Abstract
Background/Aim
This study evaluated esophageal radioprotection by the Gramicidin S (GS) derived-nitroxide, JP4-039, a mitochondrial targeting peptide-isostere covalently-linked to 4-amino-Tempo, delivered in a novel swallowed oil-based (F15) formulation.
Materials and Methods
C57BL/6HNsd female mice received intraesophageal F15 formulation containing JP4-039 (4 mg/ml in 100 μl volumes) 10 minutes before 28 or 29 Gy upper body irradiation compared to MnSOD-PL (100 μl containing 100 μg plasmid) 24 hours prior to irradiation. Subgroups received 1×107 C57BL/6HNsd, GFP+ male bone marrow cells intravenously 5 days after irradiation.
Results
JP4-039/F15 or MnSOD-PL increased survival compared to irradiated controls (p<0.0001 for either). Marrow injection further increased survival (p=0.0462 and 0.0351, respectively). Esophagi removed at 1, 3, 7, 14, 24, or 60 days showed bone marrow-derived cells in the esophagi.
Conclusion
Intraesophageal GS-nitroxide radioprotection is mediated primarily through recovery of endogenous esophageal progenitor cells.
Keywords: Radiation protection, antioxidant therapy, progenitor cells, gramicidin S, GS-nitroxide, esophagitis
Radiation therapy of non-small cell lung cancer and esophageal cancer is accompanied by the significant side effect of esophagitis (1, 2). Radiotherapy induced esophagitis also contributes to the morbidity of chemoradiotherapy of metastatic malignancies, and also limits dose escalation protocols due to dehydration, esophageal ulceration and the requirement for treatment breaks (1-2). Local therapeutic strategies to minimize esophagitis have been attempted and include swallowed administration of manganese superoxide dismutase-plasmid liposomes (MnSOD-PL) (2-10). Intraesophageal administration of MnSOD-PL decreases radiation-induced esophageal cellular DNA double-strand breaks (10), stem cell apoptosis (8), lipid peroxidation (6), esophageal ulceration (3), and dehydration with reduced morbidity of single fraction and fractionated thoracic irradiation in an animal model (5). In a recent phase I clinical trial, MnSOD-PL administration twice weekly to patients receiving seven and a half weeks chemoradiotherapy for unresectable non-small cell lung cancer was shown to be safe (11). A phase II clinical trial is currently in progress.
Intraesophageal administration of MnSOD-PL provides radioprotection associated with migration to the esophagus of bone marrow-derived progenitors of esophageal squamous epithelium (9, 22-23). Due to the required 24-hour interval between the time of administration of MnSOD-PL and expression of transgene product (3), which allows for transgene transport to the nucleus, transcription of transgene message, protein production and localization at the mitochondria (12-13), an alternative, more rapid action radioprotector was developed. MnSOD transgene product acts by dismutation of superoxide to hydrogen peroxide (14), thereby, decreasing the availability of superoxide to combine with nitric oxide to produce the lethal radical peroxynitrite (14). The present study tested the hypothesis that a small molecule SOD mimic, if targeted to the mitochondria, may be able to provide esophageal radioprotection by a similar but more rapid mechanism compared to the administration of MnSOD-PL. Furthermore, it was hypothesized that a small molecule may offer the advantage of more rapid drug uptake in the esophagus.
Nitroxide radicals, such as 4-amino-Tempo (4-AT), can be effective radioprotectors; however, high systemic doses are required and noted to reduce toxicity (15, 16). Recent studies have demonstrated the mitochondrial localization and increased drug effectiveness of a novel Gramicidin S (GS)-derived nitroxide, JP4-039, which targets 4-AT to the mitochondria, by linking it covalently to a peptide isostere analog of the cyclopeptide antibiotic GS (17-21). The present study tested the effectiveness of JP4-039 administered intraesophageally in a formulation designed to provide enhanced concentration of the drug in the esophagus. The results demonstrated the effectiveness of intraesophageal administration against ionizing irradiation-induced esophagitis.
Materials and Methods
Preparation of JP4-039 in F-15 formulation
The GS-nitroxide JP4-039 has been described in detail previously (17-21). JP4-039 (21) was formulated at final drug concentrations of 8 mg/ml in cationic mutilamellar liposomes termed F-15. F-15 is a unique form of multilamellar liposome (N,N-dioleylamine amido-L-glutamate), which was utilized as a solvent for JP4-039 in order to facilitate adherence of the drug to the esophageal mucosa. The drug is entrapped between lipid bilayers and allows slow release over time from the liposome particles. F-15 was cationically charged to facilitate surface coating and retention for esophageal mucosa. Its composition was: soy PC: Tween-80: N,N-dioleylamine amido-L-glutamate (4:1:1w/w) with a final drug concentration of 8 mg/ml in PBS. It has low toxicity to cultured mammalian cells (>0.5 mg/ml).
Soy phosphatidyl choline, Lissamine rhodamine-phycoerythrin were obtained from Avanti Polar Lipids (Alabaster, AL, USA); Tween-80, tert-boc-L-glutamic acid, oleylamine, dicyclohexylcarbodiimide, N-hydroxysuccinimide, trifluoroacidic acid were obtained from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco's phospate-buffered saline (d-PBS) was obtained from Lonza (Walkersville, MD, USA). A cationic lipid, L-glutamic acid-1,5,-dioleyl amide [NH2-L-Glu(NHC18H36)2] was synthesized using a modified route as previously described (37), by coupling tert-boc-L-glutamic acid and oleylamine with dicyclohexylcarbodiimide and N-hydroxysuccinimide as the coupling agents, followed by use of trifluoroacidic acid as the deprotecting agent.
The lipid mixture (6 mg) and drug to be encapsulated (1 mg) were dissolved in 100 μl tert-butanol, frozen on dry ice, and lyophilized overnight into a cake. The next day, a 62.5 μl d-PBS was added to the lipid cake which was allowed to hydrate for 24 h at room temperature. Cationic liposomes were prepared from the hydrated lipid suspension by manual homogenization using a pair of custom-made tight-fit tube and pestle until a homogeneous consistency were reached. Finally, the liposome suspension was removed from the tube and another 62.5 μl d-PBS was used to rinse the tube and pestle and the wash solution was combined with the liposome suspension. Thus, 1 mg JP4-039 was formulated in 225 μl volumes. The final particle sizes were measured by a laser dynamic scattering method (NP-4 Particle Sizer, Beckman Coutler, Inc., Brea, CA, USA) and found to be in the range of 200-300 nm with a mean of ~255 nm in diameter. Each mouse received an intraesophageal injection of 110 μls of F15 formulation containing 400 μg JP4-039. To determine whether Tween-80 was required for effective uptake, an identical formulation (F14) without Tween-80 was tested.
Animals and animal care
C57BL/6HNsd female and C57BL/6JHNsd-GFP male mice (22-22 gm) were housed, five per cage, and fed standard laboratory chow according to previous publications (3), according to IACUC protocols at the Hillman Cancer Center, University of Pittsburgh Cancer Institute. All protocols were approved by the IACUC of the University of Pittsburgh. Veterinary care was provided by the Division of Laboratory Animal Research of the University of Pittsburgh. C57BL/6NHsd mice (15 per group) were irradiated (details are given in the next section) and received swallowed JP4-039 or MnSOD-PL pre- or post-radiation. The mice were monitored for development of esophagitis.
Irradiation
Mice were irradiated to 28 or 29 Gy to the upper body using a JL Shepherd Mark I cesium irradiator (J.L. Shepherd and Associates, San Fernando, CA, USA) (70 cGy/mm), according to published methods (6). The head and abdomen were shielded, as described previously (3), so that only the thoracic cavity received irradiation.
Intraesophageal drug administration
The methods for preparation of MnSOD-plasmid liposomes using PNVL3 lipid have been published previously (4). Briefly, 100 μl liposomes containing 100 mg plasmid were injected by syringe intraesophageally into non-anesthetized mice immediately after they received 100 μl distilled water.
Measurement of JP4-039 nitroxide in serum and in tissues by electron paramagnetic resonance
Electron paramagnetic resonance (EPR) spectra of nitroxide radicals in cells or in mitochondrial fractions were recorded after mixing with acetonitrile (1:1 v/v) after 5 min incubation with 2 mM K3Fe(CN)6 using a JEOLRE1XEPR spectrometer (JEOL, USA, Inc., Peabody, MA, USA) under the following conditions: 3350 G center field, 25 G scan range, 0.79 G field modulation, 20 mW microwave power, 0.1 s time constant, 4 min scan time. Under these experimental conditions, nitroxides were not detectably oxidized by K3F3(CN)6 to EPR-silent oxoamminium cations. Mitochondria-enriched fractions were obtained by differential centrifugation. Briefly, cells were suspended in a mitochondria isolation buffer (210 mM mannitol, 70 mM sucrose, 10 mM Hepes-KOH, pH 7.4, 1 mM EDTA, 0.1% BST and cocktail protease inhibitor) and disrupted by Dounce homogenization. Unbroken cells, nuclei, and debris were removed by 10 min centrifugation at ×700 g at 4°C. Mitochondria-rich fractions were obtained by 10 min centrifugation at 5,000 ×g and washed twice with an isolation buffer. Partitioning efficiency was calculated as a percentage of the initial signal. The amounts of nitroxide radicals integrated into mitochondria were normalized to the content of cytochrome c oxidase subunit IV. For nitroxide integration in whole cells, tissue, or isolated mitochondria, tissue or mitochondria (1 μg/μl) were incubated with 10 μM nitroxides in an incubation buffer (210 mM sucrose, 10 mM Hepes-KOH, pH 7.4, 70 mM KCI, 0.5 mM EGTA, 3 mM phosphate) for 15 min at room temperature in the presence or absence of 5 mM succinate. After that, samples were centrifuged at 10,000 ×g for 5 min, and the pellets washed twice with the incubation buffer and analyzed by EPR as described previously (38-39).
Bone marrow transplantation, esophageal excision and cell sorting
Five days after irradiation, the time shown to optimize marrow cell homing to the irradiated esophagus (6-7, 9, 22-23), mice received intravenous injection of 1.0×107 C57BL/6HNsd GFP+ male bone marrow cells prepared as single-cell suspension from donor male mice according to published methods (10, 23). At serial time points after marrow transplantation, esophageal specimens were removed, and single-cell suspensions prepared according to published methods (10, 23). The esophageal cell suspensions were sorted for GFP+ cells. The number of GFP+ cells per 106 was calculated as described previously (10, 23). (GFP+) cells were placed on slides, and stained for detection of donor cell markers (10, 23).
Statistics
In vitro data analysis and estimation of survival of mice were performed using published statistical methods (4). The Kruskal-Wallis test and post-hoc Mann-Whitney test were used to evaluate donor marrow cells in the esophagus as described elsewhere (10). A SAS statistical program was used to perform the statistical analysis (SAS Institute, Cary, NC, USA).
Results
Intravenous JP4-039 systemic pharmacokinetics and intraesophageal formulation-mediated delivery to esophagus
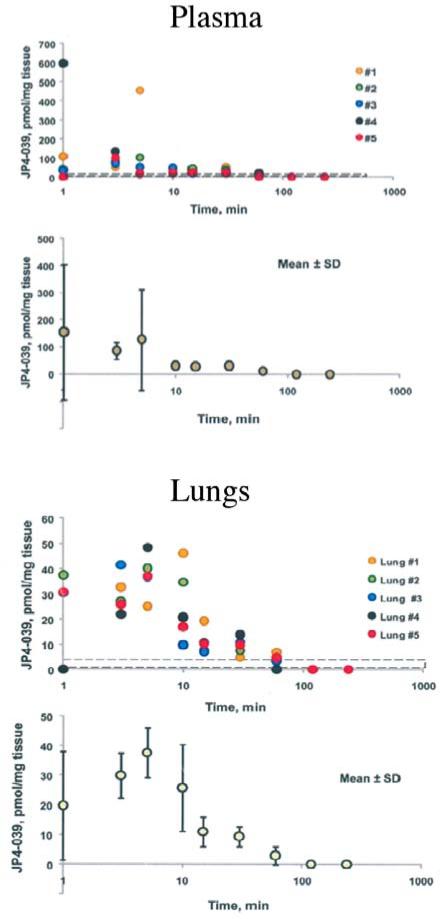
First, the clearance of JP4-039 from plasma was tested after the intravenous injection of 10 mg/kg JP4-039 in 100 μl volumes of diluents (Figure 1) using EPR measurements. JP4-039 was cleared from plasma by 10 min, but was detected in lung (and intestine) for over 30 minutes.
Figure 1.
Pharmacokinetics of clearance of JP4-039 intravenously injected into C57BL/6JHNsd mice. Mice were injected with 4 mg/kg JP4-039 in cremphor EL/ethanol 50% to 50%. Serum samples were collected and assayed. Each symbol represents an individual mouse. The methods for assay of nitroxide by EPR have been published previously (38, 39).
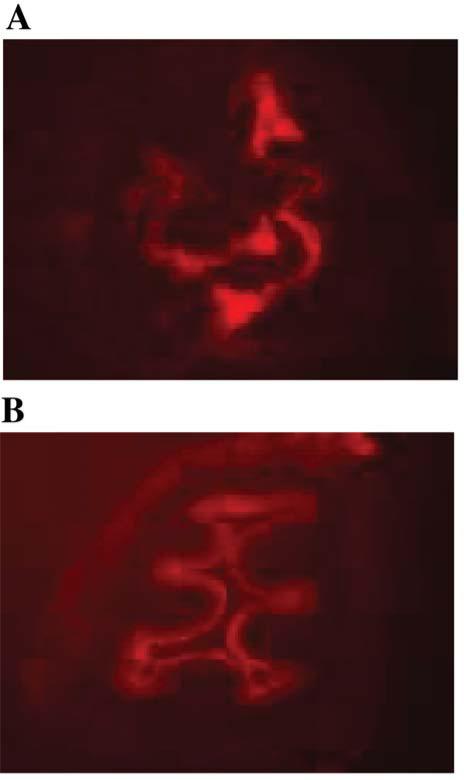
Intraesophageal administration of a red phycoerythrin dye by F15 formulation
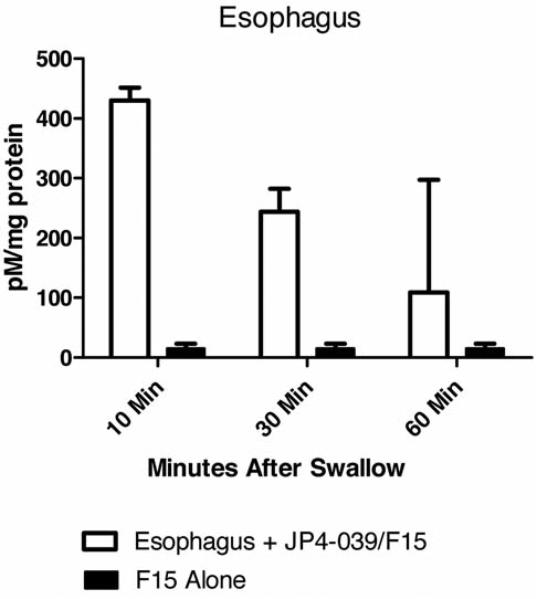
F15 emulsion containing Tween-80 was taken up by the esophagus (Figure 2). Next, the nitroxide signal of JP4-039 in the esophagus was measured in vivo after giving JP4-039/F15 by swallow. The nitroxide signal, as detected by EPR, in esophageal explants existed for up to 60 min after swallow (Figure 3).
Figure 2.
Superior penetration of cationic multilamellur liposome F15 containing 0.5 mole percent of Lissamine Rhodamine B-DOPE into the murine esophagus by swallowed F15 as compared to control formulation that did not contain dioleylamine amido-L-glutamate. Images of esophageal cross-sections taken at 10 minutes after swallow of 4 mg/kg of protein in 100 μl formulation are shown (magnification: ×100). A: F15 formulation; B: Control formulation.
Figure 3.
Quantitation of mitochondrial targeted nitroxide JP4-039 for several time points over a 60-minute period after swallow in the esophagus by EPR. The results represent mean and standard error of n=5 per group. Controls included non phycoerythrin-treated esophagi. The experimental procedures are described in Materials and Methods and in (38, 39).
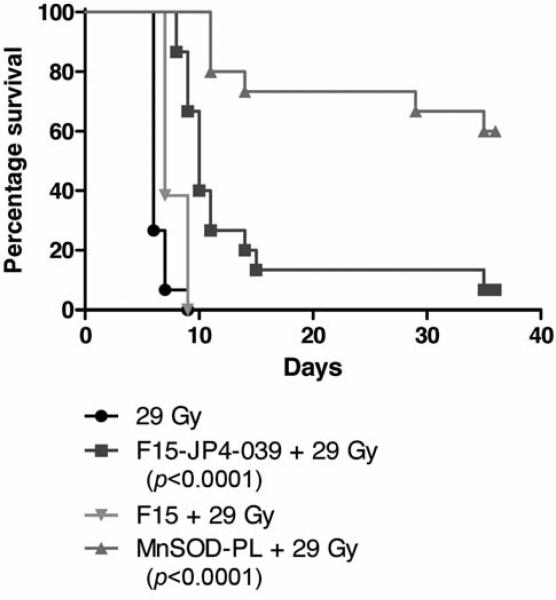
Esophageal administration of JP4-039/F15 formulation improves survival of thoracic-irradiated mice
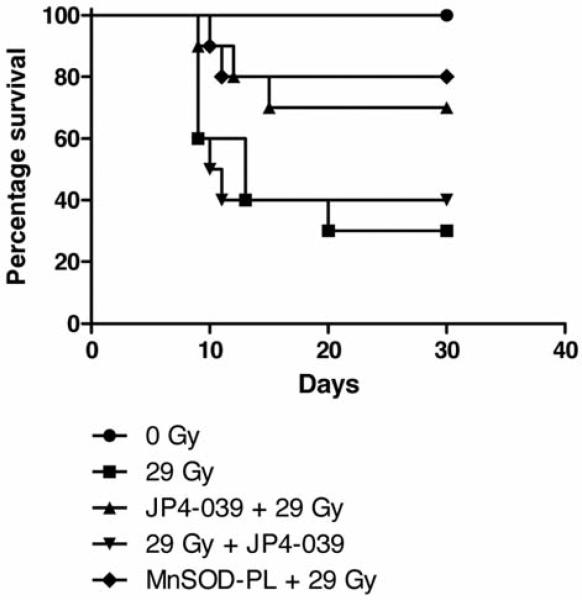
Groups of mice received JP4-039/F15, or F15 formulation alone, then 10 min later 28 Gy to the thoracic cavity and were then followed for survival. Subgroups receiving MnSOD-PL or JP4-039 in F15 formulation showed a significant increase in survival compared to mice receiving F15 formulation alone (Figure 4). Survival was improved significantly but was not sustained as with mice receiving MnSOD-PL 24 hours prior to irradiation (Figure 4).
Figure 4.
Effect of JP4-039/F15 on esophageal irradiation toxicity. Female mice (15 per group) received MnSOD-PL, JP4-039 in F15 formulation, F15 formulation, or 29 Gy upper body irradiation alone as described in the Materials and Methods. P-Values showed a significant effect of pre-irradiation intraesophageal MnSOD-PL or JP4-039/F15 compared to F15 emulsion alone against 29 Gy.
Intraesophageal JP4-039/formulation improves survival through recovery of endogenous esophageal progenitor cells
To determine whether esophageal radioprotection by JP4-039 may be increased by facilitating migration to the esophagus of bone marrow-derived cells, experimental methods were used, which previously demonstrated the bone marrow origin of progenitors of esophageal squamous epithelium (22, 23). Five groups of 15 mice each received 29 Gy to the upper body. One group received MnSOD-PL 24 hours prior to irradiation. Two groups received JP4-039/F15 formulation either 10 min prior to irradiation or JP4-039/F15 immediately after irradiation.
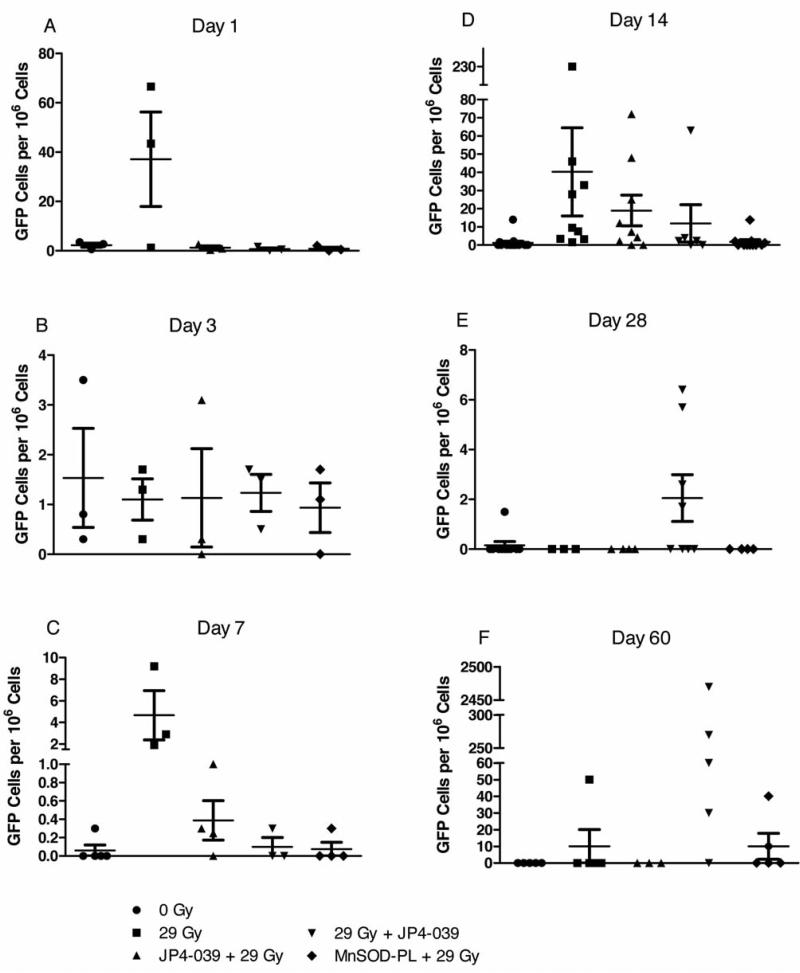
All mice received GFP+ male marrow injected intravenously at day five after irradiation. Mice receiving either MnSOD-PL or JP4-039 before irradiation showed improved survival (Figure 5). Furthermore, the survival of the JP4-039/F15 group was sustained compared to the MnSODPL group. Mice that received 1×107 GFP+ bone marrow cells intravenously five days after irradiation survived at a 30% level by being given bone marrow (Figure 5); an improvement over mice without bone marrow donation (Figure 4). At time points including 1, 3, 7, 14, 28, and 60 days after bone marrow injection, esophageal samples were removed from subgroups of mice, and single-cell suspensions sorted for the number of GFP+ cells. At days 1 and 3, five esophagi were pooled for sorting of GFP+ cells. At later days, each esophagus was kept separate. As shown in Figure 6, GFP+ cells were detected in some esophageal samples at all time points. There were low numbers at days 1, 3, 7, and 28. At days 14 and 60, individual mice had high numbers of GFP+ cells, but there was significant variation between mice. These results confirm and extend those in previous publications demonstrating that bone marrow-derived progenitors of esophageal squamous epithelium migrate into the irradiated esophagus and persist up to 60 days after irradiation, prominent in the 29 Gy + JP4-039 group (Table I).
Figure 5.
Effect of GFP+ male marrow intravenous injection and JP4-039/F15 on esophageal irradiation toxicity. Female mice (15 per group) received 29 Gy upper body irradiation on day 0, then on day 5 they received1 ×107 GFP+ marrow cells intravenously from male donors. P-values showed a significant effect of pre-irradiation intraesophageal MnSOD-PL or JP4-039/F15 on increasing the survival; p=0.0315 and p=0.0462, respectively.
Figure 6.
Detection of GFP+ marrow-derived cells in the irradiated mouse esophagus after intravenous transplant. Mice were irradiated to 29 Gy to the esophagus on day 0, and then injected with 1×107 GFP+ marrow cells intravenously on day 5 according to published methods (5-7). Five esophagus samples were removed from each animal in the various subgroups on days 1 (A), 3 (B), 7 (C), 14 (D), 28 (E) and 60 (F) after marrow injection. Samples of excised esophagi were prepared as single cell suspensions and then analyzed by cell sorting for GFP+ cells/106 esophagus cells. Each symbol represents one esophagus.
Table I.
Median and inter-quartile range (in parentheses) for the number of GFP+ cells per 106 cells in the esophagus of mice in each of the treatment groups at each day of measurement.
| Day | Treatment |
||||
|---|---|---|---|---|---|
| 0 Gy | 29 Gy | JP4-039 + 29 Gy | 29 Gy + JP4-039 | MnSOD-PL + 29 Gy | |
| 1 | 2.7 (0.6-3.5) | 43.3 (1.3-66.6) | 0.9 (0.3-2.6) | 0.5 (0-1.6) | 0.5 (0-2) |
| N=3 | N=3 | N=3 | N=3 | N=3 | |
| 3 | 0.8 (0.3-3.5) | 1.3 (0.3-1.7) | 0.3 (0-3.1) | 1.5 (0.5-1.7) | 1.1 (0-1.7) |
| N=3 | N=3 | N=3 | N=3 | N=3 | |
| 7 | 0 (0-0) | 2.9 (1.9-9.2) | 0.28 (0.13-0.65) | 0 (0-0.3) | 0 (0-0.15) |
| N=5 | N=3 | N=4 | N=3 | N=4 | |
| p1=0.018* | |||||
| 14 | 0 (0-0) | 9.6 (3.5-33) | 7.3 (2.1-25) | 2.35 (0-3.9) | 0 (0-2) |
| N=15 | N=9 | N=9 | N=6 | N=11 | |
| p1<0.0001 | p1=0.0028 | p1=0.017 | p2<0.0001** | ||
| 28 | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0.85 (0-4.15) | 0 (0-0) |
| N=10 | N=3 | N=4 | N=8 | N=4 | |
| 60 | 0 (0-0) | 0 (0-0) | 0 (0-0) | 60 (30-270) | 0 (0-0) |
| N=5 | N=5 | N=3 | N=5 | N=5 | |
| p1=0.048 | |||||
p1 is the p-value for the comparison with 0 Gy group using Mann-Whitney U-test
p2 is the p-value for the comparison with 29 Gy group using Mann-Whitney U-test.
Statistical evaluation was next carried out. At days 1, 3 and 28, the Kruskal-Wallis test gave p-values of 0.18, 0.94, and 0.060 respectively, indicating that all these groups had an equal number of GFP+ cells at these days (Table I). At day 7, the Kruskal-Wallis test gave a p-value of 0.035, indicating that these groups did not have equal number of GFP+ cells. The post-hoc Mann-Whitney test revealed that the 29 Gy group had a significantly higher number of GFP+ cells than the 0 Gy group (p=0.018). At day 14, the Kruskal-Wallis test gave a p-value of 0.0002, indicating that these groups did not have equal numbers of GFP+ cells. The post-hoc Mann-Whitney test revealed that each of the 29 Gy, JP4-039 + 29 Gy, and 29 Gy + JP4-039 groups had significantly higher numbers than the 0 Gy group (p<0.0001, p=0.0028 and p=0.017, respectively). The MnSOD-PL + 29 Gy group had a significantly lower number than the 29 Gy group (p<0.0001). The results at day 60 showed a persistent increase in donor marrow-derived cells in the 29 Gy + JP4-039 group. At day 60, the Kruskal-Wallis test gave a p-value of 0.035, indicating that these groups did not have an equal number of GFP+ cells. The post-hoc Mann-Whitney test revealed that the 29 Gy + JP4-039 group had a significantly higher number than the 0 Gy group (p=0.048).
Discussion
As improved techniques of radiotherapy become available including stereotactic frameless radiosurgery, image-guided radiotherapy, intensity modulated radiotherapy and gated respiratory cycle controlled radiotherapy, radiation oncologists will seek to escalate the radiotherapy dose to thoracic tumor target volumes (1, 2). Of necessity, such treatment approaches will require increased radiation doses to healthy tissue transit volumes including the esophagus. For the management of patients with non-small cell lung cancer and esophageal cancer, radiation delivery to significant volumes of healthy esophagus continues to be a problem for management of toxicity (25-27).
Organ-specific radiation protection has been a challenge for several reasons including maximizing efficient uptake of a radioprotector drug to the target organ, and prevention of systemic delivery of the protector drug which may produce systemic side-effects, or tumor radioprotection. In studies with MnSOD-plasmid liposomes, effective organ-specific uptake has been demonstrated in a mouse model in which esophagus, as well as lung, and other organs were removed and assayed for MnSOD transgene product by RT-PCR. In these experiments transgene uptake was detected in the esophagus and oral cavity, with low levels in the lung due to inhalation during administration (28-32). Another concern has been the potential radioprotection of tumor. Intraesophageal or intrapulmonary delivery of MnSOD-PL did not result in detectable radioprotection of orthotopic tumors (30, 33-34). Intraoral administration of MnSOD-PL did not protect but rather radiosensitized squamous cell carcinoma (SCC7) cell orthotopic tumors in the cheek pouch (30). The mechanism of healthy tissue radioprotection and simultaneous radiosensitization of tumors by MnSOD transgene product was attributed to differences in redox balance in tumor cells which show increased toxicity of the H2O2 product of superoxide dismutation by MnSOD (35). While organ-specific MnSOD-PL has been shown to be safe in a recently completed phase I clinical trial (11), the expense of production of plasmid liposomes, and the continuing concern for transgene integration at sites outside of the esophagus remain obstacles to widespread clinical use. Therefore, the development of an alternative organ-specific, small-molecule radioprotector drug has been a high priority.
The present study demonstrated that a mitochondrial targeted 4-AT derivative, JP4-039 (in which mitochondrial localization is achieved by linkage of the active nitroxide molecule to a peptide isostere, based on a mitochondrial targeting segment of the cyclopeptide antibiotic Gramicidin-S) is a highly effective radiation protector and mitigator in vitro and in vivo (17-21). To determine whether organ-specific radioprotection was achievable using JP4-039, this study developed a novel formulation (F15) and JP4-039 was dispersed in this formulation for intra-esophageal (swallowed) administration. Delivery of JP4-039/F15 intraesophageally before or after thoracic irradiation provided significant protection of the esophagus and improved survival. These results established that a small molecule, mitochondrial-targeted nitroxide, can be an effective esophageal radioprotector. In previous studies, local skin administration of Tempol in an alcohol formulation was an effective topical radioprotector for the scalp and prevented scalp injury or hair loss in patients receiving ten fractions of whole brain irradiation for brain metastasis (36). Local delivery and enhancement of the protective effect of Tempol was maximized when contact with the affected skin was maintained by covering the scalp with a specific applicator (36). Further studies are required to determine whether prolonged dwell time and contact with the esophagus, using an improved JP4-039/F15 formulation will enhance uptake in the esophagus and extend the radioprotective capacity.
The present results demonstrated that esophageal radioprotection is mediated in large part by protection of endogenous esophageal progenitor cells, with minimal contribution of bone marrow-derived progenitors of esophageal epithelium. The observation that higher numbers of donor marrow cells were detected in the explanted esophagi of the same mice at days 7, 14 and 60 after transplant may reflect the growth and expansion of rare foci of single marrow-derived esophageal cells into discrete foci as described previously (10, 22, 23). Whether these foci are derived from rare stem cells growing in rare niches is not yet known.
Previous studies showed enhanced migration to the irradiated esophagus of bone marrow-derived progenitors of esophageal squamous epithelium in mice receiving MnSODPL 24 hours prior to irradiation (22, 23). The difference between the MnSOD-PL-mediated radioprotection and that mediated by the small molecule JP4-039 is not yet known, but a low-level contribution of bone marrow-derived progenitors was detected in the esophagi of mice treated by each agent. The best survival of GFP+ cells at day 60 was with JP4-039 delivered after irradiation. Swallowed MnSODPL-treated mice may have experienced persistent gene product protection (3-4) and may have effectively protected true stem cells and their niches. Therefore, irradiation protection by MnSOD-PL may have been greater, allowing for homing of only bone marrow-derived short-term repopulating progenitors. In contrast, intraesophageal injected JP4-039 may have reached both esophageal stem cells and their microenvironment, but cleared rapidly. While JP4-039 may have prevented quiescent stem cell apoptosis (21), reduced stromal microenvironmental protection due to more rapid drug clearance may have allowed irradiation killing of more primitive esophageal stem cells and facilitated homing of bone marrow-derived primitive progenitors that protected and persisted to day 60. Further studies are required to test these hypotheses.
Acknowledgements
This work was supported by NIH grant T32AG21885, NIH/NCI grants RO1-CA83876 and NIAID/NIH grant U19-A1068021.
References
- 1.Movsas B, Moughan J, Sarna L, Langer C, Werner-Wasik M, Nicolaou N, Komaki R, Machtay M, Wasserman T, Watkins Bruner D. Quality of life supersedes the classic prognosticators for long-term survival in locally advanced non-small cell lung cancer: An analysis of RTOG 9801. J Clin Oncol. 2009;27:5816–5821. doi: 10.1200/JCO.2009.23.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel AB, Edelman MJ, Kwok Y, Krasna MJ, Suntharalingam M. Predictors of acute esophagitis in patients with non-small cell lung carcinoma treated with concurrent chemotherapy and hyperfractionated radiotherapy followed by surgery. Int J Radiation Oncol Biol Phys. 2004;60:1106–1112. doi: 10.1016/j.ijrobp.2004.04.051. [DOI] [PubMed] [Google Scholar]
- 3.Stickle RL, Epperly MW, Klein E, Bray JA, Greenberger JS. Prevention of irradiation-induced esophagitis by plasmid/liposome delivery of the human manganese superoxide dismutase (MnSOD) transgene. Radiat Oncol Invest Clinical Basic Res. 1999;7:204–217. doi: 10.1002/(SICI)1520-6823(1999)7:4<204::AID-ROI2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.Epperly MW, Gretton JA, DeFilippi SJ, Sikora CA, Liggitt D, Koe G, Greenberger JS. Modulation of radiation-induced cytokine elevation associated with esophagitis and esophageal stricture by manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) gene therapy. Radiat Res. 2001;155:2–14. doi: 10.1667/0033-7587(2001)155[0002:morice]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 5.Epperly MW, Kagan VE, Sikora CA, Gretton JE, DeFilippi SJ, Bar-Sagi D, Greenberger JS. Manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) administration protects mice from esophagitis associated with fractionated irradiation. Int J Cancer (Radiat Oncol Invest) 2001;96:221–233. doi: 10.1002/ijc.1023. [DOI] [PubMed] [Google Scholar]
- 6.Epperly MW, Zhang X, Nie S, Cao S, Kagan V, Tyurin V, Greenberger JS. MnSOD-plasmid liposome gene therapy effects on ionizing irradiation induced lipid peroxidation of the esophagus. In Vivo. 2005;19:997–1004. [PubMed] [Google Scholar]
- 7.Epperly MW, Shen H, Zhang X, Nie S, Cao S, Greenberger JS. Protection of esophageal stem cells from ionizing irradiation by MnSOD-plasmid liposome gene therapy. In Vivo. 2005;19:965–974. [PubMed] [Google Scholar]
- 8.Epperly MW, Carpenter M, Agarwal A, Mitra P, Nie S, Greenberger JS. Intra-oral manganese superoxide dismutase plasmid liposome radioprotective gene therapy decreases ionizing irradiation-induced murine mucosal cell cycling and apoptosis. In Vivo. 2004;18:401–410. [PubMed] [Google Scholar]
- 9.Epperly MW, Shen H, Jefferson M, Greenberger JS. In vitro differentiation capacity of esophageal progenitor cells with capacity for homing and repopulation of the ionizing irradiation damaged esophagus. In Vivo. 2004;18:675–685. [PubMed] [Google Scholar]
- 10.Niu Y, Wang H, Wiktor-Brown D, Rugo R, Shen H, Huq MS, Engelward B, Epperly M, Greenberger JS. Irradiated esophageal cells are protected from radiation-induced recombination by MnSOD gene therapy. Rad Res. 2010;173:453–461. doi: 10.1667/RR1763.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarhini AA, Belani C, Luketich JD, Ramalingam SS, Argiris A, Gooding W, Petro D, Kane K, Liggitt D, Greenberger JS. A phase I study of concurrent chemotherapy (paclitaxel and carboplatin) and thoracic radiotherapy with swallowed manganese superoxide dismutase (MnSOD) plasmid liposome (PL) protection in patients with locally advanced stage III non-small cell lung cancer. Human Gene Therapy. doi: 10.1089/hum.2010.078. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epperly MW, Sikora C, DeFilippi S, Gretton J, Zhan Q, Kufe DW, Greenberger JS. MnSOD inhibits irradiation-induced apoptosis by stabilization of the mitochondrial membrane against the effects of SAP kinases p38 and Jnk1 translocation. Rad Res. 2002;157:568–577. doi: 10.1667/0033-7587(2002)157[0568:msdsir]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Epperly MW, Gretton JE, Bernarding M, Nie S, Rasul B, Greenberger JS. Mitochondrial localization of copper/zinc superoxide dismutase (Cu/ZuSOD) confers radioprotective functions in vitro and in vivo. Rad Res. 2003;160:568–578. doi: 10.1667/rr3081. [DOI] [PubMed] [Google Scholar]
- 14.Epperly MW, Osipov AN, Martin I, Kawai K, Borisenko GG, Jefferson M, Bernarding M, Greenberger JS, Kagan VE. Ascorbate as a ‘redox-sensor’ and protector against irradiation-induced oxidative stress in 32D cl 3 hematopoietic cells and subclones overexpressing human manganese superoxide dismutase. Int J Radiat Oncol Biol Phys. 2004;58:851–861. doi: 10.1016/j.ijrobp.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 15.Xavier S, Yamada K, Samuni AM, Sumuni A, DeGraff W, Krishna MC, Mitchell JB. Differential protection by nitroxides and hydroxylamines to radiation-induced and metal ion-catalyzed oxidative damage. Biochim Biophys Acta. 2002;1573:109–120. doi: 10.1016/s0304-4165(02)00339-2. [DOI] [PubMed] [Google Scholar]
- 16.Hahn SM, Krishna MC, DeLuca AM, Coffin D, Mitchell JB. Evaluation of the hydroxylamine Tempol-H as an in vivo radioprotector. Free Radical Biol Med. 2000;28:953–958. doi: 10.1016/s0891-5849(00)00176-3. [DOI] [PubMed] [Google Scholar]
- 17.Jiang J, Belikova NA, Xiao J, Zhao Q, Greenberger JS, Wipf P, Kagan VE. A mitochondria-targeted nitroxide/hemigramicidin S conjugate protects mouse embryonic cells against γ-irradiation. Int J Radiat Oncol Biol Phys. 2008;70:816–825. doi: 10.1016/j.ijrobp.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fink M, Macias CA, Xiao J, Tyurina YY, Delude RL, Greenberger JS, Kagan VE, Wipf P. Hemigramicidin-TEMPO conjugates: Novel mitochondria-targeted antioxidants. Crit Care Med. 2007;35:5461–5470. doi: 10.1097/01.CCM.0000279192.96303.E7. [DOI] [PubMed] [Google Scholar]
- 19.Fink MP, Macias CA, Xiao J, Tyurina YY, Delude RL, Greenberger JS, Kagan VE, Wipf P. Hemigramicidin-TEMPO Conjugates: Novel mitochondria-targeted antioxidants. Biochemical Pharmacology. 2007;74:801–809. doi: 10.1016/j.bcp.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Jiang J, Kurnikov I, Belikova NA, Xiao J, Zhao Q, Vlasova IL, Amoscato AA, Braslau R, Studer A, Kagan VE. Structural requirements for optimized delivery, inhibition of oxidative stress and anti-apoptotic activity of targeted nitroxides. J Pharmacology, Exp Therapeutics. 2007;320:1050–1060. doi: 10.1124/jpet.106.114769. [DOI] [PubMed] [Google Scholar]
- 21.Rajagopalan MS, Gupta K, Epperly MW, Franicola D, Zhang X, Wang H, Zhao H, Tyurin VA, Kagan VE, Greenberger JS. The mitochondria-targeted nitroxide JP4-039 augments potentially lethal irradiation damage repair. In Vivo. 2009;23:717–726. [PMC free article] [PubMed] [Google Scholar]
- 22.Epperly MW, Goff JP, Sikora CA, Shields DS, Greenberger JS. Bone marrow origin of cells with capacity for homing and differentiation to esophageal squamous epithelium. Rad Res. 2004;162:233–240. doi: 10.1667/rr3224. [DOI] [PubMed] [Google Scholar]
- 23.Niu Y, Epperly MW, Shen H, Smith T, Lewis D, Gollin S, Greenberger JS. Intraesophageal MnSOD-plasmid liposome administration enhances engraftment and self-renewal capacity of bone marrow derived progenitors of esophageal squamous epithelium. Gene Therapy. 2008;15:347–356. doi: 10.1038/sj.gt.3303089. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Shen MH. Polyethylene glycol-mediated cell fusion. Methods Mol Biol. 2006;325:59–66. doi: 10.1385/1-59745-005-7:59. [DOI] [PubMed] [Google Scholar]
- 25.Liao ZX, Komaki RR, Thames HD, Liu HH, Tucker SL, Mohan R, Martel MK, Wei X, Yang K, Cox JD. Influence of technologic advances on outcomes in patients with unresectable locally advanced non-small cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiation Oncol Biol Phys. 2010;76:775–781. doi: 10.1016/j.ijrobp.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 26.Chapet O, Thomas E, Kessler ML, Fraass BA, Ten Haken RK. Esophagus-sparing with IMRT in lung tumor irradiation: and EUD-based optimization technique. Int J Radiation Oncol Biol Phys. 2005;63:179–187. doi: 10.1016/j.ijrobp.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 27.Rosenman JG, Halle JS, Socinski MA, Deschesne K, Moore DT, Johnson H, Fraser R, Morris DE. High-dose conformal radiotherapy for treatment of stage IIIA/IIIB non-small cell lung cancer: technical issues and results of a phase I/II trial. Int J Radiation Oncol Biol Phys. 2002;54:348–356. doi: 10.1016/s0360-3016(02)02958-9. [DOI] [PubMed] [Google Scholar]
- 28.Epperly MW, Bray JA, Kraeger S, Zwacka R, Engelhardt J, Travis E, Greenberger JS. Prevention of late effects of irradiation lung damage by manganese superoxide dismutase gene therapy. Gene Therapy. 1998;5:196–208. doi: 10.1038/sj.gt.3300580. [DOI] [PubMed] [Google Scholar]
- 29.Guo HL, Seixas-Silva JA, Epperly MW, Gretton JE, Shin DM, Greenberger JS. Prevention of irradiation-induced oral cavity mucositis by plasmid/liposome delivery of the human manganese superoxide dismutase (MnSOD) transgene. Rad Res. 2003;159:361–370. doi: 10.1667/0033-7587(2003)159[0361:porioc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 30.Epperly MW, Wegner R, Kanai AJ, Kagan V, Greenberger EE, Nie S, Greenberger JS. Irradiated murine oral cavity orthotopic tumor antioxidant pool destabilization by MnSOD-plasmid liposome gene therapy mediates tumor radiosensitization. Rad Res. 2007;267:289–297. doi: 10.1667/RR0761.1. [DOI] [PubMed] [Google Scholar]
- 31.Greenberger JS, Epperly M. Bone marrow-derived stem cells and radiation response. Semin Radiat Oncol. 2009;19:133–139. doi: 10.1016/j.semradonc.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenberger JS. Gene therapy approaches for stem cell protection. Gene Therapy. 2008;15:100–108. doi: 10.1038/sj.gt.3303004. [DOI] [PubMed] [Google Scholar]
- 33.Guo H, Epperly MW, Bernarding M, Nie S, Gretton J, Jefferson M, Greenberger JS. Manganese superoxide dismutaseplasmid/liposome (MnSOD-PL) interatracheal gene therapy reduction of irradiation-induced inflammatory cytokine does not protect orthotopic lewis lung carcinomas. In Vivo. 2003;17:13–22. [PubMed] [Google Scholar]
- 34.Epperly MW, DeFilippi S, Sikora C, Gretton J, Kalend K, Greenberger JS. Intratracheal injection of manganese superoxide dismutase (MnSOD) plasmid/liposomes protects normal lung but not orthotopic tumors from irradiation. Gene Therapy. 2000;7:1011–1018. doi: 10.1038/sj.gt.3301207. [DOI] [PubMed] [Google Scholar]
- 35.Oberley LW. Superoxide dismutase and cancer. In: Oberley LW, editor. Superoxide Dismutase. II. CRC Press; 1982. [Google Scholar]
- 36.Metz JM, Smith D, Mick R, Lustig R, Mitchell J, Cherakuri M, Glatstein E, Hahn SM. A phase I study of topical tempol for the prevention of alopecia induced by whole brain radiotherapy. Clin Cancer Res. 2004;10:6411–6417. doi: 10.1158/1078-0432.CCR-04-0658. [DOI] [PubMed] [Google Scholar]
- 37.Lee KC, Lukyanov AN, Gelb MH, Yager P. Formation of high axial ratio microstructures from peptides modified with glutamic acid dialkyl amides. Biochim Biophys Acta. 1998;1371:168–184. doi: 10.1016/s0005-2736(97)00267-8. [DOI] [PubMed] [Google Scholar]
- 38.Borisenko GG, Martin I, Zhao Q, Amoscato AA, Kagan VE. Nitroxides scavenge myeloperoxidase-catayzed thiyl radicals in model systems and in cells. J Am Chem Soc. 2004;4(30):9221–9232. doi: 10.1021/ja0495157. [DOI] [PubMed] [Google Scholar]
- 39.Jiang J, Stoyanovsky DA, Belikova NA, Tyurina YY, Zhao Q, Tungekar MA, Kapralova V, Huang Z, Mintz AH, Greenberger JS, Kagan VE. A mitochondria-targeted triphenylphosphoniumconjugated nitroxide functions as a radioprotector/mitigator. Radiat Res. 2009;172(6):706–717. doi: 10.1667/RR1729.1. [DOI] [PMC free article] [PubMed] [Google Scholar]