Abstract
One of the most exciting new avenues of research to repair the injured spinal cord is to combine cells for implantation with scaffolds that protect the cells and release growth factors to improve their survival and promote host axonal regeneration. To realize this goal, we fabricated biodegradable, photocurable gelatin tubes and membranes for exploratory in vitro studies. Detailed methods are described for their fabrication with a high gelatin concentration. Gelatin membranes fabricated in the same way as tubes and photo-co-immobilized with rhBDNF or rhNT-3, with or without Schwann cells (SCs), showed an initial burst of neurotrophin release within 24h, with release diminishing progressively for 21 days thereafter. SCs attained their typical bipolar conformation on membranes without neurotrophins but adhesion, alignment and proliferation were improved with neurotrophins, particularly rhBDNF. When dorsal root ganglion explants were cultured on membranes containing laminin and fibronectin plus both neurotrophins, neurite outgrowth was lengthier compared to combining one neurotrophin with laminin and fibronectin. Thus, these gelatin membranes allow SC survival and effectively release growth factors and harbor extracellular matrix components to improve cell survival and neurite growth. These scaffolds, based on the combination of cross-linked gelatin technology and incorporation of neurotrophins and extracellular matrix components, are promising candidates for spinal cord repair.
Keywords: Gelatin, photo-co-immobilization, drug sustained release, tissue engineering, Schwann cells, dorsal root ganglia, spinal cord injury
1. INTRODUCTION
Gelatin was selected for scaffold fabrication because of its high viscosity and adhesive strength [1-2], non-cytotoxic, biodegradable, local drug release potential [3, 4], particularly of growth factors [5, 6], cell attachment and low inflammatory cell response without side effects in the host tissue [2-6]. A previous study led to tube fabrication with 40% photocurable styrene-derivatized gelatin (PSDG) that produced a flexible and elastomeric conduit suitable for peripheral nerve regeneration [5, 6]. The use of these conduits in a 10 mm gap rat sciatic nerve model led to recovery of locomotor and ankle dorsiflexion to almost normal levels, improved somatosensory evoked potentials and regeneration of myelinated and unmyelinated axons across the gap [6].
These improvements prompted us to test the use of these gelatin tubes in completely transected adult rat thoracic spinal cord. Finding that they did not remain intact after a few weeks in the spinal cord, we decided to test higher gelatin concentrations along with increased photo-cross-linker, carboxylated camphorquinone (CQ), water-soluble carbodiimide (WSC) [3-6], visible light irradiation time and dehydration steps to achieve improvements in mechanical properties. These modifications resulted in tubular scaffolds that displayed improved durability after transplantation into the transected spinal cord, results that will be reported in a subsequent publication. Here we report details of fabrication of scaffolds and the results of in vitro studies using two-dimensional membranes fabricated using the same conditions as those used for the improved tubes. These membranes were used to check the viability of Schwann cells (SCs) and outgrowth of neurites from dorsal root ganglia (DRG) in vitro. In this approach a photocured styrene-modified gelatin membrane was used as a platform for local and sustained release of recombinant human brain derived neurotrophic factor (rhBDNF) and recombinant human neurotrophic factor-3 (rhNT-3) and to contain extracellular matrix (ECM) (laminin and fibronectin) components. These molecules were photo-co-immobilized into the styrene-derivatized gelatin molecules by chemical and physical crosslinking technology [5, 6].
Differing therapeutic approaches using neurotrophic factors have been tested in the injured spinal cord to promote axonal regeneration: fibrin supplemented with growth factors [7], direct intrathecal injection of nerve growth factor [8], viral vectors [9], and osmotic minipumps [10]. Because of fear of viral transmission of hepatitis from fibrin (a human blood product) [11], using it for drug delivery could be a risk for spinal cord injury (SCI) or peripheral nerve injury repair. The viral vector systems may be problematic in that they may elicit immunogenic and cytotoxic responses and the transgene needs to be turned off after a certain time [12]. Extensive scarring may be induced by the chronic placement of intrathecal tubing, thereby augmenting cord tissue damage [13]. Because these varying growth factor delivery approaches have limitations for human SCI repair, we sought to devise a non-cytotoxic gelatin scaffold material in the form of a tube or membrane as an alternative delivery system for local and sustained drug release of neurotrophins. Extracellular matrix (ECM) components were added to improve enhanced green fluorescent protein-labeled SC (EGFP-SC) adhesion and axonal outgrowth from DRG explants placed on the PSDG membrane surface in vitro.
2. MATERIALS AND METHODS
2.1. Materials
The following materials were used in this study: Gelatin derived from bovine bone type I collagen (Wako Pure Chem. Ind., Ltd., Osaka, Japan); 4-vinylbenzoic acid (4VBA, TCI America, Portland, OR); water-soluble carbodiimide [WSC, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride, Dojindo Molecular Technologies, Inc. Rockville, MD], Stericup® bottle filter (0.45 μm, HV Durapore Membrane Millipore Corporation, Billerica, MA); Dulbecco’s modified Eagles Medium with low glucose (DMEM) containing gentamicin (50μg/ml, GIBCO, Carlsbad, CA); fetal bovine serum (FBS, HyClone Thermo, Fisher Scientific Inc. Waltham, MA; R&D Systems Inc. Minneapolis, MN), recombinant human brain-derived neurotrophic factor (rhBDNF, R & D Systems, Minneapolis, MN) lyophilized from 0.2 μm filtered solution in sodium citrate and NaCl, recombinant human neurotrophin-3 (rhNT-3, R&D systems, Minneapolis, MN) lyophilized from 0.2μm filtered solution in acetonitrile and trifluoroacetic acid, and Quantikine rhBDNF and rhNT-3 enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN) used according to the manufacturer’s instructions. Mouse laminin (Engelbreth-Holm-Swarm Sarcoma in 50mM Tris- HCl, pH 7.4, and 0.15 M NaCl), and human plasma fibronectin (in 20 mM Tris-HCl, pH 7.0) were purchased from Invitrogen Life Technologies (Carlsbad, CA). Carboxylated camphorquinone (CQ; 1 wt%; [(IS)- 7, 7- dimethyl-2-3, dioxobicyclo (2.2.1) heptane-1-carboxylic acid], was synthesized in our laboratory and used as a photoinitiator according to previous publications [3-6].
2.2. Methods
2.2.1. Preparation of styrene-derivatized gelatin (SDG)
SDG was synthesized as published earlier [3-6]. Gelatin (20 g) was dissolved in 1 L PBS (pH 7.4) and stirred for 30 min at 60°C and then cooled to room temperature. The source of styrene groups, 4-vinylbenzoic acid (4VBA, 11.4 g; 38.5 mM), was dissolved in 600 ml 0.1N NaOH and neutralized to pH 7.4 with 6 N HCl. Powder carbodiimide (WSC, 29.6 g; 77.2 mM) was added and mixed with 4VBA solution and stirred for 30 min on ice (0°C). This mixture was added to the gelatin solution and stirred for 24 h at room temperature. The mixture was dialyzed for 72 h (Spectra/ Por Dialysis Membrane tube, 29.0 mm, 12-14 kDa; Spectrum Laboratories, Inc., Rancho Dominguez, CA) to remove unbound or excess of WSC crosslinker using distilled water at room temperature [3-6]. The resulting solution was filtered and sterilized using a Stericup® filter and stored (4°C) until used. The sterile solution was frozen in a benchtop freezer (FreeZone Benchtop Shell Freezer, LABCONCO Corporation, Kansas, MO) for 4 h at -42°C and lyophilized by using a manifold lyophilization method [5, 6]. Then these samples were frozen dry (Vacuum; 4Mpa; FreeZone 2.5 Liter Benchtop Freeze Dry Systems, LABCONCO Corporation) for 72 h at −50°C under reduced pressure. The white foamy SDG was collected and stored in a sterile falcon tube at 4°C. The trinitrobenzene sulfonate (TNBS) method [14] was used to determine the amount of free (ie, unreacted) amines present in the product. The degree of derivatization (DD) of conjugated styrene groups was calculated based on its absorbance with a UV/Vis Spectrophotometer (268 nm, DU530, Beckman, Fullerton, CA) to measure the amount of free amines present in the starting material and then subtracting the amount of free amines present in the product from the amount of free amines present in the starting material. The degree of derivatization was ~ 80% as previously established [3-6].
2.2.2. PSDG tube fabrication
A 3 ml volume of PBS (pH 7.4) with dry SDG (40, 45 or 60 wt%) and CQ (1wt%) was prepared (Fig. 1). These components were mixed and emulsified manually by using two syringes connected to a flow device until a milky gel solution was obtained. This was further stirred thoroughly with a high-speed rotating shaker for 15 min (3x), degassing in a water bath at 40°C. A yellow gel was obtained with very high adhesive and viscosity properties. Remaining air bubbles were removed by incubation in a water bath (38°C) for 48 h in the dark. At this point, the material is ready for photopolymerization to fabricate tubes or membranes. Therefore this material is hereafter referred to as photocurable styrene-derivatized gelatin (PSDG). For tube fabrication, a mold device consisting of a glass tube and inner glass rod (Friedrich & Dimmock Inc. Millville, NJ, see Fig. 2A) was used. Both the glass tube and rod were coated with a thin layer of silicone to facilitate subsequent gelatin tube removal from the glass rod. In a sterile hood, the aqueous PSDG solution containing CQ (Fig. 1A), was injected into the gap formed between the rod and tube by using a KDS210 infusion pump (100 μl/min; KD Scientific Inc. Holliston, MA) and sealed with silicone rubber rings at both ends. The glass assembly was secured to a high-speed rotator stirrer (RW 16, Basic S1, IKA® Works, Inc, Wilmington, NC) and oriented horizontally in a metal stand (Fig. 2A). Blue visible light irradiation (BVLI energy) was applied to the mold device containing PSDG using a metal halide lamp (MME-250, MORITEX USA Inc, San Jose, CA, maximum wavelength, 420-450 nm, light intensity, 1.5 × 106 Lux). This irradiation activates CQ as a photoinitiator that produces hydrogen and oxygen free radicals (Fig. 1A). These react with the double bonds to induce inter- and intra-molecular polymerization of styrene-gelatin molecules [3-6]. The photopolymerization treatment was carried out in a dark fume hood as the mold device was rotated at 100 rpm for 15-20 minutes. Photopolymerization using visible light is a method employed in tissue engineering to enable the rapid conversion of a photoreactive monomer or macromer solution into gel or solid structures under physiological conditions for in vitro, in vivo and in situ applications (local drug delivery, scaffold fabrication or cell entrapment) with minimal invasion [5-6].
Fig. 1. Photocurable styrene-modified gelatin formulation.
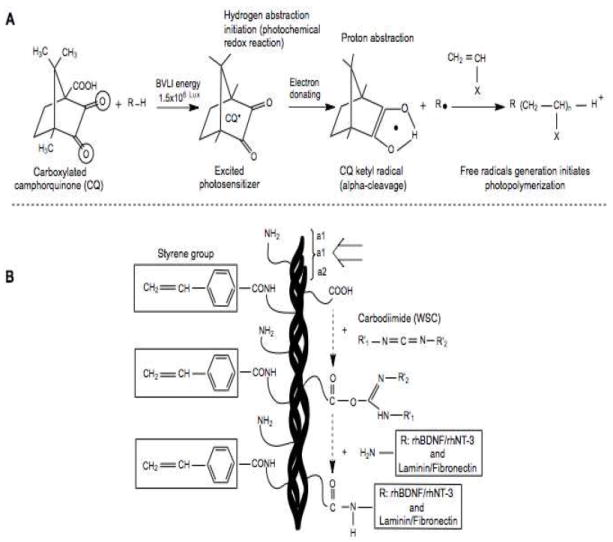
(A) Photo-crosslinking reaction underlying the polymerization of styrene groups after blue visible light irradiation (BVLI, energy) in the presence of carboxylated camphorquinone (CQ) with oxygen free radical release. (B) Water-soluble carbodiimide (WSC) was added to the gelatin molecules to initiate strong crosslinking between the amino groups of rhBDNF, rhNT-3, laminin and fibronectin with carboxyl groups of gelatin molecules. (White arrow; shorter triple helix gelatin subunits formed by two α1 and one α2 chains). =O, primary oxygen electron transfer; R–H, organic compound (4VBA) hydrogen donors; ●, free radical; R●, active free radical capable of initiating photopolymerization; X, monomer.
Fig. 2. Fabrication of gelatin tubes.
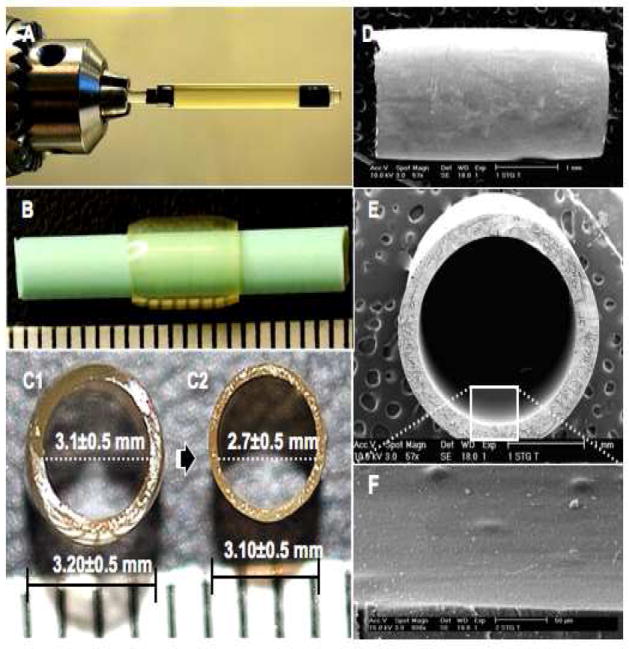
(A) A glass tube (ID; 3.2±0.5mm, OD; 5.0mm) containing a glass rod (ID; 3.1±0.5mm), both siliconized, were supported horizontally by a rotator stirrer (1000 rpm) and irradiated using blue visible light. (B) The resulting transparent gelatin tube (wall thickness, 500μm) was then reinserted (see text) on a thinner Teflon rod (OD; 2.7±0.5mm). The tube diameter before (C1) and after (C2) dehydration is shown. Longitudinal view of the tube outer surface (D), a cross-sectional view (E), and the inner surface (F) are illustrated in scanning electron micrographs.
After photopolymerization the glass assembly was removed and a swollen, semi-rigid tube was pulled from the siliconized glass rod and inserted again but on a thinner Teflon rod (OD, 2.7 mm; Fig. 2B) to reduce the inner diameter and wall thickness (Fig. 2C1-C2). The inner diameter became 2.7 mm, similar to the width of the adult Fischer rat thoracic spinal cord in order to better enclose the spinal cord stumps after complete transection in future studies. The gelatin tube was dehydrated in serial ethanol concentrations (40, 50, 70, 80%) for three min. The dry gelatin tubes were stored in sterile vials at 4°C. This method of dehydration (rather than lyophilization) produced stiff, non-collapsible, and transparent tubes (Fig. 2B, C1, C2). Transparency is advantageous for spinal cord repair because air bubbles that would interfere with axonal growth, cyst formation and the extent of spinal cord stump insertion into the tubes can be seen. The benefits of using transparent and rigid scaffolds with elastomeric mechanical properties included visualizing that the suturing needle does not damage the spinal cord stumps after entubulation and that the tube could be handled without wall collapse or fracturing during suturing [6].
2.2.3. Lateral compression measurements
Three different concentrations of PSDG were tested to select the best concentration for implanting tubes in vivo: 1) 40 (40 wt%, n = 6), 2) 45 (45 wt%, n = 6), and 3) 60 (60 wt%, n = 6. Sample tubes were introduced into a custom-made chamber filled with PBS (pH.7.4) and tested for lateral compression with a mechanical testing system (INSTRON®, Model 3342, Norwood, MA) (Fig. 3A-1). Lateral compression occurred at room temperature (22°C) using a 3.0 mm wide indenter with a compression rate of 5%/min until the tube ruptured. The length of each gelatin tube was 7-8 mm (Fig. 3A-2). The outer diameter (OD) of each tube was measured by applying a pre-load of 10 mN; the average OD was ~3.1±0.5 mm while the inner diameter (ID) was ~2.7±0.5 mm. The tube was deformed after mechanical compression was applied (Fig. 3A-3).
Fig. 3. Mechanical compression test of gelatin tubing.
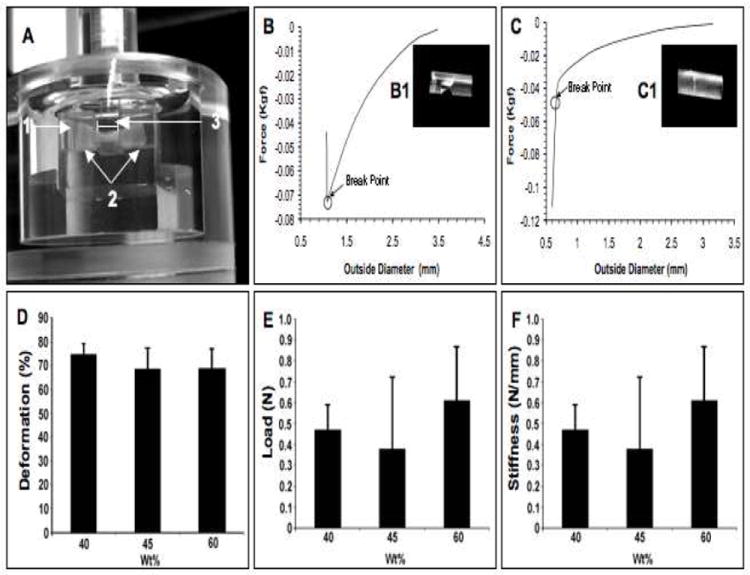
(A) A custom-made chamber was filled with PBS (1) and the gelatin tube was placed horizontally (2). Lateral compression was initiated using a 3 mm wide indenter (3) The load-deformation curve and break points of transverse failure (B), axial failure (C) and the characteristic rupture of the tube after loading (B1-C1) are shown. The graphs present data for mechanical deformation (D), ultimate load (E) and stiffness (F).
2.2.4. Preparation of PSDG membrane with bioactive molecules
The chemical formulation of the PSDG solution with or without neurotrophins and ECM proteins is shown in Fig. 1. First, this procedure was conducted in an aseptic environment in a dark laminar flow hood to prevent contamination or initial crosslinking induced by visible light; the CQ could respond to the light in the fume hood. CQ (1wt%; 18 mg) was dissolved in 1 ml PBS (pH, 7.4) and thoroughly stirred with a high-speed rotating shaker for 15 min (3x) in the dark at 4°C. Subsequently, to this PBS containing the photoinitiator, 30 μg/ml of rhNT-3 and rhBDNF (10μg/ml final concentration) in combination or separately were added and stirred for 5 min at 4°C. Fibronectin and laminin (I mg of each), dissolved in cold PBS (0.33μg/ml final concentration) were added in combination or separately and mixed slowly in the polypropylene tube containing the PBS, CQ, and neurotrophins by pippetting slowly (on ice) to avoid clumping or ECM precipitation. This mixture was kept at 4°C for an additional 2h in the dark to ensure complete mixing. Next a 2 ml volume of PBS containing 1800 mg of dry styrene derivatized gelatin (SDG; 60 wt%) was added at room temperature. This solution was emulsified manually by using two syringes connected to a key flow device until a milky gel solution was obtained. This was further stirred thoroughly with a high-speed rotating shaker for 15 min at room temperature, degassing in a water bath at 35°C for 30 min. A semitransparent yellow gel was obtained with very high adhesive and viscous properties, ready to use for photopolymerization under visible irradiation (3-6).
2.2.5. PSDG membrane fabrication
Membrane discs (1.0 mm thick and 2.0 cm in diameter) were created with the construction of a special device. 1) Holes (2.0 cm wide) were punched in a thin disc of rubber (thickness: 1.0 mm, diameter: 20.0 cm) (Fig. 4A). 2) This was glued to a Teflon sheet with screw holes (Fig 4B). 3) Two ml PSDG, containing in some cases neurotrophins and/or ECM, were added to each hole (Fig. 4C). 4) The rubber disc/Teflon assembly was covered with a transparent polyvinyl plastic sheet and secured with 4 small screws (Fig. 4D). 5) This assembly was then photoirradiated using blue visible light as described in section 2.2.2 for tube fabrication (Fig. 4E) for 20 min at room temperature in a sterile fume hood. The membranes with or without neurotrophins and ECM were immediately soaked in PBS at 40°C for 20 min to remove the non-reacted substances, sterilized by rinsing with 70% ethanol (3x) and with PBS (3x), dried in a sterile fume hood over night under gamma irradiation, and sealed in individual sterile plastic dishes at -10°C until used. After 24h the sterile membranes were equilibrated in culture with DMEM containing 10% FBS (D10) and antibiotics and placed in a humidified incubator at 37°C, with 5% CO2 in air. After 2h, the medium was removed and replaced with new medium to be ready for seeding with SCs or DRG explants.
Fig. 4. Diagram of gelatin membrane fabrication.
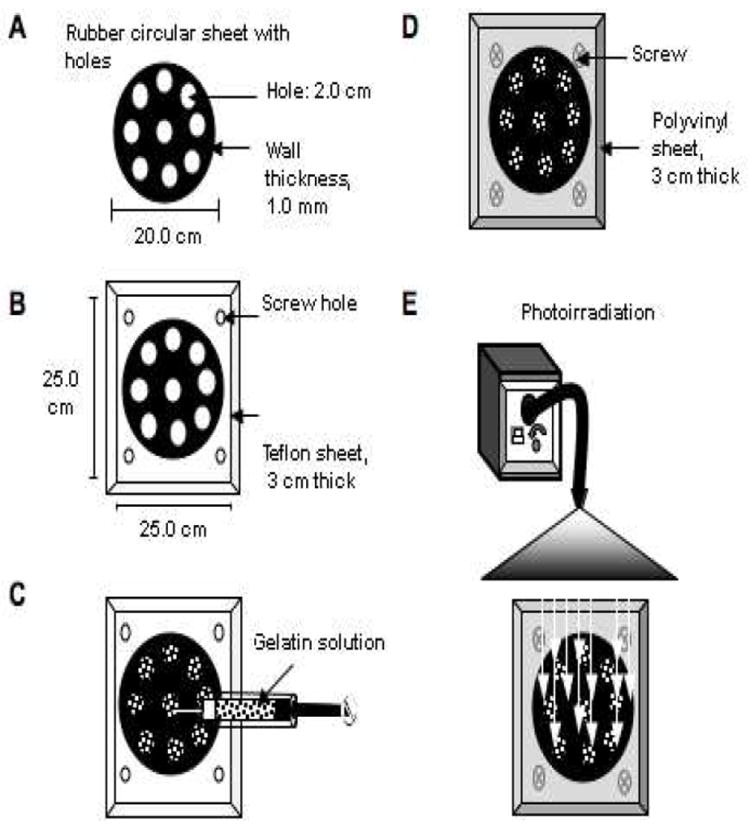
A thin circular rubber sheet with holes (A) was glued to a rectangular Teflon sheet (B). The gelatin solution, emulsified with or without neurotrophins and with or without ECM, was injected manually into the holes created in the rubber sheet (C), then covered with a transparent polyvinyl plastic sheet and secured with metal screws (D). The gelatin membranes were next photoirradiated using a metal halide lamp (E).
2.2.6. Culture of Schwann cells
SCs were harvested from sciatic nerves of adult female Fischer rats (Harlan Co., Indianapolis, IN) [15, 16]. Briefly, nerves were cut into small pieces and placed in culture dishes containing DMEM/heat inactivated (HI) FBS (D10) without mitogens. After two weeks, the pieces were transferred to new dishes where they were enzymatically dissociated and then maintained in DMEM/10% HIFBS supplemented with three mitogens: bovine pituitary extract (2 mg/ml; Invitrogen Corporation), forskolin (0.8 μg/ml), and heregulin (2.5 nM; Genentech, San Francisco, CA) (D10-3M). The SCs were detached with trypsin/EDTA at passage three, collected and concentrated to 1×106 cells/ml in D10-3M. 98% purified SCs were obtained with minimal fibroblast contamination (2-5%) based on p75 immunostaining.
2.2.7. Lentiviral vector-enhanced green fluorescent protein (LV-EGFP) vector transduction of SCs
LV-EGFP was generated in the Miami Project Viral Vector Core by methods previously described [17-18]. Briefly, the gene encoding EGFP was subcloned into an LV plasmid (p156RRLsinPPThCMVMCSpre) generously provided by Dr. L. Naldini (University of Torino). This plasmid contained the cytomegalovirus promoter and the Woodchuck post-transcriptional regulatory element. The purified plasmid (1μg/μl) was dissolved in 10mM Tris-HCl (pH 8.0) and 1 mM EDTA. The transfection and viral harvesting process took place over a 5-day period. For virus production, 293T cells cultured in T75 culture flasks were passaged to four flasks and grown to maximum confluency. These flasks were then passaged to dishes containing Iscove’s modified Dulbecco’s medium (GIBCO) supplemented with 10% HIFBS and gentamicin (basal Iscove’s medium, IMDM/10%) (day 1). Calcium phosphate-mediated transfection was performed by introducing the VSV-G, p 874 packaging, and LV plasmid (day 2). The medium was replaced with IMDM/2% (day 3), the first harvest of virus was performed (day 4), and IMDM/2% was replaced for a second harvest (day 5). After filtering, virus was concentrated by ultracentrifugation and resuspended in PBS to titer the vectors for transducing units on 293T cells or using ELISA (Promega, Madison, WI) for quantifying p24 core protein concentrations [19].
Virus concentrated in PBS was used to infect SCs according to an earlier published protocol [18]. Virus in DMEM/10% with three mitogens was added to SC cultures. A multiplicity of infection (MOI) of 10-20 was used in the cultures to ensure high transduction efficiency (Fig. 5).
Fig. 5. Transduced SCs express EGFP.
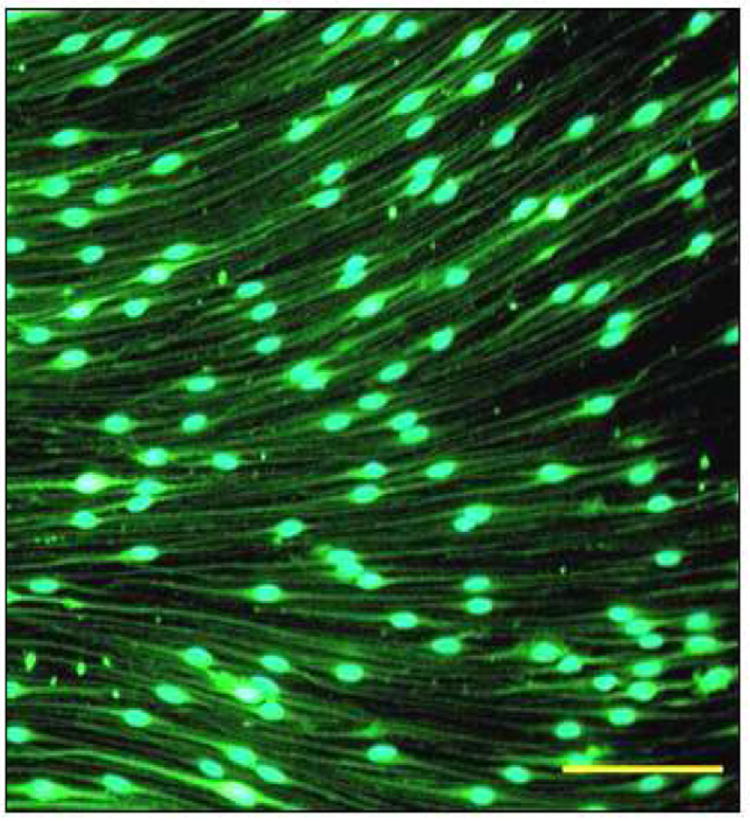
SCs were transduced with EGFP to be able to follow them in culture. Details are in Materials and Methods. Scale bar, 100μm.
2.2.8. Culture of EGFP-SCs on gelatin membranes
EGFP-SCs were seeded in the center of gelatin membranes at a density of 50,000 cells/mm2 in 0.5 ml D10-3M culture medium in 6-well plates and incubated at 37°C in 95% humidified air and 5% CO2 for 30 min to allow the cells to adhere before adding the remaining medium (3 ml D10-3M). The purity of the SCs harvested at each period of culture time varied between 96-98%. Mitogens were added for one week and withdrawn thereafter and antibiotic (gentamicin, 25 μg/μl) was present for the 21 days. The groups were: 1) EGFP-SCs on membranes without neurotrophins, 2) EGFP-SCs on membranes with rhBDNF or 3) rhNT-3. EGFP-SCs was examined for alignment, adhesion, migration and proliferation in vitro. The cultures were analyzed at 1, 3, 6, 9, 12, 15, 18, and 21 days. EGFP-SCs were visualized in living cultures (484 nm wave length) in an inverted Olympus 1×70 fluorescence microscope (Olympus America Inc, Melville, NY) equipped with a Cooke SensiCam ER camera (COOKE Corporation, Auburn Hills, MI). Representative fluorescence photographs were taken at 3, 7, and 21 days.
Proliferation of the cells was determined as follows. The use of EGFP-SCs permitted tracking and quantification. To eliminate dead or unattached EGFP-SCs, the culture dishes were rinsed with PBS (3x), leaving just the adherent cells. The rinsed membranes were transferred to new plastic dishes containing trypsin (0.05%)/EDTA (0.02%) and incubated for 5 min to allow the cells to detach completely from the membranes for counting. Cells were collected in micro centrifuge tubes, centrifuged and resuspended in DMEM. After mechanical dissociation through glass pipettes, homogenous cell suspensions were centrifuged at 235 × g for 5 min at 21°C. Trypan blue (0.4%, w/v) in PBS was used to identify dead cells; 500μl of trypan blue were transferred to a test tube (15ml) and 300μl of PBS were added and mixed thoroughly. After this, 200μl of the cell suspension were added, mixed and let stand for 8 minutes (dilution factor = 5). Approximately 10μl of the trypan blue/cell mixture was loaded into a Neubauer hemocytometer chamber. For live EGFP-SC quantification analysis, GFP-SC+ labeled cells were counted and the SC concentration was determined following the hemocytometer instructions. The criteria for GFP-SC vitality was expression of clear green fluorescent color with oval-round cell bodies and elongated cell processes. The SC purity was indicated by reference to the total number of cells; SC nuclei were counterstained with Hoechst 33342, which stains all nuclei (100 mM; Invitrogen, Molecular Preobes/360 nm; Eugene, OR). This method allowed the comparison of the numbers of EGFP-SC-positive cells with Hoechst-labeled cells. The counting was repeated (3x) to ensure reproducibility (+/- 15%). Calculations were performed as follows: Cell per ml = (the average count per square) × (dilution factor) × (10) [10 squares]; total cells = (cells per ml) × (the original volume of fluid from which the cell sample was removed).
2.2.9. Neurotrophin release in vitro
Neurotrophin release from membranes with or without SCs was determined in D-10 medium up to 21 days at 37°C in 95% humidified air and 5% CO2. Membranes photo-co-immobilized with or without rhBDNF and rhNT-3 were rinsed (3x) with PBS and then incubated in D-10 for 2 hr and rinsed again. They were seeded or not with EGFP-SCs (50,000 cells/ml in D10-3M). The medium was collected at 1, 3, 6, 9, 12, 15, 18 and 21 days and centrifuged at 5000 rpm for 5 min at room temperature. The supernatant was replaced with the same volume of fresh D-10 (3 ml). The supernatant was fast frozen with liquid nitrogen and kept at −80 °C. The groups were: 1) membrane only (control), membrane immobilized with 2) rhBDNF or 3) rhNT-3, and EGFP-SCs on membranes containing 4) EGFP-SCs/rhBDNF or 5) EGFP-SCs/rhNT-3. The amounts of neurotrophin released into the supernatant at each time point were measured by the ELISA method [29].
2.2.10. Scanning electron microscopy (SEM)
Membranes seeded with EGFP-SCs were fixed in 1% glutaraldehyde in 0.1M phosphate buffer and sucrose (4%; pH 7.2) overnight at 4°C and rinsed with the same buffer (4x). They were postfixed in 1% osmium tetroxide (Electron Microscopy Sciences, Hatfield, Pa) in 0.1M phosphate buffer for 1 h in a fume hood, and washed with buffer (5x). They were dehydrated in a graded series of ethanol, dried to the critical point, and sputter-coated with gold (Sputter Coater 108auto, Cressington, Watford, UK). Tubes were fixed in 4% glutaraldehyde in 0.2M PBS (pH 7.2) overnight at 4°C and then in osmium tetroxide (1%) in PBS for 1 h, and washed with the same buffer (5x). Subsequently, they were dehydrated as above, dried to the critical point, sputter coated with gold and analyzed by SEM. SEM images were obtained in an Environmental Scanning Electron Microscope (XL30 ESEM-FEG, FEI Company, Hillsborg, OR) operating in high vacuum mode with a beam intensity of 20 kV. A standard gray-scale threshold method was applied to SEM images taken at 75-1000X [5, 6].
To determine the extent of EGFP-SC migration on the membranes at 21 days, 10 parallel and longitudinal oriented arrays of SCs were selected from each group using representative SEM photographs and their lengths were measured using image analysis software (ImageJ 1.46g, NIH). The starting point for this measurement was at the edge of the original drop of cells on the membrane with the end point being the distal end of the arrays. The measurements was conducted as follows. First, three representative SEM images of EGFP-SCs from each group that adhered, to the membranes at 21 days were selected and converted to an 8-bit grayscale by clicking the converting grayscale tool bar in the ImageJ window. Second, we created a ‘threshold’ binary grayscale on the appropriate section in the ImageJ window to complete the conversion. Third, the setting measurement was done by drawing a line perfectly between two points on the bar scale of a known distance on the SEM photograph. Once, in the set scale window of ImageJ, the length of the line was set in microns according to the SEM bar scale measurement and the pixels of each photograph, the mean distance values (μm) of EGFP-SCs including their bipolar morphology were measured. The mean values were calculated on the SEM photographs by using the freehand lines of the ImageJ on the selected longitudinal strands of cells from the center to the periphery, because the cells was seeded initially in the center. In this way, we determined the migrational extension from the starting point over the membranes. The respective values were obtained and exported to a data sheet (Microsoft Excel, 2004 for Mac, Version 11.5, Bloomsbury Publishing Plc. USA). Statistical analysis was based on at least three independent experiments, using one-way ANOVA single factor. Data (mean ± SD) were considered to be statistically significant when the p-value was p < 0.01 (histogram not shown).
2.2.11. Culture of dorsal root ganglia (DRG) on membranes
Briefly, Sprague-Dawley rat (Harlan Co.) embryos at 15 days of gestation were transferred to cold Leibovitz’s (L-15) medium (Invitrogen, Grand Island, NY), and the spinal cords and then meningeal membranes were removed [16]. The spinal cords with ganglia attached were placed in 35 mm dishes with L-15/10% HIFBS. Dorsal root ganglia (DRG) were pulled off with forceps and transferred to fresh L-15 medium before placing on gelatin membranes. Membranes photo-co-immobilized with neurotrophin and laminin and/or fibronectin (1mg/ml each) were utilized to study neurite outgrowth up to two weeks in vitro. To fabricate these membranes with ECM molecules, laminin (L, mouse,) and fibronectin (F, human serum plasma,) were diluted in cold PBS (pH 7.4) for 30 min without shaking and combined with CQ (1 wt%) for photo-co-immobilization by light intensity irradiation. One DRG explant per gelatin membrane was cultured in 6-well plates containing 2 ml neurobasal medium (NBL, GIBCO) supplemented with B27 (2 ml/500 ml, GIBCO), L-glutamine (200 mM/0.5 ml/ml) and gentamicin (25 μg/ml) and maintained at 36°C in 93.5% O2 /6.5% CO2 in a humidified atmosphere. The medium was changed once a week. The groups were: 1) DRG; 2) DRG+rhNT-3+L; 3) DRG+rhBDNF+F and 4) DRG+rhNT-3+rhBDNF+L/F.
The Sprague-Dawley pregnant rats (for embryonic day 15 (E15) DRG and adult Fischer rats (for harvest SCs) were housed according to NIH and the Guide for the Care and Use of Animals. The Institutional Animal Care and Use Committee of the University of Miami approved all animal procedures. The rats were sacrificed after overdoses of ketamine (60 mg/kg) and xylazine (25 mg/kg) given by intraperitoneal injection.
2.2.12. Axonal length measurements
DRG cultures on membranes were preserved after 1, 3, 6, 9, 12, 15 days for 20 min using 2.5% paraformaldehyde in PBS at 4°C. Cultures were blocked with 5% normal goat serum (NGS) and 0.1% Triton X-100 in PBS prior to incubation for 1 hour at room temperature with monoclonal mouse anti-βIII tubulin antibody (1:200, Sigma Chemical Co.) and rabbit anti-neurofilament antibody (NF-H, NF-M, NF-L; 1:200, EnCor Biotechnology Inc.). Secondary antibody was conjugated to Alexa 594 (red) or 680 (blue) goat anti-mouse IgG (Invitrogen, Life Technologies, Molecular Probes). The cultures were then rinsed with 1-2% NGS/PBS for 3 × 5 min, and incubated with the appropriate secondary antibodies (Alex 594, 1:200) or Alexa 680 (1:300) in 1-2% NGS/PBS respectively, for 30-45 min at room temperature. Following a final wash in PBS, Citifluor was added to the samples, coverslipped and viewed in an inverted fluorescence microscope (Olympus 1 × 70) equipped with a Cooke SensiCam ER camera. Photoshop software was used to assemble the images because only one plane of focus could be photographed at a time; some axons were not in focus over their entire length. The mean maximal outgrowth lengths (μm) from the edge of the DRG explants to the tips of the six longest axons on each membrane were determined using a micrometer in one of the eyepieces; thus, with three membranes per group, a total of 18 axons per group were measured and plotted in histograms as means ± SD. Data were analyzed to determine the significance of differences in axonal outgrowth from DRG cultured on the membranes (error bars, SD, ** p < 0.01, * p < 0.05). Average values were calculated by using descriptive statistics analysis of ANOVA single factor (Microsoft Excel Version 11.5.).
2.2.13. Statistical analysis
All results were evaluated by using Microsoft Excel. Experiments were repeated at least 6 X unless otherwise stated. Differences between groups were evaluated using one way ANOVA and considered significant at **p < 0.01 and *p < 0.05. All data are displayed as means ± standard deviations unless otherwise noted. EGFP-SC numbers were determined in six samples in three independent experiments under fluorescence microscopy at 250X using a 400×400 μm frame.
3. Results
3.1. Fabrication of gelatin tubes and membranes
The gelatin tubes fabricated in the same way as those utilized in a peripheral nerve regeneration model [5, 6] did not prove strong enough when transplanted into a complete transection rat SCI model (unpublished studies). Perhaps due to mechanical forces generated by movements of the animal after transplantation, small pieces of the conduit were found in the tube lumen with the result that the interior space and numbers of SCs were diminished although the tube had not collapsed. The conduit had been fabricated with 40wt% gelatin concentration containing 4VBA and WSC as chemical crosslinker agents (20.0 styrene groups per gelatin molecule) without immobilization of growth factors or ECM. We attempted to test the mechanical response of the conduit, the survival of SCs in Matrigel in the lumen of the conduit and axonal regeneration through the conduit up to two months post implantation. Axonal regeneration crossing the conduit was low with only some SC survival.
For the present study, three different concentrations of PSDG solution (40, 45 and 60 wt%), therefore, were chosen to form new tubes. The composition of PSDG and the presence of CQ as a physical crosslinker responding to visible light irradiation and the incorporation of WSC as a chemical crosslinker into the gelatin molecules are illustrated in Fig. 1. Gelatin tubes were fabricated by injection of SDG/CQ solution into a glass tube mold assembly (Fig. 2A) as described in Materials and Methods. The tubes, 3.1±0.5 mm in outer diameter and 2.7±0.5 mm in inner diameter with a wall thickness of 200 μm (Fig. 2C2), were transparent (Fig. 2B). Longitudinal SEM views of the outer (Fig. 2D) and inner (Fig. 2E) surfaces of the PSDG tube revealed a smooth and compact structure (Fig. 2F) due to the high degree of derivatization, high PSDG concentration and ethanol dehydration.
Gelatin membranes were prepared as detailed in Materials and Methods and Fig.4. They were 1.0 mm thick and 2.0 cm in diameter and also transparent (Fig. 8A). rhBDNF, rhNT-3, laminin and fibronectin or their combination were sometimes incorporated into the membranes (Fig. 1B).
Fig. 8. Behavior of DRG explants on membranes with neurotrophins and ECM.
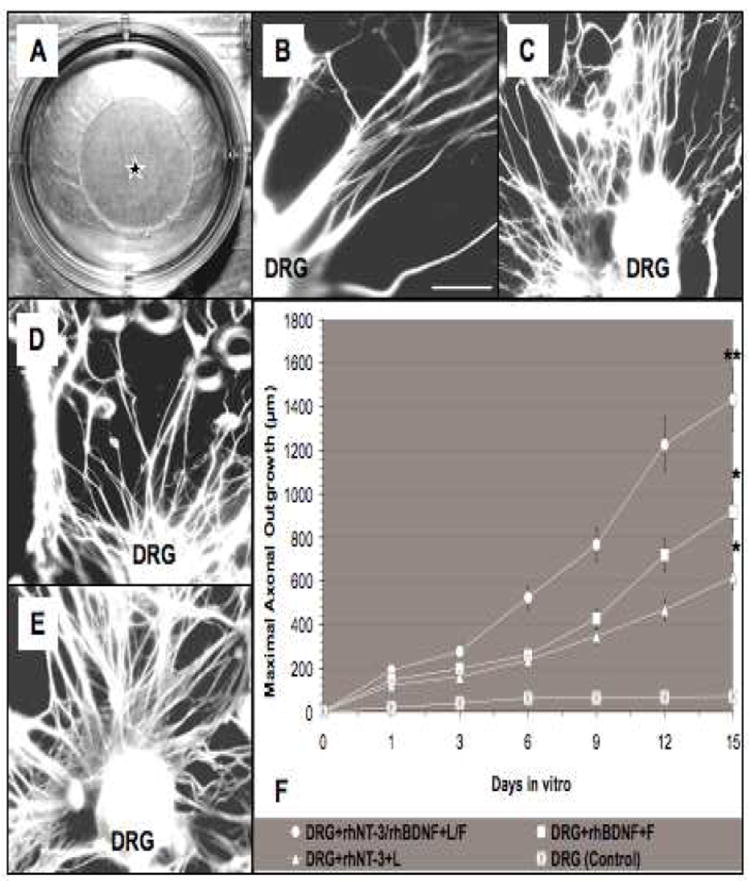
(A) Digital micrograph of a transparent gelatin membrane disc (star) in a plastic dish. (B) Axonal outgrowth from a DRG explant seeded on a membrane without ECM or neurotrophins (control). Panels B-E, images following BIII tubulin and neurofilament immunostaining illustrate the axonal outgrowth distribution on membranes containing rhNT-3+laminin (rhNT-3+ L) (C), rhBDNF+fibronectin (rhBDNF+F) (D), and a combination of L+F and neurotrophins (rhNT-3/rhBDNF+L/F) (E), including the mean length values of the six longest outgrowth axons measured (μm) over the top surface of PSDG membrane up to 15 days (F). Significant statistical differences were accepted at **p < 0.01 and *p < 0.05. Error bars: means ± SD. Scale bar for B-E, 100μm.
3.2. Mechanical properties of gelatin tubes
Mechanical behavior of the scaffold (i.e., tubes) was determined in the wet condition. The aim of this measurement was to determine the rigidity of the scaffold as a function of wall thickness and outer diameter (OD) of the tubes by applying a lateral load (stress) to compress the tube (Fig. 3A). This test gives an insight into what might happen when the tube is implanted into the spinal cord after a complete transection, where mechanical forces generated from the animal (spine, spinal canal or muscles) could disrupt or collapse the tube. The gelatin tube was placed in a PBS-filled custom-made chamber (Fig. 3A). Lateral compression of the tubes produced two distinct types of mechanical failure. Cracks were observed in the transverse direction (Fig. 3B1) on the dorsal wall of the tube while maintaining the integrity of the ventral wall (transverse failure) and in the axial direction (Fig. 3C1) after loading (axial failure). The deformation before rupture was calculated as follows: Deformation (%) = 100x (initial OD-OD at failure)/initial OD. Examination of maximum deformation (Fig. 3D) before rupture did not reveal statistical differences among the three groups of samples tested. Failure (rupture) occurred when deformation approached 70%. The ultimate load (maximum load before failure) is shown in Fig. 3E. Rupture occurred when the load approached ~0.5 N (Fig. 3C). The stiffness of gelatin tubes was evaluated (Fig. 3F). Stiffness is defined as the ratio of the ultimate load to the deformation. No statistical difference was found among the groups. Despite this, the remaining work was performed using the 60wt% PSDG as an initial trial of tubes with this concentration showed that they were durable for weeks after implantation into the injured spinal cord (unpublished data).
3.3. EGFP-SC behavior on gelatin membranes
The membranes did not show any visible signs of degradation up to 21 days in vitro, the longest period examined. To test EGFP-SC survival, adhesion, and proliferation on gelatin membranes, 50,000 purified cells/mm2 were seeded on the center area and observed by fluorescence microscopy and SEM (Fig. 6) at different incubation times. In the EGFP-SC group, SCs adhered to the membrane for 1-3 days in culture, but to a far lesser extent in mono/bi-layers with random orientation (Fig. 6A) than with neurotrophins (Fig. 6B, C). Some EGFP-SCs remained attached for the 21-day period but by 7 days they were only present near the edge of the membrane. The EGFP-SC/rhNT-3 group (Fig. 6B) displayed improved SC adhesion and alignment from 3 to 21 days in culture, in comparison to the EGFP-SC group, with a greater proportion of the membrane covered by a network of SCs (Fig. 6B). There was a much higher number of SCs in the EGFP-SC/rhNT-3 group (1,283,667 ± 52,322 cells/mm2) than in EGFP-SC cultures (20,633 ± 153 cells/ mm2) (Fig. 6D). In the EGFP-SC/rhNT-3 group SCs attached and grew on the membrane surface forming a dense three-dimensional network (Fig. 6B, long black arrow). The inner layer of SCs adhered strongly to the membrane surface, forming longitudinal bands; in the second and third layers, SCs adopted a random orientation (Fig. 6B/SEM) and displayed bipolar or tri-polar shapes. Some clusters of cells were observed in the membrane center (Fig. 6B, white squares). The EGFP-SC/rhBDNF group (Fig. 6C) contained more longitudinally arranged cells (1,967,233 ± 41,258 cells/mm2) than the rhNT-3 group (1,283,667 ± 52,322 cells/mm2) at 7-21 days (Fig. D). Between the second and third weeks, SCs became more oriented and adhered strongly to the membrane surface with long bipolar cytoplasmic processes (Fig. 6C/SEM/GFP). They were arranged from the center to the membrane periphery. These results indicate that the membranes containing rhBDNF provided the best support for adhesion, alignment and proliferation of EGFP-SCs in vitro. The extents of SC migration [34] in the EGFP-SC/rhBDNF (2856 ± 110.61 μm) and EGFP-SC/rhNT-3 (1200 ± 140 μm) groups were significantly greater than the extent in the control group (membrane/EGFP-SC; 325 ± 31.3 μm) (histogram not shown).
Fig. 6. Scanning electron micrographs of EGFP-SCs on gelatin membranes 21 days in vitro.
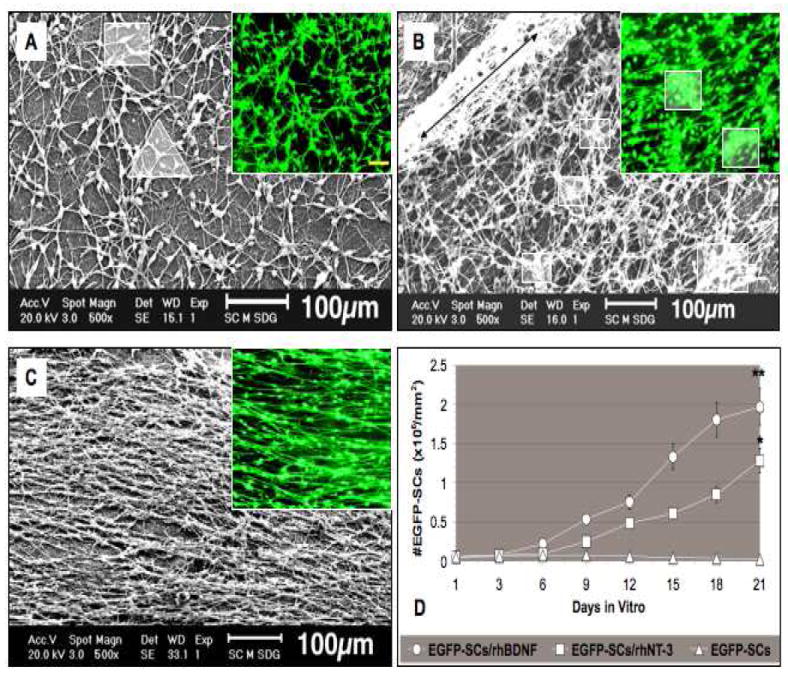
(A) EGFP-SCs seeded on membranes without neurotrophins were typically bipolar (square in A) but were not aligned; tri-polar cells (triangle in A) were sometimes observed in the SEM. Green flourescent cells are shown in the smaller panels. (B) rhNT-3 membranes contain high numbers of EGFP-SCs strongly attached to their surfaces, forming a compact network sometimes piled up in layers (long black double arrowhead) with some clumping of cells (white squares in smaller panels). (C) Membranes containing rhBDNF and EGFP-SCs supported a highly aligned cell array. Mean numbers of EGFP-SCs in the three groups are presented in panel D. Error bars represent means ± SD (n = 6 per group). Significant statistical differences were accepted at *p < 0.05 and **p < 0.01 compared to control cultures. Scale bar; 100μm in fluorescence photographs.
3.4. Sustained release of neurotrophins from membranes
The rhBDNF and rhNT-3 release profiles from the gelatin membranes, with or without EGFP-SCs, were determined by an enzyme-linked immunoabsorbent assay (ELISA) [29], at 1, 3, 6, 9, 12, 15, 18 and 21 days in vitro (Fig. 7). A first burst release of rhBDNF, seen within the first 24h, totaled 110.15 pg/ml (mean of triplicate determinations) and 86.5 pg/ml from rhNT-3 membranes. Significant differences were found in the release profiles of growth factors at 24 hr with EGFP-SCs/ rhBDNF (141.14 pg/ml) and EGFP-SCs/rhNT-3 (120.27 pg/ml) (Fig. 7), when compared to the groups with neurotrophins but without SCs. However, even after 21 days neurotrophins remained within the membrane. These results demonstrate the feasibility of fabricating PSDG scaffolds to release factors such as neurotrophins for local and sustained release.
Fig. 7. Neurotrophin release from membranes with or without EGFP-SCs over 21 days in vitro.
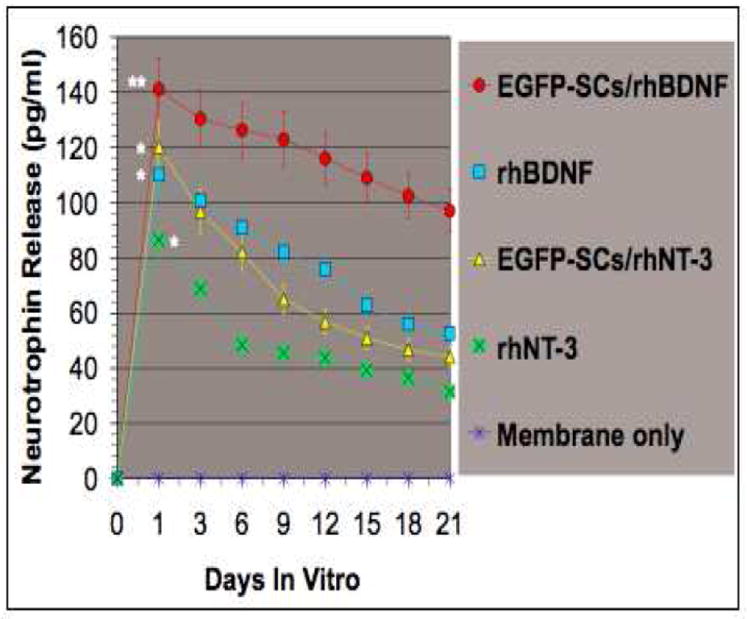
The greatest release of neurotrophin was observed when membranes contained rhBDNF and were seeded with cells. Statistical significance was observed at **p < 0.01 and *p < 0.05. Error bars represent mean ± SD (n = 6).
3.5. Axonal outgrowth on membranes
The transparent gelatin membrane inserted into a culture dish (black star) is illustrated in Fig. 8A. DRG explants placed on a membrane without incorporation of growth factors and ECM exhibited shorter and more limited axonal outgrowth (Fig. 8B) compared to membranes in which ECM and growth factors had been photo-immobilized. The presence of rhNT-3 plus laminin (Fig. 8C) and rhBDNF plus fibronectin (Fig. 8D) led to increased neurite growth. However, the combination of both laminin and fibronectin with neurotrophins in the membranes (DRG+rhNT-3/rhBDNF+L/F) resulted in a significant increase and robust axonal outgrowth (Fig. 8E) compared with the neurotrophins or ECM alone or the control group. The mean length of the six longest axons measured over the surface of PSDG membrane with the photo-co-immobilization of both neurotrophins and ECM (DRG+rhNt-3/rhBDNF+L/F; ** p < 0.01) resulted in a significant increase in mean maximal axonal outgrowth length compared with DRG+rhBDNF+F (*p < 0.05), DRG+rhNT-3+L (* p < 0.05) or control groups (Fig. 8F). Therefore, the combination of ECM and neurotrophins by chemical and physical crosslinking into the network gelatin molecules promoted the greatest length of axonal outgrowth (** p < 0.01) over the surface of PSDG membrane fabricated as an artificial substrate in vitro.
4. Discussion
The pathophysiology of SCI is complex and multiphasic including an inflammatory cascade response, scar and cavity formation with the presence of a dense meshwork of hypertrophied astrocytic processes (reactive gliosis) and their associated matrices [20]. Because the transplantation of SCs, the myelinating glial cells that promote axonal regeneration in the PNS after injury, can support CNS axonal regeneration, either as peripheral nerve transplants [21, 22] or purified cell populations [23] it is a promising therapeutic strategy for SCI repair. However, to date transplanted SCs may survive poorly, undergoing both necrosis and apoptosis, with an approximate 75% reduction in SC number within the first week [24, 25]. A probable cause, at least in part, is “anoikis” that results when cells are removed from their contact with ECM, thus disrupting integrin binding [26, 27]. Thus, to repair the injured cord with SCs, their transplantation with an appropriate biodegradable scaffold with ECM may improve survival [28-30]. The complexity of the tissue response to SCI also calls for a combination strategy to overcome many and varied deleterious effects [31]. Combining SCs with additional strategies leads to better outcomes [28-34].
Scaffolds in combination with other strategies found to be salutary could lead to improved efficacy in spinal cord repair [33-36]. The biodegradable scaffold could contain ECM components similar to those in the basal lamina that surrounds SCs associated with regenerating peripheral axons [6]. Incorporating neuroprotective agents and neurotrophic growth factors into scaffolds would not only promote cell survival but also foster axonal regeneration and guidance [35-37]. Up to now, neuroprotection agents and growth factors have been applied using varied delivery systems as mentioned in the Introduction. Systemic injection also has been attempted [36, 39] but at present peptide growth factors are unsatisfactory as systemically applied therapeutic agents. For example, both CNTF and BDNF have been found to be ineffective in human clinical trials in amyotrophic lateral sclerosis therapy for a variety of reasons, such as lack of efficacy, toxicity, immunologic complications, limited absorption, rapid biodegradation and their half lives with limited diffusion due to the blood/spinal cord barrier and rapid renal clearance [39]. High doses of BDNF and NT-3 are required to cross the blood/spinal cord barrier and often only a short period of delivery is possible due to drug clearance and absorption [39].
An ideal scaffold needs to be strong with mechanical properties that resist the intrinsic/extrinsic forces generated from the spine and surrounding muscles without collapsing or breaking in vivo. It should be non-toxic, non-antigenic and tissue-adhesive. If biodegradable, the released products should be non-toxic as well. The scaffold should persist for an appropriate time, protecting the interior from connective tissue invasion. It should be of a material that allows slow and continuous release of incorporated substances such as neurotrophic factors and ECM components [5, 6]. The fabrication of compatible scaffolds should be possible without heating or the use of toxic solvents that would degrade or denature the incorporated biological substances. Techniques used for generating scaffolds should be geared towards creating a small enough size for introduction into peripheral nerves or the CNS [40].
Scaffolds built of gelatin as described here fulfill many of these requirements. They are non-cytotoxic and non-antigenic [5, 6]. The biodegradable rate can be adjusted with controlled cross-linking and the released products are not toxic [3-6]. This contrasts with polyglycolic and polylactic acids that upon breakdown produce glycolic and lactic acids leading to a lowering of the local pH that could compromise tissue repair [41]. The complex scaffolds described here were formed by physical and chemical cross-linking with non-toxic agents, water-soluble CQ and WSC that couple carboxyl to amine groups resulting in a stable bond in the styrene-modified gelatin molecules [5-6]. The immobilization strategy for PSDG with highly tissue-adhesive properties enables SC survival and axonal outgrowth. A significant advantage of using styrene-modified gelatin and photo-co-immobilization compared to other immobilization techniques is the superior temporal and spatial control of gelatin that proceeds only when blue visible light is applied [3-6]. Also, this approach using a chemical and physical crosslinking method allows the incorporation of proteins such as growth factors and ECM to be reversibly bound to the derivatized gelatin, by using visible light irradiation without protein degradation or cell damage [5, 6].
First, the growth factors and photo-crosslinking agents were dissolved separately in PBS (pH.7.4) to ensure that the growth factors maintained their biological activity when they were added to the gelatin solution. Thus, taking the advantage of WSC crosslinker chemistry was added to gelatin molecular structure led to a covalent and stable binding between the free amine groups of rhBDNF and rhNT-3 and the carboxyl groups incorporated on the styrene-modified gelatin [42-44]. Second, the subsequent addition of the photocrosslinker CQ, as a radical initiator added to the derivatized gelatin solution, was subjected to irradiation to produce a transparent and stiff scaffold (tube and membrane) without degrading the incorporated growth factors or ECM (membrane only). (The photocrosslinker CQ has been used in the dental field to prepare composites for humans without toxic side effects [45]). For all these reasons, these tubes will be translatable to human SCI repair [46]. In addition, it will be easier to fabricate such tubes of larger diameter than the smaller one required for the rat spinal cord (8.5-11.5 mm vs. 2.7-3.0 mm for the rat). Additional agents can be provided without additional interventions due to the release from the tubes.
This material provided a suitable substrate for adhesion, migration, and proliferation of the EGFP-SCs. Neurotrophin was detected in the culture medium by ELISA upon release from the membranes in vitro up to 21 days. Higher levels of rhBDNF were found when SCs were present, suggesting that SCs themselves released rhBDNF as well as the membranes [45, 46] and providing, at least in part, an explanation for improved cell survival and proliferation.
PSDG has been used for a wide range of biomedical tissue engineering applications including drug release [3-6], peripheral nerve regeneration [5, 6], hemostatic glue [49], cartilage regeneration [50], arterial grafts [51] and hydrogel-coated metallic stents [52]. The results using these PSDG tubes in peripheral nerve injury showing robust axonal regeneration and functional improvements [6] led us to test these scaffolds in completely transected adult rat thoracic spinal cord. Finding that they did not remain intact after weeks in the spinal cord (data not published), we tested new formulations one of which is detailed here. Preliminary in vivo data (to be published elsewhere) suggests to us that the 60% PSDG tubes do not collapse and are maintained for weeks after implantation into a complete transection SCI model. To our knowledge, the use of this unique material has not yet been reported for SCI repair.
The SCs in primary cultures [15, 16] were labeled with LV-EGFP [17-19] to permit tracking the fluorescing cells without staining while in vitro for 21 days and were examined by SEM. The absence of growth factor release caused low numbers of cells to spread in random directions (Fig. 6A) with poor adhesion, rounding up of the cells, and loss of longitudinal strands of typical bipolar cells. Fast release was observed from both growth factor groups after 24 h in culture with lower sustained release for 21 days. EGFP-SCs on membranes with rhBDNF adhered significantly better (Fig. 6C), were in parallel alignment and proliferated more than cells on rhNT-3 membranes (Fig. 6B). DRG explants were placed on gelatin membranes photo-co-immobilized with or without ECM and growth factors to analyze neurite outgrowth. That axonal outgrowth was best in the presence of both ECM and the neurotrophins is in agreement with a large body of in vitro and in vivo data [5, 6, 23, 33, 34, 53-57].
5. Conclusions
We point out here advantages of using physical and chemical photo-cross-linking to fabricate gelatin membranes and tubes. The methods for generating the tubes are detailed. SCs and DRG neurons were seeded on gelatin membranes without cytotoxic side effects. The neurotrophins, BDNF and NT-3, and the ECM components, laminin and fibronectin, could be incorporated into the gelatin, with release of the neurotrophins occurring over the next three weeks. The photofabrication of this 2D-artificial scaffold with neurotrophins and ECM-containing substrates promoted the adhesion, alignment, and proliferation of the SCs and more robust neurite outgrowth from DRG explants. These tissue-engineered PSDG scaffolds appear promising for transplanting SCs into the injured spinal cord to improve repair and for supplying neurotrophic substances and ECM components to enhance cell survival and axonal regeneration after injury.
Acknowledgments
We gratefully acknowledge the Miami Project Viral Vector Core for providing the green fluorescent protein lentiviral vector and Yelena Pressman for the vector-transduced Schwann cells; Michael P. McLeod a research fellow University of Miami Miller School of Medicine for DRG dissection and immunostaining; Luis Gonzalez Graduate Teaching Assistant, University of Miami, Miller school of Medicine Dept of Microbiology & Immunology for ELISA test performance. This research was funded by NINDS grant 09923, The Christopher and Dana Reeve foundation, The Miami Project to Cure Paralysis, and the Buoniconti Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Young S, Wong M, Tabata Y, Mikos AG. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release. 2005;109(1-3):256–274. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 2.Fujita N, Matsushita T, Ishida K, Sasaki K, Kubo S, Matsumoto T, et al. An analysis of bone regeneration at a segmental bone defect by controlled release of bone morphogenetic protein 2 from a biodegradable sponge composed of gelatin and beta-tricalcium phosphate. J Tissue Eng Regen Med. 2012;6(4):291–298. doi: 10.1002/term.432. [DOI] [PubMed] [Google Scholar]
- 3.Okino H, Manabe T, Tanaka M, Matsuda T. Novel therapeutic strategy for prevention of malignant tumor recurrence after surgery: Local delivery and prolonged release of adenovirus immobilized in photocured, tissue-adhesive gelatinous matrix. J Biomed Mater Res A. 2003;66(3):643–651. doi: 10.1002/jbm.a.10016. [DOI] [PubMed] [Google Scholar]
- 4.Masuda T, Furue M, Matsuda T. Photocured, styrenated gelatin-based microspheres for de novo adipogenesis through corelease of basic fibroblast growth factor, insulin, and insulin-like growth factor I. Tissue Eng. 2004;10(3-4):523–535. doi: 10.1089/107632704323061889. [DOI] [PubMed] [Google Scholar]
- 5.Gamez E, Goto Y, Nagata K, Iwaki T, Sasaki T, Matsuda T. Photofabricated gelatin-based nerve conduits: nerve tissue regeneration potentials. Cell Transplant. 2004;13(5):549–564. doi: 10.3727/000000004783983639. [DOI] [PubMed] [Google Scholar]
- 6.Gamez E, Ikezaki K, Fukui M, Matsuda T. Photoconstructs of nerve guidance prosthesis using photoreactive gelatin as a scaffold. Cell Transplant. 2003;12(5):481–490. doi: 10.3727/000000003108747046. [DOI] [PubMed] [Google Scholar]
- 7.Johnson PJ, Tatara A, McCreedy DA, Shiu A, Sakiyama-Elbert SE. Tissue-engineered fibrin scaffolds containing neural progenitors enhance functional recovery in a subacute model of SCI. Soft Matter. 2010;6(20):5127–5137. doi: 10.1039/c0sm00173b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cirillo G, Cavaliere C, Bianco MR, De Simone A, Colangelo AM, Sellitti S, et al. Intrathecal NGF administration reduces reactive astrocytosis and changes neurotrophin receptors expression pattern in a rat model of neuropathic pain. Cell Mol Neurobiol. 2010;30(1):51–62. doi: 10.1007/s10571-009-9430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blits B, Oudega M, Boer GJ, Bartlett Bunge M, Verhaagen J. Adeno-associated viral vector-mediated neurotrophin gene transfer in the injured adult rat spinal cord improves hind-limb function. Neuroscience. 2003;118(1):271–281. doi: 10.1016/s0306-4522(02)00970-3. [DOI] [PubMed] [Google Scholar]
- 10.Lee TT, Green BA, Dietrich WD, Yezierski RP. Neuroprotective effects of basic fibroblast growth factor following spinal cord contusion injury in the rat. J Neurotrauma. 1999;16(5):347–356. doi: 10.1089/neu.1999.16.347. [DOI] [PubMed] [Google Scholar]
- 11.Bove JR. Fibrinogen--is the benefit worth the risk? Transfusion. 1978;18(2):129–136. doi: 10.1046/j.1537-2995.1978.18278160573.x. [DOI] [PubMed] [Google Scholar]
- 12.Blits B, Boer GJ, Verhaagen J. Pharmacological, cell, and gene therapy strategies to promote spinal cord regeneration. Cell Transplant. 2002;11(6):593–613. [PubMed] [Google Scholar]
- 13.Zhang SX, Huang F, Gates M, White J, Holmberg EG. Extensive scarring induced by chronic intrathecal tubing augmented cord tissue damage and worsened functional recovery after rat spinal cord injury. J Neurosci Methods. 2010;191(2):201–207. doi: 10.1016/j.jneumeth.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 14.Habeeb AF. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- 15.Morrissey TK, Kleitman N, Bunge RP. Isolation and functional characterization of Schwann cells derived from adult peripheral nerve. J Neurosci. 1991;11(8):2433–2442. doi: 10.1523/JNEUROSCI.11-08-02433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood PM. Separation of functional Schwann cells and neurons from normal peripheral nerve tissue. Brain Res. 1976;115(3):361–375. doi: 10.1016/0006-8993(76)90355-3. [DOI] [PubMed] [Google Scholar]
- 17.Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci U S A. 1996;93(21):11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barakat DJ, Gaglani SM, Neravetla SR, Sanchez AR, Andrade CM, Pressman Y, et al. Survival, integration, and axon growth support of glia transplanted into the chronically contused spinal cord. Cell Transplant. 2005;14(4):225–240. doi: 10.3727/000000005783983106. [DOI] [PubMed] [Google Scholar]
- 19.Blits B, Kitay BM, Farahvar A, Caperton CV, Dietrich WD, Bunge MB. Lentiviral vector-mediated transduction of neural progenitor cells before implantation into injured spinal cord and brain to detect their migration, deliver neurotrophic factors and repair tissue. Restor Neurol Neurosci. 2005;23(5-6):313–324. [PubMed] [Google Scholar]
- 20.Puckett WR, Hiester ED, Norenberg MD, Marcillo AE, Bunge RP. The astroglial response to Wallerian degeneration after spinal cord injury in humans. Exp Neurol. 1997;148(2):424–432. doi: 10.1006/exnr.1997.6692. [DOI] [PubMed] [Google Scholar]
- 21.Cote MP, Hanna A, Lemay MA, Ollivier-Lanvin K, Santi L, Miller K, et al. Peripheral nerve grafts after cervical spinal cord injury in adult cats. Exp Neurol. 2010;225(1):173–182. doi: 10.1016/j.expneurol.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cote MP, Amin AA, Tom VJ, Houle JD. Peripheral nerve grafts support regeneration after spinal cord injury. Neurotherapeutics. 2011;8(2):294–303. doi: 10.1007/s13311-011-0024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fortun J, Hill CE, Bunge MB. Combinatorial strategies with Schwann cell transplantation to improve repair of the injured spinal cord. Neurosci Lett. 2009;456(3):124–132. doi: 10.1016/j.neulet.2008.08.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill CE, Hurtado A, Blits B, Bahr BA, Wood PM, Bartlett Bunge M, et al. Early necrosis and apoptosis of Schwann cells transplanted into the injured rat spinal cord. Eur J Neurosci. 2007;26(6):1433–1445. doi: 10.1111/j.1460-9568.2007.05771.x. [DOI] [PubMed] [Google Scholar]
- 25.Hill CE, Moon LD, Wood PM, Bunge MB. Labeled Schwann cell transplantation: cell loss, host Schwann cell replacement, and strategies to enhance survival. Glia. 2006;53(3):338–343. doi: 10.1002/glia.20287. [DOI] [PubMed] [Google Scholar]
- 26.Taddei ML, Giannoni E, Fiaschi T, Chiarugi P. Anoikis: an emerging hallmark in health and diseases. J Pathol. 2012;226(2):380–393. doi: 10.1002/path.3000. [DOI] [PubMed] [Google Scholar]
- 27.Koda M, Someya Y, Nishio Y, Kadota R, Mannoji C, Miyashita T, et al. Brain-derived neurotrophic factor suppresses anoikis-induced death of Schwann cells. Neurosci Lett. 2008;444(2):143–147. doi: 10.1016/j.neulet.2008.07.055. [DOI] [PubMed] [Google Scholar]
- 28.Novikova LN, Pettersson J, Brohlin M, Wiberg M, Novikov LN. Biodegradable poly-beta-hydroxybutyrate scaffold seeded with Schwann cells to promote spinal cord repair. Biomaterials. 2008;29(9):1198–1206. doi: 10.1016/j.biomaterials.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 29.Gros T, Sakamoto JS, Blesch A, Havton LA, Tuszynski MH. Regeneration of long-tract axons through sites of spinal cord injury using templated agarose scaffolds. Biomaterials. 2010;31(26):6719–6729. doi: 10.1016/j.biomaterials.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 30.Tabesh H, Amoabediny G, Nik NS, Heydari M, Yosefifard M, Siadat SO, et al. The role of biodegradable engineered scaffolds seeded with Schwann cells for spinal cord regeneration. Neurochem Int. 2009;54(2):73–83. doi: 10.1016/j.neuint.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Bunge MB. Novel combination strategies to repair the injured mammalian spinal cord. J Spinal Cord Med. 2008;31(3):262–269. doi: 10.1080/10790268.2008.11760720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Straley KS, Foo CW, Heilshorn SC. Biomaterial design strategies for the treatment of spinal cord injuries. J Neurotrauma. 2010;27(1):1–19. doi: 10.1089/neu.2009.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bamber NI, Li H, Lu X, Oudega M, Aebischer P, Xu XM. Neurotrophins BDNF and NT-3 promote axonal re-entry into the distal host spinal cord through Schwann cell-seeded mini-channels. Eur J Neurosci. 2001;13(2):257–268. [PubMed] [Google Scholar]
- 34.Xu XM, Guenard V, Kleitman N, Aebischer P, Bunge MB. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp Neurol. 1995;134(2):261–272. doi: 10.1006/exnr.1995.1056. [DOI] [PubMed] [Google Scholar]
- 35.Olson HE, Rooney GE, Gross L, Nesbitt JJ, Galvin KE, Knight A, et al. Neural stem cell- and Schwann cell-loaded biodegradable polymer scaffolds support axonal regeneration in the transected spinal cord. Tissue Eng Part A. 2009;15(7):1797–1805. doi: 10.1089/ten.tea.2008.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bregman BS, Coumans JV, Dai HN, Kuhn PL, Lynskey J, McAtee M, et al. Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury. Prog Brain Res. 2002;137:257–273. doi: 10.1016/s0079-6123(02)37020-1. [DOI] [PubMed] [Google Scholar]
- 37.Kang CE, Poon PC, Tator CH, Shoichet MS. A new paradigm for local and sustained release of therapeutic molecules to the injured spinal cord for neuroprotection and tissue repair. Tissue Eng Part A. 2009;15(3):595–604. doi: 10.1089/ten.tea.2007.0349. [DOI] [PubMed] [Google Scholar]
- 38.Yuen EC, Mobley WC. Therapeutic applications of neurotrophic factors in disorders of motor neurons and peripheral nerves. Mol Med Today. 1995;1(6):278–286. doi: 10.1016/s1357-4310(95)91189-8. [DOI] [PubMed] [Google Scholar]
- 39.Henriques A, Pitzer C, Schneider A. Neurotrophic growth factors for the treatment of amyotrophic lateral sclerosis: where do we stand? Front Neurosci. 2010;4(32):1–14. doi: 10.3389/fnins.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedman JA, Windebank AJ, Moore MJ, Spinner RJ, Currier BL, Yaszemski MJ. Biodegradable polymer grafts for surgical repair of the injured spinal cord. Neurosurgery. 2002;51(3):742–51. discussion 751-2. [PubMed] [Google Scholar]
- 41.Agrawal CM, Athanasiou KA. Technique to control pH in vicinity of biodegrading PLA-PGA implants. J Biomed Mater Res. 1997;38(2):105–114. doi: 10.1002/(sici)1097-4636(199722)38:2<105::aid-jbm4>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 42.Chen PR, Chen MH, Sun JS, Chen MH, Tsai CC, Lin FH. Biocompatibility of NGF-grafted GTG membranes for peripheral nerve repair using cultured Schwann cells. Biomaterials. 2004;25(25):5667–5673. doi: 10.1016/j.biomaterials.2004.01.052. [DOI] [PubMed] [Google Scholar]
- 43.Haniu M, Montestruque S, Bures EJ, Talvenheimo J, Toso R, Lewis-Sandy S, et al. Interactions between brain-derived neurotrophic factor and the TRKB receptor. Identification of two ligand binding domains in soluble TRKB by affinity separation and chemical cross-linking. J Biol Chem. 1997;2(40):25296–25303. doi: 10.1074/jbc.272.40.25296. [DOI] [PubMed] [Google Scholar]
- 44.Hanthamrongwit M, Reid WH, Grant MH. Chondroitin-6-sulphate incorporated into collagen gels for the growth of human keratinocytes: the effect of cross-linking agents and diamines. Biomaterials. 1996;17(8):775–780. doi: 10.1016/0142-9612(96)81414-1. [DOI] [PubMed] [Google Scholar]
- 45.Okada N, Muraoka E, Fujisawa S, Machino M. Effects of visible light-irradiated camphorquinone and 9-fluorenone on murine oral mucosa. Dent Mater J. 2008;27(6):809–813. doi: 10.4012/dmj.27.809. [DOI] [PubMed] [Google Scholar]
- 46.Louis R. Spinal stability as defined by the three-column spine concept. Anat Clin. 1985;7(1):33–42. doi: 10.1007/BF01654627. [DOI] [PubMed] [Google Scholar]
- 47.Zhang JY, Luo XG, Xian CJ, Liu ZH, Zhou XF. Endogenous BDNF is required for myelination and regeneration of injured sciatic nerve in rodents. Eur J Neurosci. 2000;12(12):4171–4180. [PubMed] [Google Scholar]
- 48.Peng HB, Yang JF, Dai Z, Lee CW, Hung HW, Feng ZH, et al. Differential effects of neurotrophins and schwann cell-derived signals on neuronal survival/growth and synaptogenesis. J Neurosci. 2003;23(12):5050–5060. doi: 10.1523/JNEUROSCI.23-12-05050.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li C, Sajiki T, Nakayama Y, Fukui M, Matsuda T. Novel visible-light-induced photocurable tissue adhesive composed of multiply styrene-derivatized gelatin and poly(ethylene glycol) diacrylate. J Biomed Mater Res B Appl Biomater. 2003;66(1):439–446. doi: 10.1002/jbm.b.10025. [DOI] [PubMed] [Google Scholar]
- 50.Hoshikawa A, Nakayama Y, Matsuda T, Oda H, Nakamura K, Mabuchi K. Encapsulation of chondrocytes in photopolymerizable styrenated gelatin for cartilage tissue engineering. Tissue Eng. 2006;12(8):2333–2341. doi: 10.1089/ten.2006.12.2333. [DOI] [PubMed] [Google Scholar]
- 51.Miyazu K, Kawahara D, Ohtake H, Watanabe G, Matsuda T. Luminal surface design of electrospun small-diameter graft aiming at in situ capture of endothelial progenitor cell. J Biomed Mater Res B Appl Biomater. 2010;94(1):53–63. doi: 10.1002/jbm.b.31623. [DOI] [PubMed] [Google Scholar]
- 52.Nakayama Y, Ji-Youn K, Nishi S, Ueno H, Matsuda T. Development of high-performance stent: gelatinous photogel-coated stent that permits drug delivery and gene transfer. J Biomed Mater Res. 2001;57(4):559–566. doi: 10.1002/1097-4636(20011215)57:4<559::aid-jbm1202>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 53.Chernousov MA, Yu WM, Chen ZL, Carey DJ, Strickland S. Regulation of Schwann cell function by the extracellular matrix. Glia. 2008;56(14):1498–1507. doi: 10.1002/glia.20740. [DOI] [PubMed] [Google Scholar]
- 54.Jurga M, Dainiak MB, Sarnowska A, Jablonska A, Tripathi A, Plieva FM, et al. The performance of laminin-containing cryogel scaffolds in neural tissue regeneration. Biomaterials. 2011;32(13):3423–3434. doi: 10.1016/j.biomaterials.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 55.King VR, Hewazy D, Alovskaya A, Phillips JB, Brown RA, Priestley JV. The neuroprotective effects of fibronectin mats and fibronectin peptides following spinal cord injury in the rat. Neuroscience. 2010;168(2):523–530. doi: 10.1016/j.neuroscience.2010.03.040. [DOI] [PubMed] [Google Scholar]
- 56.Lykissas MG, Batistatou AK, Charalabopoulos KA, Beris AE. The role of neurotrophins in axonal growth, guidance, and regeneration. Curr Neurovasc Res. 2007;4(2):143–151. doi: 10.2174/156720207780637216. [DOI] [PubMed] [Google Scholar]
- 57.Tonge D, Edstrom A, Ekstrom P. Use of explant cultures of peripheral nerves of adult vertebrates to study axonal regeneration in vitro. Prog Neurobiol. 1998;54(4):459–480. doi: 10.1016/s0301-0082(97)00072-5. [DOI] [PubMed] [Google Scholar]


