SUMMARY
Long-term usage of rosiglitazone, a synthetic PPARγ agonist, increases fracture rates among diabetic patients. PPARγ suppresses osteoblastogenesis while activating osteoclastogenesis, suggesting that rosiglitazone decreases bone formation while sustaining or increasing bone resorption. Using mouse models with genetically altered PPARγ, PGC1β or ERRα, here we show that PGC1β is required for the resorption-enhancing effects of rosiglitazone. PPARγ activation indirectly induces PGC1β expression by down-regulating β-catenin and derepressing c-jun. PGC1β in turn functions as a PPARγ coactivator to stimulate osteoclast differentiation. Complementarily, PPARγ also induces ERRα expression, which coordinates with PGC1β to enhance mitochondrial biogenesis and osteoclast function. ERRα knockout mice exhibit osteoclast defects, revealing ERRα as an important regulator of osteoclastogenesis. Strikingly, PGC1β deletion in osteoclasts confers complete resistance to rosiglitazone-induced bone loss. These findings identify PGC1β as an essential mediator for the PPARγ stimulation of osteoclastogenesis by targeting both PPARγ itself and ERRα, thus activating two distinct transcriptional programs.
INTRODUCTION
Bone is a dynamic tissue that constantly remodels by balancing osteoclast-mediated bone resorption and osteoblast-mediated bone formation. Osteoclasts are derived from hematopoietic progenitors (Ash et al., 1980) in the monocyte/macrophage lineage (Scheven et al., 1986; Tondravi et al., 1997) and differentiates in response to the tumor necrosis factor family cytokine Receptor Activator of NFκB Ligand (RANKL) (Lacey et al., 1998; Yasuda et al., 1998); in contrast, osteoblasts are of mesenchymal lineage (Pittenger et al., 1999). Bone homeostasis is normally maintained by the tight coupling of bone resorption and bone formation (Edwards and Mundy, 2008). Pathological increases in osteoclast activity and bone resorption, thus the uncoupling of bone remodeling, can cause several diseases including osteoporosis, arthritis and bone metastasis of cancers (Novack and Teitelbaum, 2008).
Peroxisome proliferator-activated receptor γ (PPARγ) is a member of the nuclear receptor family of transcription factors that can be activated by lipophilic ligands (Evans et al., 2004; Tontonoz and Spiegelman, 2008). It regulates a diverse spectrum of physiological processes including adipogenesis (Tontonoz et al., 1994), lipid metabolism (Chawla et al., 2001; Tontonoz et al., 1998), insulin sensitivity (Agostini et al., 2006; Barroso et al., 1999; He et al., 2003) and inflammation (Jiang et al., 1998; Ricote et al., 1998; Wan et al., 2007b), as well as diseases such as diabetes, obesity and atherosclerosis (Evans et al., 2004; Lehrke and Lazar, 2005; Tontonoz and Spiegelman, 2008). Its importance is accentuated by the widespread use of thiazolidinediones (TZDs), synthetic PPARγ ligands, as drugs for insulin resistance and type 2 diabetes, including Avandia (rosiglitazone or BRL 49653) and Actos (pioglitazone) (Lehmann et al., 1995; Nolan et al., 1994; Tontonoz and Spiegelman, 2008). Epidemiological studies suggest that skeletal fragility is increased in type 2 diabetes mellitus (Grey, 2009; Strotmeyer and Cauley, 2007). Recent clinical trials report that long-term use of rosiglitazone increased fracture rates among diabetic patients (Grey, 2009; Home et al., 2009; Kahn et al., 2006; Kahn et al., 2008). Thus, TZD administration exacerbates skeletal fragility in a population already at increased fracture risk; and it is of paramount importance and urgency to elucidate the cellular and molecular mechanisms by which PPARγ and TZDs regulate bone remodeling. Moreover, TZD treatment causes bone loss in mice and rats, indicating that these animal models represent relevant experimental systems to dissect TZD actions in bone (Ali et al., 2005; Lazarenko et al., 2007; Li et al., 2006; Sottile et al., 2004).
Emerging evidence suggest that PPARγ plays important roles in skeletal homeostasis. PPARγ suppresses osteoblast differentiation from mesenchymal stem cells (Akune et al., 2004; Barak et al., 1999; Cock et al., 2004; Kubota et al., 1999; Rosen et al., 1999). Our recent study reveals that PPARγ also promotes osteoclast differentiation from hematopoietic stem cells (Wan et al., 2007a). Loss of PPARγ function in mouse hematopoietic lineages causes osteoclast defects manifested as osteopetrosis, a disease characterized by increased bone mass and extramedullary hematopoiesis in the spleen. Gain of PPARγ function by rosiglitazone (BRL) activation enhances osteoclastogenesis and bone resorption in vitro and in vivo. Thus, TZDs increase skeletal fragility by inhibiting bone formation while sustaining or increasing bone resorption, leading to the uncoupling of bone remodeling and a net loss of bone (Lazarenko et al., 2007; Wahli, 2008; Wan et al., 2007a). A recent clinical study examining bone biomarkers in participants randomized to rosiglitazone in A Diabetes Outcome Progression Trial (ADOPT) demonstrated that bone resorption was significantly increased in women taking rosiglitazone (Zinman et al., 2009). In this study, we aim to determine the molecular mechanisms by which PPARγ stimulates osteoclastogenesis.
PPARγ modulates transcription through ligand-mediated recruitment of coactivators. Tissue-specific differences in coactivator expression can affect PPARγ function, adding another dimension to the complexity of its gene- and cell-specific transcriptional regulation (Yu and Reddy, 2007). To determine the PPARγ coactivator in osteoclasts, we measured the expression of several nuclear receptor coactivators, and found that PGC1β (peroxisome proliferator-activated receptor-gamma coactivator 1β, Ppargc1β) is highly upregulated during osteoclast differentiation. Thus, we hypothesize that PGC1β may be involved in PPARγ regulation of osteoclastogenesis. PGC1β is a transcriptional coactivator that regulates energy metabolism by stimulating mitochondrial biogenesis and respiration of cells (Kamei et al., 2003; Lai et al., 2008; Lelliott et al., 2006; Lin et al., 2005; Sonoda et al., 2007b; Vianna et al., 2006). Intriguingly, a recent study reported that PGC1β deletion causes defects in both osteoclasts and osteoblasts. PGC1β was induced during osteoclast differentiation by reactive oxygen species. Knockdown of PGC1β in vitro inhibited osteoclast differentiation and mitochondria biogenesis, and global PGC1β deletion in mice resulted in increased bone mass. However, the mechanisms underlying PGC1β regulation of osteoclasts was underexplored. In addition, defects were also observed in PGC1β-deficient osteoblasts (Ishii et al., 2009), thus it was unclear whether PGC1β deletion in osteoclasts was sufficient to cause a resorption defect. Here we report that PGC1β is highly induced by BRL during osteoclast differentiation in a PPARγ-dependent manner. Moreover, PGC1β is required for the pro-osteoclastogenic effect of BRL in vivo and ex vivo. PGC1β functions as a PPARγ coactivator to stimulate osteoclast differentiation. Complementarily, PGC1β also coordinates with the BRL-induced ERRα to enhance mitochondrial biogenesis and osteoclast function. Importantly, using conditional PGC1β knockout mice, we have investigated the specific requirement for osteoclastic PGC1β in rosiglitazone-induced bone loss.
RESULTS
PPARγ Activation Induces PGC1β Expression during Osteoclast Differentiation
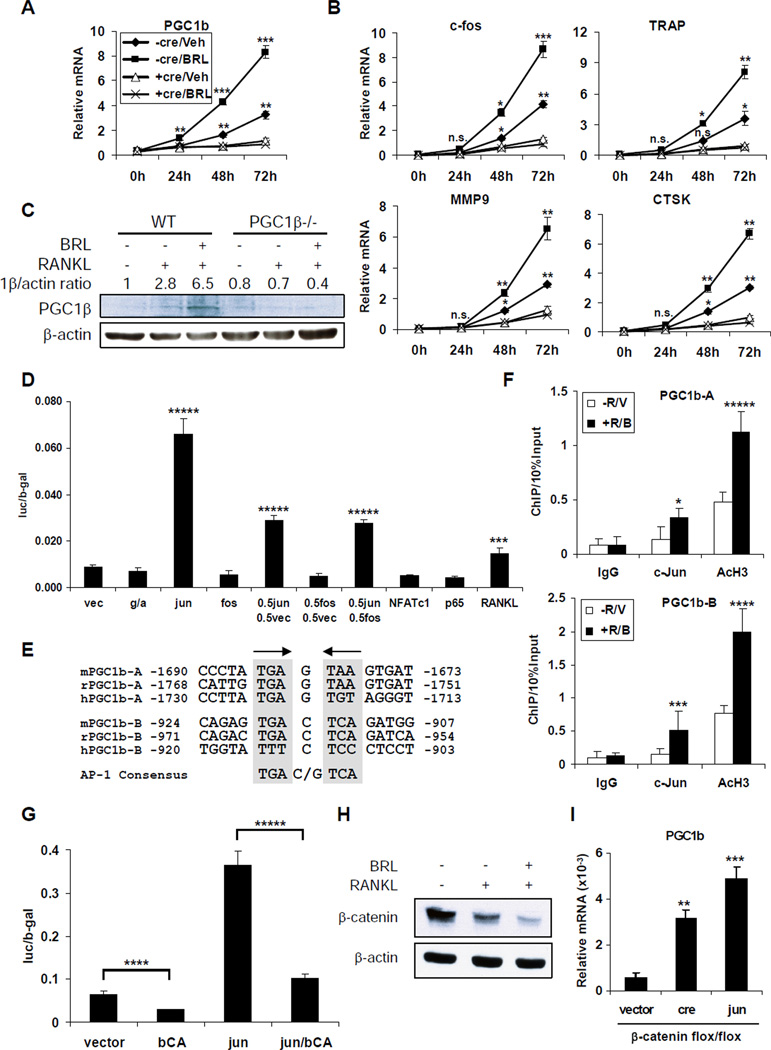
In our previous study, we generated the PPARγflox/flox; Tie2cre+/− (gf/fTie2cre) mice in which PPARγ was deleted in hematopoietic lineages, but not in mesenchymal lineages; thus in osteoclasts but not in osteoblasts (Wan et al., 2007a). We isolated bone marrow cells from gf/fTie2cre (+cre) mutants or gf/f (−cre) controls, and performed ex vivo osteoclast differentiation in the presence of macrophage colony stimulating factor (MCSF) and RANKL, with or without BRL (rosiglitazone) treatment. The induction of PGC1β during RANKL-mediated osteoclast differentiation was markedly potentiated by BRL (Figure 1A). PGC1β induction by BRL and RANKL was PPARγ-dependent, because it was abolished in the PPARγ−/− cells differentiated from the bone marrow of gf/fTie2cre mutants (Figure 1A). In contrast, a closely related coactivator PGC1α was not induced by BRL or RANKL (not shown). The BRL induction of PGC1β preceded the BRL induction of osteoclast marker genes (Figure 1B). Furthermore, PGC1β protein induction by BRL and RANKL was confirmed by western blot analysis (Figure 1C). These results showed that ligand activation of PPARγ strongly stimulates PGC1β expression during osteoclast differentiation.
Figure 1. PPARγ Activation Induces PGC1β Transcription during Osteoclast Differentiation.
(A) RNA expression of PGC1β was induced during a 72h time course of RANKL treatment, and further stimulated by BRL. Bone marrow cells from gf/f control mice (−cre) or gf/fTie2cre mutant mice (+cre) were cultured with MCSF for 3 days, in the presence of BRL or vehicle. On day 4 (0h), the macrophage precursors were differentiated with RANKL and MCSF for 3 days (72h), in the presence of BRL or vehicle. “Veh” indicates RANKL alone; “BRL” indicates RANKL+BRL. The p values were calculated by comparing each time point with “0h” baseline control (n=3).
(B) The BRL induction of PGC1β preceded the BRL induction of osteoclast marker genes.
(C) Western blot analysis showed that PGC1β protein level was induced by RANKL and further elevated by BRL in the WT but not PGC1β−/− bone marrow differentiation culture after 72 hrs.
(D) Transient transfection assays showed that the PGC1β promoter was induced by c-jun. HEK293 cells were co-transfected with a PGC1β-luc reporter and each indicated transcription factor, or alternatively treated with RANKL overnight, 24 hrs after transfection (RANKL, right). The same amount of total DNA was transfected; thus half c-jun was transfected in “0.5jun 0.5fos” compared to “jun”. The p values were calculated by comparing each condition to the vector transfected and untreated control (left) (n=3). g, PPARγ; a, RXRα; vec, vector.
(E) Alignment of mouse, rat, and human PGC1b-A and PGC1b-B AP-1 binding regions, together with the AP-1 consensus.
(F) ChIP analysis of c-jun binding to the endogenous mouse PGC1b-A and PGC1b-B promoter regions in bone marrow differentiation cultures with or without BRL and RANKL treatment (n=3).
(G) Both basal expression and c-jun induction of PGC1β promoter were significantly inhibited by β-catenin. An expression vector encoding a constitutive active β-catenin mutant (bCA) was co-transfected (n=3).
(H) Western blot analysis of bone marrow-differentiation culture showed that the β-catenin protein level was down-regulated by RANKL and further diminished by BRL.
(I) β-catenin deletion or c-jun overexpression induced PGC1β expression. Macrophages were differentiated from the bone marrow of β-cateninflox/flox mice retrovirally transduced with cre, c-jun or vector control. PGC1β mRNA expression was measured by RT-QPCR. Bars in (A), (B), (D), (F), (G) and (I) represent means ± SD.
To determine how PPARγ and BRL induced PGC1β transcription, we cloned a 1.8Kb PGC1β promoter into a luciferase reporter. Transient transfection analyses in several cell lines indicated that BRL activation of PPARγ had no significant effect on the luciferase readout, suggesting that PPARγ induced PGC1β via indirect mechanisms. Interestingly, we observed that BRL stimulated PGC1β expression only during RANKL-induced osteoclast differentiation, but not in the macrophage precursors before RANKL treatment (Figure 1A, time point 0h). This suggested that there was a functional crosstalk between PPARγ and RANKL signaling, and that BRL induction of the PGC1β promoter required component(s) in the RANKL pathway. To identify this component, we co-transfected various RANKL-activated transcription factors, including c-fos, c-jun, NFAT-c1 and the p65 subunit of NFκB, to determine which one(s) could induce the PGC1β promoter. RANKL treatment of the cells transfected with PGC1β-luc alone indeed activated the PGC1β promoter by 1.4 fold. However, this induction was largely conferred by c-jun, which robustly activated the PGC1β promoter by 7 fold; while the other factors tested had no significant effect (Figure 1D). Moreover, c-jun induced PGC1β promoter in a dose-dependent manner because the luciferase output was reduced by 56% in cells transfected with half the amount of c-jun. In addition, c-fos exerted neither activity nor interference because c-fos had no effect on either the basal or the c-jun induced PGC1β promoter expression, suggesting that c-jun functioned as homodimers (Figure 1D). Two conserved AP-1 sites were identified in the PGC1β promoter (Figure 1E). Chromatin-immunoprecipitation (ChIP) assay confirmed c-jun binding to these sites at the endogenous mouse PGC1β promoter in bone marrow-derived osteoclast precursors upon BRL and RANKL treatment, which was associated with increased histone H3 acetylation indicating activation of PGC1β transcription (Figure 1F).
Surprisingly, co-transfection of a constitutively active β-catenin mutant repressed both the basal expression and the c-jun induction of the PGC1β promoter by 56% and 72%, respectively (Figure 1G). β-catenin is the downstream effectors in the canonical Wnt signaling pathway and is regulated by protein degradation. Western blot analysis revealed that the β-catenin protein level was reduced upon RANKL treatment, which was further diminished by BRL in bone marrow-derived osteoclast precursors (Figure 1H). Furthermore, β-catenin deletion or c-jun overexpression is sufficient to induce PGC1β expression in bone marrow-derived macrophages (Figure 1I). Together, these results demonstrated that BRL-activation of PPARγ indirectly induced PGC1β expression by down-regulating β-catenin protein level, thus derepressing c-jun, which directly activates the PGC1β promoter.
PGC1β Is Required For Rosiglitazone Stimulation of Osteoclast Differentiation Ex Vivo
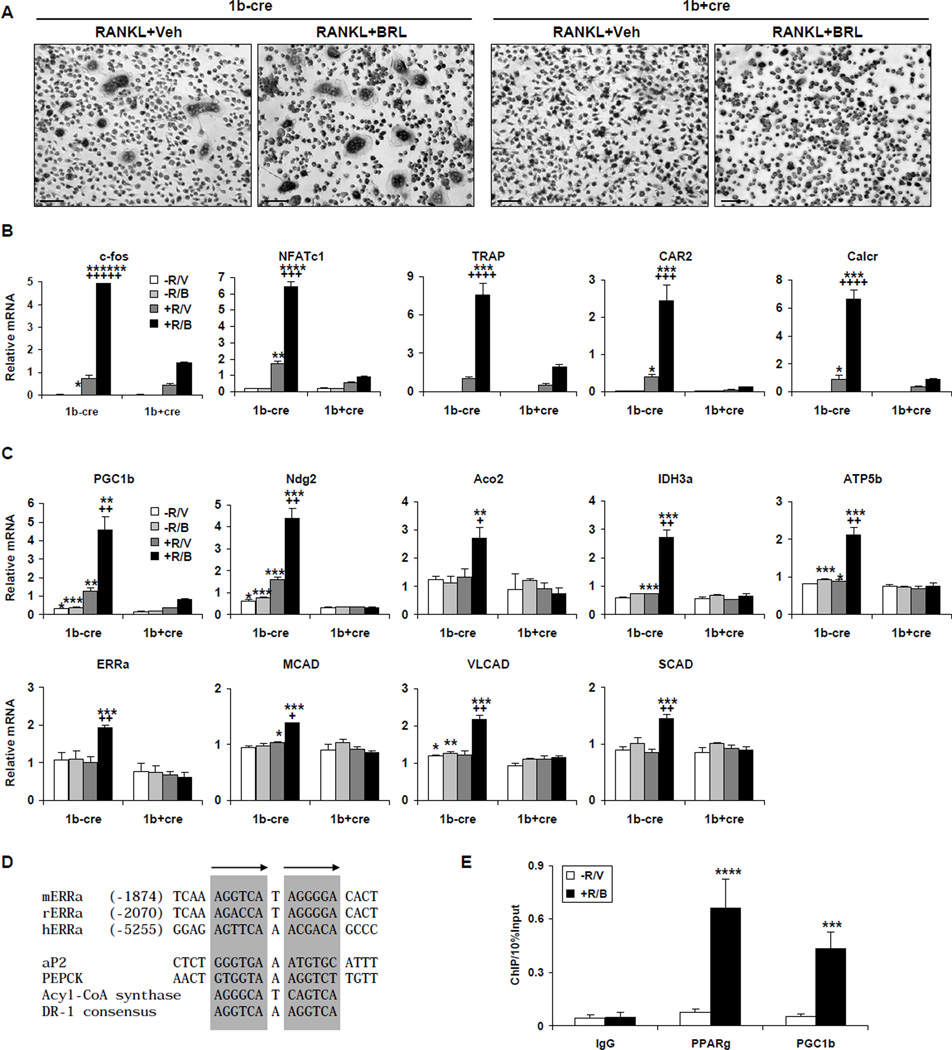
To determine the functional significance of the PPARγ induction of PGC1β, we examined whether PGC1β deletion affected PPARγ stimulation of osteoclastogenesis (Wan et al., 2007a). We generated conditional PGC1β KO mice by crossing PGC1β flox mice (Sonoda et al., 2007b) (1bf/f, Figure S1) with Tie2cre transgenic mice (Constien et al., 2001; Wan et al., 2007a). Our previous studies showed that Tie2cre deletes flox alleles in all hematopoietic lineages but not in mesenchymal lineages; thus in osteoclasts but not in osteoblasts in bone (Wan et al., 2007a; Wan et al., 2007b). Bone marrow cells were isolated from 1bf/fTie2cre mutants (1b+cre) or 1bf/f littermate controls (1b−cre), and differentiated ex vivo in the presence of MCSF and RANKL, with or without BRL treatment. The result demonstrated that PGC1β deletion severely impaired the pro-osteoclastogenic effect of BRL. In the control differentiation culture, BRL robustly stimulated the formation of multinucleated TRAP+ (Tartrate-Resistant Acid Phosphatase) mature osteoclasts, while this effect was abolished in the 1b+cre mutant culture (Figure 2A). Furthermore, PGC1β deletion resulted in a markedly reduced ability of BRL to potentiate the expression of RANKL-induced transcription factors (c-fos and NFATc1) and osteoclast function genes (TRAP, CAR2 and Calcr) (Figure 2B). This indicated that PGC1β was required for BRL stimulation of osteoclast differentiation, possibly as a coactivator for either PPARγ or other transcription factors in the specific context of osteoclastogenesis.
Figure 2. PGC1β Is Required For Rosiglitazone Stimulation of Osteoclast Differentiation.
Bone marrow cells were isolated from PGC1βflox/floxTie2cre+/− (1b+cre) mutants or PGC1βflox/flox (1b−cre) controls, and differentiated ex vivo with RANKL and MCSF, in the presence or absence of BRL treatment.
(A) Representative images of the TRAP-stained osteoclast differentiation culture. Mature osteoclasts were identified as multinucleated TRAP+ (purple) cells. Scale bar, 25µm.
(B) BRL induction of osteoclast marker genes was impaired during osteoclast differentiation from PGC1β−/− bone marrow isolated from 1b+cre mutants. R, RANKL; V, vehicle; B, BRL.
(C) BRL induction of ERRα and PGC1β, as well as their downstream targets of mitochondrial genes was abolished during osteoclast differentiation from the PGC1β−/− bone marrow. Truncated PGC1β transcripts were detected using primers specific for sequences in exon 8 (Sonoda et al., 2007b). The p values designated as * were calculated by comparing 1b−cre controls and 1b+cre mutants under the same treatment conditions; the p values designated as + were calculated by comparing +R/B and +R/V treatment conditions in the 1b−cre cells (n=3).
(D) Alignment of mouse, rat and human ERRα promoter PPRE region, together with known PPREs for ap2, PEPCK, ACS and DR-1 consensus.
(E) ChIP analysis of PPARγ and PGC1β binding to the endogenous mouse ERRα promoter PPRE region in bone marrow differentiation cultures with or without BRL and RANKL treatment (n=3). Bars in (B), (C) and (E) represent means ± SD.
PGC1β Coordinates With ERRα To Activate Mitochondrial Function in Osteoclasts
Previous studies have shown that PGC1β functions as a ligand-independent coactivator (or protein ligand) for estrogen receptor-related receptor α (ERRα) to induce the expression of medium-chain acyl-CoA dehydrogenase (MCAD), a pivotal enzyme in mitochondrial fatty acid β-oxidation (FAO) (Kamei et al., 2003). PGC1β activation of ERRα also induces other mitochondrial target genes involved in the tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS), such as Ndg2 (Nur77 downstream gene 2), Aco2 (aconitase 2), IDH3a (isocitrate dehydrogenase 3) and ATP5b (ATP synthase 5b) (Sonoda et al., 2007a). In light of the role of PGC1β in mitochondrial biogenesis during osteoclast activation (Ishii et al., 2009), we examined whether BRL could induce these ERRα/PGC1β target genes. As shown in Figure 2C, BRL in conjunction with RANKL, but not BRL or RANKL alone, increased the expression of ERRα, thus resulting in the significant induction of its target genes, including Ndg2, Aco2, IDH3a, ATP5b, MCAD, VLCAD and SCAD. Strikingly, BRL induction of these genes was completely abolished in PGC1β−/− differentiation culture, demonstrating that it was PGC1β-dependent. The fact that most of these genes were only induced when ERRα was increased suggested that they were ERRα targets. Furthermore, we identified a conserved PPAR-Response-Element (PPRE) in the ERRα promoter (Fig. 2D). ChIP assay demonstrated that both PPARγ and PGC1β were associated with this PPRE during BRL and RANKL-induced osteoclast differentiation (Fig. 2E), suggesting that ERRα is a direct PPARγ target gene in the specific context of osteoclastogenesis. Together, these results showed that BRL activation of PPARγ induced the expression of both PGC1β and ERRα, which coordinately up-regulated mitochondrial genes during osteoclastogenesis.
ERRα Deletion Causes Osteoclast Defects and Decreased Bone Resorption
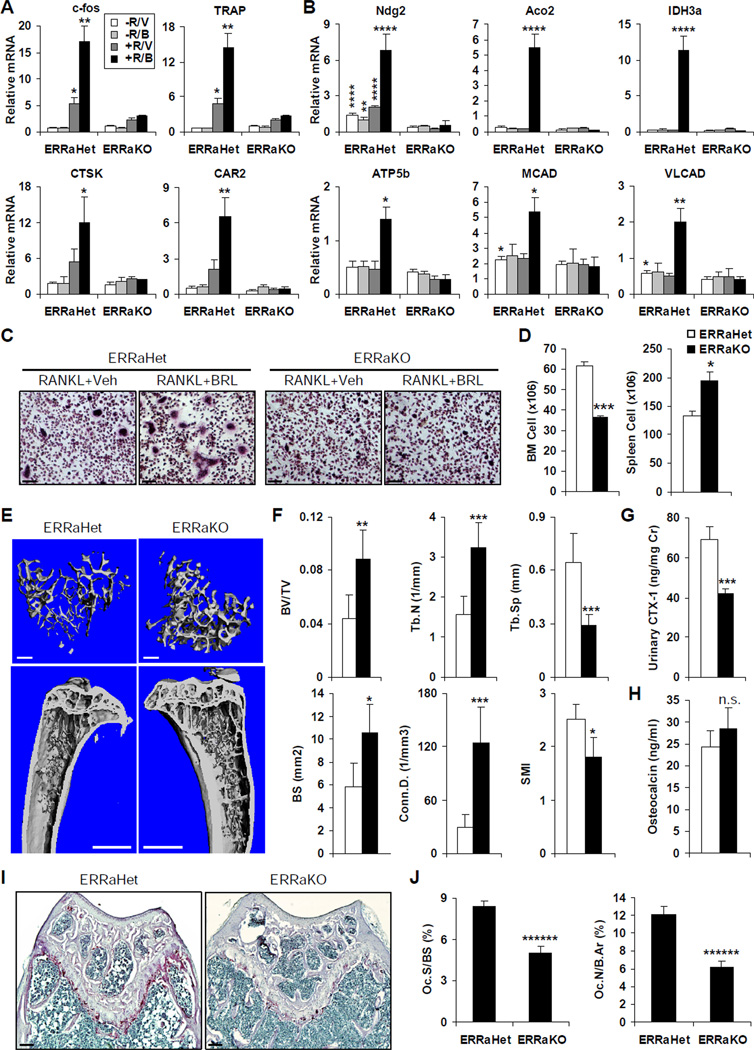
To determine the role of ERRα in osteoclast differentiation ex vivo, we compared the osteoclastogenic potential of the bone marrow cells isolated from ERRα KO mice or ERRα heterozygous (Het) control mice (Luo et al., 2003). ERRα deletion severely compromised RANKL induction of several key osteoclast genes; more strikingly, it blunted their stimulation by BRL (Figure 3A). In addition, ERRα deletion also completely abolished the BRL induction of the aforementioned mitochondrial genes, further demonstrating that they were ERRα/PGC1β targets during osteoclastogenesis (Figure 3B). Consequently, the RANKL-mediated and BRL-stimulated formation of mature osteoclasts was impaired in the ERRα KO differentiation culture (Figure 3C). These data suggested that ERRα deletion caused osteoclast defects.
Figure 3. ERRα Deletion Results in Osteoclast Defects and Decreased Bone Resorption.
(A–C) Bone marrow cells were isolated from ERRαKO mice or ERRαHet controls, and differentiated ex vivo with RANKL and MCSF, in the presence or absence of BRL treatment. RANKL-mediated and BRL-stimulated induction of osteoclast markers (A), as well as BRL induction of mitochondrial genes (B) were severely impaired in ERRαKO differentiation culture compared to ERRαHet controls (n=4); R, RANKL; V, vehicle; B, BRL. (C) Representative images of the TRAP-stained osteoclast differentiation culture. Mature osteoclasts were identified as multinucleated TRAP+ (purple) cells. Scale bar, 25µm.
(D) ERRα KO mice exhibited extramedullary hemetopoiesis in the spleen, evidenced by reduced bone marrow cell numbers and increased splenocyte numbers compared to littermate ERRαHet controls (n=4).
(E–F) ERRα KO mice displayed increased bone mass. Tibiae from ERRαKOs or littermate ERRαHet controls (10–12 month old, male, n=4) were scanned and analyzed by µCT35.
(E) Representative images of the trabecular bone of the tibial metaphysis (top, scale bar, 10µm), and the entire proximal tibia (bottom, scale bar, 1mm).
(F) Quantification of trabecular bone volume and architecture. BV/TV, bone volume/tissue volume ratio; Tb.N, trabecular number; Tb.Sp, trabecular separation; BS, bone surface; Conn.D., connectivity density; SMI, structure model index.
(G) Urinary concentration of a bone resorption marker CTX-1 (normalized to urinary creatinine concentration) was significantly decreased in ERRα KO mice (n=4).
(H) Serum concentration of a bone formation marker osteocalcin was increased in ERRα KO mice, but the difference was statistically non-significant (n=4).
(I–J) Histomorphometric analysis showed decreased osteoclasts in the ERRα KO mice (n=4).
(I) Representative images of TRAP-stained femoral sections from ERRαKO or littermate ERRαHet controls. Osteoclasts were identified as multinucleated TRAP+ (purple) cells. Scale bar, 100µm.
(J) Quantification of osteoclast surface (Oc.S/BS) and osteoclast number (Oc.N/B.Ar); B.Ar, bone area. Bars in (A), (B), (D), (F), (G), (H) and (J) represent means ± SD.
Consistently, the ERRα KO mice exhibited osteopetrosis and extramedullary hematopoiesis in the spleen similar to the gf/fTie2cre mice (Wan et al., 2007a). Bone marrow cell number was decreased by 41% in ERRαKOs compared to ERRαHet controls (Figure 3D, left), which was compensated by a 46% increase in spleen cell numbers (Figure 3D, right). MicroCT analysis of the trabecular bone in the proximal tibia (Figure 3E) revealed that the ERRα KO mice displayed higher bone volume. Quantification of bone structure and architecture demonstrated that the trabecular bone volume/tissue volume ratio (BV/TV) was increased by 102% in ERRαKOs compared to ERRαHet controls, accompanied by 79% greater bone surface (BS), 106% greater trabecular number (Tb.N), 55% less trabecular separation (Tb.Sp), 3.3-fold greater connectivity density (Conn.D), and 28% less Structure Model Index (SMI) (Figure 3F). This observation was confirmed by the statistically significant increases in both the trabecular apparent density and the BV/TV of the entire tibia, although the BV/TV of the cortical bone was not significantly altered (Figure S2).
To determine whether the bone defects in ERRα KO mice resulted from decreased bone resorption and/or increased bone formation, we measured urinary CTX-1 (C-terminal telopeptides of type-1 collagen) and serum osteocalcin levels, respectively. CTX-1 was significantly reduced (−39%, Figure 3G), while osteocalcin was elevated (+18%), although statistically non-significant (Figure 3H). Consistently, histomorphometric analysis of femoral metaphyses showed that ERRα KO mice exhibited significantly less osteoclast surface (Oc.S/BS, −39%) and osteoclast number (Oc.N./B.Ar, −49%) (Figure 3I–J); in contrast, they had greater osteoblast surface and number (Figure S3). Moreover, BRL-induced bone resorption and bone loss were severely diminished in ERRα KO mice (Figure S4). Together, these results demonstrated that the osteopetrosis-like phenotype in the ERRαKOs was mainly caused by decreased bone resorption but also contributed by increased bone formation, leading to the uncoupling of bone remodeling and a net gain of bone. Importantly, these results have identified a previously unrecognized role for ERRα as a critical regulator of osteoclastogenesis, and provided in vivo evidence for ERRα as a key PGC1β target to promote bone resorption.
PGC1β Functions As A PPARγ Coactivator to Promote Osteoclast Differentiation
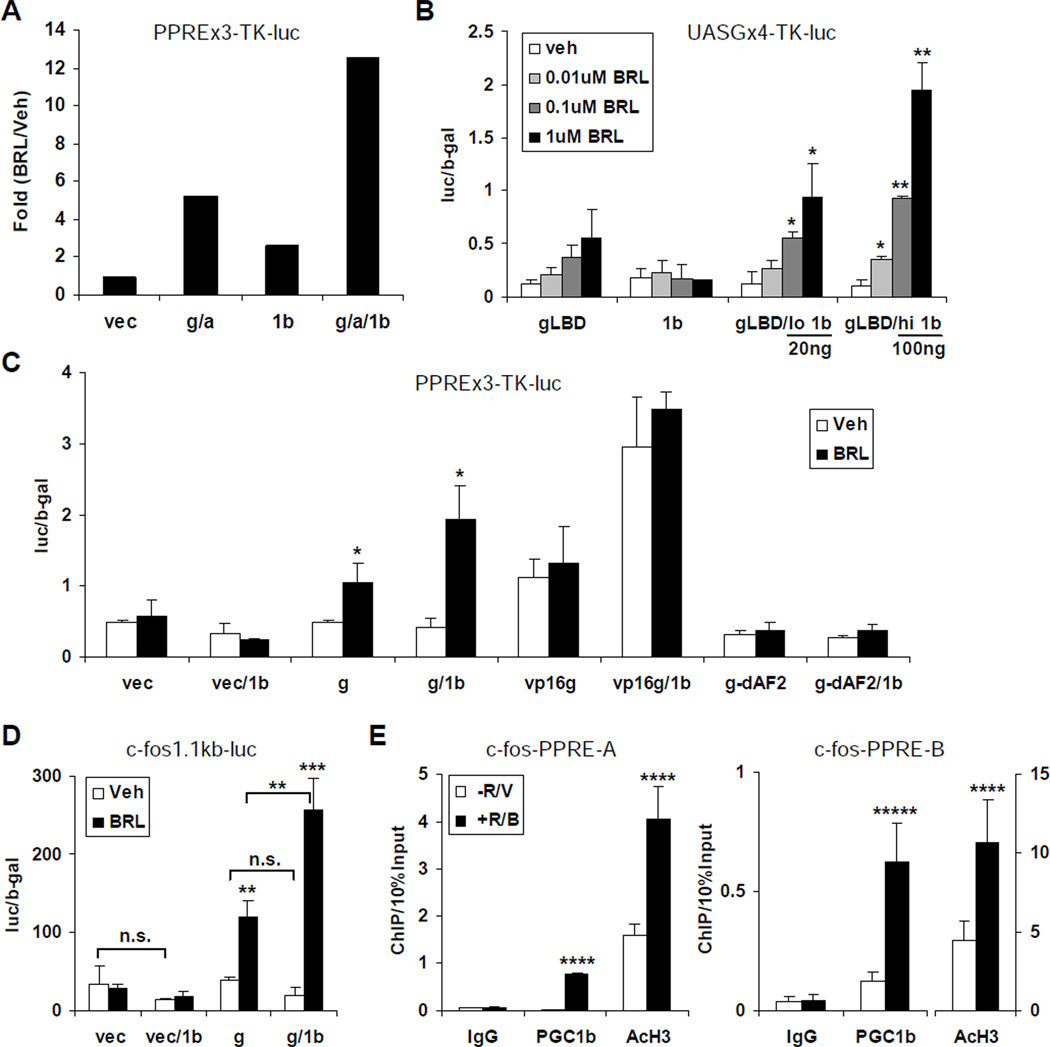
Next, we examined whether PGC1β also functioned as a transcriptional coactivator for PPARγ. First, we tested the effect of PGC1β on PPARγ activation of a consensus PPAR Response Element (PPRE)-driven luciferase reporter in a transient transfection assay of the RAW 264.7 macrophage cell line. Co-transfection of PGC1β with PPARγ and RXRα potentiated the BRL activation of PPRE, resulting in a 12.5-fold induction, compared to a 5.2-fold induction for the receptors alone and a 2.6-fold induction for PGC1β alone (Figure 4A). Second, we tested whether PGC1β functioned through the PPARγ ligand binding domain (LBD), by examining its ability to stimulate the BRL activation of a Gal4DBD-PPARγLBD fusion protein (Forman et al., 1995). To determine whether PGC1β could increase the potency of BRL to activate PPARγ LBD, we conducted a dose curve of BRL treatment (0.01, 0.1 and 1µM). To investigate whether the coactivation was PGC1β dose-dependent, PGC1β expression plasmid was transfected at a low (20ng) or high (100ng) amount. The results demonstrated that PGC1β indeed acted through PPARγ LBD, and sensitized PPARγ LBD to ligand activation at a lower BRL concentration in a PGC1β dose-dependent manner (Figure 4B). Third, PGC1β enhanced the PPRE activation by a constitutively active VP16-PPARγ fusion protein (Saez et al., 2004), but not an AF2 domain-deleted PPARγ mutant (Figure 4C); further confirming that PPARγ LBD was required for PGC1β coactivation.
Figure 4. PGC1β Functions As A PPARγ Transcriptional Coactivator.
(A) PGC1β potentiated the BRL activation of the consensus PPAR response element (PPRE). Expression plasmids for PPARγ (g), RXRα (a), and PGC1β (1b) or vector control (vec) were transfected into the RAW 264.7 macrophage cell line, along with the reporter PPREx3-TK-luc.
(B) PGC1β potentiated the BRL activation of a Gal4DBD-PPARγLBD fusion protein, and sensitized PPARγ LBD to ligand activation at lower BRL concentration in a PGC1β dosedependent manner. Expression plasmids for Gal4DBD-PPARγLBD (gLBD) and/or PGC1β (1b) were transfected as indicated, along with the Gal4 reporter UASGx4-TK-luc. PGC1β were transfected at 20ng (low 1b) or 100ng (hi 1b). BRL treatment was at the indicated concentration.
(C) PGC1β increased the PPRE activation by a constitutively active VP16-PPARγ fusion protein (VP16g), but not an AF2 domain deleted PPARγ mutant (g-dAF2).
(D) PGC1β enhanced the ability for PPARγ to induce the c-fos promoter upon BRL stimulation.
(E) ChIP analysis of PGC1β recruitment to the endogenous mouse cfos-A and cfos-B PPRE regions in bone marrow differentiation cultures with or without BRL and RANKL treatment. The p values were calculated by comparing BRL and vehicle treatment (n=3). Bars in (A)–(E) represent means ± SD.
In our previous study, we identified c-fos, a key regulator of osteoclast differentiation (Grigoriadis et al., 1994), as a direct PPARγ target (Wan et al., 2007a). Thus, we tested whether PGC1β could enhance the ability for PPARγ to induce the c-fos promoter in RAW 264.7 cells upon BRL stimulation. The results showed that PGC1β potentiated the BRL induction of c-fos by 2.1 fold (Figure 4D). Furthermore, ChIP assays demonstrated that PGC1β was indeed recruited to the two previously identified PPREs (Wan et al., 2007a) in the endogenous c-fos promoter upon BRL stimulation of the bone marrow-derived osteoclast precursors, resulting in increased histone H3 acetylation and thus c-fos transcriptional activation (Figure 4E). Together, these data provided strong evidence that PGC1β also functions as a PPARγ coactivator to effectively mediate the BRL stimulation of osteoclast differentiation.
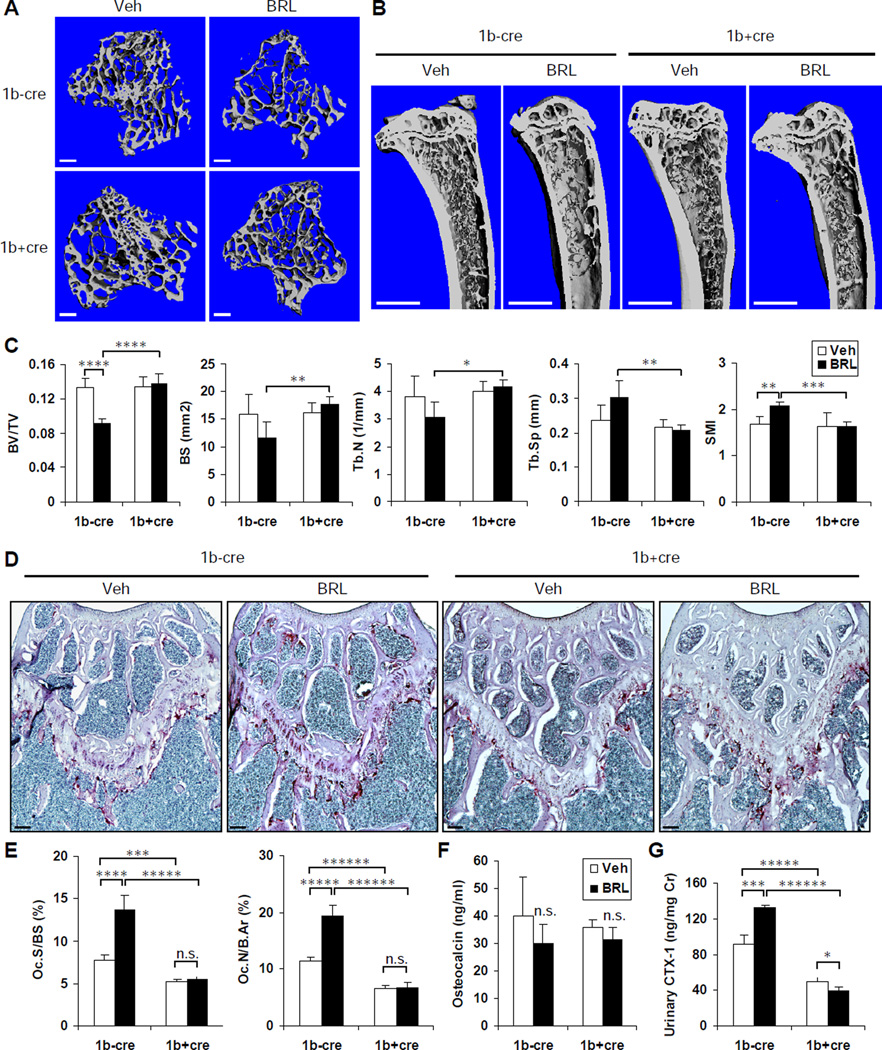
Osteoclastic PGC1β Is Required For Rosiglitazone-Induced Bone Loss In Vivo
To further delineate the specific requirement for osteoclastic PGC1β in rosiglitazone-induced bone resorption and bone loss in vivo, we treated 8-month-old 1bf/fTie2cre mutants (1b+cre) and 1bf/f littermate controls (1b−cre) with BRL (10mg/kg/day) or vehicle daily by oral gavage for 8 weeks. MicroCT imaging of the proximal tibiae revealed that the BRL-mediated reduction in trabecular bone in control mice was completely abolished in the 1b+cre mutants (Figure 5A–B). Quantification of bone parameters showed that BRL significantly decreased the BV/TV in the controls (−31%); while a statistically non-significant increase (+2%) was found in the mutants. Consistently, in the control mice, BRL resulted in a 27% less bone surface (BS) along with an 18% greater bone surface/bone volume ratio (BS/BV, not shown), as well as a 19% less trabecular number (Tb.N) along with a 28% greater trabecular separation (Tb.Sp), all indicating a lesser trabecular bone apparent density. Moreover, BRL also led to skeletal fragility in the control mice, evidenced by a 23% greater Structure Model Index (SMI), a parameter that quantifies the characteristic form of a three-dimensional structure in terms of the relative amount of plates (SMI=0, strong bone) and rods (SMI=3, fragile bone) independent of the physical dimensions (Hildebrand and Ruegsegger, 1997). Strikingly, all these parameters were unaltered by BRL in the 1b+cre mutants (Figure 5C). Interestingly, no statistically significant differences were found in any structural measurements between the mutants and controls under vehicle treated conditions (Figure 5C).
Figure 5. Osteoclastic PGC1β Is Required For Rosiglitazone-Induced Bone Loss In Vivo.
PGC1βf/fTie2cre mutant mice (1b+cre) and PGC1βf/f littermate control mice (1b−cre) (8-monthold, male) were treated with BRL at 10mg/kg/day or vehicle daily by oral gavage for 8 weeks (n=4 or 5 in each group).
(A–C) MicroCT imaging and analysis of the tibiae.
(A) Representative images of the trabecular bone of the tibial metaphysis. Scale bar, 10µm.
(B) Representative images of the entire proximal tibia. Scale bar, 1mm.
(C) Quantification of trabecular bone volume and architecture. BV/TV, bone volume/tissue volume ratio; BS, bone surface; BS/BV, bone surface/bone volume ratio; Tb.N, trabecular number; Tb.Sp, trabecular separation; SMI, structure model index.
(D–E) Histomorphometric analysis showed that BRL increased osteoclast surface and number in control mice (1b−cre) but not in mutants (1b+cre).
(D) Representative images of TRAP-stained femoral sections. Osteoclasts were identified as multinucleated TRAP+ (purple) cells. Scale bar, 100µm.
(E) Quantification of osteoclast surface (Oc.S/BS) and osteoclast number (Oc.N/B.Ar). B.Ar, bone area.
(F) Serum concentration of a bone formation marker osteocalcin.
(G) Urinary concentration of a bone resorption marker CTX-1 (normalized to urinary creatinine concentration). Bars in (C), (E), (F) and (G) represent means ± SD.
Histomorphometric analysis of femoral metaphyses revealed that BRL significantly increased both osteoclast surface (Oc.S/BS, +76%) and osteoclast number (Oc.N/B.Ar, +69%) in the controls, but this induction was abolished in the 1b+cre mutants (Figure 5D–E). In contrast, osteoblast surface and number were not significantly altered (Figure S5). Consistently, although serum osteocalcin was non-significantly decreased in both controls (−25%) and mutants (−13%) by BRL (Figure 5F), urinary CTX-1 was significantly increased in the controls (+45%) but decreased in the mutants (−22%) by BRL (Figure 5G). This demonstrated that, in the control mice, bone resorption and bone formation were uncoupled by BRL, thus leading to a net loss of bone. In contrast, in the 1b+cre mutants, loss of PGC1β in hematopoietic progenitors rendered them refractory to the osteoclast-stimulating effect of BRL; therefore the coupling of bone resorption and bone formation was maintained and bone loss was prevented. Intriguingly, there was a reduction in both CTX-1 (−45%) and osteocalcin (−11%) in the vehicle-treated 1b+cre mutants compared to the 1b−cre controls (Figure 5F–G) despite the unaltered BV/TV (Figure 5C). This indicated that osteoclastic PGC1β deletion indeed suppressed basal bone resorption, yet this defect was largely compensated by a simultaneous reduction in bone formation, presumably through the coupling mechanism, thus preserving skeletal homeostasis. Collectively, these results provided compelling evidence that PGC1β deletion in the osteoclast lineage confers a complete resistance to BRL-induced bone resorption and bone loss; therefore PGC1β is an essential mediator of PPARγ activation of osteoclastogenesis in vivo.
DISCUSSION
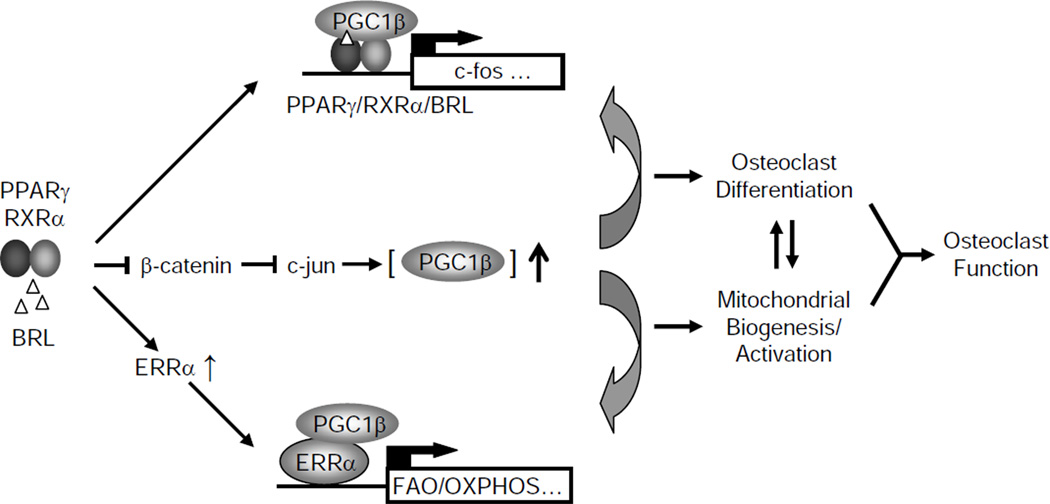
This study has elucidated the molecular mechanisms for how PPARγ and rosiglitazone stimulate osteoclastogenesis by orchestrating the downstream targets PGC1β and ERRα. Using several mouse models with genetically altered PPARγ, PGC1β or ERRα, we have provided in vitro, ex vivo and in vivo evidence that PGC1β is required for the pro-osteoclastogenic and bone resorption-enhancing effects of PPARγ and rosiglitazone. PPARγ and PGC1β form a positive feedback loop: on one hand, PPARγ activation indirectly induces PGC1β expression by down-regulating β-catenin protein level, thus derepressing c-jun, which directly activates the PGC1β promoter; on the other hand, PGC1β functions as a PPARγ coactivator to stimulate the transcription of its target genes such as c-fos, thus promoting osteoclast differentiation (Figure 6). Moreover, PGC1β also coordinates with ERRα to induce genes required for mitochondrial biogenesis and fatty acid oxidation, thereby activating osteoclast function (Figure 6). Strikingly, targeted deletion of PGC1β in the osteoclast lineage results in complete resistance to rosiglitazone-induced bone loss. Together, these findings demonstrate that PGC1β mediates the pro-osteoclastogenic function of PPARγ by targeting both PPARγ itself and ERRα, thus activating two distinct transcriptional programs (Figure 6).
Figure 6. A Simplified Model for How PGC1β Mediates PPARγ Activation of Osteoclast Differentiation and Bone Resorption.
Rosiglitazone activated PPARγ, in concert with RANKL signaling, indirectly induces PGC1β expression by down-regulating β-catenin protein, thus stimulating both basal and c-jun induced PGC1β transcription. PGC1β in turn forms a positive feedback loop by functioning as a PPARγ coactivator to induce PPARγ target genes such as c-fos, thereby stimulating osteoclast differentiation. Complementarily, rosiglitazone activated PPARγ also induces ERRα expression during osteoclast differentiation. PGC1β acts as an ERRα coactivator (or protein ligand) to induce mitochondrial genes involved in fatty acid β-oxidation (FAO) and oxidative phosphorylation (OXPHOS), thereby promoting mitochondrial biogenesis and activation. By coordinating two distinct transcriptional programs enhancing osteoclast differentiation and mitochondrial activation, PGC1β mediates PPARγ stimulation of osteoclastogenesis and rosiglitazone-induced bone loss.
As acid- and proteinase-secreting polykaryons, osteoclasts are in a state of high energy demand and possess abundant mitochondria (Brown and Breton, 1996; Ishii et al., 2009). A recent study revealed that PGC1β coordinates with iron uptake to orchestrate mitochondrial biogenesis during osteoclast development (Ishii et al., 2009). However, the molecular mechanism by which PGC1β exerts this function was unknown, and how PGC1β interacts with the osteoclast differentiation program provoked by RANKL and PPARγ remained underexplored. Our present study demonstrates that PGC1β functions as a transcriptional coavtivator for both PPARγ and ERRα to induce the expression of c-fos and mitochondrial genes, thus linking osteoclast differentiation with osteoclast activation.
Osteoclast differentiation and mitochondrial activation crosstalk with each other. For example, reactive oxygen species generated by the mitochondria can stimulate osteoclast differentiation by inducing Ca2+ oscillations and NFATc1 activation (Kim et al.); conversely, transcription factors activated during osteoclast differentiation induce target gene expression to promote osteoclast function and mitochondrial biogenesis (Ishii et al., 2009; Novack and Teitelbaum, 2008). Our proposed model (Fig. 6) illustrates that the direct downstream targets of ERRα are mitochondrial genes, but ERRα also indirectly regulates osteoclast differentiation. Moreover, we have previously shown that PPARγ deletion impairs osteoclast differentiation, demonstrating that basal PPARγ activity, potentially induced by endogenous PPARγ ligands, is required for efficient osteoclastogenesis (Wan et al., 2007a). Therefore, both PGC1β and ERRα deletions compromise the basal PPARγ activity, resulting in fewer osteoclasts induced by RANKL ex vivo and lower bone resorption in vivo.
A recent report suggested that ERRα inhibits osteoblastogenesis and enhances adipogenesis (Delhon et al., 2009). Our present study reveals a previously unrecognized role for ERRα in promoting osteoclastogenesis by inducing the expression of mitochondrial genes via a PGC1β-dependent mechanism. Furthermore, a polymorphic autoregulatory hormone response element on the human ERRα promoter (Laganiere et al., 2004) has been found to be associated with bone mineral density (Laflamme et al., 2005). Together, these findings not only identify ERRα as a critical regulator of skeletal and mineral homeostasis, but also highlight a functional link between PPARγ and ERRα pathways, converging at the transcriptional coactivator PGC1β.
Rosiglitazone induces bone loss by stimulating bone resorption and inhibiting bone formation, both of which are required for the uncoupling of bone remodeling. However, the relative effect of rosiglitazone on osteoclast and osteoblast is age-dependent. In old mice, rosiglitazone increases bone resorption while sustaining bone formation; in contrast, in young mice, rosiglitazone decreases bone formation while sustaining bone resorption (Lazarenko et al., 2007). In our study, 8-week rosiglitazone treatment significantly increased osteoclast number (Fig. 5E) and bone resorption (Fig. 5G) in 8–10 month old mice. If the coupling of bone remodeling was intact, there should have been an increase in osteoblast number and bone formation as well, yet we observed a reduction in the osteocalcin bone formation marker (Fig. 5F) and unaltered osteoblast number (Fig. S5). Therefore, relatively speaking, rosiglitazone indeed suppressed osteoblast number and bone formation in our study. This is consistent with previous findings that ligand activation of PPARγ inhibits osteoblastogenesis from the mesenchymal stem cells by favoring adipogenesis (Akune et al., 2004; Barak et al., 1999; Cock et al., 2004; Kubota et al., 1999; Rosen et al., 1999); on the other hand, repression of PPARγ by canonical or noncanonical Wnt signaling enhances osteoblastogenesis by reducing adipogenesis (Kang et al., 2007; Takada et al., 2007). Our study identifies osteoclastic PGC1β as an essential mediator of the bone-resorption enhancing effect by rosiglitazone, which acts in concert with the bone-formation suppressing effect by rosiglitazone to induce uncoupling and bone loss.
In summary, this study demonstrates that rosiglitazone stimulates osteoclastogenesis and bone resorption via a transcriptional network comprised of PPARγ, PGC1β and ERRα. Provocatively, rosiglitazone-mediated activation of adipogenesis and suppression of osteoblastogenesis has been shown to be partially attributed to the coactivator SRC-2 (Modder et al., 2009). Therefore, PPARγ recruits distinct transcriptional coactivators in hematopoietic and mesenchymal lineages to confer differential regulation of osteoclast and adipocyte development. Importantly, this mechanistic understanding of cell type-specific gene regulation by PPARγ will facilitate the design of improved diabetic drugs such as Selective PPARγ Modulators (SPPARMs) that retain the insulin-sensitizing benefits but dampen the detrimental bone loss effects.
EXPERIMENTAL PROCEDURES
Mice
PPARγflox/flox; Tie2cre+/− mice (Wan et al., 2007a), PGC1βflox/flox mice (Sonoda et al., 2007b), ERRα KO mice (Luo et al., 2003) and β-cateninflox/flox mice (Brault et al., 2001) have been described. To specifically delete PGC1β in hematopoietic lineages and endothelial cells, we bred PGC1βflox/flox (1bf/f) mice (backcrossed to C57BL/6J for at least 6 generations) with Tie2cre transgenic mice (Kisanuki et al., 2001) to generate 1bf/fTie2cre+/− mice and 1bf/f littermate controls. All protocols for mouse experiments were approved by the Institutional Animal Care and Use Committee of University of Texas Southwestern Medical Center.
Bone Analyses
To evaluate bone volume and architecture by Micro-Computed Tomography (microCT), mouse tibiae were fixed in 70% ethanol and scanned using a Scanco µCT-35 instrument (SCANCO Medical) at several resolutions for both overall tibial assessment (14 micron resolution) and the structural analysis of trabecular and cortical bone (7 micron resolution). Trabecular bone parameters were calculated using the Scanco software to analyze the bone scans from the trabecular region directly distal to the proximal tibial growth plate. Histomorphometric analyses were conducted using the BIOQUANT Image Analysis software (Bioquant). TRAP staining of osteoclasts was performed using the Leukocyte Acid Phosphatase staining kit (Sigma). ALP staining of osteoblasts was performed using the Alkaline Phosphatase staining kit (Sigma). As a bone resorption marker, urinary C-terminal telopeptide fragments of the type I collagen (CTX-1) was measured with the RatLaps™ EIA kit (Immunodiagnostic Systems), and normalized by urinary creatinine measured by the Infinity Creatinine Reagent (Thermo Scientific). As a bone formation marker, serum osteocalcin was measured with the mouse osteocalcin EIA kit (Biomedical Technologies Inc.).
Ex Vivo Osteoclast Differentiation
Osteoclasts were differentiated from mouse bone marrow cells as described (Kawano et al., 2003; Wan et al., 2007a). Briefly, cells were differentiated with 40ng/ml of M-CSF (R&D Systems) in α-MEM containing 10% FBS for 3 days, then with 40ng/ml of MCSF and 100ng/ml of RANKL (R&D Systems) for 3 days, in the presence or absence of BRL (1µM, unless otherwise stated). Retroviral gene transduction was performed as previously described (Wan et al., 2007a). Mature osteoclasts were identified as multinucleated (>3 nuclei) TRAP+ cells. Osteoclast differentiation was quantified by the RNA expression of RANKL-induced transcription factors and osteoclast function genes using RT-QPCR analysis.
Gene Expression Analyses
RNA was reverse transcribed into cDNA using an ABI High Capacity cDNA RT Kit, and analyzed using real-time quantitative PCR (SYBR Green) in triplicate. All RNA expression was normalized by L19. Antibodies used for western blots were: PGC1β (Santa Cruz), β-catenin (BD Biosciences), β-actin (Sigma).
Promoter Analyses
For transient transfection, a luciferase reporter was co-transfected into the mouse macrophage cell line RAW264.7 cells or HEK293 cells with expression plasmids for β-gal and factors to be tested using FuGENE HD reagent (Roche). Vector alone served as a negative control. Next day, the cells were treated with BRL or DMSO vehicle control overnight. Luciferase activity was normalized by β-gal activity. All transfection experiments were performed in triplicates and repeated for at least three times. Luciferase reporters PPREx3-TK-luc, UASGx4-TK-luc and c-fos1.1kb-luc have been previously described (Forman et al., 1995; Wan et al., 2007a). A 1.8Kb genomic DNA fragment upstream of the mouse PGC1β transcription start site was cloned into the pGL3Basic vector to generate the PGC1β-luc reporter. Expression plasmids for PPARγ, RXRα, PGC1β, VP16-PPARγ and PPARγ-dAF2 mutant were previously described (Saez et al., 2004; Sonoda et al., 2007b; Wan et al., 2007a). An expression plasmid for a constitutively active β-catenin mutant was generously provided by Dr. Chi Zhang (Texas Scottish Rite Hospital for Children). Expression plasmids for c-jun, c-fos, NFATc1, p65 were purchased from Open Biosystems. Promoter sequence alignment was performed using Vector NTI Advanced 11 AlignX software (Invitrogen). ChIP assays were performed using mouse bone marrow-derived osteoclast precursors that were treated with BRL/RANKL/MCSF or MCSF alone for 3 days as previously described (Wan et al., 2007a). Antibodies used were: c-jun (Cell Signaling), PGC1β (Santa Cruz), acetyl-Histone H3 (Upstate/Millipore), PPARγ (Santa Cruz) and IgG negative control (BD Biosciences). ChIP output was quantified by real-time PCR in triplicates and normalized by 10% input.
Statistical Analyses
All statistical analyses were performed with Student's t-Test and represented as mean ± standard deviation (s.d.). The p values were designated as: *, p<0.05; **, p<0.01; ***, p<0.005; ****, p<0.001; *****, p<0.0005; ******, p<0.0001; n.s. non-significant (p>0.05).
Supplementary Material
HIGHLIGHTS.
PPARγ Activation Induces PGC1β during Osteoclast Differentiation
PGC1β Acts as a PPARγ Coactivator to Stimulate Osteoclast Differentiation
PGC1β Coordinates with ERRα to Enhance Osteoclast Function
Osteoclastic PGC1β Is Required For Rosiglitazone-Induced Bone Loss In Vivo
ACKNOWLEDGEMENTS
We would like to thank R. Evans (Salk Institute) for providing the PGC1βflox/flox mice; V. Giguère (McGill University) for providing the ERRα KO mice; D. Mangelsdorf, S. Kliewer, J. Zerwekh, O. Oz (University of Texas Southwestern Medical Center) and J. Sonoda (Salk Institute) for helpful discussion; A. Gray for administrative assistance. Y. Wan is a Virginia Murchison Linthicum Scholar in Medical Research. This work was supported by the University of Texas Southwestern Medical Center Endowed Scholar Startup Fund, a BD Biosciences Research Grant Award and CPRIT funding (RP100841) NIH grant (DK057978, RME).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no financial conflict of interest.
REFERENCE
- Agostini M, Schoenmakers E, Mitchell C, Szatmari I, Savage D, Smith A, Rajanayagam O, Semple R, Luan J, Bath L, Zalin A, Labib M, Kumar S, Simpson H, Blom D, Marais D, Schwabe J, Barroso I, Trembath R, Wareham N, Nagy L, Gurnell M, O'Rahilly S, Chatterjee K. Non-DNA binding, dominant-negative, human PPARgamma mutations cause lipodystrophic insulin resistance. Cell Metab. 2006;4:303–311. doi: 10.1016/j.cmet.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology. 2005;146:1226–1235. doi: 10.1210/en.2004-0735. [DOI] [PubMed] [Google Scholar]
- Ash P, Loutit JF, Townsend KM. Osteoclasts derived from haematopoietic stem cells. Nature. 1980;283:669–670. doi: 10.1038/283669a0. [DOI] [PubMed] [Google Scholar]
- Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O'Rahilly S. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-***Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Brown D, Breton S. Mitochondria-rich, proton-secreting epithelial cells. J Exp Biol. 1996;199:2345–2358. doi: 10.1242/jeb.199.11.2345. [DOI] [PubMed] [Google Scholar]
- Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, Liao D, Nagy L, Edwards PA, Curtiss LK, Evans RM, Tontonoz P. A PPAR gamma-LXR-***ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- Cock TA, Back J, Elefteriou F, Karsenty G, Kastner P, Chan S, Auwerx J. Enhanced bone formation in lipodystrophic PPARgamma(hyp/hyp) mice relocates haematopoiesis to the spleen. EMBO Rep. 2004;5:1007–1012. doi: 10.1038/sj.embor.7400254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constien R, Forde A, Liliensiek B, Grone HJ, Nawroth P, Hammerling G, Arnold B. Characterization of a novel EGFP reporter mouse to monitor Cre recombination as demonstrated by a Tie2 Cre mouse line. Genesis. 2001;30:36–44. doi: 10.1002/gene.1030. [DOI] [PubMed] [Google Scholar]
- Delhon I, Gutzwiller S, Morvan F, Rangwala S, Wyder L, Evans G, Studer A, Kneissel M, Fournier B. Absence of estrogen receptor-related-alpha increases osteoblastic differentiation and cancellous bone mineral density. Endocrinology. 2009;150:4463–4472. doi: 10.1210/en.2009-0121. [DOI] [PubMed] [Google Scholar]
- Edwards CM, Mundy GR. Eph receptors and ephrin signaling pathways: a role in bone homeostasis. Int J Med Sci. 2008;5:263–272. doi: 10.7150/ijms.5.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- Grey A. Thiazolidinedione-induced skeletal fragility--mechanisms and implications. Diabetes Obes Metab. 2009;11:275–284. doi: 10.1111/j.1463-1326.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, Wagner EF. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266:443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100:15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand T, Ruegsegger P. Quantification of Bone Microarchitecture with the Structure Model Index. Comput Methods Biomech Biomed Engin. 1997;1:15–23. doi: 10.1080/01495739708936692. [DOI] [PubMed] [Google Scholar]
- Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJ. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009 doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- Ishii KA, Fumoto T, Iwai K, Takeshita S, Ito M, Shimohata N, Aburatani H, Taketani S, Lelliott CJ, Vidal-Puig A, Ikeda K. Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med. 2009;15:259–266. doi: 10.1038/nm.1910. [DOI] [PubMed] [Google Scholar]
- Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Zinman B, Lachin JM, Haffner SM, Herman WH, Holman RR, Kravitz BG, Yu D, Heise MA, Aftring RP, Viberti G. Rosiglitazone-associated fractures in type 2 diabetes: an Analysis from A Diabetes Outcome Progression Trial (ADOPT) Diabetes Care. 2008;31:845–851. doi: 10.2337/dc07-2270. [DOI] [PubMed] [Google Scholar]
- Kamei Y, Ohizumi H, Fujitani Y, Nemoto T, Tanaka T, Takahashi N, Kawada T, Miyoshi M, Ezaki O, Kakizuka A. PPARgamma coactivator 1beta/ERR ligand 1 is an ERR protein ligand, whose expression induces a high-energy expenditure and antagonizes obesity. Proc Natl Acad Sci U S A. 2003;100:12378–12383. doi: 10.1073/pnas.2135217100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, Macdougald OA. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:14515–14524. doi: 10.1074/jbc.M700030200. [DOI] [PubMed] [Google Scholar]
- Kawano H, Sato T, Yamada T, Matsumoto T, Sekine K, Watanabe T, Nakamura T, Fukuda T, Yoshimura K, Yoshizawa T, Aihara K, Yamamoto Y, Nakamichi Y, Metzger D, Chambon P, Nakamura K, Kawaguchi H, Kato S. Suppressive function of androgen receptor in bone resorption. Proc Natl Acad Sci U S A. 2003;100:9416–9421. doi: 10.1073/pnas.1533500100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Yang YM, Son A, Tian YS, Lee SI, Kang SW, Muallem S, Shin DM. RANKL-mediated ROS pathway that induces long-lasting Ca2+ oscillations essential for osteoclastogenesis. J Biol Chem. doi: 10.1074/jbc.M109.051557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, Eto K, Tsubamoto Y, Okuno A, Murakami K, Sekihara H, Hasegawa G, Naito M, Toyoshima Y, Tanaka S, Shiota K, Kitamura T, Fujita T, Ezaki O, Aizawa S, Kadowaki T, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Giroux S, Loredo-Osti JC, Elfassihi L, Dodin S, Blanchet C, Morgan K, Giguere V, Rousseau F. A frequent regulatory variant of the estrogen-related receptor alpha gene associated with BMD in French-Canadian premenopausal women. J Bone Miner Res. 2005;20:938–944. doi: 10.1359/JBMR.050203. [DOI] [PubMed] [Google Scholar]
- Laganiere J, Tremblay GB, Dufour CR, Giroux S, Rousseau F, Giguere V. A polymorphic autoregulatory hormone response element in the human estrogen-related receptor alpha (ERRalpha) promoter dictates peroxisome proliferator-activated receptor gamma coactivator-1alpha control of ERRalpha expression. J Biol Chem. 2004;279:18504–18510. doi: 10.1074/jbc.M313543200. [DOI] [PubMed] [Google Scholar]
- Lai L, Leone TC, Zechner C, Schaeffer PJ, Kelly SM, Flanagan DP, Medeiros DM, Kovacs A, Kelly DP. Transcriptional coactivators PGC-1alpha and PGC-lbeta control overlapping programs required for perinatal maturation of the heart. Genes Dev. 2008;22:1948–1961. doi: 10.1101/gad.1661708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarenko OP, Rzonca SO, Hogue WR, Swain FL, Suva LJ, Lecka-Czernik B. Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology. 2007;148:2669–2680. doi: 10.1210/en.2006-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Lelliott CJ, Medina-Gomez G, Petrovic N, Kis A, Feldmann HM, Bjursell M, Parker N, Curtis K, Campbell M, Hu P, Zhang D, Litwin SE, Zaha VG, Fountain KT, Boudina S, Jimenez-Linan M, Blount M, Lopez M, Meirhaeghe A, Bohlooly YM, Storlien L, Stromstedt M, Snaith M, Oresic M, Abel ED, Cannon B, Vidal-Puig A. Ablation of PGC-1beta results in defective mitochondrial activity, thermogenesis, hepatic function, and cardiac performance. PLoS Biol. 2006;4:e369. doi: 10.1371/journal.pbio.0040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Pan LC, Simmons HA, Li Y, Healy DR, Robinson BS, Ke HZ, Brown TA. Surface-specific effects of a PPARgamma agonist, darglitazone, on bone in mice. Bone. 2006;39:796–806. doi: 10.1016/j.bone.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Lin J, Yang R, Tarr PT, Wu PH, Handschin C, Li S, Yang W, Pei L, Uldry M, Tontonoz P, Newgard CB, Spiegelman BM. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell. 2005;120:261–273. doi: 10.1016/j.cell.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Luo J, Sladek R, Carrier J, Bader JA, Richard D, Giguere V. Reduced fat mass in mice lacking orphan nuclear receptor estrogen-related receptor alpha. Mol Cell Biol. 2003;23:7947–7956. doi: 10.1128/MCB.23.22.7947-7956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modder UI, Monroe DG, Fraser DG, Spelsberg TC, Rosen CJ, Gehin M, Chambon P, O'Malley BW, Khosla S. Skeletal consequences of deletion of steroid receptor coactivator-2/transcription intermediary factor-2. J Biol Chem. 2009;284:18767–18777. doi: 10.1074/jbc.M109.000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan JJ, Ludvik B, Beerdsen P, Joyce M, Olefsky J. Improvement in glucose tolerance and insulin resistance in obese subjects treated with troglitazone. N Engl J Med. 1994;331:1188–1193. doi: 10.1056/NEJM199411033311803. [DOI] [PubMed] [Google Scholar]
- Novack DV, Teitelbaum SL. The osteoclast: friend or foe? Annu Rev Pathol. 2008;3:457–484. doi: 10.1146/annurev.pathmechdis.3.121806.151431. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- Saez E, Rosenfeld J, Livolsi A, Olson P, Lombardo E, Nelson M, Banayo E, Cardiff RD, Izpisua-Belmonte JC, Evans RM. PPAR gamma signaling exacerbates mammary gland tumor development. Genes Dev. 2004;18:528–540. doi: 10.1101/gad.1167804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheven BA, Visser JW, Nijweide PJ. In vitro osteoclast generation from different bone marrow fractions, including a highly enriched haematopoietic stem cell population. Nature. 1986;321:79–81. doi: 10.1038/321079a0. [DOI] [PubMed] [Google Scholar]
- Sonoda J, Laganiere J, Mehl IR, Barish GD, Chong LW, Li X, Scheffler IE, Mock DC, Bataille AR, Robert F, Lee CH, Giguere V, Evans RM. Nuclear receptor ERR alpha and coactivator PGC-1 beta are effectors of IFN-gamma-induced host defense. Genes Dev. 2007a;21:1909–1920. doi: 10.1101/gad.1553007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda J, Mehl IR, Chong LW, Nofsinger RR, Evans RM. PGC-1beta controls mitochondrial metabolism to modulate circadian activity, adaptive thermogenesis, and hepatic steatosis. Proc Natl Acad Sci U S A. 2007b;104:5223–5228. doi: 10.1073/pnas.0611623104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottile V, Seuwen K, Kneissel M. Enhanced marrow adipogenesis and bone resorption in estrogen-deprived rats treated with the PPARgamma agonist BRL49653 (rosiglitazone) Calcif Tissue Int. 2004;75:329–337. doi: 10.1007/s00223-004-0224-8. [DOI] [PubMed] [Google Scholar]
- Strotmeyer ES, Cauley JA. Diabetes mellitus, bone mineral density, and fracture risk. Curr Opin Endocrinol Diabetes Obes. 2007;14:429–435. doi: 10.1097/MED.0b013e3282f1cba3. [DOI] [PubMed] [Google Scholar]
- Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, Igarashi M, Youn MY, Takeyama K, Nakamura T, Mezaki Y, Takezawa S, Yogiashi Y, Kitagawa H, Yamada G, Takada S, Minami Y, Shibuya H, Matsumoto K, Kato S. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat Cell Biol. 2007;9:1273–1285. doi: 10.1038/ncb1647. [DOI] [PubMed] [Google Scholar]
- Tondravi MM, McKercher SR, Anderson K, Erdmann JM, Quiroz M, Maki R, Teitelbaum SL. Osteopetrosis in mice lacking haematopoietic transcription factor PU.1. Nature. 1997;386:81–84. doi: 10.1038/386081a0. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- Vianna CR, Huntgeburth M, Coppari R, Choi CS, Lin J, Krauss S, Barbatelli G, Tzameli I, Kim YB, Cinti S, Shulman GI, Spiegelman BM, Lowell BB. Hypomorphic mutation of PGC-1beta causes mitochondrial dysfunction and liver insulin resistance. Cell Metab. 2006;4:453–464. doi: 10.1016/j.cmet.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahli W. PPAR gamma: ally and foe in bone metabolism. Cell Metab. 2008;7:188–190. doi: 10.1016/j.cmet.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Wan Y, Chong LW, Evans RM. PPAR-gamma regulates osteoclastogenesis in mice. Nat Med. 2007a;13:1496–1503. doi: 10.1038/nm1672. [DOI] [PubMed] [Google Scholar]
- Wan Y, Saghatelian A, Chong LW, Zhang CL, Cravatt BF, Evans RM. Maternal PPAR gamma protects nursing neonates by suppressing the production of inflammatory milk. Genes Dev. 2007b;21:1895–1908. doi: 10.1101/gad.1567207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Reddy JK. Transcription coactivators for peroxisome proliferator-activated receptors. Biochim Biophys Acta. 2007;1771:936–951. doi: 10.1016/j.bbalip.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Zinman B, Haffner SM, Herman WH, Holman RR, Lachin JM, Kravitz BG, Paul G, Jones NP, Aftring RP, Viberti G, Kahn SE. Effect of Rosiglitazone, Metformin, and Glyburide on Bone Biomarkers in Patients with Type 2 Diabetes. J Clin Endocrinol Metab. 2009 doi: 10.1210/jc.2009-0572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.