Abstract
Viral infections characteristically induce a cytokine-driven activated natural killer (NK) cell response that precedes an antigen-driven T cell response. These NK cells can restrain some but not all viral infections by attacking virus-infected cells and can thereby provide time for an effective T cell response to mobilize. Recent studies have revealed an additional immunoregulatory role for the NK cells, where they inhibit the size and functionality of the T cell response, regardless of whether the viruses are themselves sensitive to NK cells. This subsequent change in T cell dynamics can alter patterns of immunopathology and persistence and implicates NK cells as rheostat-like regulators of persistent infections.
Introduction
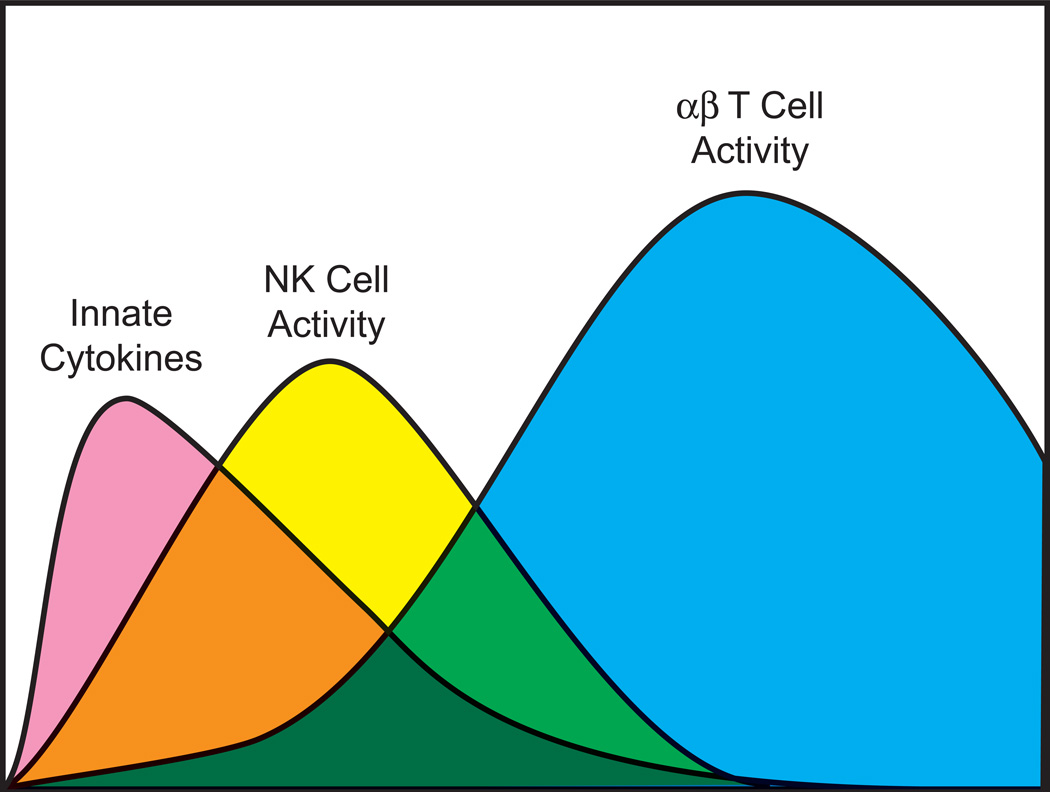
Natural killer (NK) cells and T cells are regulatory and effector lymphocytes that get mobilized into the host response to viral infections. NK cells are cytotoxic antiviral cytokine-producing lymphocytes whose activities are regulated by cytokines and by a number of stochastically-expressed positive- and negative- signaling NK receptors (NKR) that recognize cellular stress-related molecules, adhesion molecules, and major histocompatibility complex (MHC) proteins (Lanier, 2008; Raulet, 2003). Some NKR have even evolved to directly recognize certain viral proteins (Daniels et al., 2001; Lee et al., 2001; Brown et al., 2001; Voigt et al., 2003). T cells, on the other hand, express randomly generated and clonally distributed T cell receptors (TCR) that recognize processed viral peptide epitopes presented to them in the grooves of MHC molecules expressed on the surface of antigen-presenting cells (Wilson et al., 2004). The activated T cells can be similar to NK cells in their acquisition of cytotoxic and cytokine-producing effector functions; this is especially true for the CD8 T cells, which recognize peptide epitopes presented by class 1 MHC molecules. The activated CD4 T cells, which recognize peptides presented by class 2 MHC molecules, can secrete factors that regulate the T cells and the rest of the immune response in positive or negative ways. NK cells patrol the host at a moderate state of activation and at a relatively high frequency (~ 15% of peripheral blood lymphocytes), but will proliferate and become even more active during a viral infection (Biron et al., 1983; Welsh, 1978). However, immunologically naïve T cells specific to any peptide epitope exist at low frequency (~1/50,000) and in an inactive naïve state and require a substantial clonal expansion to increase in numbers and functions sufficient to control of infection (Blattman et al., 2002; Seedhom et al., 2009). Innate cytokines such as the type 1 interferons (IFN), IL-12, and IL-15 are rapidly induced during viral infections and can stimulate the activation and proliferation of NK cells and greatly augment the proliferation of T cells (Biron, 1995). The dynamics of this process follow the innate and adaptive immune response paradigm, first described in the 1970s: an early cytokine-driven activated NK cell innate response followed by a peak in clonally expanded T cells (Figure 1) (Welsh, 1978).
Figure 1. Innate and adaptive host response to infection.
This figure portrays the timing of the peaks in innate cytokines (type 1 IFN, etc), NK cell cytolytic activity (not cell number), and T cell number and activity during an acute viral infection, based on (Welsh, 1978).
The temporal relationship between the early activated NK cell vs. late T cell peak has historically engendered questions about whether these cell populations were influencing each other. Certainly, the T cell response may clear the pathogen that induces the cytokines that the NK cells need to stay highly active and proliferating. That is probably not the entire explanation of the waning of the NK cell response, however, as some work has shown that TGFβ made late in the response has a more suppressive effect on NK cells than T cells (Su et al., 1991). In the other direction, a number of papers described later have proposed that NK cells may either enhance or inhibit the T cell response, and earlier papers even suggested that the NK cells may turn into T cells! We can dismiss that latter suggestion, as it is now clear that NK cells and T cells represent different lineages, but the question of how well NK cells control T cells has recently come to the forefront. It would not be out of the question to think that NK cells could promote T cell proliferation, as they produce IFNγ, which itself can promote CD8 T cell expansion (Whitmire et al., 2007). Also, NK cells might indirectly promote T cell expansion by directly controlling viral load early in infection, thereby inhibiting the levels of virus that might cause immune suppression (Bukowski et al., 1984). It should also be of no surprise that NK cells could affect T cells in a negative way. T cell targets such as mouse YAC-1 cells were among the earliest target cells used in cytotoxicity assays to detect the activity of NK cells (Salazar-Onfray et al., 1997), and primary thymocytes were among the first documented targets in vivo (Hansson et al., 1980; Hansson et al., 1979). Recent work has indicated that in the context of a viral infection the NK cells have the capacity to directly kill or indirectly regulate the numbers and activities of antiviral CD4 and CD8 T cells (Su et al., 2001a; Waggoner et al., 2010; Waggoner et al., 2012; Lang et al., 2012; Narni-Mancinelli et al., 2012; Andrews et al., 2010; Robbins et al., 2007; Mitrovic et al., 2012; Ge et al., 2012; Lee et al., 2009; Stadnisky et al., 2011). As a consequence of this activity, NK cells may serve as rheostats regulating the T cells that control whether an infection becomes resolved, persistent, or lethal.
Patterns of viral pathogenesis and persistence
Viral infections can present themselves in many forms. Many acute viral infections induce sterilizing T and B cell immune responses that clear the infection, form long term memory, and leave the host resistant to re-infection. Other infections, such as those with a variety of herpesviruses, are mostly cleared by the immune response, but residual foci of low level infection remain, with the possibility of an occasional reactivation. Other viruses can cause long term persistent infections associated with high levels of virus due to some compromise of immunological function. Examples of these abound, including hepatitis B and C and HIV in humans and lymphocytic choriomeningitis virus (LCMV) in the mouse. The balance between the functional immune response and the viral load is crucial in determining the pattern of viral persistence. An overzealous immune response in the presence of high antigen load can lead to severe immune pathology such as that which occurs during fulminant viral hepatitis. This means that in some cases it might be good to generate a very strong immune response to quickly eradicate viral antigen, but in other cases, in order to protect the host from severe immune pathology, a more tempered response may be better.
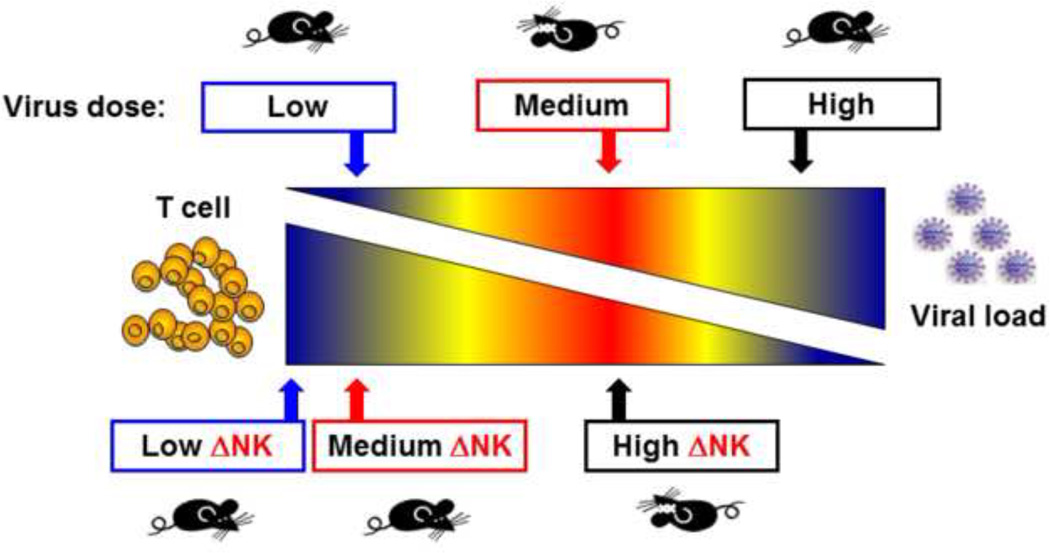
This paradox can perhaps best be illustrated in mice intravenously infected with the clone 13 strain of LCMV (Waggoner et al., 2012). This strain, in part due to its high affinity binding to its alpha dystroglycan receptor on host cells (Cao et al., 1998), disseminates rapidly in adult mice and can cause a persistent infection when inoculated at high dose (Ahmed et al., 1984). This persistent infection can occur because the virus is not directly cytopathic and because the functions of the T cells that would normally control the infection become compromised. The T cell response becomes clonally exhausted, in part by the activation-induced death of high affinity T cells (Zhou et al., 2002) and in part by inhibitory T cell signaling molecules that provide negative signals to T cells challenged with high antigen load (Zajac et al., 1998; Barber et al., 2006). These include molecules such as programmed death-1 (PD-1/CD279), 2B4 (CD244), lymphocyte activation gene-3 (LAG-3/CD233), CD160, and cytotoxic T lymphocyte antigen 4 (CTLA-4) (Wherry, 2011). At low virus dose a rapid and fully functional T cell response clears LCMV clone 13 with little immune pathology (Figure 2). However, at an intermediate dose of virus there is a high enough antigen load and a sufficiently functional T cell response that severe immune pathology develops in the liver and lungs (Waggoner et al., 2012; Stamm et al., 2012). Thus the magnitude and functionality of a T cell response will determine whether a viral infection results in clearance, persistence, or sometimes lethal immune pathology. The host has mechanisms to regulate such responses, and new findings indicate that NK cells can act like rheostats to monitor antiviral T cell responses. In the LCMV model described above, depletion of NK cells prevents the severe pathology in the mid-dose model and leads to viral clearance; here we can say that the presence of NK cells was bad for the host (Figure 2). On the other hand, depletion of NK cells in the high dose model results in high mortality (Waggoner et al., 2012). There the NK cells are apparently needed for the persistent infection to develop. Otherwise the T cell response is so severe that lethal immune pathology occurs.
Figure 2. Pathogenesis of LCMV infection at different viral doses in normal or NK cell-depleted mice.
The red area is the zone of severe immune pathology, where there are sufficient amounts of virus and T cells to cause tissue damage. At a higher dose of virus the T cells exhaust, and at a lower dose the virus is cleared. NK cell depletion results in a stronger T cell response, which changes the dose at which immune pathology occurs (Waggoner et al., 2012).
What we know about how NK cells control viral infections
NK cells are thought to directly control infections with some viruses if the NK cells become positively activated by the up-regulation of stress-related proteins in virus-infected cells or if they fail to respond to negative signals that may occur, for example, by the down regulation of class 1 MHC antigens on virus-infected cells (Raulet, 2003; Brutkiewicz & Welsh, 1995). As a consequence, viruses may encode proteins to interact with these negative and positive signaling triggering molecules or their ligands to alter the dynamics of this process. This sometimes can be tricky, as T cells require MHC molecules for recognition of targets. Thus, protecting targets from T cells by the down modulation of MHC antigens might make those targets more sensitive to NK cells. Type 1 IFN, which is induced at high levels during viral infection, up-regulates expression of class 1 MHC, and in so doing increases target cell sensitivity to CTL while decreasing sensitivity to NK cells (Trinchieri & Santoli, 1978; Welsh et al., 1981; Ljunggren & Karre, 1990; Bukowski & Welsh, 1986). Interestingly, HIV nef protein down regulates the HLA- A and B MHC molecules, with which CD8 T cells prefer to react, while allowing cell surface expression of HLA-C, with which NK cells prefer to react (Cohen et al., 1999; Swann et al., 2001). When NK cells do directly control viral infections they do so by way of perforin-dependent cytotoxicity or by the secretion of IFNγ, which has many downstream consequences, including the induction of nitric oxide synthase, whose metabolite, nitric oxide, has dramatic anti-microbial properties. With murine cytomegalovirus (MCMV) for which the most is known about the NK cell-mediated control of viral infections, a perforin-dependent mechanism is preferred in the spleen, and an IFNγ- and NO-dependent mechanism is preferred in the liver (Tay & Welsh, 1997).
The MCMV infection of the C576BL/6 mouse is the best studied model for the control of viral infections by NK cells. The genetics of resistance maps to the NK cell complex on chromosome 6 (Scalzo et al., 1995). This gene complex codes for a series of proteins of interest to NK cells, including the positive signaling molecules NKR-P1c (NK1.1), a target for a useful depleting antibody, and NKG2D. It also codes for CD94 and NKG2A, B, C, and E proteins, which interact to form negatively signaling heterodimers, and a group of c-type lectin molecules of the Ly49 series, which deliver negative or positive signals when engaging discrete mouse MHC molecules (Brown et al., 1997). The NKG2 and CD94 molecules have human homologs, as does the positively signaling receptor NKp46, which is encoded on chromosome 7 (Biassoni et al., 1999). The Ly49 molecules do not have human homologues, but human killer cell immunoglobulin-like receptors (KIRs) perform the same function of recognizing MHC molecules (Lanier, 1998). What is unusual in the C57BL/6 mice is that the genetic resistance to MCMV maps to a Cmv-1 resistance locus that is congruent with Ly49H, a positive signaling NKR that reacts with the MCMV-encoded glycoprotein m157, thereby initiating an NK cell attack on MCMV-infected cells (Brown et al., 2001; Smith et al., 2002; Daniels et al., 2001; Lee et al., 2001; Arase et al., 2002). Passage of MCMV in Ly49H-expressing mice selects for m157 negative viral mutants (Voigt et al., 2003). m157, however, is just one of a family of similar molecules encoded by MCMV, and other molecules of this family interact with other Ly49 molecules, such that the genetics of resistance to MCMV differs between mouse strains (Kielczewska et al., 2009; Orr & Lanier, 2011; Lanier, 2008). MCMV also encodes proteins that alter the expression of cellular class I proteins, presumably to confuse both NK cells and T cells (Babic et al., 2010). Further, MCMV encodes proteins that inhibit expression of the ligands for NKG2D (Arapovic et al., 2009). The complexity of the MCMV system may be due to the fact that MCMV causes a persistent infection and has co-evolved with its host for millions of years. Human CMV is similar in regards to its complex relationship with its host (Lanier, 2008).
Less is known about the NK cell recognition of other viruses. Genetic resistance to ectromelia virus also maps to the NK complex, and this resistance seems in part to be mediated by NKG2D (Fang et al., 2008) and by CD94-NKG2E heterodimers (Fang et al., 2011). Resistance to influenza A virus (IAV) has been attributed in part to NKp46, as this molecule is reported to interact with the IAV hemagglutinin (Glasner et al., 2012), and NKp46 knock-out mice have enhanced sensitivity to IAV (Gazit et al., 2006). NK cells do not seem to directly control mouse polyomavirus, but NK cells lyse polyomavirus-induced tumors by an NKG2D-dependent mechanism, and tumor growth is enhanced in NK cell-deficient mice (Mishra et al., 2010).
Other viruses resist the direct anti-viral effects of NK cells. An example is LCMV, which grows to equal titers during the first three days of infection in the spleens of mice depleted or not of NK cells, and which grows similarly in SCID mice deleted or not of NK cells for many weeks (Welsh et al., 1991). The relatively noncytopathic LCMV infection does not cause appreciable stress to cells nor does it down-regulate class I MHC antigens (Bukowski & Welsh, 1985). Although IFNγ production by NK cells may slightly restrict LCMV replication at the peripheral inoculation site (Mack et al., 2011), we can generally categorize LCMV as a relatively NK-resistant virus and contrast it to MCMV, the prototypic NK-sensitive virus. Nevertheless, depletion of NK cells under certain conditions can greatly alter the patterns of LCMV pathogenesis and persistence (Figure 2). This effect of NK cells is not, however, because of any direct effect of NK cells on LCMV. Rather, it reflects the ability of the NK cells to modulate the T cell response.
Influence of NK cells on T cell responses
Some studies in the 1980s using reagents inferior to what we have now suggested that CTL development may be dependent on NK cells (Burlington et al., 1984; Suzuki et al., 1985), whereas other reports had implicated NK cells as being natural suppressors of immunological function, inhibiting hematopoiesis (Kiessling et al., 1977; Thomsen et al., 1986) and B cell proliferation and differentiation (Arai et al., 1983; Kuwano et al., 1986). One of the earliest seminal papers on NK cells by Kiessling and coworkers linked NK cells to the control of bone marrow allografts (Kiessling et al., 1977). A subpopulation of class 1 MHC low thymocytes were shown to be highly sensitive to NK cell-mediated lysis (Hansson et al., 1979; Hansson et al., 1980), and in vivo generated NK cells were shown to lyse other NK cells or cell lines mediating NK-like cytotoxic activity. Even IL-2- stimulated “lymphokine activated killer (LAK) cells,” sometimes used in tumor therapy regimens, could be killed by activated host NK cells when inoculated in vivo (Brubaker et al., 1991). A number of studies have also focused on the potential ability of NK cells to lyse dendritic cells (Wilson et al., 1999; Gilbertson et al., 1986; Andrews et al., 2003), which not only provide cytokines for the activation of NK cells but also present class 1 and 2 MHC antigens and stimulate CD8 and CD4 T cell responses. Thus, the potential is there for NK cells to be effective modulators of immune response function.
Nevertheless, the literature on whether NK cells can have an impact on viral pathogenesis by an immunoregulatory mechanism has been murky until recently. Most of the studies had been done in the MCMV system, where depletion of NK cells causes elevations in viral titers and virus-induced cytokines simply due to the removal of the direct NK cell control of the virus. This obfuscates the interpretation of downstream events after NK cell depletion. Some reports using such NK cell depletion strategies have suggested that NK cell depletion enhances MCMV-induced T cell responses (Su et al., 2001b; Andrews et al., 2010) and other reports suggest that NK cell depletion suppresses MCMV-induced T cell responses (Bukowski et al., 1984; Robbins et al., 2007). We feel that a possible explanation for this dichotomy may relate to the effect of NK cell depletion on viral load in the animal. A certain threshold of viral antigen and virus-induced cytokines is needed to induce a strong CD8 T cell response. Thus, if the NK cell depletion enhances a low virus load to a moderate virus load, an elevated T cell response may occur. On the other hand, if the NK cell depletion converts a moderate viral load into a high viral load, then the viral antigens and high cytokine levels may cause a general immune suppression and reduced T cell response, as detailed by us previously (Bukowski et al., 1984). In a recent elegant though unusual mutagenesis study in mice, hyperactive NK cells were generated due to a mutation in the Ncr1 gene that encodes the positively signaling NK cell receptor NKp46. These mice had enhanced NK cell activity, enhanced resistance to MCMV, and a reduced CD8 T cell response to MCMV (Narni-Mancinelli et al., 2012). Some work with MCMV has indicated that the activated NK cells may be affecting T cell responses by killing off the dendritic cells or other antigen-presenting cells (Andrews et al., 2010). However, in this case the DC would be infected with MCMV and be susceptible to a virus-specific NK cell attack. The consequential loss of DC may well alter the T cell response, but it is difficult to attribute this to a true immunoregulatory role for NK cells rather than just to their ability to kill virus-infected cells. Thus, for a less ambiguous read out, studies have recently been done with a virus, LCMV, that is not directly controlled by NK cells.
NK cells regulate viral pathogenesis of an NK-“resistant” virus by regulating the T cell response
For many years the Armstrong strain of LCMV was routinely studied by our group in NK-depleted vs. normal mice, and only modest differences in T cell activity were observed in the presence vs. the absence of NK cells. Beige mice, which have a defect in NK cell cytolytic activity, had higher levels of T cells than wild type mice after LCMV-Armstrong infection, but that could have been due to a slower clearance of the virus by the T cells, which were also cytolytically compromised (Biron et al., 1987). More recently Su et al found little difference in anti-viral T cell responses in LCMV-Armstrong infected wild type mice depleted of NK cells, but, surprisingly, they found that the CD4 T cell response in β2 microglobulin knock-out mice, which have very few CD8 T cells, was elevated in the absence of NK cells (Su et al., 2001b). Our recent studies have shown that NK cells can dramatically regulate the T cell responses in other models or conditions of LCMV infection and that they do so by cytolytically attacking the CD4 T cells, consistent with the findings with the β2 microglobulin knock-out mice (Waggoner et al., 2012).
By exploiting the various pathogenic models of the clone 13 variant of LCMV, we were able to demonstrate profound impacts of the NK cells on T cell responses and viral pathogenesis, as mentioned earlier in this review (Waggoner et al., 2012) (Figure 2). At a low virus dose NK cell depletion resulted in a higher CD8 T cell response which had no major role in the already effective clearance of the virus. At the medium dose, which normally resulted in severe and often lethal T cell-dependent immune pathology, the deletion of NK cells prevented the disease. The reason that the disease was prevented was that the absence of the NK cells allowed for a much greater CD4 and CD8 T cell response in terms of virus-specific T cell number and functionality. Many of the CD4 and CD8 T cells present in medium dose-infected untreated mice had a partially exhausted phenotype and produced few cytokines. The reason for the reduction in pathology in the NK cell-depleted medium-dose infected mice was simply due to the enhanced clearance of virus by the more vigorous T cell response. Thus, at this dose the presence of NK cells was detrimental to the host, because it inhibited a T cell response required to clear virus before the acquisition of severe immune pathology. It should be emphasized that all the pathology was mediated by the T cells. In medium dose-infected T cell knock-out mice there was no weight loss, no mortality, no significant immune pathology, and no clearance of virus, and no differences in viral titers, consistent with the fact that LCMV is an NK-resistant virus and that the effects of the NK cell depletion were mediated by T cells.
Paradoxically, depletion of NK cells at a high virus dose that would normally induce a several month persistent infection without mortality resulted in enhanced pathology and mortality. Briefly put, for this well-established model for viral persistence, NK cells were needed for the persistence to occur. The reason for the increased mortality in the absence of NK cells appeared to be due to the fact that the larger and more effective T cell response in the absence of NK cells could not be properly exhausted before severe damage was done.
These changes in viral pathogenesis were all accomplished by a single injection of a limiting amount of NK cell depleting antibody at the beginning of infection. This regimen depleted classic NK cells and not NK/T cells. Further, this could be accomplished with either of two NK cell-depleting antibodies and similarly occurred in CD1d KO mice which lack many NK-T cells and in γδ TCR KO mice, which lack NK1.1-expressing γδ T cells (Waggoner et al., 2012). Thus, eliminating NK cells at their peak of activation had a profound effect. We more recently have examined whether depletion of NK cells several weeks into a persistent infection could resurrect T cell responses and stimulate viral clearance. Our preliminary data indicate that this treatment can indeed have therapeutic value, and we are testing this further.
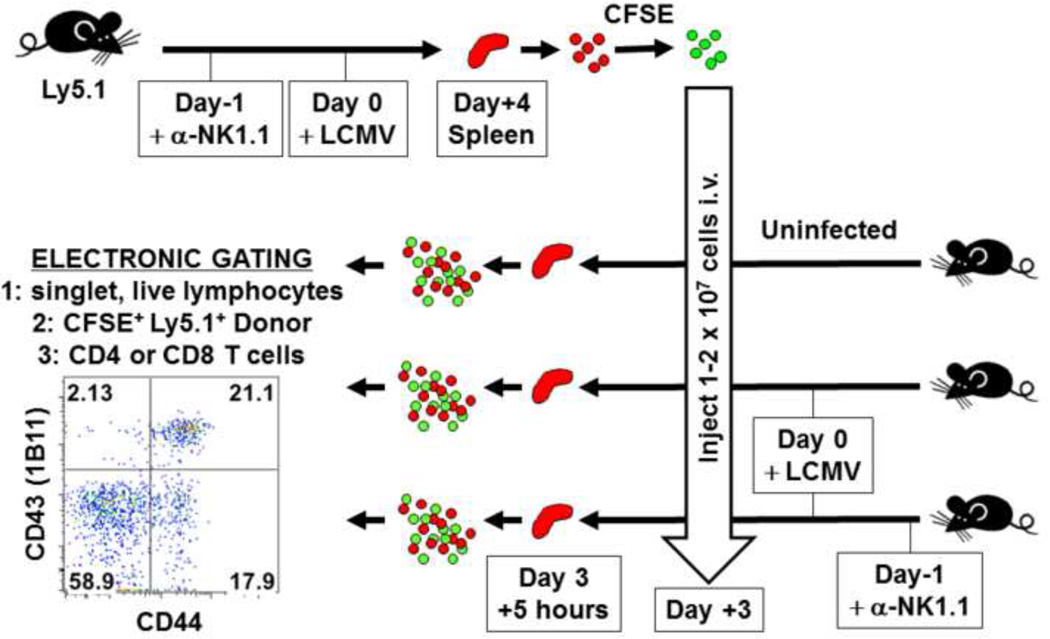
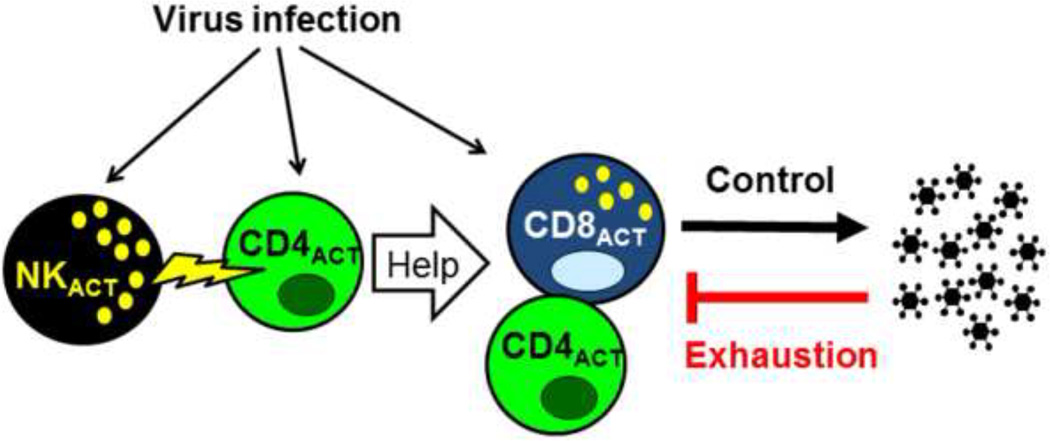
We explored the mechanism by which the NK cells controlled the T cell response in this LCMV model and found, using in vivo cytotoxicity assays described in Figure 3, that the NK cells cytolytically attacked activated CD4 T cells in a perforin-dependent manner (Waggoner et al., 2012). Further, in the medium dose model, depletion of NK cells affected CD8 T cell numbers and function only if the CD4 T cells were present. From these data we proposed a model whereby the effects of NK cells on CD4 T cells were mostly direct, due to NK cell-dependent cytotoxicity, but the effects of NK cells on the CD8 T cells were indirect. Thus, the complete model, as shown in Figure 4, indicates that a) a virus infection induces cytokines and antigen to activate the NK cells and T cells, b) the activated NK cells cytolytically eliminate some of the activated CD4 T cells, which are needed to provide help for the c) CD8 T cells, which control the viral load that can, in turn, d) functionally exhaust the T cells if the antigen load gets too high. The pathological consequences of these events are dependent on a numbers game between the degree of antigen load and the magnitude of the T cell response, and the rheostat-like effect the NK cells can have on these dynamics.
Figure 3. In vivo cytotoxicity assay.
Splenocytes from NK cell-depleted, virus-infected Ly5.1+ mice are labeled with CFSE and transferred into infected or uninfected recipients deleted or not of NK cells. After 5 hours donor target cells are gated for CD4 or CD8, and the numbers of cells expressing activation antigens (e.g. CD43 and CD44) are quantified (Waggoner et al., 2012).
Figure 4. NK cells regulate the T cell response by acting on CD4 T cells.
This diagrams how NK cells can regulate the overall T cell response by attacking activated helper CD4 T cells. This allows for excess viral antigen that can exhaust both CD4 and CD8 T cells.
A story with some similarities has been reported using a different persistent infection-inducing strain of LCMV, the Docile variant of strain WE (Lang et al., 2012). NK cell-deficient (Nfil3−/−, E4BP4−/−) mice infected with this virus and wild type mice depleted of NK cells with antibody developed enhanced T cell responses to LCMV. Under the dose of virus used, the absence of NK cells enabled a stronger T cell response that prevented the persistent infection. This report focused on the effects of NK cell depletion on CD8 T cell activity and showed that perforin-containing NK cells could limit the proliferative expansion of CD8 T cells. This conclusion was based on an indirect assay, and a role for CD4 T cells was not addressed.
NK cell lysis of CD4 T cells is a general feature of viral infections
NK cell-mediated regulation of T cell activity may be a general property of viral infections, but the impact of NK cells on infections with certain viruses may be complicated by the fact that NK cells can possess direct anti-viral activity and at the same time suppress the T cell response. Thus, the positive and negative effects of the NK cells have the potential to cancel each other out. This could be a particular problem in the analysis of the MCMV system, where the virus is clearly sensitive to control by NK cells, CD4 T cells, and CD8 T cells. The other issue in evaluating what might be enhanced T cell responses after NK cell depletion is that a more rapid T cell-dependent clearance of antigen in the absence of NK cells might reduce the antigen load needed to further increase the T cell response. This may well be the story with the Armstrong strain of LCMV, which has a relatively restrained replication and with which there had not been a noticeable phenotype found after NK cell depletion. We reinvestigated this and found that if one looked earlier after infection (day 6), there was an enhanced CD8 T cell response as well as enhanced clearance of the virus. By day 98 there was little difference in the T cell response, as the T cell response in the untreated animals, which bore a higher early viral load, “caught up.” Thus, by examining the T cell response at its peak the impact of NK cell depletion had gone unnoticed.
Given all these issues, we sought to directly examine the effects of NK cells induced by several viruses on activated CD4 T cell targets induced by several viruses. By employing the in vivo cytotoxicity assay (Figure 3) we could mix and match target and effector populations and bypass issues presented by the direct NK cell control of viral infections or changes in antigen clearance dynamics by the activated T cells. The results showed that NK cells activated by LCMV, Pichinde virus, mouse hepatitis virus, or the IFN-inducer poly I:C all could lyse LCMV-induced activated CD4 T cells in vivo. Further, CD4 T cells activated by infections with LCMV, MCMV, Pichinde virus, mouse hepatitis virus, or vaccinia virus were all susceptible to lysis by LCMV-activated NK cells (Waggoner et al., 2012). This indicates that activated NK cell-mediated killing of activated CD4 T cells is a universal phenomenon occurring during viral infections.
NKR involved in the NK cell regulation of T cell responses
In vitro studies have shown that NK cells can kill activated CD8 T cells by way of NKG2D, which recognizes stress-related ligands on target cells (Rabinovich et al., 2003), and Lang et al have reported that NKG2D is responsible for the NK cell regulation of CD8 T cell responses in their system with the Docile LCMV strain (Lang et al., 2012). They report modest expression of NKG2D ligands on the CD8 T cells and a higher CD8 T cell response in mice treated with an anti-NKG2D blocking antibody. In our Clone 13 LCMV system, which employed the in vivo cytotoxicity assay, there was very little expression of NKG2D ligands on activated CD4 or CD8 T cells, and the NK cell-dependent killing of the activated CD4 T cells occurred normally in the presence of anti-NKG2D or in NKG2D KO mice (Waggoner et al., 2012). It is quite possible that different NKR are used in different infection systems, but this awaits further clarification.
Regulation of NK cell killing of T cells by negative-signaling receptors, however, seems more clear to us. 2B4 (CD244) is an NKR that can deliver negative signals to NK cells after engaging its ligand, CD48, which is expressed on many cell types (Lee et al., 2004). In the clone 13 system, where activated CD4 T cells are more sensitive to lysis than activated CD8 T cells, expression of CD48 was much higher on the CD8 T cells (Waggoner et al., 2012). Further, during infection of 2B4 KO mice, the NK cells aggressively and directly lysed activated CD8 T cells (Waggoner et al., 2010).
Having described how the presence or absence of NK cells can affect viral persistence, we used 2B4 KO mice to examine how NK cells without their normal inhibitions would act in the context of a persistent infection. High dose clone 13 infection of 2B4 KO mice resulted in reduced T cell responses, thymic atrophy, splenomegaly, chronic hepatitis, and a long term persistent infection that never resolved (Waggoner et al., 2010). All of these pathologies were ablated by a single injection of anti-NK1.1 in the first three days of infection, and the infection ultimately resolved, like that in wild type mice. Thus, in wild type mice the presence of NK cells enable a persistent infection that resolves in a few months; the absence of NK cells in wild type mice causes an aggressive immune response and severe immune pathology and death; and hyperactive NK cells in 2B4 KO mice severely ablate the T cell response and result in a persistent infection that does not resolve. Hence, the activity of NK cells very early in the infection process can have a long lasting impact on viral persistence.
Genetic studies linking NKR to persistent infections in humans
Many human viral infections result in long term persistence, and HIV, HBV, and HCV are highly problematic infections associated with high virus loads. All of these infections are thought to be controlled at least in part by T cells, but why different HIV-infected individuals have different antigen load set points and why some hepatitis virus infections resolve and others do not has remained a mystery. A series of human genetic studies has been performed by Carrington and co-workers in an attempt to correlate the pathogenesis of these infections with the presence of certain NKR, notably the KIR, and the MHC antigens with which they engage (Carrington & Alter, 2012). Strikingly, several KIR-MHC combinations have shown correlations with control of infection, and the question is why, with the answer probably being quite complex. There appears to be KIR-mediated selection of HIV variants that might suggest direct antiviral effects mediated by the NK cells (Alter et al., 2011), and HIV-infected T cells can be directly susceptible to lysis by NK cells (Ruscetti et al., 1986). On the other hand, some KIR that correlate with better prognosis of HIV are those with negative signaling capacity (Alter et al., 2009; Martin et al., 2002; Jennes et al., 2006), and negatively signaling KIR have also been associated with resolution of hepatitis C virus infection (Khakoo et al., 2004). These findings about the negatively signaling KIR may be consistent with the concept that the suppression of NK cell activity by negative signaling NKR would allow for greater T cell responses to control the infection. In fact, an inverse relationship was found between NK cell responses and HIV-specific T cell responses in a cohort of elite HIV controllers (Tomescu et al., 2012).
It has also been speculated that higher innate host responses, such as those involving IFN, a potent activator of NK cells, might contribute to differences in viral load set points that characterize HIV patients (Carrington & Alter, 2012), and that this set point decision may thus be made very early in infection. HIV RNA triggers more IFN through TLR7/8 in DC from women than from men, and women tend to have lower HIV set points (Meier et al., 2009). Likewise, the development of AIDS-like disease in SIV-infected macaques is associated with a prolonged type 1 IFN inflammatory response that is substantially curtailed during the non-pathogenic SIV infections of sooty mangabEy and African green monkeys (Bosinger et al., 2009; Jacquelin et al., 2009). These findings are consistent with studies in the LCMV model showing that the functions of NK cells activated during the first three days of infection could have long term consequences on the levels and duration of viral persistence (Waggoner et al., 2010; Waggoner et al., 2012).
Influence of NK cells on the generation of T cell memory
Because the magnitude of the T cell memory response is proportional to the burst size of the acute response (Hou et al., 1994), which is impaired by the activity of NK cells, it stands to reason that NK cells should suppress vaccine efficiency and the development of immunological memory. Indeed, depletion of NK cells by administration of diphtheria toxin (DT) to mice expressing the DT receptor on NK cells or in wild type mice depleted of NK cells with anti-NK1.1 prior to immunization with ovalbumin (OVA) resulted in the generation of considerably higher numbers of OVA-specific transgenic T cells, which protected against an OVA-expressing tumor line (Soderquest et al., 2011; Walzer et al., 2007). Correspondingly, the frequencies of OVA-specific memory cells after immunization with an OVA-expressing strain of Listeria monocytogenes were reduced in NKp46 mutant mice harboring hyperactive NK cells (Narni-Mancinelli et al., 2012). Our own studies have found increased CD8 memory cell frequencies to LCMV and to Pichinde virus in NK cell-depleted mice (unpublished). Collectively these results illustrate a dynamic equilibrium between NK cells and T cells, and it is possible that suppression of the NK cell response might lead to more effective vaccination. Many adjuvants are strong stimulators of cytokines such as IFN, which can both activate NK cells and promote T cell proliferation. Under some conditions, however, T cell proliferation can be greatly reduced by IFN (Marshall et al., 2011), indicating that the timing of these stimulating events may be important to consider for optimal immunization.
Conclusions
The immune system is the only organ system in the body that can expand and contract several-fold all within a few days, and it is therefore very important to have mechanisms in place to keep things from getting out of control and causing toxic shock-like conditions, autoimmunity, or leukemias and lymphomas. As a result, macrophages and regulatory T cells may make suppressive cytokines like TGFβ and IL-10 to restrain responses extrinsically, and the T cells themselves can intrinsically restrain their responses by undergoing apoptosis or clonal exhaustion through the up-regulation of inhibitory molecules (Wherry, 2011). It is now clear that NK cells are another cell type that can regulate T cell responses, and this regulation of T cells may be particularly strong under the conditions of viral infections, which induce NK cell-activating cytokines like IFN, IL-12, and IL-15. We showed here that the NK cell response early in viral infections can have lasting effects on viral pathogenesis and persistence. We speculate that prophylactic depletion of NK cells may enhance vaccination and that therapeutic depletion of NK cells in the midst of a persistent infection may resurrect T cell responses and reduce viral titers.
Highlights.
Activated NK cells lyse activated CD4 T cells and in so doing inhibit both CD4 and CD8 T cell responses
NK cell mediated suppression of T cell responses changes patterns of viral pathogenesis and persistence
NK cells indirectly regulate the clonal exhaustion of T cells and may be needed to maintain persistent infections
Acknowledgements
This work was supported by NIH Training Grant T32 AI07349 and an Ellison Medical Foundation grant to SNW and by NIH research grants AI017672, CA34461, AI081675, and AI046629 to RMW. The views expressed are those of the authors and are not necessarily the views of the NIH. We thank Keith Daniels for help in the preparation of the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MBA. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice: role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter G, Heckerman D, Schneidewind A, Fadda L, Kadie CM, Carlson JM, Oniangue-Ndza C, Martin M, Li B, Khakoo SI, Carrington M, Allen TM, Altfeld M. HIV-1 adaptation to NK-cell-mediated immune pressure. Nature. 2011;476:96–100. doi: 10.1038/nature10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter G, Rihn S, Walter K, Nolting A, Martin M, Rosenberg ES, Miller JS, Carrington M, Altfeld M. HLA class I subtype-dependent expansion of KIR3DS1+ and KIR3DL1+ NK cells during acute human immunodeficiency virus type 1 infection. J. Virol. 2009;83:6798–6805. doi: 10.1128/JVI.00256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews DM, Estcourt MJ, Andoniou CE, Wikstrom ME, Khong A, Voigt V, Fleming P, Tabarias H, Hill GR, van der Most RG, Scalzo AA, Smyth MJ, Degli-Esposti MA. Innate immunity defines the capacity of antiviral T cells to limit persistent infection. J. Exp. Med. 2010;207:1333–1343. doi: 10.1084/jem.20091193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol. 2003;4:175–181. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- Arai S, Yamamoto H, Itoh K, Kumagai K. Suppressive effect of human natural killer cells on pokeweed mitogen-induced B cell differentiation. J. Immunol. 1983;131:651–657. [PubMed] [Google Scholar]
- Arapovic J, Lenac RT, Reddy AB, Krmpotic A, Jonjic S. Promiscuity of MCMV immunoevasin of NKG2D: m138/fcr-1 down-modulates RAE-1epsilon in addition to MULT-1 and H60. Mol. Immunol. 2009;47:114–122. doi: 10.1016/j.molimm.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- Babic M, Pyzik M, Zafirova B, Mitrovic M, Butorac V, Lanier LL, Krmpotic A, Vidal SM, Jonjic S. Cytomegalovirus immunoevasin reveals the physiological role of "missing self" recognition in natural killer cell dependent virus control in vivo. J. Exp. Med. 2010;207:2663–2673. doi: 10.1084/jem.20100921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Biassoni R, Pessino A, Bottino C, Pende D, Moretta L, Moretta A. The murine homologue of the human NKp46, a triggering receptor involved in the induction of natural cytotoxicity. Eur. J. Immunol. 1999;29:1014–1020. doi: 10.1002/(SICI)1521-4141(199903)29:03<1014::AID-IMMU1014>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Biron CA. Cytokines in the generation of immune responses to, and resolution of virus infection. Curr. Opin. Immunol. 1995;6:530–538. doi: 10.1016/0952-7915(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Biron CA, Pedersen KF, Welsh RM. Aberrant T cells in beige mutant mice. J. Immunol. 1987;138:2050–2056. [PubMed] [Google Scholar]
- Biron CA, Turgiss LR, Welsh RM. Increase in NK cell number and turnover rate during acute viral infection. J. Immunol. 1983;131:1539–1545. [PubMed] [Google Scholar]
- Blattman JN, Antia R, Sourdive DJ, Wang X, Kaech SM, Murali-Krishna K, Altman JD, Ahmed R. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J Exp Med JID - 2985109R. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, Francella N, Sidahmed A, Smith AJ, Cramer EM, Zeng M, Masopust D, Carlis JV, Ran L, Vanderford TH, Paiardini M, Isett RB, Baldwin DA, Else JG, Staprans SI, Silvestri G, Haase AT, Kelvin DJ. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J. Clin. Invest. 2009;119:3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MG, Dokun AO, Heusel JW, Smith HR, Beckman DL, Blattenberger EA, Dubbelde CE, Stone LR, Scalzo AA, Yokoyama WM. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 2001;292:934–937. doi: 10.1126/science.1060042. [DOI] [PubMed] [Google Scholar]
- Brown MG, Scalzo AA, Matsumoto K, Yokoyama WM. The natural killer gene complex: a genetic basis for understandig natural killer cell function and innate immunity. Immunol. Rev. 1997;155:53–65. doi: 10.1111/j.1600-065x.1997.tb00939.x. [DOI] [PubMed] [Google Scholar]
- Brubaker JO, Chong KT, Welsh RM. Lymphokine-activated killer (LAK) cells are rejected in vivo by activated natural killer cells. J. Immunol. 1991;147:1439–1444. [PubMed] [Google Scholar]
- Brutkiewicz RR, Welsh RM. Major histocompatibility complex class I antigens and the control of viral infections by natural killer cells. J. of Virol. 1995;69:3967–3971. doi: 10.1128/jvi.69.7.3967-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski JF, Welsh RM. Interferon enhances the susceptibility of virusinfected fibroblasts to cytotoxic T cells. J. Exp. Med. 1985;161:257–262. doi: 10.1084/jem.161.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski JF, Welsh RM. Enhanced susceptibility to cytotoxic T lymphocytes of target cells isolated from virus-infected or interferon-treated mice. J. Virol. 1986;59:735–739. doi: 10.1128/jvi.59.3.735-739.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski JF, Woda BA, Welsh RM. Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J. Virol. 1984;52:119–128. doi: 10.1128/jvi.52.1.119-128.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlington DB, Djeu JY, Wells MA, Kiley SC, Quinnan GV., Jr Large granular lymphocytes provide an accessory function in the in vitro development of influenza A virus-specific cytotoxic T cells. J Immunol. 1984;132:3154–3158. [PubMed] [Google Scholar]
- Cao W, Henry MD, Borrow P, Yamada H, Elder JH, Ravkov EV, Nichol ST, Compans RW, Campbell KP, Oldstone MB. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282:2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- Carrington M, Alter G. Innate Immune Control of HIV. Cold Spring Harb. Perspect. Med. 2012;2 doi: 10.1101/cshperspect.a007070. a007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- Daniels KA, Devora G, Lai WC, O'Donnell CL, Bennett M, Welsh RM. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J. Exp. Med. 2001;194:29–44. doi: 10.1084/jem.194.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Lanier LL, Sigal LJ. A role for NKG2D in NK cell-mediated resistance to poxvirus disease. PLoS. Pathog. 2008;4:e30. doi: 10.1371/journal.ppat.0040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Orr MT, Spee P, Egebjerg T, Lanier LL, Sigal LJ. CD94 is essential for NK cell-mediated resistance to a lethal viral disease. Immunity. 2011;34:579–589. doi: 10.1016/j.immuni.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit R, Gruda R, Elboim M, Arnon TI, Katz G, Achdout H, Hanna J, Qimron U, Landau G, Greenbaum E, Zakay-Rones Z, Porgador A, Mandelboim O. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat. Immunol. 2006;7:517–523. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- Ge MQ, Ho AW, Tang Y, Wong KH, Chua BY, Gasser S, Kemeny DM. NK Cells Regulate CD8+ T Cell Priming and Dendritic Cell Migration during Influenza A Infection by IFN-gamma and Perforin-Dependent Mechanisms. J. Immunol. 2012;189:2099–2109. doi: 10.4049/jimmunol.1103474. [DOI] [PubMed] [Google Scholar]
- Gilbertson SM, Shah PD, Rowley DA. NK cells suppress the generation of Lyt-2+ cytolytic T cells by suppressing or eliminating dendritic cells. J Immunol. 1986;136:3567–3571. [PubMed] [Google Scholar]
- Glasner A, Zurunic A, Meningher T, Lenac RT, Tsukerman P, Bar-On Y, Yamin R, Meyers AF, Mandeboim M, Jonjic S, Mandelboim O. Elucidating the mechanisms of influenza virus recognition by Ncr1. PLoS. ONE. 2012;7:e36837. doi: 10.1371/journal.pone.0036837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson M, Karre K, Kiessling R, Roder J, Andersson B, Hayry P. Natural NK-cell targets in the mouse thymus: characteristics of the sensitive cell population. J. Immunol. 1979;123:765–771. [PubMed] [Google Scholar]
- Hansson M, Kiessling R, Andersson B, Welsh RM. Effect of interferon and interferon inducers on the NK sensitivity of normal mouse thymocytes. J Immunol. 1980;125:2225–2231. [PubMed] [Google Scholar]
- Hou S, Hyland L, Ryan W, Portner A, Doherty PC. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature. 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, Dillies MA, Roques P, Butor C, Silvestri G, Giavedoni LD, Lebon P, Barre-Sinoussi F, Benecke A, Muller-Trutwin MC. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J. Clin. Invest. 2009;119:3544–3555. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennes W, Verheyden S, Demanet C, Adje-Toure CA, Vuylsteke B, Nkengasong JN, Kestens L. Cutting edge: resistance to HIV-1 infection among African female sex workers is associated with inhibitory KIR in the absence of their HLA ligands. J. Immunol. 2006;177:6588–6592. doi: 10.4049/jimmunol.177.10.6588. [DOI] [PubMed] [Google Scholar]
- Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, Cheng J, Goedert JJ, Vlahov D, Hilgartner M, Cox S, Little AM, Alexander GJ, Cramp ME, O'Brien SJ, Rosenberg WM, Thomas DL, Carrington M. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- Kielczewska A, Pyzik M, Sun T, Krmpotic A, Lodoen MB, Munks MW, Babic M, Hill AB, Koszinowski UH, Jonjic S, Lanier LL, Vidal SM. Ly49P recognition of cytomegalovirus-infected cells expressing H2-Dk and CMV-encoded m04 correlates with the NK cell antiviral response. J. Exp. Med. 2009;206:515–523. doi: 10.1084/jem.20080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling R, Hochman PS, Haller O, Shearer GM, Wigzell H, Cudkowicz G. Evidence for a similar or common mechanism for natural killer cell activity and resistance to hemopoietic grafts. Eur. J. Immunol. 1977;7:655–663. doi: 10.1002/eji.1830070915. [DOI] [PubMed] [Google Scholar]
- Kuwano K, Arai S, Munakata T, Tomita Y, Yoshitake Y, Kumagai K. Suppressive effect of human natural killer cells on Epstein-Barr virus-induced immunoglobulin synthesis. J. Immunol. 1986;137:1462–1468. [PubMed] [Google Scholar]
- Lang PA, Lang KS, Xu HC, Grusdat M, Parish IA, Recher M, Elford AR, Dhanji S, Shaabani N, Tran CW, Dissanayake D, Rahbar R, Ghazarian M, Brustle A, Fine J, Chen P, Weaver CT, Klose C, Diefenbach A, Haussinger D, Carlyle JR, Kaech SM, Mak TW, Ohashi PS. Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc. Natl. Acad. Sci. U. S. A. 2012;109:1210–1215. doi: 10.1073/pnas.1118834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier LL. NK cell receptors. Annu. Rev. Immunol. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- Lanier LL. Evolutionary struggles between NK cells and viruses. Nat. Rev. Immunol. 2008;8:259–268. doi: 10.1038/nri2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavenworth JW, Wang X, Wenander CS, Spee P, Cantor H. Mobilization of natural killer cells inhibits development of collagen-induced arthritis. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14584–14589. doi: 10.1073/pnas.1112188108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, McNerney ME, Stepp SE, Mathew PA, Schatzle JD, Bennett M, Kumar V. 2B4 acts as a non-major histocompatibility complex binding inhibitory receptor on mouse natural killer cells. J Exp Med. 2004;199:1245–1254. doi: 10.1084/jem.20031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Girard S, Macina D, Busa M, Zafer A, Belouchi A, Gros P, Vidal SM. Susceptibility to mouse cytomegalovirus is associated with deletion of an activating natural killer cell receptor of the C-type lectin superfamily. Nat Genet. 2001;28:42–45. doi: 10.1038/ng0501-42. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kim KS, Fodil-Cornu N, Vidal SM, Biron CA. Activating receptors promote NK cell expansion for maintenance, IL-10 production, and CD8 T cell regulation during viral infection. J. Exp. Med. 2009;206:2235–2251. doi: 10.1084/jem.20082387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren HG, Karre K. In search of the 'missing self': MHC molecules and NK cell recognition. Immunology Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- Mack EA, Kallal LE, Demers DA, Biron CA. Type 1 interferon induction of natural killer cell gamma interferon production for defense during lymphocytic choriomeningitis virus infection. M Bio. 2011;2 doi: 10.1128/mBio.00169-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall HD, Urban SL, Welsh RM. Virus-induced transient immune suppression and the inhibition of T cell proliferation by type I interferon. J. Virol. 2011;85:5929–5939. doi: 10.1128/JVI.02516-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MP, Gao X, Lee JH, Nelson GW, Detels R, Goedert JJ, Buchbinder S, Hoots K, Vlahov D, Trowsdale J, Wilson M, O'Brien SJ, Carrington M. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat. Genet. 2002;31:429–434. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, Wen TF, Lindsay RJ, Orellana L, Mildvan D, Bazner S, Streeck H, Alter G, Lifson JD, Carrington M, Bosch RJ, Robbins GK, Altfeld M. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat. Med. 2009;15:955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R, Chen AT, Welsh RM, Szomolanyi-Tsuda E. NK cells and gammadelta T cells mediate resistance to polyomavirus-induced tumors. PLoS. Pathog. 2010;6 doi: 10.1371/journal.ppat.1000924. e1000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrovic M, Arapovic J, Jordan S, Fodil-Cornu N, Ebert S, Vidal SM, Krmpotic A, Reddehase MJ, Jonjic S. The NK cell response to mouse cytomegalovirus infection affects the level and kinetics of the early CD8(+) T-cell response. J. Virol. 2012;86:2165–2175. doi: 10.1128/JVI.06042-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narni-Mancinelli E, Jaeger BN, Bernat C, Fenis A, Kung S, De GA, Mahmood S, Gut M, Heath SC, Estelle J, Bertosio E, Vely F, Gastinel LN, Beutler B, Malissen B, Malissen M, Gut IG, Vivier E, Ugolini S. Tuning of natural killer cell reactivity by NKp46 and Helios calibrates T cell responses. Science. 2012;335:344–348. doi: 10.1126/science.1215621. [DOI] [PubMed] [Google Scholar]
- Orr MT, Lanier LL. Inhibitory Ly49 receptors on mouse natural killer cells. Curr. Top. Microbiol. Immunol. 2011;350:67–87. doi: 10.1007/82_2010_85. [DOI] [PubMed] [Google Scholar]
- Rabinovich BA, Li J, Shannon J, Hurren R, Chalupny J, Cosman D, Miller RG. Activated, but not resting, T cells can be recognized and killed by syngeneic NK cells. J Immunol. 2003;170:3572–3576. doi: 10.4049/jimmunol.170.7.3572. [DOI] [PubMed] [Google Scholar]
- Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat. Rev. Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- Robbins SH, Bessou G, Cornillon A, Zucchini N, Rupp B, Ruzsics Z, Sacher T, Tomasello E, Vivier E, Koszinowski UH, Dalod M. Natural killer cells promote early CD8 T cell responses against cytomegalovirus. PLoS. Pathog. 2007;3:e123. doi: 10.1371/journal.ppat.0030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti FW, Mikovits JA, Kalyanaraman VS, Overton R, Stevenson H, Stromberg K, Herberman RB, Farrar WL, Ortaldo JR. Analysis of effector mechanisms against HTLV-I- and HTLV-III/LAV-infected lymphoid cells. J. Immunol. 1986;136:3619–3624. [PubMed] [Google Scholar]
- Salazar-Onfray F, Charo J, Petersson M, Freland S, Noffz G, Qin Z, Blankenstein T, Ljunggren HG, Kiessling R. Down-regulation of the expression and function of the transporter associated with antigen processing in murine tumor lines expressing IL-10. J. Immunol. 1997;159:3195–3202. [PubMed] [Google Scholar]
- Scalzo AA, Lyons PA, Fitzgerald NA, Forbes CA, Yokoyama WM, Shellam GR. Genetic mapping of Cmv-1 in the region of mouse chromosome 6 encoding NK gene complex-associated loci Ly49 and musNKR-P1. Genomics. 1995;27:435–441. doi: 10.1006/geno.1995.1074. [DOI] [PubMed] [Google Scholar]
- Seedhom MO, Jellison ER, Daniels KA, Welsh RM. High frequencies of virus-specific CD8+ T-cell precursors. J. Virol. 2009;83:12907–12916. doi: 10.1128/JVI.01722-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HR, Heusel JW, Mehta IK, Kim S, Dorner BG, Naidenko OV, Iizuka K, Furukawa H, Beckman DL, Pingel JT, Scalzo AA, Fremont DH, Yokoyama WM. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc. Natl. Acad. Sci. U. S. A. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderquest K, Walzer T, Zafirova B, Klavinskis LS, Polic B, Vivier E, Lord GM, Martin-Fontecha A. Cutting edge: CD8+ T cell priming in the absence of NK cells leads to enhanced memory responses. J. Immunol. 2011;186:3304–3308. doi: 10.4049/jimmunol.1004122. [DOI] [PubMed] [Google Scholar]
- Stadnisky MD, Xie X, Coats ER, Bullock TN, Brown MG. Self MHC class I-licensed NK cells enhance adaptive CD8 T-cell viral immunity. Blood. 2011;117:5133–5141. doi: 10.1182/blood-2010-12-324632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm A, Valentine L, Potts R, Premenko-Lanier M. An intermediate dose of LCMV clone 13 causes prolonged morbidity that is maintained by CD4+ T cells. Virology. 2012;425:122–132. doi: 10.1016/j.virol.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HC, Leite-Morris KA, Braun L, Biron CA. A role for transforming growth factor-beta 1 in regulating natural killer cell and T lymphocyte proliferative responses during acute infection with lymphocytic choriomeningitis virus. J. Immunol. 1991;147:2717–2727. [PubMed] [Google Scholar]
- Su HC, Nguyen KB, Salazar-Mather TP, Ruzek MC, Dalod MY, Biron CA. NK cell functions restrain T cell responses during viral infections. Eur. J Immunol. 2001a;31:3048–3055. doi: 10.1002/1521-4141(2001010)31:10<3048::aid-immu3048>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Su HC, Nguyen KB, Salazar-Mather TP, Ruzek MC, Dalod MY, Biron CA. NK cell functions restrain T cell responses during viral infections. Eur. J Immunol. 2001b;31:3048–3055. doi: 10.1002/1521-4141(2001010)31:10<3048::aid-immu3048>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Suzuki S, Ebina N, Kumagai K. Suppression of alloimmune cytotoxic T lymphocyte (CTL) generation by depletion of NK cells and restoration by interferon and/or interleukin 2. J Immunol. 1985;134:2139–2148. [PubMed] [Google Scholar]
- Swann SA, Williams M, Story CM, Bobbitt KR, Fleis R, Collins KL. HIV-1 Nef blocks transport of MHC class I molecules to the cell surface via a PI 3- kinase-dependent pathway. Virology. 2001;282:267–277. doi: 10.1006/viro.2000.0816. [DOI] [PubMed] [Google Scholar]
- Tay CH, Welsh RM. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J. Virol. 1997;71:267–275. doi: 10.1128/jvi.71.1.267-275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen AR, Pisa P, Bro-Jorgensen K, Kiessling R. Mechanisms of lymphocytic choriomeningitis virus-induced hemopoietic dysfunction. J. Virol. 1986;59:428–433. doi: 10.1128/jvi.59.2.428-433.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomescu C, Duh FM, Hoh R, Viviani A, Harvill K, Martin MP, Carrington M, Deeks SG, Montaner LJ. Impact of protective KIR/HLA genotypes on NK Cell and T cell function in HIV-1 infected controllers. AIDS. 2012 doi: 10.1097/QAD.0b013e32835861b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G, Santoli D. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J Exp Med. 1978;147:1314–1333. doi: 10.1084/jem.147.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt V, Forbes CA, Tonkin JN, Degli-Esposti MA, Smith HR, Yokoyama WM, Scalzo AA. Murine cytomegalovirus m157 mutation and variation leads to immune evasion of natural killer cells. Proc. Natl. Acad. Sci. U. S. A. 2003;100:13483–13488. doi: 10.1073/pnas.2233572100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2012;481:394–398. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner SN, Taniguchi RT, Mathew PA, Kumar V, Welsh RM. Absence of mouse 2B4 promotes NK cell-mediated killing of activated CD8+ T cells, leading to prolonged viral persistence and altered pathogenesis. J. Clin. Invest. 2010;120:1925–1938. doi: 10.1172/JCI41264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer T, Blery M, Chaix J, Fuseri N, Chasson L, Robbins SH, Jaeger S, Andre P, Gauthier L, Daniel L, Chemin K, Morel Y, Dalod M, Imbert J, Pierres M, Moretta A, Romagne F, Vivier E. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc. Natl. Acad. Sci. U. S. A. 2007;104:3384–3389. doi: 10.1073/pnas.0609692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh RM. Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. I. Characterization of natural killer cell induction. J. Exp. Med. 1978;148:163. doi: 10.1084/jem.148.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh RM, Brubaker JO, Vargas-Cortes M, O'Donnell CL. Natural killer (NK) cell response to virus infections in mice with severe combined immunodeficiency. The stimulation of NK cells and the NK cell-dependent control of virus infections occur independently of T and B cell function. J. Exp. Med. 1991;173:1053–1063. doi: 10.1084/jem.173.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh RM, Karre K, Hansson M, Kunkel LA, Kiessling RW. Interferon-mediated protection of normal and tumor target cells against lysis by mouse natural killer cells. J. Immunol. 1981;126:219–225. [PubMed] [Google Scholar]
- Wherry EJ. T cell exhaustion. Nat. Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- Whitmire JK, Eam B, Benning N, Whitton JL. Direct interferon-gamma signaling dramatically enhances CD4+ and CD8+ T cell memory. J Immunol. 2007;179:1190–1197. doi: 10.4049/jimmunol.179.2.1190. [DOI] [PubMed] [Google Scholar]
- Wilson DB, Wilson DH, Schroder K, Pinilla C, Blondelle S, Houghten RA, Garcia KC. Specificity and degeneracy of T cells. Mol. Immunol. 2004;40:1047–1055. doi: 10.1016/j.molimm.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Wilson JL, Heffler LC, Charo J, Scheynius A, Bejarano MT, Ljunggren HG. Targeting of human dendritic cells by autologous NK cells. J Immunol. 1999;163:6365–6370. [PubMed] [Google Scholar]
- Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJD, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Ou R, Huang L, Moskophidis D. Critical role for perforin-, Fas/FasL-, and TNFR1-mediated cytotoxic pathways in down-regulation of antigen-specific T cells during persistent viral infection. J Virol. 2002;76:829–840. doi: 10.1128/JVI.76.2.829-840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]