Abstract
Commensal bacterial sensing by Toll-like receptors (TLRs) is critical for maintaining intestinal homeostasis, but can lead to colitis in the absence of IL-10. While TLRs are expressed in multiple cell types in the colon, the cell type(s) responsible for the development of colitis currently unknown. Here, we generated mice that are selectively deficient in MyD88 in various cellular compartments in an IL-10−/− setting. While epithelial expression of MyD88 was dispensable, MyD88 expression in the mononuclear phagocyte (MNP) compartment was required for colitis development. Specifically, phenotypically distinct populations of colonic MNPs expressed high levels IL-1β, IL-23 and IL-6 and promoted Th17 responses in the absence of IL-10. Thus, gut bacterial sensing through MyD88 in MNPs drives inflammatory bowel disease (IBD) when unopposed by IL-10.
Increasing evidence supports the notion that (IBD) results from a dysregulated interaction between the host immune system and its commensal microbiota1. Commensal bacteria are most abundant in the colon and they normally co-exist with the host in a mutually beneficial relationship. At the same time, the mammalian host must maintain the ability to recognize pathogenic microbes in the colon and respond appropriately to contain and eliminate them. While the exact mechanism by which commensal bacteria and pathogenic bacteria are distinguished is unclear, recent evidence indicates that multiple mechanisms exist to limit inflammatory immune responses against commensal microbes. One such mechanism involves secretion of IL-10 in the intestinal mucosa. In the absence of IL-10, mice develop severe colitis with variable kinetics depending on the facility 2, 3. MyD88 deficiency completely rescues colitis in IL-10−/− mice4. However, the cell types responsible for inducing colitogenic signals in IL-10−/− mice remain unknown.
Both hematopoietic and non-hematopoietic cell types express MyD88. In the intestinal mucosa, enterocytes express MyD88 and respond to a variety of TLR ligands5. Transgenic expression of MyD88 by Paneth cells in MyD88−/− mice is sufficient to induce antimicrobial peptides upon sensing of commensal bacteria and prevent bacterial translocation6. Another important cell type that recognizes bacteria through the TLR-MyD88 pathway is the MNP consisting of macrophages and dendritic cells (DCs). The colonic lamina propria contains distinct macrophages and DC populations with partially overlapping phenotypic and functional properties7, 8. The colonic MNPs can be divided into macrophages that are CX3CR1+ CD11b+, and DCs which contain the CD103+ CD11b−, CD103+ CD11b+ and CD103− CD11b+ subsets9. Colonic macrophages contain at least two subsets, CD11c+ and CD11c−, and are both considered to be anti- inflammatory, as they predominantly secrete IL-10 but not IL-12 or TNF-α, and suppress inflammatory responses 9–12. Under inflammatory conditions in a T-cell mediated colitis model, monocyte-derived E-cadherin+ CD103− DCs predominate the colon and secrete colitogenic cytokines such as IL-23 and IL-6 9, 13. However, very little is known about the role of specific MNP subsets in chronic spontaneous colitis that arise in the absence of IL-10.
In order to interrogate the cell types responsible for mediating spontaneous colitis that arises in IL-10 deficient mice, we generated mice that are selectively deficient in MyD88 in various cellular compartments and followed colitis progression over time. We further characterized the phenotype and function of MNPs in which MyD88-dependent signals drive IBD in IL-10−/− mice. Our results reveal the selective importance of gut bacterial sensing by MNPs through the MyD88-dependent pathway in mediating inflammatory bowel disease, and place the colonic MNPs as the key initiator of colitogenic inflammation.
Results
Cell type specific MyD88 function in colitis development
IL-10−/− mice develop spontaneous colitis around 4–8 weeks of age in our facility, which depends on intestinal bacteria sensing through MyD88-dependent signals4. We first tested the existence of Helicobacter spp., which is known to drive colitis development in IL-10 deficient mice. Mice were screened by PCR and 16S rRNA sequences, and H. typhlonicus, H. Hepaticus, H. ganmani and H. rodentium were detected in our colony (data not shown). To confirm that colitis is induced by commensal bacteria, we treated IL-10−/− mice with antibiotics, and indeed pathology measured by endoscopic colitis score, histopathologic score and mesenteric lymph nodes enlargement were all reversed after 2 weeks of treatment (Supplementary Figure S1).
To determine the cell types responsible for colitis development in IL-10−/− mice, mice deficient in MyD88 expression in various cell types were generated. Mice carrying MyD88 floxed allele(s) were crossed to IL-10−/− background, and further crossed to different cre-recombinase expressing mouse lines, namely, MyD88FL; Villin-Cre(+); IL- 10−/− (Villin-MyD88/IL-10 KO; targeting MyD88 deletion in intestinal epithelial cells), MyD88FL; CD11c-Cre(+); IL-10−/− (CD11c-MyD88/IL-10 KO; targeting dendritic cells14), and MyD88FL; LysM-Cre(+); IL-10−/− (LysM-MyD88/IL-10 KO; targeting phagocytes). The deletion efficacy in the respective cell types was found to be 100 %, 99 % and 83 % respectively (Supplementary Figure S2 a–d).
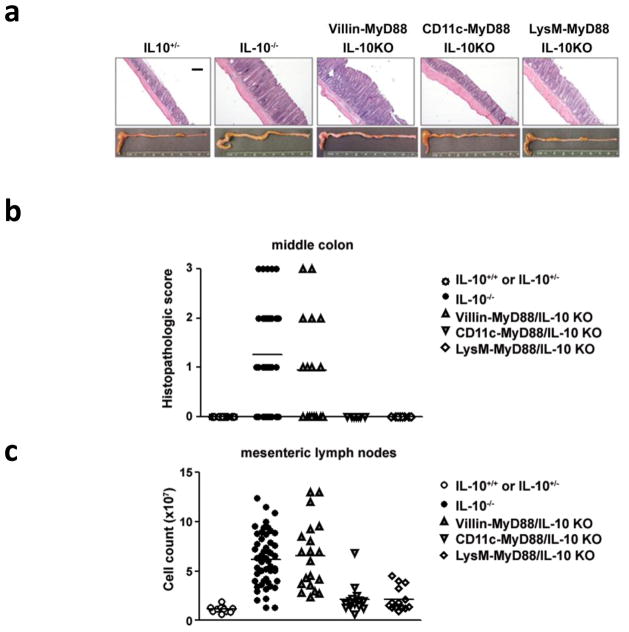
As expected, over 91% of IL-10−/− mice showed severe inflammation and an increase in the number of mesenteric lymph node (MLN) cells by 15 weeks of age (Figure 1). Macroscopically, Villin-MyD88/IL-10 KO showed signs of colitis comparable to IL-10−/− mice, including intestinal wall thickening, unformed stools and MLN hypertrophy. In contrast, minimal signs of inflammation were detected in either CD11c-MyD88/IL-10 KO or LysM-MyD88/IL-10 KO mice (Figure 1a). In addition, histological examination detected pathological changes including epithelial hyperplasia and massive leukocytic infiltration only in the tissue of IL10−/− and Villin-MyD88/IL-10 KO but not in CD11c-MyD88/IL-10 KO or LysM-MyD88/IL-10 KO mice (Figure 1b). Consistently, MLN hypertrophies was detected in IL-10−/− mice and in Villin-MyD88/IL-10 KO but cell numbers in the MLN were only slightly above the WT control in CD11c-MyD88/IL-10 KO or LysM-MyD88/IL-10 KO mice (Figure 1c). Taken together, these data indicated that MyD88 signals in CD11c+ and LysM+ cells, but not in epithelial cells, are required for the onset of colitis in the absence of IL-10.
Figure 1. Cell type specific MyD88 deletion in IL-10−/− colitis.
(a) H&E staining on middle colon tissue and photograph of presentative colon of 15weeks old mice. Scale bar, 200 μm (b) Histopathological score using middle colon of IL-10+/+ or IL10+/− (n=15; age raging 10–15 weeks old), IL-10−/− (n=32; age raging 9–18 weeks old), Villin- MyD88/IL-10 KO (n=16; 9–15 weeks old), CD11c-MyD88/IL10-KO (n=6; age raging 15–18 weeks old) and LysM-MyD88/IL-10 KO (n=8; age raging 10–15 weeks old). Samples were harvested only from sets of littermates for the comparison between control IL-10−/− and each conditional knockout strains. (c) Cell number of mesenteric lymph nodes cells of IL-10+/+ or IL10+/− (n=11), IL-10−/− (n=50), Villin-MyD88/IL-10 KO (n=20), CD11c-MyD88/IL10-KO (n=14) and LysM-MyD88/IL-10 KO (n=14) at 15 weeks old.
MyD88 in colonic MNPs is required for cytokine responses
As CD11c and LysM are expressed by antigen-presenting cells (APCs) such as DCs and macrophages, the absence of colitis seen in CD11c-MyD88/IL-10 KO and LysM-MyD88/IL-10 KO mice implicate the importance of these cell types in secreting pro-inflammatory cytokines and inducing colitogenic T cell responses. To this end, we measured the secretion of IL-12 p40 from the supernatant of colon tissue explant cultures. IL-12 p40 is a cytokine subunit necessary to form intact IL-12 p70 and IL-23, which play a key role in Th1 and Th17 responses, respectively15–19. Colonic tissue explants from Villin-MyD88/IL-10 KO produced similar levels of IL-12 p40 as the control IL-10−/− tissue (Figure 2a). In contrast, colonic tissues taken from CD11c-MyD88/IL-10 KO and LysM-MyD88/IL-10 KO failed to secrete appreciable levels of IL-12 p40 (Figure 2a). To extend this finding to other proinflammatory cytokines, we next measured the levels of IL-1β, IL-6 and TNF-α mRNA from colonic tissues by quantitative PCR. Again, mRNA levels of all of these cytokines were significantly reduced in the colons of CD11c-MyD88/IL-10 KO and LysM-MyD88/IL-10 KO mice compared to IL-10−/− mice, whereas Villin-MyD88/IL-10 KO mice expressed similarly high levels of these cytokines as the IL- 10−/− controls (Figure 2b). These results indicate that MNPs responsible for the onset of colitis rely on MyD88-depent signals for secretion of proinflammatory cytokines in the colon.
Figure 2. Regulation of inflammatory cytokines in IL-10−/− mice.
(a) ELISA analysis for IL-12/23 p40 level of colon explants cultures. IL-10+/+ or IL10+/− (n=5), IL-10−/− (n=21), Villin-MyD88/IL-10 KO (n=11), CD11c-MyD88/IL10-KO (n=6) and LysM-MyD88/IL-10 KO (n=5) at 12–15 weeks old were used. (b) Relative mRNA expression levels in the colon tissue measured by quantitative PCR. At least 6 sets of littemates were used in each genotypes. Error bars represent mean ± s.e.m. *, p < 0.05 (unpaired Studen’t t-t test).
MyD88 in colonic MNPs drives Th1 and Th17 expansion
To determine the cellular mechanism of colonic inflammation in IL-10−/− mice, we next examined the phenotype of CD4 T cells in the lymph nodes draining the colon. Cytokine secretion from MLN T cells stimulated with anti-CD3 antibody indicated that, while high levels of IL-17A and IFN-γ are produced from MLN of IL-10−/− and Villin-MyD88/IL-10 KO mice, these cytokines remained barely detectable in CD11c-MyD88/IL-10 KO and LysM-MyD88/IL-10 KO (Figure 3a). We also tested the cytokine secretion from lamina propria T cells stimulated with anti-CD3 antibody, the result was similar to that of MLN cells (Figure 3b). Accordingly, both IL-17A and IFN-γ mRNA levels were much lower in the colonic tissue from CD11c-MyD88/IL-10 KO and LysM-MyD88/IL-10 KO mice compared to IL-10−/− control (Figure 3c), indicating that such Th1/Th17 expansion occurred within the tissue only upon MyD88-dependent stimulation of MNPs. Although the lack of colitis development in CD11c-MyD88/IL-10 KO and LysM-MyD88/IL-10 KO mice was likely due to the absence of intestinal bacterial sensing in these mice, it was also possible that expansion of the Th2 and/or Treg compartments may have contributed to protection from disease by inhibiting the colitogenic IL-17 and IFN-γ response. To address the latter possibility, mRNA levels of IL-4 and the frequency of Tregs in the colon were measured. There was no change in IL-4 levels (Figure 3c) or the frequency of Treg cells in CD11c-MyD88/IL-10 KO and LysM-MyD88/IL-10 KO mice compared to IL-10−/− control group (Figure 3d). These data suggested that Th1 and Th17 expansion occurs in the colon and in the MLN of IL-10−/− mice in a manner dependent on bacterial sensing by CD11c+ and LysM+ MNPs, without affecting the frequency of Th2 or Treg subsets. However, the suppressive function of Tregs is abrogated by the absence of IL-1020–22.
Figure 3. Regulatory and effector T cells in IL-10−/− mice.
(a) Mesenteric lymph nodes cells were cultured in the presence of plate bound anti-CD3ε antibody for 48 hrs. IL-17A and IFN-γ levels in the culture superrnatants were mesured by ELISA. Sets of littermates at 13–22 weeks old were used for the comparison between control IL-10−/− and each conditional knokout strains. IL-10+/+ or IL-10+/− mice were of same age range as IL-10−/− experimental mice (of which some mouse samples are shared among Villin-, CD11c- and LysM-Cre experiments). Each plot indicates indivisual mouse. Shown is the combined data of at least 5 repeated experiments. (b) Colonic lamina propria cells were cultured in the presence of plate bound anti-CD3ε antibody for 48 hrs. IL-17A and IFN-γ levels in the culture superrnatants were mesured by ELISA. WT plot represents the pooled sample of 3 mice. Only sets of littermates were used for the comparison between control IL-10−/− (each plot represent individual mouse) and CD11c-MyD88/IL-10 KO mice (each plot is either individual mouse or pooled sample of 2 mice). (c) Relative mRNA expression levels of colon tissue measured by quantitative PCR. At least 6 sets of littermates at 13–17 weeks old were used. IL-10+/+ or IL-10+/− mice samples (n=7) are shared among Villin-, CD11c- and LysM-Cre experiments. (d) Frequency of Foxp3+ in MLNs and colonic lamina propria were analysed by flow cytometry. Each plot indicates an individual mouse. Error bars represent mean ± s.e.m. *, p < 0.05 (unpaired Studen’t t-t test).
Cell specificity of MyD88 deletion
We next investigated the MNP cell types in which MyD88 is depleted in our conditional knockout mice. Consistent with the previous studies of small intestine and colon MNPs23–27, we detected two major CD11c+ populations, CD11c+ CD11b− (P1) and CD11c+ CD11b+ (P2). The CD11c− CD11b+ population consisted of 2 separate groups, CD11c− CD11b+ side scatter (SSC) l0 Siglec-F− macrophages (P3), and CD11c− CD11b+ SSChi Siglec-F+ eosinophils (Figure 4a). Representative Diff-Quick staining of cytospin preparations revealed that while P1 and P3 were morphologically homogeneous, P2 population was morphologically heterogeneous. Analysis of cell surface markers (Figure 4b) indicated that P1 is CD103+ CX3CR1− DEC205+ F4/80−, corresponding to the DC4 population found in isolated lymphoid follicles in the colon9. P3 expression profile (DEC205− F4/80+ CX3CR1+ CD103− F4/80+ CD11b+) is consistent with the MP1 (CD11c− macrophages) that are distributed in the lamina propria of mouse colon9. As observed from the heterogeneous morphology (Figure 4a), the P2 population consisted of three separate cell types; 1) CD103− CD11b+ CD11c+ DCs (DC3 CD103−), 2) CD103+ CD11b+ CD11c+ DCs (DC3 CD103+), and 3) CD11c+ CD103− CX3CR1+ macrophages (MP2) (Supplementary Figure S3) 9. To investigate which cell populations are targeted by CD11c-Cre and LysM-Cre transgene expression, we crossed these Cre-recombinase expressing alleles to Rosa26-STOPFL-EYFP reporter mice28. As expected, CD11c-Cre was highly expressed in the CD11c+ population MNPs represented by P1 and P2 subsets. In contrast, LysM-Cre was expressed primarily in P2 and P3 subsets. Of note, neither CD11c-Cre nor LysM-Cre was expressed by P4 cells, excluding the possibility of eosinophils being responsible for colitis development in IL-10−/− hosts. Expression of LysM, a myeloid cell marker, in P2 and P3 populations is consistent with the fact that the P2 population consists of the CX3CR1+ CD11c+ MP2 cells, which are of monocyte origin24, 25, and P3 representing the MP1 macrophages (Figure 4b–c). These data suggest that, while P1 and P3 may contribute to colitogenesis in IL-10−/− mice, the expression of MyD88 in P1 (in LysM-MyD88/IL-10 KO mice) or P3 (in CD11c-MyD88/IL-10 KO mice) is not sufficient to drive colitis in the respective mouse lines. Therefore, the key colitogenic potential appears to rest with the MNP populations within the P2 group of cells.
Figure 4. Analysis of MyD88 deletion specificity.
(a) 4 subsets of colonic lamina propria cells separated using CD11b, CD11c, Siglec-F and SSC. Pictures are Diff-Quick staining of each of sorted subsets. Scale bar, 20 μm (b) Cell surface phenotype of 4 populations. Filled histograms are the isotype control. (c) Frequency of EYFP+ cells in the colonic lamina propria of CD11c-Cre(+)/Rosa26-STOPFL EYFP (n=10) and LysM-Cre(+)/Rosa26-STOPFL EYFP (n=5)
Pro-inflammatory role of colonic CD11b+CD11c+ cells
To characterize the functional relevance of P1–P3 populations, we first sorted P1–P3 MNP subsets from IL-10 sufficient (WT) non-colitic mice and examined their relative expression levels for TLRs and cytokine genes by quantitative PCR at steady state (Figure 5). P2 population expressed a wide range of TLRs and proinflammatory cytokine mRNA, including IL-1β, IL-6 and IL-23 p19 which are important for Th17 cell differentiation and expansion 27, 29–31. Moreover, the P2 cells expressed highest levels of IL-10 at steady state. In addition, P3 expressed several TLRs and moderate expression of cytokines including IL-1β and IL-6. In contrast, P1 cells had minimal expression of TLRs or cytokines, except for TLR9. Importantly, all subsets expressed comparable levels of MyD88. These data suggest that P2 contains both immunoregulatory IL-10 secreting cells, as well as cells capable of responding to various TLR ligands and secreting innate proinflammatory cytokines that can skew naïve CD4 T cells to the Th17 phenotype.
Figure 5. Expression of TLRs and cytokines by P2 population.

P1–P3 populations were sorted from pooled colon sample of over 10 B6 mice. mRNA levels were measured by quantitative PCR. Combined data from 2–4 independent experiments is shown. Error bars represent mean ± s.e.m.
CCR2 deficiency does not reverse colitis in IL-10−/− mice
Results thus far suggest that the P2 population contains cells capable of inducing colitis development in IL-10−/− mice upon microbial sensing upstream of MyD88. To determine the MNP constituency in IL-10−/− colitic mice, we examined surface phenotype of MNPs from colitic IL-10−/− mice. Compared to non-colitic IL-10+/− mice, P2 cells are slightly increased in number and MP2 CX3CR1+ subset was increased in frequency in IL-10−/− mice (Figure 6a, b and Supplementary Figure S4) suggesting that the MP2 CX3CR1+ subset may be responsible for colitis phenotype in IL-10 deficient mice. We also found that the CD11c− CD11b+ cells with very low SSC were predominant in the inflamed colon of IL-10−/− mice (Figure 6a, b). Furthermore, this inflammation dependent P3 population expressed higher levels of Gr-1 and lower levels of CX3CR1 than the P3 population from the non-colitic IL-10+/− mice (Figure 6a), suggesting that they are derived from inflammatory monocytes32. Previous studies in the T-cell transfer colitis model reported the recruitment of inflammatory monocytes that secrete Th17-inducing cytokines9, 13. Further, CCR2-dependent migration of inflammatory monocytes is critical for the pathogenesis of DSS-induced colitis33. To test whether CCR2-dependent migration of MNPs is required for colitis development in IL-10−/− mice, IL-10−/− mice were crossed to CCR2−/− mice to generate IL-10−/− CCR2−/− double KO mice. Spontaneous colitis that develops in IL-10−/− mice was not reversed by CCR2 deficiency, based on multiple parameters including the severity and kinetics of colitis disease, and the Th17 or Th1 responses in the MLNs (Supplementary Figure S5). This prompted us to examine the MNP subsets in the colitic colon of CCR2−/− IL-10−/− mice. There was no significant difference in the overall frequencies of P1 or P3 MNPs in the inflamed colons of CCR2−/− IL-10−/− mice compared to IL-10−/− mice. These data indicated that inflammatory monocytes have CCR2-independent redundant mechanisms of migration, and CCR2 deficiency is not sufficient to reverse the colitis in IL-10−/− mice.
Figure 6. P2 and P3 populations and Th17 induction.
(a) Flow cytometry analysis. Filled histogram in CX3CR1 panel is the negative control using CX3CR1+/+/IL-10+/+ mice. (b) Cell numbers of P1–P3 populations per 1.0 × 105 of total colonic lamina propria cells were calculated using flow cytometry data. Each plot indicates an individual mouse. *, p < 0.05 (unpaired Studen’t t-t test) (c) P1–P3 populations from pooled colon samples of IL-10 sufficient mice and colitic IL10−/− were sorted, and relative mRNA expression levels were measured by quantitative PCR. Data shown are respresentative of 2 independent experiments with similar result. (d) P1–P3 populations sorted from colitic IL10−/− mice were co-cultured with naive CD4 T cells in the presence of anti-CD3ε antibody. Culture supernatants were used to measure IL-17A by ELISA. Data shown is total of 4 independent experiments. *, p < 0.05 (unpaired Studen’t t-t test) (e) P1–P3 population sorted from pooled colon samples of control IL-10−/− and CD11c-MyD88/IL- 10KO mice were co-cultured with naive CD4 T cells in the presence of anti-CD3ε antibody. Culture supernatants were used to measure IL-17A by ELISA. Each value was normalized to that of P1(Cre-) sample. Data shown are the total of 3 independent experiments.
MyD88 in CD11c+CD11b+ cells amplifies colonic inflammation
Th17 cells play a key pathogenic role in colitis that develops in IL-10−/− mice, indicated by the fact that colitis develops in IL-12 p35−/−/IL-10−/− mice but not in IL-12 p40−/−/IL-10−/− nor IL-23 p19−/−/IL-10−/− mice 17. To determine which of the MNP subsets are involved in Th17 differentiation in the colon of IL-10−/− mice, P1-P3 populations were sorted from IL- 10−/− colitic mice and cytokine mRNA expression was analyzed. Of note, not only the P2 but also the P3 population showed high levels of Th17 skewing cytokines, including IL- 1β, IL-23 p19 and IL-6 in the inflamed colon (Figure 6c). To test the ability of these cell populations to support Th17 differentiation, sorted P1-P3 populations from IL-10−/− mice were co-cultured with naïve splenic CD4 T cells and IL-17A secretion was measured from the supernatant upon anti-CD3 Ab stimulation. Consistent with the mRNA profile, both P2 and P3, but not P1, populations induced robust IL-17A secretion from CD4 T cells (Figure 6d). Further, to examine whether the Th17 inducing phenotype of MNPs in IL-10−/− mice indeed depends on MyD88 expression by the respective MNP populations, we sorted MNP subsets from CD11c-MyD88/IL-10 KO and MyD88FL/+ IL-10−/− control mice, and co-cultured these cells with naïve splenic CD4 T cells. Importantly, in the absence of MyD88 expression in the CD11c+ MNP subsets, neither P2 nor P3 MNP subsets supported IL-17A production from naive CD4 T cells (Figure 6e). These data suggested that the P2 population initiates colonic inflammation through bacterial sensing via MyD88, and subsequently recruits inflammatory macrophages to the tissue. Together, P2 and inflammatory macrophages in the P3 gate support the induction of colitogenic Th17 cells in situ in the absence of IL-10.
Discussion
The TLR-MyD88 signalling pathway, which is engaged upon commensal microbiota recognition, plays an important role in maintaining intestinal homeostasis34. In the absence of the key suppressive cytokine IL-10, MyD88-dependent signals lead to inflammation and colitis 4. In this study, we examined the cell types responsible for inducing colitis in IL-10−/− hosts. Our data revealed that MyD88 deletion in intestinal epithelial cells did not affect colitis progression in IL-10−/− mice. However, deletion of MyD88 in CD11c+ or LysM+ MNPs led to a near complete abrogation of colitis in IL-10−/− hosts. Further analyses showed that CD11c+CD11b+ cell compartment contain cells which likely act as initiators of colitis through the secretion of proinflammatory cytokines, which are normally suppressed by IL-10. Our results are consistent with the previous report demonstrating that myeloid-specific deletion of Stat3 – a key intermediate of IL-10 signalling, develop colitis that can be prevented by TLR4 deficiency35. Helicobacter Spp. are known to drive colitis in IL-10 deficient mice36, 37 and are present in our colony. Therefore, we speculate that MyD88 expression in CD11c+CD11b+ cell compartment is required to sense those species (or other microorganisms that have similar colitis-inducing properties) to trigger inflammatory response in the colon.
One question that arises from our data is the cell type responsible for secreting IL-10 and suppressing inflammatory responses in the colon. IL-10 secretion from Tregs plays an essential role in maintaining homeostasis in the intestinal mucosa20. In addition to Tregs, IL-10 is produced by local MNPs. Previous studies have demonstrated that the two CX3CR1+ F4/80+ CD11b+ non-migratory macrophage subsets, CD11c− (MP1) and CD11c+ (MP2), spontaneously secrete IL-10, even in response to TLR agonists9, 10.
These macrophages are thought to be anti-inflammatory, as they actively suppress IL-12 p35 and IL-23 p19 production from colonic LP MNP subsets under non-inflammatory conditions in vivo 9. Spontaneous IL-10 secretion by these macrophages depends on the presence of commensal flora, but is independent of MyD88 9. We also observed IL-10 mRNA expression from MP1 CD11c− macrophages (P3) and the P2 population that contains MP2 CD11c+ macrophages (Figure 5). Taken together, these results suggest that IL-10 secretion that normally suppresses inflammatory responses likely comes from Tregs and the immunoregulatory macrophages. It is interesting to note that by genetic ablation of IL-10, these colonic macrophages become highly pathogenic, by secreting cytokines that promote Th17 differentiation.
Our data also begin to uncover the nature of the colitogenic MNP populations in the colon. Based on our observations, we propose a two-step model of colitis progression in IL-10−/− mice. In the first step, P2 MNPs sense intestinal microbiota and induce proinflammatory cytokines in a MyD88-dependent manner. This is followed by a second step in which inflammatory monocytes are recruited, giving rise to a large number of P3 macrophages capable of secreting more inflammatory cytokines and driving colitic Th17 responses. Evidence for this model comes from our results that in CD11c-MyD88/IL-10KO mice lacking MyD88 in P1 and P2 but not P3 population, colitis progression is completely halted, indicating that P2-dependent sensing of microbiota provides a crucial initiating signal and that MyD88 signalling in the P3 population alone is not sufficient to promote colitis. The second line of evidence comes from our observation that P3 macrophages are robustly recruited to the colitic tissue, which outnumber P1 or P2 cells by about 4.3 and 2.2 folds respectively (Figure 6b), suggesting that the inflammatory P3 cells contribute to intestinal inflammation at later stages of colitis. Due to the lack of a mouse system in which MyD88 can be selectively deleted from the P3 cells, it is currently not possible to provide definitive evidence for this hypothesis.
The next question that follows is which of the P2 MNP subsets contribute to colitogenesis. We showed that in the IL-10−/− mice, P2 and P3, but not P1 population upregulate expression of IL-1b, IL-23 p19 and IL-6 and induce Th17 differentiation ex vivo (Figure 6). These results are highly consistent with a recently published report by Kelsall and colleagues, where MP1 and MP2 macrophages and CD11b+ DC subsets (CD103− CD11b+ and CD103+ CD11b+), but not the CD103+ CD11b− DCs, upregulated mRNA expression of p35 and p19 in IL-10−/− mice 9. Taken together, the P2 subset that are likely responsible for initiating colitis are the collection of all three MNP types, CD11c+ macrophages (MP2), CD103− CD11b+ DCs and CD103+ CD11b+ DCs.
Inflammatory diseases, once initiated, are often exacerbated by an amplification loop. As discussed above, the amplifier in the IL-10−/− colitis appears to be the P3 macrophages. Thus, we observed a most dramatic increase in the number of P3 CD11c− macrophages (MP1), which exhibited a highly proinflammatory signature and a Th17 inducing phenotype. Consistent with this finding is a recent report, which showed that intestinal MP1 macrophages (CD11b+ CD11c−) secrete IL-1β in a MyD88-dependent manner and promote Th17 differentiation upon sensing of microbiota in the absence of inflammation 27. We also observed significant expression of IL-1β in P3 macrophages at steady state, although the level was less than the P2 population (Figure 5). Collectively, the P3 macrophages are constitutively able to induce IL-1β responses in the gut 27, but are also recruited to the inflamed colon and amplify colitis in IL-10−/− mice. However, this may not be universal to all chronic colitis models, as CD103−CD11b+ DCs, but not P3 macrophages, were found to differentiate from circulating inflammatory monocytes, predominate the colon, and drive both Th1 and Th17 responses in the T-cell transfer colitis model 9.
Although IL10−/− and most other models of colitis depend on the presence of ‘commensal’ bacteria, different components of microbiota are not equivalent in terms of their ability to cause the disease. Indeed, it is increasingly recognized that colitis development is dependent on the presence of ‘triggering’ bacteria, sometime also referred to as ‘pathobionts’ 38, 39. It is the presence or absence and the relative abundance of triggering bacteria that determines the timing of onset of colitis and affects the variability in colitis susceptibility in different animal facilities. The bacterial species capable of triggering colitis development in IL10−/− mice (for example, Helicobacter spp) are normally harmless in unmanipulated wild type animals. And yet, these animals do mount immune responses to intestinal pathogens. Thus, IL-10 appears to suppress the unwanted immune responses against otherwise harmless bacteria, yet permits the immune response against bacterial pathogens. How this distinction is made by the immune system is unclear, but one possibility is that sensing of pathogen-specific behaviors, such as invasion or production of pore-forming toxins, maybe linked with downregulation of IL-10 production, thus permitting pathogen-specific immune responses.
It should also be noted that both commensal and pathogenic bacteria can induce immune responses, but these responses are qualitatively and quantitatively different. It is well known, for example, that the mammalian host generates carefully orchestrated immune responses to commensal bacteria, such as localized sIgA and antimicrobial peptide production40, 41. Such immune responses are likely important to manage the location, composition and density of intestinal bacteria41. Importantly, these responses have low tissue damage potential and therefore their constitutive engagement is well tolerated by the host. These responses however can be insufficient to suppress the growth or invasion of pathogenic bacteria, which require a far more aggressive immune response, such as Th17 and Th1-mediated response, which is also associated with immunopathology. It is these latter types of immune responses that appear to be suppressed by IL-10 and unleashed in its absence. Activation of these responses, however still requires the presence of triggering bacteria in the intestines.
Interestingly, deletion of A20, a potent negative regulator of TLR signalling, results in lethal inflammation that is triggered by commensal bacteria and is dependent on MyD8842. Thus the outcome of IL-10 and A20 deletion differs in severity and illustrates non-redundant nature of anti-inflammatory mechanisms.
In summary, our study demonstrated a key role for microbiota sensing by colonic MNPs in colitis development in IL-10−/− mice. While human genome wide association studies reveal key inflammatory genes being linked to IBD43, treatment options for IBD are limited. One of the key questions in this field relates to the initiation signal that leads to the onset of colitis. Our study provides a clue to the initiating events that underlie disease pathogenesis when the regulatory circuit is dysfunctional. In addition to host genetic lesions, dysbiosis of intestinal microbiota can result in spontaneous transmissible colitis44. Defining key anti-inflammatory and proinflammatory circuits that govern our host-microbiota relationship could open promising therapeutic approaches to treating human IBD.
Methods
Mice
A brief summary of the generation of the conditional allele of MyD88 (MGI: 108005) has been published previously45, and the detail will be described elsewhere. MyD88FLOX, MyD88−/−, IL10−/−, Villin-Cre, LysM-Cre, CD11c-Cre14, wild-type C57BL/6, Rosa-STOPFLOX EYFP, and CX3CR1gfp mice were bread and maintained under specific pathogen-free conditions at the animal facility of Yale University School of Medicine. To generate MyD88 conditional knockout mice on IL10−/− background, MyD88FL/FL mice were crossed to IL10−/− and further crossed to villin-cre, CD11c- Cre mice. For the study of LysM-MyD88/IL-10 KO mice, MyD88 FL/FL were first crossed to MyD88−/− mice to generate MyD88 FL/− mice and then crossed to LysM-Cre mice. Accordingly, bothMyD88 FL/−; LysM-Cre; IL-10−/− and MyD88FL/FL; LysM-Cre; IL-10−/− are included as LysM-cre conditional knockout mice (described as LysM-MyD88/IL-10 KO) in this study. Mice were screened for Helicobacter spp. by PCR and 16S rRNA sequences. H. typhlonicus, H. Hepaticus, H. ganmani and H. rodentium were detected in our colony. Studies were approved by the institutional Animal Care and Use Committee of Yale University
Colonic lamina propria cell preparation and flow cytometry
Colons were excised and mesentery was carefully cleaned. Tissue was opened longitudinally, washed of intestinal contents and then was cut into 2–3 pieces. Tissue was then transferred into 50 ml conical tubes and shaken in PBS containing 2% FCS and 5mM EDTA for 20–30 min at 37 °C to remove intestinal epithelial cells. To further remove residual epithelial cells, colon tissues were placed in petri dish and extensively washed in PBS several times using forceps until the supernatants become clear. The remaining tissue was transferred to new 50 ml tubes and digested in PBS containing 2% FCS, 1 unit/ml of type VIII collagenase (Sigma-Aldrich), and 10 μg/ml of DNase I (Roche) with constant shaking for 60 min at 37 °C. The cells were filtered through 70μm cell strainer and washed in PBS 2 times. Cells were stained with a combination of anti-CD11c (clone HL3), anti-CD11b (clone M170), anti-Siglec-F (clone E50-2440), anti-CD103 (clone M290), anti-CD86 (GL-1), anti-I-A/I-E (M5/114.15.2), anti-DEC205 (205yekta), anti-F4/80 (clone BM8), anti-CD4 (clone L3T4) and anti-Foxp3 (clone FJK-16s) after blockade of Fc receptors. Flow cytometry was performed on a LSR II (BD Biosciences). For some experiments, cells were further sorted on a Moflo (DakoCytomation) or iCyte Reflection (Sony), and were used as lamina propria leukocytes in assays.
Histological scoring
Colons were excised and divided into three equal segments to be named proximal, middle and distal colon. Middle colon tissue was fixed in with Bouin’s fixative (Sigma-Aldrich) for 30 min, paraffin embedded, sectioned and stained with hematoxylin and eosin. Sections were analyzed and scored for the levels of inflammation by a pathologist in a blind manner. The score was evaluated on the bases of epithelial hyperplasia, mononuclear infiltrate and polymorphonuclear infiltrate (0 = none; 1 = mild; 2 = moderate; 3 = severe).
Colon tissue culture
1 cm segment of proximal colon were excised and washed in cold PBS supplemented with penicillin and streptomycin. Tissue was placed in 24-well plate with 1 ml of serum-free RPMI 1640 medium supplemented with penicillin and streptomycin. After 24 hours, supernatants were collected as samples.
Mesenteric lymph node cell culture
MLNs were excised and grinded by sterile frosted slides and the filtered through 70 μm cell strainer to make single cell suspension. 2 × 106 cells were plated into 24-well plates and incubated in the presence of plate-bound anti-CD3ε (1μg/ml; clone 145-2C11) in 1 ml of complete medium (RPMI 1640 containing 10% FCS, 100 U/ml penicillin-streptomycin, 2 mM L-glutamine, 1 mM sodium pyruvate, 10 mM HEPES and 50 μMβ-mercaptoethanol). After 48 hours, supernatants were collected and cytokines were measured by ELISA.
Colonic lamina propria cell culture
Bulk colonic lamina propria cells obtained after collagenase treatment described in “Colonic lamina propria cell preparation” were subjected to density-gradient centrifugation in 44% to 65% Percoll (GE Healthcare). The cells at the interface were collected and washed in PBS 2 times. Cells were plated into 24-well plates at the concentration of 7.5 × 105 cells/ml and incubated in the presence of plate-bound anti- CD3ε (1μg/ml; clone 145-2C11) for 48 hours. The supernatants were collected and cytokines were measured by ELISA.
Cytokine quantification by enzyme linked immunosorbant assay
Paired antibodies (anti-mouse purified and biotinylated; IL-12 p40; clone C17.6 and C17.8, IL-17A; clone eBio17CK15A5 and eBio17B7, and IFN-γ; clone XMG1.2 and clone R4-6A2 respectively) and recombinant standards for IL-12 p40, IL-17A and IFN-γ were used to quantify factors present in supernatants of colon tissue, MLNs, and LP and T cell cultures.
Isolation of RNA and quantitative RT-PCR
For isolation of colon tissue mRNA, the tissue was homogenized in RNA-Bee (TEL-TEST, Inc.) and total RNA was isolated according to manufacturer’s instructions. For FACS sorted cell mRNA isolation, RNeasy kit (Qiagen) was used. cDNAs were prepared using SMART MMLV reverse transcriptase (Clontech) and used for PCR with SYBR Green reagents (Qiagen or Quanta Bioscience) on a Stratagene MX3000 bioanalyzer. The abundance of mRNA of target genes was normalized to hypoxanthine guanine phosphoribosyl transferase (HPRT) expression. Sequences of the primers used in this study are shown in the Supplementary Table S1.
In vitro T cell stimulation
Naive T cells from the spleens of C57BL/6 were purified by magnetic sorting with mouse anti-CD4 beads (clone L3T4, Miltenyi Biotec). 1× 105 T cell were co-cultured with 2 × 104 purified colonic lamina propria leukocytes in 200 μl complete medium (RPMI 1640 containing 10% FCS, 100 U/ml penicillin-streptomycin, 2 mM L-glutamine, 1 mM sodium pyruvate, 10 mM HEPES and 50 μM β-mercaptoethanol) in the presence of anti-CD3ε antibody in 96-well plates. After 72 hr incubation, supernatants were collected as samples.
Endoscopic analysis
Colonoscopy was performed in a blinded fashion for colitis scoring via the Coloview system (Karl Storz, Germany) 21. In brief, colitis scoring was based on granularity of mucosal surface, stool consistence, vascular pattern, translucency of the colon, and fibrin visible (0–3 points for each).
Statistical analysis
Statistical significance was evaluated with an unpaired two-tailed student’s t-test. A p value of less than 0.05 was considered significant (Prism; GraphPad software, Inc.).
Supplementary Material
Acknowledgments
We would like to thank S. Rakoff-Nahoum and Y. Kumamoto for technical advice and discussions; C. Annicelli and S. Cronin for animal care. This study was supported by the Howard Hughes Medical Institute and grants from US National Institutes of Health (DK071754, AI046688, AI055502) to R.M. and (RO1OD011141) to J.G.F. D.S. was supported first by a training grant of the NIH and then by an Irvington Fellowship of the Cancer Research Institute.
Footnotes
Author contributions
N.H. conducted the experiments; D.S. generated the MyD88FL mice and designed some experiments; N.H. and S.N. designed the experiments; Z.W. performed histological scoring; N.G and R.F. provided the endoscopic system and performed endoscopic analysis; B.R. provided CD1c-Cre mice; Z.S. and J.F. tested helicobacter PCR in mice colony; N.H., A.I. and R.M. prepared the manuscript; R.M. directed the research.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Sun L, Nava GM, Stappenbeck TS. Host genetic susceptibility, dysbiosis, and viral triggers in inflammatory bowel disease. Curr Opin Gastroenterol. 2011;27:321–327. doi: 10.1097/MOG.0b013e32834661b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 3.Sellon RK, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rakoff-Nahoum S, Hao L, Medzhitov R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006;25:319–329. doi: 10.1016/j.immuni.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinalfunction. Nat Rev Immunol. 2010;10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 6.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci U SA. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bar-On L, Zigmond E, Jung S. Management of gut inflammation through the manipulation of intestinal dendritic cells and macrophages? Semin Immunol. 2011;23:58–64. doi: 10.1016/j.smim.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Varol C, Zigmond E, Jung S. Securing the immune tightrope: mononuclear phagocytes in the intestinal lamina propria. Nat Rev Immunol. 2010;10:415–426. doi: 10.1038/nri2778. [DOI] [PubMed] [Google Scholar]
- 9.Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J Exp Med. 2012;209:139–155. doi: 10.1084/jem.20101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 11.Murai M, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takada Y, et al. Monocyte chemoattractant protein-1 contributes to gut homeostasis and intestinal inflammation by composition of IL-10-producing regulatory macrophage subset. J Immunol. 2010;184:2671–2676. doi: 10.4049/jimmunol.0804012. [DOI] [PubMed] [Google Scholar]
- 13.Siddiqui KR, Laffont S, Powrie F. E-cadherin marks a subset of inflammatory dendritic cells that promote T cell-mediated colitis. Immunity. 2010;32:557–567. doi: 10.1016/j.immuni.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signallingcontrols the homeostasis of CD8-dendritic cells in the spleen. J Exp Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh CS, et al. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 16.Macatonia SE, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 17.Yen D, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGeachy MJ, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 19.Langrish CL, et al. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 20.Rubtsov YP, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Chaudhry A, et al. Interleukin-10signallingin regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34:566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber S, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3 and Foxp3+ regulatory CD4+ T cells inan interleukin-10-dependent manner. Immunity. 2011;34:554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uematsu S, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 24.Bogunovic M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varol C, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 26.Manicassamy S, et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw MH, Kamada N, Kim YG, Nunez G. Microbiota-induced IL-1beta, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J Exp Med. 2012;209:251–258. doi: 10.1084/jem.20111703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu W, Troutman TD, Edukulla R, Pasare C. Priming microenvironments dictate cytokine requirements for T helper 17 cell lineage commitment. Immunity. 2012;35:1010–1022. doi: 10.1016/j.immuni.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 31.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 32.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 33.Andres PG, et al. Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J Immunol. 2000;164:6303–6312. doi: 10.4049/jimmunol.164.12.6303. [DOI] [PubMed] [Google Scholar]
- 34.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi M, et al. Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J Clin Invest. 2003;111:1297–1308. doi: 10.1172/JCI17085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kullberg MC, et al. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12-and gamma interferon-dependent mechanism. Infect Immun. 1998;66:5157–5166. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chichlowski M, Sharp JM, Vanderford DA, Myles MH, Hale LP. Helicobacter typhlonius and Helicobacter rodentium differentially affect the severity of colon inflammation and inflammation-associated neoplasia in IL10-deficient mice. Comp Med. 2008;58:534–541. [PMC free article] [PubMed] [Google Scholar]
- 38.Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313–338. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- 39.Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santaolalla R, Abreu MT. Innate immunity in the small intestine. Curr Opin Gastroenterol. 2012;28:124–129. doi: 10.1097/MOG.0b013e3283506559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 42.Turer EE, et al. Homeostatic MyD88-dependent signals cause lethal inflamMation in the absence of A20. J Exp Med. 2008;205:451–464. doi: 10.1084/jem.20071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abraham C, Cho JH. IL-23 and autoimmunity: new insights into the pathogenesisof inflammatory bowel disease. Annu Rev Med. 2009;60:97–110. doi: 10.1146/annurev.med.60.051407.123757. [DOI] [PubMed] [Google Scholar]
- 44.Garrett WS, Glimcher LH. T-bet−/− RAG2−/− ulcerative colitis: the role of T-bet as a peacekeeper of host-commensal relationships. Cytokine. 2009;48:144–147. doi: 10.1016/j.cyto.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kleinridders A, et al. MyD88 signallingin the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 2009;10:249–259. doi: 10.1016/j.cmet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.