI] [PubMed] [Google Scholar]
- Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA. 2008;105:17046–9. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofhuis W, de Jongste JC, Merkus PJ. Adverse health effects of prenatal and postnatal tobacco smoke exposure on children. Arch Dis Child. 2003;88:1086–1090. doi: 10.1136/adc.88.12.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauniaux E, Burton GJ. Morphological and biological effects of maternal exposure to tobacco smoke on the feto-placental unit. Early Hum Dev. 2007;83:699–706. doi: 10.1016/j.earlhumdev.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Joubert BR, Haberg SE, Nilsen RM, Wang X, Vollset SE, Murphy SK, Huang Z, Hoyo C, Midttun O, Cupul-Uicab LA, et al. 450K Epigenome-wide scan identifies differential DNA methylation in newborns related to maternal smoking during pregnancy. Environ Health Perspect. 2012;120:1425–1431. doi: 10.1289/ehp.1205412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawajiri K, Nakachi K, Imai K, Yoshii A, Shinoda N, Watanabe J. Identification of genetically high risk individuals to lung cancer by DNA polymorphisms of the cytochrome P450IA1 gene. FEBS Lett. 1990;263:131–133. doi: 10.1016/0014-5793(90)80721-t. [DOI] [PubMed] [Google Scholar]
- Kim H, Kwon YM, Kim JS, Lee H, Park JH, Shim YM, Han J, Park J, Kim DH. Tumor-specific methylation in bronchial lavage for the early detection of non-small-cell lung cancer. J Clin Oncol. 2004;22:2363–2370. doi: 10.1200/JCO.2004.10.077. [DOI] [PubMed] [Google Scholar]
- Kohle C, Bock KW. Coordinate regulation of Phase I and II xenobiotic metabolisms by the Ah receptor and Nrf2. Biochem Pharmacol. 2007;73:1853–1862. doi: 10.1016/j.bcp.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lan Q, Siegfried JM, Luketich JD, Keohavong P. Aberrant promoter methylation of p16 and MGMT genes in lung tumors from smoking and never-smoking lung cancer patients. Neoplasia. 2006;8:46–51. doi: 10.1593/neo.05586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Krewski D, Shi Y, Chen Y, Burnett RT. Association between maternal exposure to ambient air pollutants during pregnancy and fetal growth restriction. J Expo Sci Environ Epidemiol. 2007;17:426–432. doi: 10.1038/sj.jes.7500503. [DOI] [PubMed] [Google Scholar]
- Liu F, Killian JK, Yang M, Walker RL, Hong JA, Zhang M, Davis S, Zhang Y, Hussain M, Xi S, et al. Epigenomic alterations and gene expression profiles in respiratory epithelia exposed to cigarette smoke condensate. Oncogene. 2010;29:3650–3664. doi: 10.1038/onc.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Murphy SK, Murtha AP, Fuemmeler BF, Schildkraut J, Huang Z, Overcash F, Kurtzberg J, Jirtle R, Iversen ES, et al. Depression in pregnancy, infant birth weight and DNA methylation of imprint regulatory elements. Epigenetics. 2012;7:735–746. doi: 10.4161/epi.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119:323–335. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan C, D'Errico N, Stees J, Hughes D. Methylation changes at NR3C1 in newborns associate with maternal prenatal stress exposure and newborn birth weight. Epigenetics. 2012;7:853–857. doi: 10.4161/epi.21180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SK, Adigun A, Huang Z, Overcash F, Wang F, Jirtle RL, Schildkraut JM, Murtha AP, Iversen ES, Hoyo C. Gender-specific methylation differences in relation to prenatal exposure to cigarette smoke. Gene. 2012;494:36–43. doi: 10.1016/j.gene.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom ML, Cnattingius S. Smoking habits and birthweights in two successive births in Sweden. Early Hum Dev. 1994;37:195–204. doi: 10.1016/0378-3782(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Nukui T, Day RD, Sims CS, Ness RB, Romkes M. Maternal/newborn GSTT1 null genotype contributes to risk of preterm, low birthweight infants. Pharmacogenetics. 2004;14:569–576. doi: 10.1097/00008571-200409000-00001. [DOI] [PubMed] [Google Scholar]
- Rogers JM. Tobacco and pregnancy. Reprod Toxicol. 2009;28:152–160. doi: 10.1016/j.reprotox.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Roos PH, Bolt HM. Cytochrome P450 interactions in human cancers: new aspects considering CYP1B1. Expert Opin Drug Metab Toxicol. 2005;1:187–202. doi: 10.1517/17425255.1.2.187. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Kondo T, Sata F, Saijo Y, Katoh S, Nakajima S, Ishizuka M, Fujita S, Kishi R. Maternal smoking during pregnancy and genetic polymorphisms in the Ah receptor, CYP1A1 and GSTM1 affect infant birth size in Japanese subjects. Mol Hum Reprod. 2006;12:77–83. doi: 10.1093/molehr/gal013. [DOI] [PubMed] [Google Scholar]
- Shimada T. Xenobiotic-metabolizing enzymes involved in activation and detoxification of carcinogenic polycyclic aromatic hydrocarbons. Drug Metab Pharmacokinet. 2006;21:257–276. doi: 10.2133/dmpk.21.257. [DOI] [PubMed] [Google Scholar]
- Simpson WJ. A preliminary report on cigarette smoking and the incidence of prematurity. Am J Obstet Gynecol. 1957;73:807–815. [PubMed] [Google Scholar]
- Soubry A, Murphy S, Huang Z, Murtha A, Schildkraut J, Jirtle R, Wang F, Kurtzberg J, Demark-Wahnefried W, Forman M, et al. The effects of depression and use of antidepressive medicines during pregnancy on the methylation status of the IGF2 imprinted control regions in the offspring. Clin Epigenetics. 2011;3:2. doi: 10.1186/1868-7083-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura H, Suzuki I, Hamada GS, Iwase T, Takahashi T, Nagura K, Iwata H, Watanabe S, Kino I, Tsugane S. Cytochrome P-450 lA1 genotype in lung cancer patients and controls in Rio de Janeiro, Brazil. Cancer Epidemiol Biomarkers Prev. 1994;3:145–148. [PubMed] [Google Scholar]
- Suter MA, Aagaard K. What changes in DNA methylation take place in individuals exposed to maternal smoking in utero? Epigenomics. 2012;4:115–118. doi: 10.2217/epi.12.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter M, Abramovici A, Aagaard-Tillery K. Genetic and epigenetic influences associated with intrauterine growth restriction due to in utero tobacco exposure. Pediatr Endocrinol Rev. 2010a;8:94–102. [PMC free article] [PubMed] [Google Scholar]
- Suter M, Abramovici A, Showalter L, Hu M, Shope CD, Varner M, Aagaard-Tillery K. In utero tobacco exposure epigenetically modifies placental CYP1A1 expression. Metabolism. 2010b;59:1481–1490. doi: 10.1016/j.metabol.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter M, Bocock P, Showalter L, Hu M, Shope C, McKnight R, Grove K, Lane R, Aagaard-Tillery K. Epigenomics: maternal high-fat diet exposure in utero disrupts peripheral circadian gene expression in nonhuman primates. Faseb J. 2011a;25:714–726. doi: 10.1096/fj.10-172080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter M, Ma J, Harris AS, Patterson L, Brown KA, Shope C, Showalter L, Abramovici A, Aagaard-Tillery KM. Maternal tobacco use modestly alters correlated epigenome-wide placental DNA methylation and gene expression. Epigenetics. 2011b;6:1284–1294. doi: 10.4161/epi.6.11.17819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessema M, Yu YY, Stidley CA, Machida EO, Schuebel KE, Baylin SB, Belinsky SA. Concomitant promoter methylation of multiple genes in lung adenocarcinomas from current, former and never smokers. Carcinogenesis. 2009;30:1132–1138. doi: 10.1093/carcin/bgp114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobi EW, Heijmans BT, Kremer D, Putter H, Delemarre-van de Waal HA, Finken MJ, Wit JM, Slagboom PE. DNA methylation of IGF2, GNASAS, INSIGF and LEP and being born small for gestational age. Epigenetics. 2011;6:171–176. doi: 10.4161/epi.6.2.13516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobi EW, Slagboom PE, van Dongen J, Kremer D, Stein AD, Putter H, Heijmans BT, Lumey LH. Prenatal famine and genetic variation are independently and additively associated with DNA methylation at regulatory loci within IGF2/H19. PLoS One. 2012;7:e37933. doi: 10.1371/journal.pone.0037933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HJ, Liu X, Mestan K, Yu Y, Zhang S, Fang Y, Pearson C, Ortiz K, Zuckerman B, Bauchner H, et al. Maternal cigarette smoking, metabolic gene polymorphisms, and preterm delivery: new insights on GxE interactions and pathogenic pathways. Hum Genet. 2008;123:359–369. doi: 10.1007/s00439-008-0485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt M, Briese V, Jorch G, Henrich W, Schneider KT, Straube S. The influence of smoking during pregnancy on fetal growth. Considering daily cigarette consumption and the SGA rate according to length of gestation. Z Geburtshilfe Neonatol. 2009a;213:194–200. doi: 10.1055/s-0029-1214405. [DOI] [PubMed] [Google Scholar]
- Voigt M, Briese V, Jorch G, Henrich W, Schneider KT, Straube S. The influence of smoking during pregnancy on fetal growth. Considering daily cigarette consumption and the SGA rate according to length of gestation. Z Geburtshilfe Neonatol. 2009b;213:194–200. doi: 10.1055/s-0029-1214405. [DOI] [PubMed] [Google Scholar]
- Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, Niu T, Wise PH, Bauchner H, Xu X. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002;287:195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Ritz B. Local variations in CO and particulate air pollution and adverse birth outcomes in Los Angeles County, California, USA. Environ Health Perspect. 2005;113:1212–1221. doi: 10.1289/ehp.7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm-Benartzi CS, Houseman EA, Maccani MA, Poage GM, Koestler DC, Langevin SM, Gagne LA, Banister CE, Padbury JF, Marsit CJ. In utero exposures, infant growth, and DNA methylation of repetitive elements and developmentally related genes in human placenta. Environ Health Perspect. 2012;120:296–302. doi: 10.1289/ehp.1103927. [DOI] [PMC free article] [PubMed] [Google Scholar]


