Abstract
High-force lengthening contractions are associated with muscle damage and pain, and the muscle–tendon junction is commonly cited as the primary area where myofiber damage occurs. We induced injury in the rat tibialis anterior muscle and acquired magnetic resonance imaging (MRI) images postinjury. We also assayed membrane damage and quantified the number of centrally nucleated myofibers throughout the injured muscles. Results suggest that myofiber injury occurs primarily in the middle portion of the muscle, with interstitial edema in the middle and distal portions.
Keywords: eccentric injury, MRI, sarcolemma
Skeletal muscles perform hundreds of submaximal lengthening (“eccentric”) contractions daily without injury, but high-force lengthening contractions are associated with muscle damage and pain.1 Maximal lengthening contractions generate more force than maximal isometric or shortening (“concentric”) contractions2 and are used frequently in strength training and in sports. The injury resulting from a high-force lengthening contraction is clinically termed a “muscle strain” and is one of the most common ailments seen by physicians.3
Inflammation is a consequence of most muscle injuries, but its role in facilitating versus obstructing muscle repair is still being defined.4,5 Structural damage is also commonly used as a marker of injury,6,7 but it is not clear where the primary area of damage is located. Some investigators have suggested that much of the inflammation and damage is located at the muscle–tendon junction (MTJ), the interface between the connective tissue and myofibers; therefore, the MTJ is commonly cited as the area where myofiber damage occurs.8–10 Such conclusions are sometimes derived from in vitro load-to-failure–type studies,11 which may not be representative of normal physiology. Moreover, in more functional in vivo models, structural damage is commonly cited in the muscle belly after a strain injury.12,13
Magnetic resonance imaging (MRI) can reliably detect the hemorrhage and edema that follow muscle injuries. Muscle strains are best revealed by T2-weighted images, where edema is observed as a bright signal against the surrounding normal tissue. A high signal intensity on T2-weighted imaging is also a reflection of an increase in the T2-relaxation of the tissue, which is a fundamental MRI tissue property suggesting a breakdown in the structural integrity of the tissue. Some MRI studies support the concept that the MTJ is the area of primary damage,14 whereas others indicated damage primarily in the midbelly of the muscle.15–17 In humans with an acute muscle strain, one limitation is that MR images might be acquired days after injury, when edema may have diffused in a gravity-dependent manner.
In mammalian skeletal muscle, the myofibers do not extend from tendon to tendon18 and sometimes do not even extend the length of the fascicle19; in some muscles the myofibers are even “in series.”20 The mean myofiber length of the rat tibialis anterior muscle (TA) has been estimated to be only 57% of the total muscle length.21,22 This means that even if injury to a single myofiber resulted in damage along the entire cell, it cannot be assumed that damage observed in cross-sections is similar throughout the whole muscle. The aim of the present study was to compare data derived from MRI to histological markers of damage after injury using an in vivo animal model. Based on previous results examining the histological changes in sections made throughout the TA after injury,7,23 we hypothesized that the greatest damage to the myofibers after lengthening contractions occurs in the middle of the muscle.
METHODS
Injury induced by repeated lengthening contractions was performed as described elssewhere.7,23,24 Briefly, male Sprague-Dawley rats (Charles River Laboratories, Wilmington, Massachusetts), weighing 315 ± 12 g (approximately 2–3 months old), were anesthetized with isoflurane (2% with oxygen flow rate of 0.5 L/min). With the animal supine, the hind-limb was stabilized and the foot was secured onto a plate, the axis of which was attached to a stepper motor (Model T8904; NMB Technologies, Chatsworth, California). A custom program based on commercial software (LabView version 4.1; National Instruments, Austin, Texas) was used to synchronize contractile activation and the onset of ankle rotation. Injury resulted from 15 forced lengthening (plantarflexion) contractions through an 80° arc of motion, superimposed onto a maximally contracting tibialis anterior muscle (TA, a dorsiflexor).
High-resolution (195 μm in-plane at 1.25-mm slice thickness) dual-echo proton density and T2-weighted rapid acquisition relaxation-enhanced (RARE) MR images (TE1/TE2/TR/ETL/NEX = 17.4 ms/52.1 ms/5000 ms/4/8) were acquired on a 7-Tesla MR system (Biospec 7T/30; Bruker Bio-spin, Billerica, Massachusetts) to assess muscle damage on the day of injury (D0) and on consecutive days for 1 week (N = 3 animals). Measurements of signal intensity were carried out using Image J v1.41 software (NIH). To confirm that the observed T2 relaxation time prolongation (i.e., signal change) was not a result of the in vivo procedural methods, MRI was also acquired on a single rat in which only isometric contractions were performed on one leg and only stretches were performed on the other leg.
TAs were harvested on day 14 to quantify the number of centrally nucleated fibers (CNFs, visualized by staining with hematoxylin–eosin [H&E]). Myonuclei in healthy skeletal muscle are restricted to the periphery of the cell, near the sarcolemma; CNFs are the myofibers that have nuclei in or near the center of the sarcoplasm and are recognized as regenerating muscle cells (the presence of CNFs is an accepted marker of myogenesis that occurs in the segment of a myofiber that is damaged, but day 14 was selected because they do not appear immediately after injury15,23). After harvesting, TAs were sectioned into proximal, middle, and distal thirds, snap frozen in liquid nitrogen, and stored at –80° C until needed.
In separate animals (N = 3), the same injury was induced, and Evans blue dye (EBD) was used to assess membrane damage. EBD, which binds to serum albumin, is detected intracellularly only in myofibers that sustain membrane damage.7,23 One day before injury, rats received an intraperitoneal injection of 1% (wt/vol) EBD (Sigma Co., St. Louis, Missouri) in phosphate-buffered saline (PBS) at a volume of 1% body mass (1 mg EBD/0.1 ml PBS/10 g body mass). This solution was sterilized by passage through a 0.22-μm filter (Millex-GP; Millipore, Bedford, Massachusetts). To assess membrane damage, TAs were harvested on D0 and cut into proximal, middle, and distal thirds, and the numbers of myofibers with and without EBD were counted.
To determine the number of EBD-positive myofibers or myofibers with CNFs, at least 15 cross-sections (10 μm thick) were examined at random from proximal, middle, and distal sections of control and injured TAs. Sections were randomized and viewed at ×20 (average of 37 ± 12 myofibers/field) in a light microscope (Axioskop; Carl Zeiss, Germany), and pictures were taken with a digital camera (AxioCam HR using AxioVision 3.0; Carl Zeiss, Germany). Differences in the number of fibers between the proximal, middle and distal segments with EBD or CNFs were compared using one-way analysis of variance (ANOVA). Significance was set at P < 0.05, and all results are reported as mean ± SD.
RESULTS
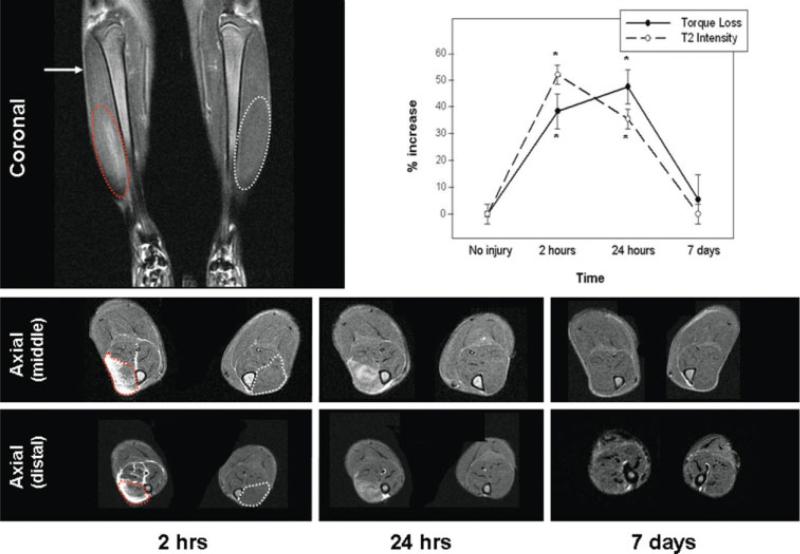
On the day of injury, the T2 changes on MRI were diffuse throughout the middle and distal aspects of the TA muscle (Fig. 1 and 1A); the signal intensity of both portions was increased compared with the control side at the same time-point. The overall pattern of T2 changes paralleled the pattern of force loss and recovery (Fig. 1B). These changes were persistent over time but resolved by day 7 (Fig. 1). The T2 signal in the proximal portion of the TA (Figure 1, 2 hours) was unaltered compared with controls. Similar to other reports,25 we did not observe changes in T2 signal intensity anywhere in the TA after sham procedures (not shown).
FIGURE 1.
MR imaging after contraction-induced injury. The top MR image (T2-weighted) shows the tibialis anterior (TA) muscles in the frontal plane (coronal view) on the day of injury (2 hours postinjury). The increased T2 signal in the injured TA is circled in red and, for orientation, the corresponding area is circled in white on the noninjured TA. The proximal portion (arrow) was unaffected. The lower MR images show cross-sections (axial) from the portions of the TA with altered T2 signals (middle and distal) at the selected time-points postinjury (2 hours, 24 hours, and 7 days). For orientation, the injured TA is circled with a dotted red line and the control TA is circled with a dotted white line at the earliest time-point. Cross-sections of the proximal portion are not shown, because, as pictured in the coronal view, there were no changes in the T2 signal in the axial views. The graph shows changes in T2 signal and muscle force. The dotted line (open circles) shows the percent increase in the T2 signal intensity in the midbelly of the TA (compared with the noninjured side), and the solid line (filled circles) shows the percent of torque loss after injury (compared with pre-injury torque in the same muscle). *P < 0.05.
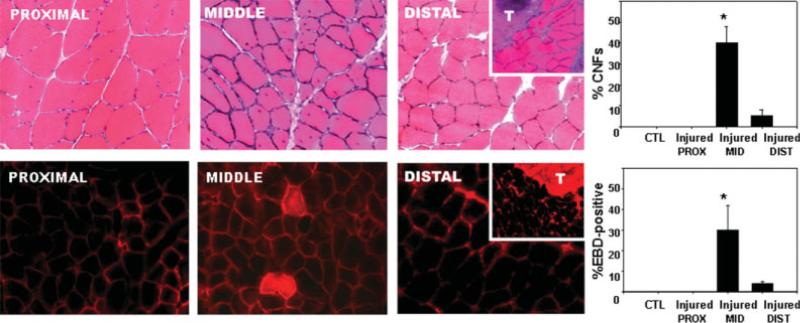
CNFs do not appear immediately after injury, but instead are found in the damaged segments of myofibers within days/weeks after injury. At 2 weeks after injury, the occurrence of CNFs was much higher in the middle of the muscle (40 ± 8% of myofibers) than in the distal (5 ± 3%) or proximal (0 ± 0%) portions of the muscle (Fig. 2A). Once the EBD enters a damaged myofiber, its distribution remains in the immediate area of the localized membrane damage, rather than running the entire length of the myofiber.5 The number of myofibers with membrane damage, as indicated by the presence of EBD inside the myofibers (Fig. 2B), was much higher in the middle of the muscle (30 ± 12% of myofibers) than in the distal (4 ± 1%) or proximal (0 ± 0%) portion of the muscle.
FIGURE 2.
Histological damage to myofibers after contraction-induced injury. Harvested muscles were cut into proximal, middle, and distal thirds. The top row of micrographs shows cross-sections stained with H&E from each portion of an injured tibialis anterior muscle. The numbers of centrally nucleated fibers (CNFs) from all three sections were quantified and are shown in the histogram (control and proximal were 0%). Inset shows cross-sections slightly more distally where the tendon (T) is clearly visible. The bottom row of micrographs shows cross-sections from the proximal, middle, and distal aspects of an injured tibialis anterior muscle from a rat injected with the low-molecular-weight diazo dye, Evans blue (EBD). The number of myofibers containing EBD (the middle panel shows one example of EBD-positive myofibers) was quantified from all three sections and is shown in the histogram (control and proximal were 0%). Inset shows cross-sections more distally where the tendon (T) begins to take up much of the optical field. *P < 0.05.
DISCUSSION
The MTJ has been cited by some investigators as the primary area of damage after a contraction-induced muscle injury.3,10 Reasons for this may include clinical findings, which often include pain and tenderness in this area, and imaging studies, which show fluid accumulation at the MTJ several days after injury.14 However, based on the literature, damage clearly can occur in the midbelly of muscles after a strain injury. In fact many, if not most, animal studies of contraction-induced injured muscle have used sections from the mid-belly to demonstrate the histopathology that ensues.12,13,15,26,27 In studies of humans, biopsies are typically harvested in the muscle belly to examine tissue damage.28 Even MRI studies have shown changes in the belly of the muscle in rodents after hind-limb suspension/reloading15 or downhill running.16 Some animal studies simply have not described from which part of the muscle the sections were made for tissue analysis, but is very common to see cross-sectional images of the muscle without the tendon, indicating that the belly of the muscle was used.29–31
Using an established in vivo model of muscle injury,7,23,24 we compared data from MRI and histological sections to assess the location of damage after lengthening contractions. Our MRI findings indicate that edema is present in the middle aspect of the TA after a contraction-induced injury. Muscle injuries are best depicted on T2-weighted images in the axial plane.14 After injury, there is an increase in the signal intensity on T2-weighted images (see Fig. 1A), suggesting a prolongation of the T2 relaxation time. This change is presumably due to edema and hemorrhage14; however, in some models of injury, the changes in T2 can last long after inflammation has resolved.17,32 The EBD and CNF data confirm that the most of the damage to myofibers occurred in the midbelly. The MR images indicate that edema was also present in the distal aspects of the muscles. Because the histological findings confirm damage primarily to the middle portion of the muscle, and the MR images were not acquired until several hours after the injury, it is possible that changes in the T2 signal in the distal aspects reflect extracellular blood and edema rather than disruption or damage to the myofibers.
The reason why damage was localized to the middle of the muscle is not clear. Although our understanding is further complicated by the lack of consensus regarding the mechanisms underlying injury due to lengthening contractions,1 our data are consistent with the hypothesis that damage after a muscle strain is caused by high shear stresses at the interface between the myofibers and the extracellular matrix.33,34 Because many skeletal muscles have a greater cross-sectional area in the mid-belly than at either end, one cause for greater damage in the midportion of the muscle might be due to the greater number of myofibers in parallel in this region. Although we can only speculate at the mechanism explaining the location of damage, the data from this study clearly show that damage is greatest in the midbelly, at least with the experimental model and injury protocol that we used.
Interestingly, the changes on MRI resolved within the same time period required for return of contractile function (Fig. 1B). We observed a peak in MR signal change in images acquired closest to the time of injury (Fig. 1). It is possible that we missed an even higher intensity of MR signal between the initial MRI and the 24-hour period, but it is clear that the signal intensity is diminished by 24 hours. Other studies have shown that T2 values gradually peak after injury, either as early as 7 hours35 (rat) or as late as 2 days36 (mouse); however, these studies employed a myotoxin to injure the muscle. Myotoxins provide a model to study necrosis, inflammation, and massive degeneration/regeneration, they but do not provide a physiological model of injury. Marqueste et al.16 used MRI to assess “eccentric type muscle damage” in rats after downhill running. Their results differ from ours in that MR changes were not temporally related to muscle contractility, and MR changes peaked well after the 24-hour period. Human studies17,37 indicate a bimodal pattern in T2 changes (i.e., an increase after injury followed by a further increase by 24 hours postinjury) after lengthening contractions, similar to the bimodal pattern seen with loss of force. It is unclear why we do not see this further increase in T2 intensity at 24 hours postinjury. Such contrasts suggest that a high-force muscle “strain” injury, as used here, cannot be considered the same as a muscle “overuse” injury, or the same as an injury induced by means other than lengthening contractions.
If localized treatments are directed at skeletal muscle to foster myogenesis and facilitate muscle growth after injury, it is important to know where the damage occurs. Because treatment of an acute muscle injury involves injection of anti-inflamma-tory agents,38,39 delivery of growth factors,29,40 or topical application of other therapeutic modalities,41 it behooves the clinician to be as precise as possible when providing treatment.
Our results suggest that small-animal MRI is a valid tool in assessing muscle injury and that much of the damage after a contraction-induced injury is located in the midbelly of the muscle. Our findings may be limited to the current injury model and protocol (e.g., number of repetitions and arc of motion). It is also possible that the same injury protocol would yield different results using a different muscle. Muscle architecture is a significant factor in muscle force development,18 and muscles that cross two joints are commonly injured.14 Fiber type is also thought to affect susceptibility to injury, with type 2 (fast) myofibers being more prone to damage.14 The rat TA is a predominantly fast muscle22 that spans only one joint, so a muscle with a mixed fiber type that spans two joints may exhibit damage that is more limited to the MTJ. Further studies are needed to determine such possibilities.
Abbreviations
- ANOVA
analysis of variance
- CNF
centrally nucleated fibers
- EBD
Evans blue dye
- MRI
magnetic resonance imaging
- MTJ
muscle–tendon junction
- PBS
phosphate-buffered saline
- TA
tibialis anterior
REFERENCES
- 1.Proske U, Allen TJ. Damage to skeletal muscle from eccentric exercise. Exerc Sport Sci Rev. 2005;33:98–104. doi: 10.1097/00003677-200504000-00007. [DOI] [PubMed] [Google Scholar]
- 2.LaStayo PC, Woolf JM, Lewek MD, Snyder-Mackler L, Reich T, Lindstedt SL. Eccentric muscle contractions: their contribution to injury, prevention, rehabilitation, and sport. J Orthop Sports Phys Ther. 2003;33:557–571. doi: 10.2519/jospt.2003.33.10.557. [DOI] [PubMed] [Google Scholar]
- 3.Garrett WE., Jr Muscle strain injuries. Am J Sports Med. 1996;24(suppl):S2–S8. [PubMed] [Google Scholar]
- 4.Toumi H, Best TM. The inflammatory response: friend or enemy for muscle injury? Br J Sports Med. 2003;37:284–286. doi: 10.1136/bjsm.37.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tidball JG, Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol. 2007;578:327–336. doi: 10.1113/jphysiol.2006.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lieber RL, Shah S, Friden J. Cytoskeletal disruption after eccentric contraction-induced muscle injury. Clin Orthop Relat Res. 2002;403(suppl):S90–S99. doi: 10.1097/00003086-200210001-00011. [DOI] [PubMed] [Google Scholar]
- 7.Lovering RM, De Deyne PG. Contractile function, sarcolemma integrity, and the loss of dystrophin after skeletal muscle eccentric contraction-induced injury. Am J Physiol Cell Physiol. 2004;286:C230–C238. doi: 10.1152/ajpcell.00199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehto MU, Jarvinen MJ. Muscle injuries, their healing process and treatment. Ann Chir Gynaecol. 1991;80:102–108. [PubMed] [Google Scholar]
- 9.Kirkendall DT, Garrett WE., Jr Clinical perspectives regarding eccentric muscle injury. Clin Orthop Relat Res. 2002;403(suppl):S81–S89. doi: 10.1097/00003086-200210001-00010. [DOI] [PubMed] [Google Scholar]
- 10.Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33:745–764. doi: 10.1177/0363546505274714. [DOI] [PubMed] [Google Scholar]
- 11.Safran MR, Garrett WE, Jr, Seaber AV, Glisson RR, Ribbeck BM. The role of warmup in muscular injury prevention. Am J Sports Med. 1988;16:123–129. doi: 10.1177/036354658801600206. [DOI] [PubMed] [Google Scholar]
- 12.Baker BA, Mercer RR, Geronilla KB, Kashon ML, Miller GR, Cutlip RG. Impact of repetition number on muscle performance and histological response. Med Sci Sports Exerc. 2007;39:1275–1281. doi: 10.1249/mss.0b013e3180686dc7. [DOI] [PubMed] [Google Scholar]
- 13.Pizza FX, Peterson JM, Baas JH, Koh TJ. Neutrophils contribute to muscle injury and impair its resolution after lengthening contractions in mice. J Physiol. 2005;562:899–913. doi: 10.1113/jphysiol.2004.073965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blankenbaker DG, De Smet AA. MR imaging of muscle injuries. Appl Radiol. 2004:14–6. [Google Scholar]
- 15.Frimel TN, Walter GA, Gibbs JD, Gaidosh GS, Vandenborne K. Noninvasive monitoring of muscle damage during reloading following limb disuse. Muscle Nerve. 2005;32:605–612. doi: 10.1002/mus.20398. [DOI] [PubMed] [Google Scholar]
- 16.Marqueste T, Giannesini B, Fur YL, Cozzone PJ, Bendahan D. Comparative MRI analysis of T2 changes associated with single and repeated bouts of downhill running leading to eccentric-induced muscle damage. J Appl Physiol. 2008;105:299–307. doi: 10.1152/japplphysiol.00738.2007. [DOI] [PubMed] [Google Scholar]
- 17.Foley JM, Jayaraman RC, Prior BM, Pivarnik JM, Meyer RA. MR measurements of muscle damage and adaptation after eccentric exercise. J Appl Physiol. 1999;87:2311–2318. doi: 10.1152/jappl.1999.87.6.2311. [DOI] [PubMed] [Google Scholar]
- 18.Lieber RL, Friden J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000;23:1647–1666. doi: 10.1002/1097-4598(200011)23:11<1647::aid-mus1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 19.Loeb GE, Pratt CA, Chanaud CM, Richmond FJ. Distribution and innervation of short, interdigitated muscle fibers in parallel-fibered muscles of the cat hindlimb. J Morphol. 1987;191:1–15. doi: 10.1002/jmor.1051910102. [DOI] [PubMed] [Google Scholar]
- 20.Heron MI, Richmond FJ. In-series fiber architecture in long human muscles. J Morphol. 1993;216:35–45. doi: 10.1002/jmor.1052160106. [DOI] [PubMed] [Google Scholar]
- 21.Wickiewicz TL, Roy RR, Powell PL, Edgerton VR. Muscle architecture of the human lower limb. Clin Orthop Rel Res. 1983:275–283. [PubMed] [Google Scholar]
- 22.Eng CM, Smallwood LH, Rainiero MP, Lahey M, Ward SR, Lieber RL. Scaling of muscle architecture and fiber types in the rat hindlimb. J Exp Biol. 2008;211:2336–2345. doi: 10.1242/jeb.017640. [DOI] [PubMed] [Google Scholar]
- 23.Lovering RM, Roche JA, Bloch RJ, De Deyne PG. Recovery of function in skeletal muscle following 2 different contraction-induced injuries. Arch Phys Med Rehabil. 2007;88:617–625. doi: 10.1016/j.apmr.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Lovering RM, Hakim M, Moorman CT, III, De Deyne PG. The contribution of contractile pre-activation to loss of function after a single lengthening contraction. J Biomech. 2005;38:1501–1507. doi: 10.1016/j.jbiomech.2004.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heemskerk AM, Drost MR, van Bochove GS, van Oosterhout MF, Nicolay K, Strijkers GJ. DTI-based assessment of ischemia–reperfusion in mouse skeletal muscle. Magn Reson Med. 2006;56:272–281. doi: 10.1002/mrm.20953. [DOI] [PubMed] [Google Scholar]
- 26.Lieber RL, Friden J. Muscle damage is not a function of muscle force but active muscle strain. J Appl Physiol. 1993;74:520–526. doi: 10.1152/jappl.1993.74.2.520. [DOI] [PubMed] [Google Scholar]
- 27.Hentzen ER, Lahey M, Peters D, Mathew L, Barash IA, Friden J, et al. Stress-dependent and -independent expression of the myogenic regulatory factors and the MARP genes after eccentric contractions in rats. J Physiol. 2006;570:157–167. doi: 10.1113/jphysiol.2005.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crameri RM, Langberg H, Magnusson P, Jensen CH, Schroder HD, Olesen JL, et al. Changes in satellite cells in human skeletal muscle after a single bout of high intensity exercise. J Physiol. 2004;558:333–340. doi: 10.1113/jphysiol.2004.061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan YS, Li Y, Foster W, Fu FH, Huard J. The use of suramin, an antifibrotic agent, to improve muscle recovery after strain injury. Am J Sports Med. 2005;33:43–51. doi: 10.1177/0363546504265190. [DOI] [PubMed] [Google Scholar]
- 30.Rathbone CR, Wenke JC, Warren GL, Armstrong RB. Importance of satellite cells in the strength recovery after eccentric contraction-induced muscle injury. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1490–R1495. doi: 10.1152/ajpregu.00032.2003. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Foster W, Deasy BM, Chan Y, Prisk V, Tang Y, et al. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol. 2004;164:1007–1019. doi: 10.1016/s0002-9440(10)63188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleckenstein JL, Weatherall PT, Parkey RW, Payne JA, Peshock RM. Sports-related muscle injuries: evaluation with MR imaging. Radiology. 1989;172:793–798. doi: 10.1148/radiology.172.3.2772190. [DOI] [PubMed] [Google Scholar]
- 33.Gao Y, Wineman AS, Waas AM. Mechanics of muscle injury induced by lengthening contraction. Ann Biomed Eng. 2008;36:1615–1623. doi: 10.1007/s10439-008-9547-3. [DOI] [PubMed] [Google Scholar]
- 34.Gao Y, Waas AM, Faulkner JA, Kostrominova TY, Wineman AS. Micromechanical modeling of the epimysium of the skeletal muscles. J Biomech. 2008;41:1–10. doi: 10.1016/j.jbiomech.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Mattila KT, Lukka R, Hurme T, Komu M, Alanen A, Kalimo H. Magnetic resonance imaging and magnetization transfer in experimental myonecrosis in the rat. Magn Reson Med. 1995;33:185–192. doi: 10.1002/mrm.1910330207. [DOI] [PubMed] [Google Scholar]
- 36.Wishnia A, Alameddine H, Tardif de GS, Leroy-Willig A. Use of magnetic resonance imaging for noninvasive characterization and follow-up of an experimental injury to normal mouse muscles. Neuromuscul Disord. 2001;11:50–55. doi: 10.1016/s0960-8966(00)00164-4. [DOI] [PubMed] [Google Scholar]
- 37.Jayaraman RC, Reid RW, Foley JM, Prior BM, Dudley GA, Weingand KW, et al. MRI evaluation of topical heat and static stretching as therapeutic modalities for the treatment of eccentric exercise-induced muscle damage. Eur J Appl Physiol. 2004;93:30–38. doi: 10.1007/s00421-004-1153-y. [DOI] [PubMed] [Google Scholar]
- 38.Levine WN, Bergfeld JA, Tessendorf W, Moorman CT., III Intramuscular corticosteroid injection for hamstring injuries. A 13-year experience in the National Football League. Am J Sports Med. 2000;28:297–300. doi: 10.1177/03635465000280030301. [DOI] [PubMed] [Google Scholar]
- 39.Hakim M, Hage W, Lovering RM, Moorman CT, III, Curl LA, De Deyne PG. Dexamethasone and recovery of contractile tension after a muscle injury. Clin Orthop Relat Res. 2005;439:235–242. doi: 10.1097/01.blo.0000177716.70404.f9. [DOI] [PubMed] [Google Scholar]
- 40.Kasemkijwattana C, Menetrey J, Bosch P, Somogyi G, Moreland MS, Fu FH, et al. Use of growth factors to improve muscle healing after strain injury. Clin Orthop Relat Res. 2000:272–285. doi: 10.1097/00003086-200001000-00028. [DOI] [PubMed] [Google Scholar]
- 41.Wilkin LD, Merrick MA, Kirby TE, Devor ST. Influence of therapeutic ultrasound on skeletal muscle regeneration following blunt contusion. Int J Sports Med. 2004;25:73–77. doi: 10.1055/s-2003-45234. [DOI] [PubMed] [Google Scholar]