Abstract
South Chattanooga has been home to foundries, coke furnaces, chemical, wood preserving, tanning and textile plants for over 100 years. Most of the industries were in place before any significant development of residential property in the area. During the 1950s and 1960s, however, the government purchased inexpensive property and constructed public housing projects in South Chattanooga. Many neighborhoods that surround the Chattanooga Creek were previous dumping grounds for industry. Polycyclic aromatic hydrocarbons (PAHs) comprised the largest component of the dumping and airborne industrial emissions. To address the human exposure to these PAHs, a broad study of South Chattanooga soil contaminant concentrations was conducted on 20 sites across the city. Sixteen priority pollutant PAHs were quantified at two depths (0-10cm and 10-20cm) and compared against reference site soils, as well as to soils from industrially-impacted areas in Germany, China, and the US. From these data, the probability that people would encounter levels exceeding EPA Residential Preliminary Remediation Goals (PRG) was calculated. Results indicate that South Chattanooga soils have relatively high concentrations of total PAHs, specifically Benzo[a]pyrene (B[a]P). These high concentrations of B[a]P were somewhat ubiquitous in South Chattanooga. Indeed, there is a high probability (88%) of encountering soil in South Chattanooga that exceeds the EPA PRG for B[a]P. However, there is a low probability (15%) of encountering a site with ∑PAHs exceeding EPA PRG guidelines.
Keywords: Benzo(a)pyrene, Polycyclic aromatic hydrocarbons, PAHs, Residential preliminary remedial goal, South Chattanooga
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous contaminants in the environment. Anthropogenic sources of PAHs include the burning of fossil fuels, coal production, oil manufacturing, oil spills, wood preservation (creosote), tobacco smoke, and various forms of cooking. Natural sources include volcanoes and forest fires (Fouchecourt et al., 1999). Of the hundreds of known PAHs, sixteen have been designated High Priority Pollutants by the Environmental Protection Agency (EPA); they include: naphthalene (NAP), acenaphthylene (ACY), acenaphthene (ACE), fluorene (FLU), phenanthrene (PHEN), anthracene (ANTH), fluoranthene (FLTH), pyrene (PYR), benzo[a]anthracene (B[a]A), chrysene (CHRY), benzo[b]fluoranthene (B[b]F), benzo[k]fluoranthene (B[k]F), benzo[a]pyrene (B[a]P), benzo[g,h,i]perylene (B[ghi]P), indeno[1,2,3-c,d]pyrene (IND), and dibenz[a,h]anthracene (D[ah]A). These 16 PAHs are of environmental concern because of their potential toxicity in humans and other organisms and their prevalence and persistence in the environment. Several PAHs are probable or known carcinogens (IARC, 2006).
The tendency for PAHs to bind to particulate matter (Means et al., 1980; Doick et al., 2005; O’Halloran, 2006) allows them to be transported by air and water and settle out in soil and sediments (Witt and Siegel, 2000; Witter et al., 2003), which serve as contaminant sinks (O’Halloran, 2006). Polycyclic aromatic hydrocarbons can accumulate to dangerous levels near their industrial sources. Over 800 hazardous waste sites have reported an elevated presence (above natural background) of PAHs (HAZDAT, 2007).
One area affected by industrial contamination of PAHs is the watershed of Chattanooga Creek. Chattanooga Creek flows northward through Chattanooga, TN (Hamilton County), before its confluence with the Tennessee River. This area of the city is both industrial and residential. For many decades, the creek and its banks received industrial effluent and waste from several facilities (Dynamac Corporation, 1991). One facility, the Tennessee Products site (TP), was located adjacent to Chattanooga Creek. The company used the creek to dispose of coal tar and coal tar pitch (Weston, Inc., 1999). In 1997, the non-time-critical remediation of Chattanooga Creek began. This remediation was known as Phase I, where a one-mile stretch of creek was dredged and approximately 25,300 cubic yards of coal-tar deposits and contaminated sediments were removed from the creek (USEPA, 2007). Phase I was completed in November 1998. The second non-time-critical remediation (Phase II) began in 2005 and was completed in late 2007. The portion of the creek downstream of the Phase I remediation, approximately 1.5 miles, was dredged and the contaminated sediments removed (USEPA, 2007).
Reuse plans for the creek have been developed by the community and the Trust for Public Land (TPL) with the aid of an EPA Superfund Redevelopment Initiative (SRI) grant awarded to the City of Chattanooga (TPL, 2002). The likely plan includes the construction of a greenway along the creek, which would connect the neighborhoods and offer recreation and exercise opportunities for the residents. Health concerns remain because the floodplain soil was not remediated and seasonal flooding can redeposit any lingering contamination on the banks and throughout the neighborhoods. It is possible the reuse plan could increase the potential for contact with the contamination and the increased risk of exposure has not been addressed.
One way to evaluate the current contamination levels is to compare the values to the guidelines for residential soil offered in the Residential Preliminary Remedial Goals (PRG) from EPA Region IX. The values are based on exceeding a cancer risk of one in a million (10−6 ;Table 1). Though the PRGs are not considered regulatory, they are useful for determining the potential for risk (Hensley et al., 2007; Weinstein et al., 2010). Residential soils exceeding the PRGs may pose elevated exposure risks to humans.
Table 1.
Assigned Preliminary Remedial Goals (PRGs; mg kg−1 soil) and Toxic Equivalency Factors (TEFs) for 7 Priority PAHs (Nibet and LaGoy, 1992). NA = value not available.
| PAH | PRG | TEF |
|---|---|---|
| Acenaphthene (ACE) | 3700 | NA |
| Acenaphthylene (ACY) | NA | NA |
| Anthracene (ANTH) | 22000 | NA |
| Benzo[a]anthracene (B[a]A) | 0.62 | 0.1 |
| Benzo[a]pyrene (B[a]P) | 0.062 | 1.0 |
| Benzo[b]fluoranthene (B[b]F) | 0.62 | 0.1 |
| Benzo[k]fluoranthene (B[k]F) | 6.2 | 0.01 |
| Benzo[g,h,i]perylene (B[ghi]P) | NA | NA |
| Chrysene (CHRY) | 62 | 0.001 |
| Dibenz[a,h]anthracene (D[ah]A) | 0.062 | 1.0 |
| Fluoranthene (FLTH) | 2300 | NA |
| Fluorene (FLU) | 2700 | NA |
| Indeno[1,2,3-c,d]pyrene (IND) | 0.62 | 0.1 |
| Phenanthrene (PHEN) | NA | NA |
| Pyrene (PYR) | 2300 | NA |
| Naphthalene (NAP) | 56 | NA |
| ∑ PAHs | 70 | 2.311 |
In human health risk assessment, media concentrations can be converted to weighted values that better represent the potential for risk. Determining the carcinogenic potential or hazard of PAHs in combination can be very complex. In order to simplify determining the carcinogenic hazard associated with these combinations, the toxicity equivalency factor (TEF) methodology was developed. Toxic equivalency factors may be applied to the assessment of carcinogenic hazards from oral exposure (USEPA, 1993). The approach assigns TEFs to compounds based on estimates of their relative toxicities compared to a reference compound, which in the case of PAHs, is the known human carcinogen B[a]P (Table 1, Nisbet and LaGoy, 1992; USEPA, 1993; Chun-The et al., 2003).
The TEF method has been used to calculate the carcinogenic potential of each carcinogenic PAH (cPAH) and combination present at National Priority List (NPL) sites (Nisbet and LaGoy, 1992). Using EPA-derived TEF values, concentrations of cPAHs are converted to an equivalent concentration of B[a]P mixtures (EPA, 1993a). Again, the TEF values are an estimate of the relative carcinogenicity of a chemical compared to B[a]P. Benzo[a]pyrene was chosen as the reference compound because its risk as potential human carcinogen is well characterized (DHHS, 2011) and highest among PAHs (EPA, 2000). The cPAH toxicity equivalent of B[a]P is determined by multiplying the concentration of each PAH in a mixture by its corresponding TEF value, also known as the toxicity equivalent concentration (TEC; ATSDR, 1995a). The B[a]P equivalencies of each PAH are then summed to obtain the total amount of risk attributed to all cPAHs present on-site (EPA, 1993).
The objective of this study is to characterize the extent of contamination of the EPA’s 16 high priority PAHs and associated ∑TEFs across South Chattanooga. The locations across South Chattanooga were selected based on public use and proximity to Chattanooga Creek.
2. Materials and Methods
2.1 Solvents and standards
Acetone and hexane were ACS reagent grade (Fisher Scientific) or better, however, all solvents were pre-screened for PAHs. Acenaphthene (99.0%), acenaphthylene (90.9%), anthracene (99.6%), benzo[a]anthracene (99.0%), benzo[b]fluoranthene (99.5%), benzo[k]fluoranthene (99.0%), chrysene (99.0%), fluoranthene (98.5%), fluorene (99.0%), naphthalene (98.8%), phenanthrene, and pyrene were purchased from ChemService (West Chester, PA). Benzo[a]pyrene (99.0%), indeno[1,2,3-c,d]pyrene (100%), benzo[g,h,i]perylene (98.0%), and dibenz[a,h]anthracene (99.5%) were purchased from AccuStandard (New Haven, CT). Naphthalene-d8, acenaphthene-d10, phenanthrene-d10, chrysene-d12, and perylene-d12 were used as internal standards (2000 ug ml−1 each; Supelco, Bellefonte, PA).
2.2 Sampling sites
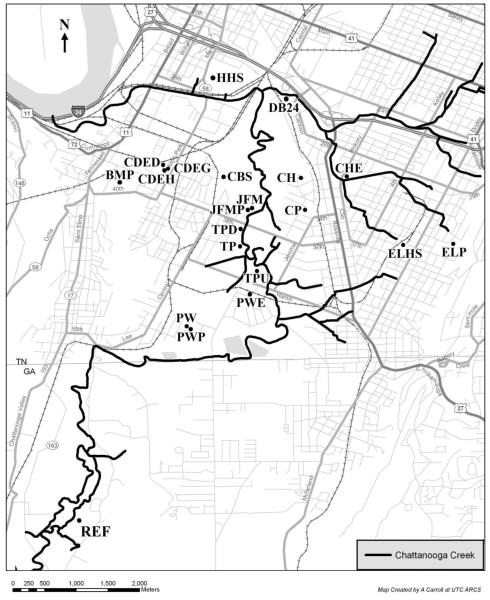
Twenty sites were selected across South Chattanooga based on public use and proximity to Chattanooga Creek (Figure 1). Nine of these sites were within the 100-year floodplain of Chattanooga Creek, 11 were not. Sampling consisted of three tiers: Event I in February 2005, Event II in May 2005, and Event III in March 2006. After each event samples were transported on dry ice and then stored at −80°C. None of the sampling events included field blanks. Event I was a broad screening of the area; all 20 sites were sampled with three replicates (for a total of 60 samples) by collecting surface (0-2 cm) soil with stainless-steel spatulas. Event II narrowed the study to soil cores at two depths (0-10 cm and 10-20 cm) at ten sites (5 replicates per depth for a total of 100 samples for Event II) and Event III focused on five of those sites (10 replicates per depth for a total of 100 samples for Event III). The purpose of this sampling strategy was to focus on the most contaminated sites and to cost-effectively increase the certainty of a conservative risk calculation (i.e., avoid false negatives) at those sites. In both Event II and III, soil cores were collected with a JMC Environmentalist’s Subsoil Probe (ESP; Clements Associates Inc., IA) with PETG Copolyester 48-inch liners. At each site, the first sampling location was randomly selected and then the subsequent samples were collected 50 m apart in two parallel transects. Additionally, a Reference Site located on Chattanooga Creek, 2.5 miles upstream of South Chattanooga in Georgia, was evaluated at the two depths by collecting three soil cores.
Figure 1.
Map of sampling locations in South Chattanooga, TN. Sites significantly greater than the reference site include (from highest to lowest): CBS, TP, BMP, DB24, CHE, TPD, TPU, CDED, ELHS, and HHS. Sites within the background level: CH, PW, and PWP.
2.3 Extraction and analysis
Soil samples were extracted by a method slightly modified from EPA SW-846 3540C. Briefly, approximately 10 g of wet soil was weighed out (±0.0001g) for each sample, mixed with 35 g anhydrous sodium sulfate, and loaded into the soxhlet extractors. Extraction solvent was 100 mL of acetone:hexane (1:1) and the soxhlet extractors cycled 4-6 times per hour for 16 hours. Solvent was reduced via rotary evaporation, quantitatively transferred into a 2 ml volumetric flask and brought to a final volume of 2 mL in hexane. Prior to final volume internal standards (IS) were added at a concentration of 1μg ml−1. The method was validated by preparing five soxhlet extractors with soil that was fortified at the second lowest reporting value and processed as stated above. Method soil extraction blanks (using previously soxhlet extracted soils) were extracted at minimum every 10 samples. Method solvent blanks were extracted at minimum every 15 samples. The frequency of the latter was dependent on the use of recently opened solvent containers.
Soil dry weights were determined with three replicates for each sample of approximately 1 g wet weight (±0.0001g) in pre-weighed aluminum weigh boats. Soils were placed in 105°C oven for 48 hours. Soils were cooled to room temperature and weighed again. Percent dry weights were determined by the following equation:
Samples were analyzed on a Varian 3400CX gas chromatograph (GC) coupled with a Saturn 2000 ion-trap mass-spectrometer (MS) (Varian, Walnut Creek, CA). Separation of the compounds was achieved with an Rxt®-5Sil MS (30 m, 0.25 internal diameter, 0.25 μm film thickness) capillary column (Restek, Bellefonte, PA). Injection volume was 2 μL (CombiPal; LTC Analytics, Switzerland) with the inlet in splitless mode. The inlet was set at 270°C, the transfer line at 280°C, the trap at 200°C, and the manifold at 80°C. The initial oven temperature was 50°C with a hold time of 2 minutes, then programmed to 160°C at 20°C/min, then to 210°C at 15°C/min, and then to 320°C at 5.5°C/min with a 3 min hold time. Total run time was 33.83 minutes.
There was a filament delay on the mass spectrometer for 6.0 min, after which the GC/MS was in full scan electron impact (EI) mode (70eV), scanning from 45- to 300 amu. Scan time was 2 μscans which translated into 0.57 seconds for a full scan (45- to 300 amu). The filament emission current was set at 25 μamps. The electron multiplier offset was set at +200 volts. The instrumental detection limit for all 16 compounds was obtained when all compounds had a signal to noise ratio of 3-5.
Instrument calibration was achieved with seven calibration standards: 0.01-, 0.05-, 0.1-, 0.5, 0.75-, 1.0-, and 2.5 ug ml−1 with peak area as the measured response Calibration and quantitation used the ratio of the IS response to the target compound response to correct for any instrumental deviations. Quantitation relied on the Varian GC/MS Workstation software operating in selected ion extraction mode using 2 to 3 major ions per compound. Additionally, intermittent (mid-range) standards were run every three to five samples to confirm the instrument’s accuracy within ±15% of the known concentration. If any compound was beyond this range, the instrument was recalibrated and all samples after the last accurate standard were reanalyzed.
2.4 Statistical analysis
Concentrations varied by several orders of magnitudes across the sites. As a result, prior to statistical analysis, data were normalized by taking the natural log of the concentrations and adding one. Two-way ANOVA was performed testing effect of site and depth on concentration and a one-way ANOVA was performed testing effect of site when depths were separated were carried out with SYSTAT® 12 (San Jose, CA). A one-tailed Dunnett’s Test was performed on both 0-10 cm and 10-20 cm layers to identify which sites were significantly greater (p=0.05) than the Reference Site (SYSTAT® 12, San Jose, CA; Zar, 1999). A Scheffé’s post-hoc test was performed between the 100-year floodplain sites (n=9) and non-floodplain sites (n=11) at a one-tailed p value of 0.05 (Zar, 1999).
3. Results and Discussion
3.1 Methods validation
The final GC temperature program was set when baseline (or 100%) separation of most compounds was achieved. In general, resolutions greater than 1.0 are considered adequate separation, although greater than 1.5 is preferred (Górecki, 2006). The three sets of compounds that did have minimal overlap were PHEN and ANTH, B[b]K and B[k]F, and B[ghi]P and D[ah]A. Their calculated resolutions and approximate percent separations were 1.27 and 98%, 1.15 and 95%, and 1.25 and 98%, respectively (Górecki, 2006).
The extraction method percent recoveries and coefficients of variation are shown in Table 2. None of the data was corrected for their percent recoveries. Pyrene had the lowest recovery at 67.4% (±5.0) and B[a]A had the highest at 94.5% (±8.8). Eleven of the 16 PAHs had extraction recoveries greater than 80%. Also of significance is the relatively low coefficient of variations demonstrating the precision of the method.
Table 2.
Method validation for the extraction technique.
| PAH | % Recovery (n = 5) |
Coefficient of Variation |
|---|---|---|
| Acenaphthene (ACE) | 85.1 | 7.1 |
| Acenaphthylene (ACY) | 83.2 | 6.9 |
| Anthracene (ANTH) | 82.8 | 6.1 |
| Benzo[a]anthracene (B[a]A) | 94.5 | 8.8 |
| Benzo[a]pyrene (B[a]P) | 88.1 | 7.5 |
| Benzo[b]fluoranthene (B[b]F) | 74.8 | 3.5 |
| Benzo[k]fluoranthene (B[k]F) | 75.3 | 5.3 |
| Benzo[g,h,i]perylene (B[ghi]P) | 85.8 | 10.6 |
| Chrysene (CHRY) | 83.6 | 8.1 |
| Dibenz[a,h]anthracene (D[ah]A) | 76.1 | 13.5 |
| Fluoranthene (FLTH) | 70.7 | 8.3 |
| Fluorene (FLU) | 90.2 | 5.8 |
| Indeno[1,2,3-c,d]pyrene (IND) | 83.4 | 5.9 |
| Phenanthrene (PHEN) | 90.4 | 7.3 |
| Pyrene (PYR) | 67.4 | 5.0 |
| Naphthalene (NAP) | 85.6 | 4.6 |
3.2 Contamination across South Chattanooga
Individual mean PAH concentrations, ∑PAHs, and ∑TEFs for each sampling site are given in Tables 3 and 4 for 0-10 cm and 10-20 cm layers, respectively. The values are reported on a dry-weight basis. To decrease the reporting of false positive results due to the varying soil composition throughout South Chattanooga, the reporting limits (RL) were set at 5 times the instrumental detection limits. The RL was 0.05 mg kg−1. Concentrations ranged over several orders of magnitude. Initially, a two-way ANOVA (p=0.05) was performed on the data testing the effect of site and depth and an interaction of the two on ∑PAH concentrations. There was not a significant interaction of site and depth (p=0.951), however, there was a significant effect of depth on concentration (p=0.009), so the depths (0-10 cm and 10-20 cm) were treated separately. One-way ANOVA was performed on each depth to test the effect of site on concentrations followed by a post-hoc Dunnett’s Test (p=0.05). The most contaminated sites included the Charles A. Bell School (CBS), the Tennessee Products Site (TP), which was the location of a coke production facility thought to be the main source of PAHs, Ben Miller Park (BMP), on Dobb’s Branch at the HWY 24 intersection before confluence with Chattanooga Creek (DB24), Clifton Hills Elementary (CHE), and both upstream and downstream of TP (TPU and TPD).
Table 3.
Mean concentrations (mg kg−1 soil) and ranges of PAHs, ∑PAHs and ∑TEFs at depth of 0-10 cm in soils collected from South Chattanooga and upstream Reference Site. ND= not detected. Bolding indicates value is significantly greater (Dunnett’s Test, p<0.05) than the Reference Site value.
| SITE | BMP | CBS | CDED | CDEG | CDEH | CH | CHE | CP | DB24 | ELHS | ELP |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| n=3a | n=18 | n=18 | n=3 | n=3 | n=3 | n=3 | n=3 | n=18 | n=8 | n=3 | |
| PAH(s) | |||||||||||
| NAP | 0.7 | 1.9 | 0.8 | <0.05 | <0.05 | <0.05 | 0.4 | <0.05 | 0.5 | 0.3 | 0.1 |
| <0.05-1.8 | ND-3.8 | 0.1-4.3 | <0.05-0.1 | <0.05-0.1 | ND-<0.05 | 0.3-0.6 | <0.05-<0.05 | <0.05-5.6 | <0.05-0.8 | <0.05-0.3 | |
| ACY | <0.05 | 2.0 | 0.1 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | 0.1 | <0.05 | <0.05 |
| <0.05-0.1 | <0.05-4.8 | <0.05-0.1 | <0.05-<0.05 | <0.05-<0.05 | ND-<0.05 | <0.05-<0.05 | <0.05-<0.05 | <0.05-0.3 | <0.05-0.1 | <0.05-<0.05 | |
| ACE | 0.7 | 0.2 | 0.2 | <0.05 | <0.05 | <0.05 | 0.2 | <0.05 | 0.2 | 0.1 | 0.2 |
| <0.05-1.3 | ND-0.6 | <0.05-0.9 | <0.05-<0.05 | <0.05-<0.05 | ND-ND | 0.1-0.2 | ND-<0.05 | ND-1.6 | ND-0.4 | ND-0.6 | |
| FLU | 0.7 | 0.3 | 0.2 | <0.05 | <0.05 | <0.05 | 0.2 | <0.05 | 0.3 | 0.1 | 0.2 |
| <0.05-1.1 | <0.05-0.8 | 0.1-0.9 | <0.05-<0.05 | <0.05-<0.05 | ND-<0.05 | 0.1-0.2 | <0.05-<0.05 | <0.05-2.2 | ND-0.4 | <0.05-0.5 | |
| PHEN | 6.6 | 2.7 | 2.7 | 0.4 | 0.4 | <0.05 | 3.0 | 0.3 | 3.1 | 1.6 | 1.2 |
| 0.2-11.5 | 0.4-6.0 | 0.7-7.6 | 0.3-0.4 | 0.3-0.5 | <0.05-0.1 | 1.9-4.0 | 0.3-0.4 | 0.3-9.4 | 0.1-6.5 | 0.1-3.5 | |
| ANTH | 1.1 | 2.2 | 0.4 | 0.1 | 0.1 | <0.05 | 0.4 | <0.05 | 0.5 | 0.2 | 0.3 |
| <0.05-1.8 | 0.1-9.9 | 0.1-1.3 | <0.05-0.1 | <0.05-0.1 | <0.05-<0.05 | 0.3-0.5 | <0.05-0.1 | <0.05-1.7 | <0.05-0.7 | <0.05-1.0 | |
| FLTH | 7.4 | 25.3 | 3.7 | 0.7 | 0.7 | 0.1 | 6.0 | 0.8 | 5.9 | 2.8 | 1.2 |
| 0.3-11.0 | 0.7-48.5 | 1.5-10.0 | 0.6-0.9 | 0.6-0.8 | 0.1-0.1 | 3.9-7.6 | 0.6-1.2 | 0.4-11.1 | 0.2-12.7 | 0.2-3.2 | |
| PYR | 5.9 | 23.8 | 2.8 | 0.7 | 0.6 | 0.1 | 5.0 | 0.7 | 4.7 | 2.6 | 1.1 |
| 0.2-8.8 | 0.2-47.5 | 1.2-6.0 | 0.5-0.8 | 0.5-0.7 | <0.05-0.1 | 3.3-6.2 | 0.5-1.1 | 0.3-8.8 | 0.2-11.6 | 0.2-2.9 | |
| B[a]A | 3.5 | 23.1 | 2.0 | 0.4 | 0.4 | <0.05 | 2.7 | 0.4 | 3.0 | 1.4 | 0.5 |
| 0.1-5.3 | 1.1-46.6 | 0.9-4.4 | 0.3-0.4 | 0.3-0.5 | <0.05-0.1 | 1.5-3.4 | 0.3-0.5 | 0.2-6.4 | 0.1-5.4 | 0.1-1.2 | |
| CHRY | 4.7 | 21.8 | 2.7 | 0.5 | 0.6 | 0.1 | 4.2 | 0.7 | 3.7 | 1.6 | 0.6 |
| 0.2-7.1 | 1.1-43.0 | 0.9-6.3 | 0.4-0.5 | 0.5-0.8 | 0.1-0.1 | 2.4-5.1 | 0.5-1.0 | 0.3-6.7 | 0.2-6.0 | 0.2-1.3 | |
| B[b]F | 3.2 | 37.1 | 2.9 | 0.3 | 0.4 | 0.1 | 2.6 | 0.7 | 4.3 | 2.2 | 0.5 |
| 0.2-4.9 | 0.8-67.0 | 1.1-6.5 | 0.3-0.4 | 0.4-0.5 | 0.1-0.1 | 1.4-3.2 | 0.6-1.0 | 0.4-7.7 | 0.2-9.7 | 0.2-1.1 | |
| B[k]F | 2.7 | 14.7 | 1.2 | 0.3 | 0.3 | <0.05 | 2.2 | 0.4 | 1.7 | 0.8 | 0.4 |
| 0.1-4.1 | 0.6-26.2 | 0.1-1.9 | 0.2-0.3 | 0.3-0.4 | <0.05-<0.05 | 1.0-2.8 | 0.3-0.6 | 0.2-3.7 | <0.05-3.2 | 0.1-0.9 | |
| B[a]P | 3.0 | 20.9 | 1.8 | 0.3 | 0.4 | <0.05 | 1.8 | 0.3 | 2.9 | 1.1 | 0.4 |
| 0.1-4.7 | 0.8-51.3 | 0.9-3.9 | 0.2-0.4 | 0.3-0.5 | <0.05-<0.05 | 1.2-2.1 | 0.2-0.4 | 0.2-6.6 | 0.2-3.8 | 0.1-1.1 | |
| B[ghi]P | 2.0 | 14.2 | 1.4 | 0.2 | 0.3 | <0.05 | 1.1 | 0.5 | 2.2 | 0.8 | 0.2 |
| 0.1-3.3 | 0.6-29.3 | 0.7-2.7 | 0.2-0.2 | 0.2-0.4 | <0.05-0.1 | 0.7-1.5 | 0.3-0.6 | 0.1-5.5 | 0.1-3.5 | 0.1-0.5 | |
| D[ah]A | 0.5 | 5.9 | 0.6 | 0.1 | 0.1 | <0.05 | 1.2 | 0.1 | 0.6 | 0.5 | 0.1 |
| 0.1-0.8 | 0.4-14.7 | 0.1-1.4 | <0.05-0.1 | 0.1-0.1 | <0.05-<0.05 | 0.1-1.9 | 0.1-0.2 | 0.2-1.1 | 0.1-1.6 | <0.05-0.2 | |
| IND | 1.7 | 11.7 | 1.2 | 0.2 | 0.3 | <0.05 | 0.9 | 0.2 | 1.7 | 0.7 | 0.3 |
| 0.1-2.8 | 0.6-22.4 | 0.5-2.6 | 0.2-0.2 | 0.3-0.4 | <0.05-<0.05 | 0.6-1.2 | 0.2-0.3 | 0.1-4.5 | 0.1-2.4 | 0.1-0.6 | |
|
| |||||||||||
| ∑PAHs | 44.5 | 208.0 | 24.6 | 4.1 | 4.7 | 0.6 | 31.9 | 5.3 | 35.4 | 16.7 | 7.4 |
| 1.7-68.0 | 7.9-405.4 | 10.7-55.9 | 3.4-4.8 | 3.8-5.7 | 0.4-0.6 | 18.8-40.3 | 3.9-7.7 | 2.9-73.6 | 1.6-68.8 | 1.3-18.6 | |
| ∑TEFs | 4.4 | 34.2 | 3.0 | 0.4 | 0.6 | 0.1 | 3.7 | 0.6 | 4.4 | 2.0 | 0.7 |
| 0.2-6.7 | 1.5-80.0 | 1.5-6.6 | 0.3-0.5 | 0.5-0.8 | 0.1-0.1 | 1.7-4.8 | 0.4-0.8 | 0.5-9.4 | 0.3-7.2 | 0.1-1.5 | |
| SITE | HHS | JFM | JFMP | PW | PWE | PWP | TP | TPD | TPU | REF |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| n=8a | n=3 | n=3 | n=3 | n=8 | n=8 | n=18 | n=8 | n=18 | n=3 | |
| PAH(s) | ||||||||||
| NAP | 0.1 | <0.05 | 0.1 | <0.05 | <0.05 | <0.05 | 0.8 | 0.2 | 0.5 | <0.05 |
| ND-0.5 | <0.05-<0.05 | <0.05-0.1 | <0.05-<0.05 | ND-<0.05 | <0.05-<0.05 | <0.05-4.0 | ND-0.9 | <0.05-1.8 | <0.05-<0.05 | |
| ACY | 0.2 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | 0.5 | 0.2 | 0.2 | <0.05 |
| ND-0.6 | <0.05-<0.05 | <0.05-<0.05 | ND-<0.05 | ND-<0.05 | ND-<0.05 | 0.1-1.0 | <0.05-0.4 | <0.05-0.7 | <0.05-<0.05 | |
| ACE | 0.1 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | 0.1 | <0.05 | 0.1 | <0.05 |
| ND-0.2 | <0.05-0.1 | ND-<0.05 | ND-ND | ND-<0.05 | ND-ND | <0.05-0.2 | ND-0.2 | ND-0.3 | <0.05-<0.05 | |
| FLU | 0.1 | 0.1 | <0.05 | <0.05 | <0.05 | <0.05 | 0.1 | 0.1 | 0.1 | <0.05 |
| ND-0.2 | <0.05-0.1 | <0.05-<0.05 | ND-ND | ND-<0.05 | ND-<0.05 | ND-0.5 | ND-0.3 | <0.05-0.3 | <0.05-<0.05 | |
| PHEN | 0.8 | 0.7 | 0.2 | <0.05 | 0.2 | 0.1 | 1.5 | 0.8 | 1.1 | 0.1 |
| 0.1-2.2 | 0.5-1.2 | 0.1-0.4 | <0.05-<0.05 | <0.05-1.4 | <0.05-0.1 | 0.2-4.0 | 0.1-1.9 | <0.05-3.1 | <0.05-0.1 | |
| ANTH | 0.3 | 0.1 | <0.05 | <0.05 | <0.05 | <0.05 | 0.6 | 0.4 | 0.4 | <0.05 |
| <0.05-0.8 | 0.1-0.2 | <0.05-<0.05 | ND-<0.05 | ND-<0.1 | ND-<0.05 | 0.1-1.5 | <0.05-1.3 | <0.05-1.6 | <0.05-<0.05 | |
| FLTH | 1.5 | 1.0 | 0.3 | <0.05 | 0.7 | 0.1 | 6.7 | 4.2 | 2.7 | 0.1 |
| 0.1-4.0 | 0.8-1.4 | 0.2-0.5 | <0.05-0.1 | <0.05-3.5 | <0.05-0.1 | 0.9-23.2 | 0.3-11.3 | 0.2-8.2 | <0.05-0.2 | |
| PYR | 1.2 | 0.8 | 0.3 | <0.05 | 0.6 | 0.1 | 5.7 | 3.6 | 2.3 | 0.1 |
| 0.1-3.1 | 07-1.1 | 0.2-0.4 | <0.05-0.1 | <0.05-2.9 | <0.05-0.1 | 1.2-17.2 | 0.3-10.1 | 0.1-6.9 | <0.05-0.1 | |
| B[a]A | 0.9 | 0.5 | 0.2 | <0.05 | 0.3 | <0.05 | 4.8 | 3.0 | 2.0 | <0.05 |
| 0.1-2.2 | 0.4-0.8 | 0.1-0.3 | ND-<0.05 | ND-1.1 | ND-<0.05 | 0.8-13.3 | 0.3-8.8 | 0.1-4.7 | <0.05-0.1 | |
| CHRY | 1.3 | 0.8 | 0.4 | <0.05 | 0.4 | 0.1 | 5.8 | 3.7 | 2.6 | 0.1 |
| 0.1-3.2 | 0.6-1.2 | 0.2-0.5 | <0.05-<0.05 | <0.05-1.6 | <0.05-0.1 | 0.9-20.2 | 0.4-11.9 | 0.2-6.3 | 0.1-0.2 | |
| B[b]F | 2.2 | 0.5 | 0.3 | <0.05 | 0.6 | 0.1 | 7.6 | 4.0 | 3.8 | 0.1 |
| 0.1-5.3 | 0.4-0.7 | 0.2-0.4 | <0.05-0.1 | ND-2.1 | <0.05-0.1 | 1.6-18.0 | 0.5-10.8 | 0.2-10.9 | 0.1-0.2 | |
| B[k]F | 0.7 | 0.4 | 0.2 | <0.05 | 0.2 | <0.05 | 3.8 | 2.5 | 1.5 | <0.05 |
| <0.05-1.8 | 0.3-0.6 | 0.1-0.3 | <0.05-<0.05 | ND-0.8 | <0.05-<0.05 | 0.8-15.4 | 0.2-8.8 | 0.1-4.6 | <0.05-<0.05 | |
| B[a]P | 1.6 | 0.5 | 0.3 | <0.05 | 0.3 | <0.05 | 5.7 | 3.4 | 2.5 | 0.1 |
| 0.1-4.3 | 0.4-0.7 | 0.2-0.3 | <0.05-0.1 | <0.05-1.3 | <0.05-0.1 | 0.6-11.6 | 0.2-9.2 | 0.1-6.2 | <0.05-0.2 | |
| B[ghi]P | 1.5 | 0.4 | 0.2 | <0.05 | 0.3 | <0.05 | 4.5 | 2.0 | 2.2 | 0.1 |
| 0.1-3.4 | 0.3-0.6 | 0.2-0.3 | <0.05-<0.05 | ND-1.1 | ND-0.1 | 0.4-9.3 | 0.1-5.6 | 0.1-6.7 | <0.05-0.2 | |
| D[ah]A | 0.5 | 0.1 | 0.1 | <0.05 | 0.2 | <0.05 | 3.1 | 1.1 | 1.2 | <0.05 |
| 0.1-1.2 | 0.1-0.1 | 0.1-0.1 | ND-<0.05 | ND-0.7 | ND-0.1 | 0.3-9.1 | 0.1-3.8 | 0.1-3.7 | <0.05-<0.05 | |
| IND | 1.4 | 0.4 | 0.3 | <0.05 | 0.2 | <0.05 | 4.4 | 1.7 | 2.0 | 0.1 |
| 0.1-3.4 | 0.3-0.6 | 0.3-0.3 | <0.05-<0.05 | <0.05-1.1 | <0.05-0.1 | 0.4-11.5 | 0.1-4.7 | 0.1-6.2 | <0.05-0.1 | |
|
| ||||||||||
| ∑PAHs | 14.4 | 6.4 | 3.0 | 0.3 | 4.2 | 0.6 | 55.6 | 30.9 | 25.1 | 1.0 |
| 0.7-33.0 | 4.9-9.4 | 2.1-3.8 | 0.1-0.5 | 0.1-17.7 | 0.2-1.1 | 9.4-140.0 | 2.7-85.2 | 1.6-69.6 | 0.4-1.4 | |
| ∑TEFs | 2.6 | 0.8 | 0.4 | 0.1 | 0.6 | 0.1 | 10.5 | 5.4 | 4.5 | 0.2 |
| 0.1-6.2 | 0.6-1.0 | 0.4-0.5 | <0.05-0.1 | <0.05-2.5 | <0.05-0.3 | 1.7-21.9 | 0.4-14.6 | 0.3-12.1 | <0.05-0.2 | |
Sites with a sample size of eight indicates the site was sample during events I and II, whereas a site with a sample size of 18 was sampled during all three events.
Table 4.
Mean concentrations (mg kg−1 soil) and ranges of PAHs, ∑PAHs and ∑TEFs at 10-20 cm depth in soils collected from South Chattanooga and upstream Reference Site. ND= not detected. Bolding indicates value is significantly greater (Dunnett’s Test, p<0.05) than the Reference Site value.
| SITE | CBS | CDED | DB24 | ELHS | HHS | PWE | PWP | TP | TPD | TPU | REF |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| n=18a | n=18 | n=18 | n=8 | n=8 | n=8 | n=8 | n=18 | n=8 | n=18 | n=3 | |
| PAH(s) | |||||||||||
| NAP | 1.2 | 0.6 | 0.2 | 0.2 | 0.1 | <0.05 | <0.05 | 0.5 | 0.1 | 0.3 | <0.05 |
| <0.05-6.3 | 0.1-3.2 | <0.05-0.5 | ND-0.4 | ND-0.5 | ND-<0.05 | ND-<0.05 | ND-1.7 | ND-0.4 | <0.05-0.9 | <0.05-<0.05 | |
| ACY | 1.5 | 0.1 | 0.1 | <0.05 | 0.2 | <0.05 | <0.05 | 0.5 | 0.1 | 0.1 | <0.05 |
| <0.05-10.6 | <0.05-0.1 | <0.05-0.2 | ND-<0.05 | <0.05-0.9 | ND-<0.05 | ND-ND | ND-2.3 | <0.05-0.3 | <0.05-0.9 | <0.05-<0.05 | |
| ACE | 0.5 | 0.1 | 0.1 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| ND-2.6 | <0.05-0.7 | <0.05-0.3 | ND-<0.05 | ND-0.1 | ND-ND | ND-ND | ND-0.2 | ND-0.1 | ND-0.1 | <0.05-<0.05 | |
| FLU | 0.5 | 0.2 | 0.2 | <0.05 | 0.1 | <0.05 | <0.05 | 0.1 | <0.05 | 0.1 | <0.05 |
| ND-2.4 | <0.05-0.9 | <0.05-0.4 | ND-0.1 | ND-0.2 | ND-<0.05 | ND-<0.05 | ND-0.3 | ND-0.1 | <0.05-0.2 | <0.05-<0.05 | |
| PHEN | 6.3 | 2.0 | 2.1 | 0.6 | 0.7 | 0.1 | <0.05 | 1.2 | 0.3 | 0.7 | 0.1 |
| <0.05-40.6 | 0.5-6.7 | <0.05-5.1 | ND-1.1 | <0.05-1.9 | <0.05-0.2 | <0.05-0.1 | <0.05-3.7 | 0.1-0.7 | <0.05-1.9 | 0.1-0.1 | |
| ANTH | 2.1 | 0.3 | 0.4 | 0.1 | 0.3 | <0.05 | <0.05 | 0.5 | 0.2 | 0.2 | <0.05 |
| <0.05-15.2 | 0.1-1.0 | <0.05-1.0 | ND-0.2 | <0.05-1.2 | ND-<0.05 | ND-<0.05 | ND-1.9 | ND-0.9 | <0.05-1.1 | <0.05-<0.05 | |
| FLTH | 21.6 | 2.8 | 4.2 | 0.7 | 1.4 | 0.2 | 0.1 | 5.8 | 1.7 | 1.8 | 0.1 |
| 0.2-127.3 | 0.8-6.5 | 0.1-10.7 | <0.05-0.9 | 0.1-4.4 | <0.05-0.4 | <0.05-0.1 | 0.1-23.8 | 0.1-5.6 | <0.05-7.0 | 0.1-0.2 | |
| PYR | 19.2 | 2.3 | 3.4 | 0.7 | 1.2 | 0.2 | <0.05 | 4.9 | 1.5 | 1.5 | 0.1 |
| 0.2-122.2 | 0.7-4.9 | 0.1-8.3 | <0.05-1.1 | 0.1-3.6 | <0.05-0.3 | <0.05-0.1 | 0.1-20.8 | 0.1-5.0 | <0.05-6.5 | 0.1-0.1 | |
| B[a]A | 15.0 | 1.6 | 2.3 | 0.5 | 1.1 | 0.1 | <0.05 | 4.6 | 1.4 | 1.5 | 0.1 |
| 0.2-104.0 | 0.5-3.0 | 0.1-5.6 | <0.05-0.9 | <0.05-3.7 | <0.05-0.3 | <0.05- <0.05 |
<0.05-21.2 | 0.1-4.0 | <0.05-5.4 | <0.05-0.1 | |
| CHRY | 13.4 | 2.0 | 2.5 | 0.4 | 1.5 | 0.1 | <0.05 | 5.0 | 1.5 | 1.9 | 0.1 |
| 0.2-86.4 | 0.6-4.1 | 0.1-6.1 | <0.05-0.6 | 0.1-5.2 | <0.05-0.3 | <0.05-0.1 | 0.1-21.1 | 0.1-4.2 | <0.05-7.0 | 0.1-0.2 | |
| B[b]F | 21.6 | 2.4 | 3.4 | 0.6 | 2.6 | 0.2 | 0.1 | 8.4 | 2.0 | 3.0 | 0.1 |
| 0.3-113.3 | 0.8-4.7 | 0.1-6.9 | <0.05-1.1 | 0.1-9.2 | <0.05-0.5 | <0.05-0.1 | 0.1-39.3 | 0.2-5.8 | <0.05-17.6 | 0.1-0.2 | |
| B[k]F | 8.6 | 0.8 | 1.2 | 0.3 | 1.0 | 0.1 | <0.05 | 3.4 | 0.8 | 1.1 | <0.05 |
| 0.1-52.3 | 0.1-1.5 | <0.05-2.9 | ND-0.7 | <0.05-3.5 | <0.05-0.2 | <0.05- <0.05 |
<0.05-16.4 | 0.1-2.1 | <0.05-5.9 | <0.05-<0.05 | |
| B[a]P | 13.0 | 1.5 | 2.1 | 0.4 | 2.1 | 0.1 | <0.05 | 6.7 | 1.4 | 2.1 | 0.1 |
| 0.1-62.6 | 0.5-2.7 | 0.1-5.0 | <0.05-1.1 | 0.1-7.7 | <0.05-0.2 | <0.05-0.1 | <0.05-34.7 | 0.1-4.3 | <0.05-34.7 | 0.1-0.1 | |
| B[ghi]P | 9.6 | 1.1 | 1.4 | 0.3 | 2.0 | 0.1 | <0.05 | 5.5 | 1.0 | 1.9 | 0.1 |
| 0.2-49.7 | 0.2-2.2 | <0.05-3.8 | ND-0.8 | 0.1-8.5 | <0.05-0.2 | <0.05-0.1 | 0.1-30.6 | 0.1-2.7 | <0.05-10.3 | 0.1-0.1 | |
| D[ah]A | 5.6 | 0.4 | 0.4 | 0.2 | 1.5 | 0.1 | <0.05 | 2.4 | 0.5 | 1.0 | <0.05 |
| 0.1-23.8 | 0.1-0.9 | <0.05-0.8 | ND-0.5 | 0.05-6.5 | ND-0.2 | <0.05-0.1 | 0.1-6.0 | 0.1-0.9 | <0.05-5.5 | <0.05-<0.05 | |
| IND | 8.8 | 1.0 | 1.1 | 0.3 | 1.9 | 0.1 | <0.05 | 4.7 | 0.8 | 1.7 | 0.1 |
| 0.1-51.0 | 0.2-1.8 | <0.05-3.0 | <0.05-0.7 | 0.1-8.0 | <0.05-0.2 | <0.05-0.1 | <0.05-23.8 | 0.1-2.3 | <0.05-9.0 | 0.1-0.1 | |
|
| |||||||||||
| ∑PAHs | 148.2 | 19.3 | 25.2 | 5.3 | 17.7 | 1.5 | 0.5 | 54.1 | 13.4 | 19.0 | 1.1 |
| 1.8-850.8 | 6.1-43.0 | 0.9-57.0 | 0.1-8.7 | 0.6-65.2 | 0.1-3.0 | 0.2-0.7 | 0.6-245.7 | 0.9-39.0 | <0.05-89.2 | 0.9-1.3 | |
| ∑TEFs | 23.2 | 2.5 | 3.2 | 0.8 | 4.1 | 0.2 | 0.1 | 10.8 | 2.4 | 3.8 | 0.2 |
| 0.3-114.0 | 0.7-4.5 | 0.2-7.1 | <0.05-1.7 | 0.1-16.3 | <0.05-0.5 | <0.05-0.2 | 0.1-48.0 | 0.2-6.3 | <0.05-18.8 | 0.1-0.2 | |
Sites with a sample size of eight indicates the site was sample during events I and II, whereas a site with a sample size of 18 was sampled during all three events.
3.3 Contamination in South Chattanooga relative to other studies
The Reference Site concentrations (∑PAHs mean = 1.1 mg kg−1; range = 0.5-1.4 mg kg−1) are characteristic of background levels. Menzie et al. (1992) reviewed several studies and found the range of PAH concentrations in rural areas to be 0.01- to 1.01 mg kg−1. Three sample sites in South Chattanooga were also in the background range for PAHs. These sites were Clifton Hills (CH), the Piney Woods Elementary School surrounding woodlot (PW), and the Piney Woods Elementary School Playground (PWP).
For a frame of reference, South Chattanooga ∑PAH and B[a]P concentrations were compared to other residential soils. Krauss and Wilcke (2003), measured ∑PAHs concentrations across different land uses in Bayreuth, Germany. Industrial sites, which included a former gas works site, three railroad areas and a landfill, had ∑PAHs concentrations (the sum of 20 PAHs) ranging from 2.4- to 48.9 mg kg−1 soil. The levels in the city-center ranged from 0.63- to 20.7 mg kg−1 soil (Krauss and Wilcke, 2003). In South Chattanooga 40.0% of sites had soil samples with ∑PAHs that exceeded Bayreuth’s highest residential ∑PAHs. Tang et al. (2005) examined soils from Beijing, China. Concentrations (the sum of 16 PAHs) in city soils were greatest on roadsides and service stations. The highest ∑PAHs concentration found in Beijing was 27.8 mg kg−1 soil, with the lowest being 0.22 mg kg−1 (Tang et al., 2005). In South Chattanooga (the sum of 16 PAHs) 35.0% of sites had soil samples with ∑PAHs exceeded Beijing’s highest ∑PAHs. New Orleans, LA, has been studied multiple times. Meilke et al. (2001) reported that inner-city soils had a total-PAH (the sum of 16 PAHs) median concentration of 3.73 mg kg−1 soil with a concentration range of 0.65- to 40.69 mg kg−1 soil (Mielke et al., 2001). Following Hurricane Katrina in September, 2005, Presley et al. (2006) measured soil from New Orleans for various contaminants and found the highest concentrations of B[a]A, B[b]F, and B[a]P to be 1.64-, 1.88-, and 1.26 mg kg−1 soil, respectively (Presley et al., 2006). The highest concentrations of those compounds in South Chattanooga were: B[a]A = 23.1 mg kg−1; B[b]F = 37.1 mg kg−1; and B[a]P = 20.9 mg kg−1, respectively. Indeed, in South Chattanooga, 36.6%, 56.6% and 53.3% of the sites had higher concentrations of B[a]A, B[b]F, and B[a]P, respectively, than the highest values found in New Orleans. In comparison to levels in other industrial areas, PAHs are at elevated concentrations at many locations across South Chattanooga.
In 2006, there was an ATSDR Health Consultation that evaluated ∑TEFs for PAHs in floodplain soils along Chattanooga Creek where the proposed greenway would be located. In the present study, the ∑TEF for TP was similar to values from samples taken near the same location for the 2006 ATSDR Health Consultation (ATSDR, 2006); however, the mean for CBS was twice the highest ∑TEF measured in that study. Several of the ∑TEF values in South Chattanooga are elevated relative to other cities. In a study by Saltiene et al. (2002), ∑TEFs were measured in soils assumed to be unaffected by industry from five different cities. In that study, the ∑TEF geometric mean values of samples collected in Chicago (USA; n=4) and London (England; n=3) were approximately 2.3- and 2.0 mg kg−1 soil with ranges 0.7- to 7.3 mg kg−1 soil and 0.4- to 11.6 mg kg−1 soil, respectively. The three other cities, Tallinn (Estonia), Helsinki (Finland), and Vilnius (Lithuania), had ∑TEF geometric means less than 0.8 mg kg−1 soil. The geometric mean of ∑TEFs across South Chattanooga was 1.6 mg kg−1 soil with a range of <0.05- to 113.9 mg kg−1 soil (Table 3) when the industrial site, TP, was excluded. The geometric mean in South Chattanooga is comparable to the geometric means of other cities; however, the upper range values are considerably higher for the ∑TEFs in the soils from the present study.
One consideration when applying TEFs is that risk may be overestimated (Nisbet and LaGoy, 1992). For example, the TEFs are based on oral exposure. If no oral exposure occurs, the risk is largely eliminated (unless inhalation or dermal exposure occurs). In addition, there are infinite combinations of PAHs within environmental mixtures and their interactions are not well understood. Bioavailability, competition for binding sites, and metabolism may also vary depending on the PAHs and their amounts present in the different mixtures (Nisbet and LaGoy, 1992).
3.4 Possible contamination source
The locations of the highest contamination are generally found within the 100-year floodplain of Chattanooga Creek (Figure 1). Of the ten sites located in the 100-year floodplain, eight sites were significantly greater than the Reference Site. Similarly, of the 11 sites not located in the 100-year floodplain, only one (Ben Miller Park) was significantly greater than the Reference Site. The Scheffé’s test compared ∑PAH concentrations in the surface layer at the 100-year floodplain sites and the non-100-year floodplain sites. There was a significant difference (p<<0.05) between the groups (with floodplain sites having the higher ∑PAH concentrations), indicating a positive relationship between contamination and flooding. Additionally, the contamination tended to be greater in the surface soils. This suggests that the input of PAHs is still occurring, providing further evidence that contamination can result from flooding. However, given that the non-floodplain concentrations were higher than the Reference Site (which is up-wind) and several other cities (discussed above), some PAH contamination must also be from atmospheric drift/deposition.
In order to address the potential risk of human exposure to relatively high concentrations of ∑PAH and B[a]P, we quantified the probability that South Chattanoogans would encounter ∑PAH or B[a]P on public property across South Chattanooga exceeding EPA Region IX Residential PRGs (Figures 2 and 3). The principle of the probabilistic approach is based on the premise that many parameters and measures are distributed in a consistent manner (following a normal or similar distribution) and that, from these distributions, it is possible to assign probabilities to a range of values (i.e., the likelihood that a measure will exceed a certain value; Bunce et al., 2000). This applies to concentrations of substances in the environment that are frequently log-normally distributed. Herein, the same premise was used to estimate the probability that a person living in South Chattanooga will be exposed to soil that exceeds EPA recommended safe limits (PRGs) for ∑PAH or B[a]P exposure. Thus, the probabilistic approach not only determines if there is a risk, but the probability of exceeding a risk threshold. This is in contrast to traditional deterministic risk assessment, which produces a binary (yes or no) answer to risk estimation. Although deterministic risk assessment is more common, the US EPA recommends using probabilistic risk assessments (USEPA, 1999).
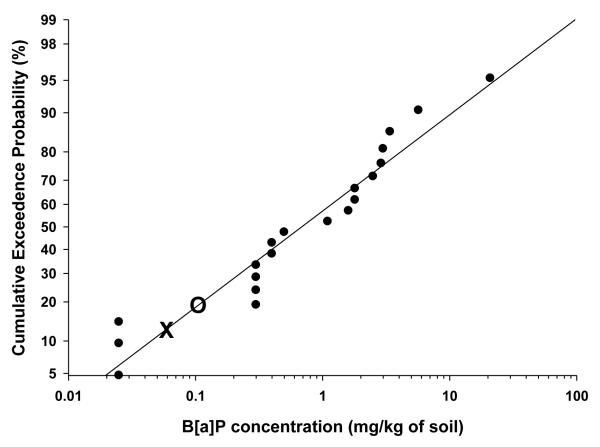
Figure 2.
Probability of encountering mean Benzo(a)pyrene (B[a]P) concentrations exceeding EPA residential EPA preliminary remediation goal (PRG). The PRG is 0.06 mg kg−1 for B[a]P (indicated by X). The mean value detected at reference sites is indicated by O. There is ≈88% probability that residents will encounter a site in South Chattanooga with total B[a]P concentrations exceeding EPA Residential PRGs.
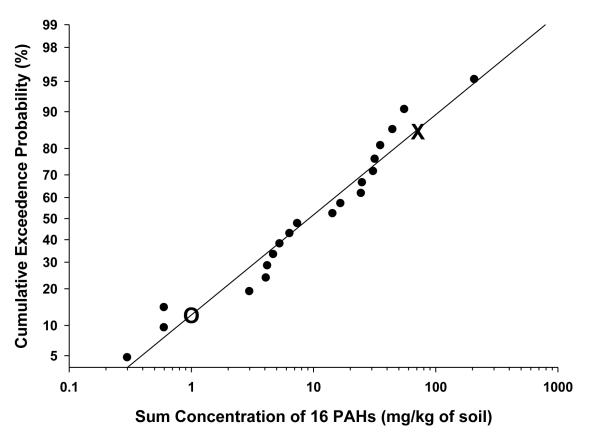
Figure 3.
Probability of encountering mean ∑PAHs (16 EPA priority pollutants) exceeding EPA residential preliminary remediation goal (PRG). The PRG is 70.0 mg kg−1 for ∑PAHs. (indicated by X). The mean value detected at reference sites is indicated by O. There is ≈15% probability that residents will encounter a site in South Chattanooga with ∑PAHs exceeding EPA Residential PRGs.
To graph the probabilistic estimate, all mean 0-10 cm B[a]P or ∑PAH concentrations were ranked from lowest to highest (e.g., CBS is highest and PW is lowest on Figure 3), converted to centiles (similar to the way that standardized testing scores are reported) and graphed against the corresponding B[a]P or ∑PAH concentration . The 0-10 cm values were used because data were available from all 20 sites (Event I), rather than just 10 sites (Events II and III) from the 10-20 cm values. Additionally, people would be most likely to contact the PAHs in the 0-10 cm soil core. The higher centile sites (those most contaminated) are represented at the top right-hand corner of Figure 2 and 3. The probability of humans encountering those higher centile sites would be the lowest. To determine the probability that someone would encounter a site in South Chattanooga with concentrations above EPA guidelines, the PRG for B[a]P or ∑PAH was superimposed on Figures 2 and 3, respectively. The percentile of sites above that PRG represents the probability of encounter.
As presented herein, the majority of locations in South Chattanooga exceed the EPA IX Residential B[a]P PRG. Indeed, based on that guideline, our data indicate there is approximately an 88% probability that a site in South Chattanooga exceeds the residential B[a]P PRG (Figure 2). However, the probability of encountering a site with ∑PAHs exceeding EPA PRG guidelines is approximately 15% (Figure 3). Again, our approach estimates the probability that people will encounter a site containing B[a]P or ∑PAH concentrations above EPA recommendations. As noted, although PRGs are not regulatory, they are useful for determining the potential for risk (Hensley et al., 2007; Weinstein et al., 2010). Thus, residential soils exceeding the PRGs may pose exposure risks to humans.
4. Conclusions
Concentrations of ∑PAHs and B[a]P are heterogeneous throughout the South Chattanooga floodplain and contamination tends to be higher in the surface (0-10 cm) soils though it exists at lower depths (10-20 cm). The more contaminated areas are closely associated with the Chattanooga Creek floodplain. Of the ten sites significantly greater than the Reference Site, eight are located in the 100-year floodplain. Similarly, of the ten sites not significantly greater than the Reference Site, only one was located in the floodplain. As a group, the floodplain sites are significantly greater than the non-floodplain sites. The present study indicates that South Chattanooga PAH soil concentrations are relatively high when compared with studies from other metropolitan areas. There is a high probability (88%) of encountering soil in South Chattanooga that exceeds the EPA Residential PRG for B[a]P. There is a low probability (15%) of encountering a site with ∑PAHs exceeding EPA PRG guidelines.
Acknowledgements
The authors would like to thank Clair Morris, Ben Paulson and Nellie Shaul from SIUE for their technical assistance with sample collection and analysis. We would also like to thank Andy Carroll from the UTC Office of Academic and Research Computing Services for his help with GIS mapping. We would finally like to thank Troy Keith from the Tennessee Department of Environment and Conservation for all of his logistical support. The study was financially supported primarily by a grant from the National Institutes of Health (1 R15 ESO13129-01). Partial funds were from a Research Grant for Graduate Students awarded by SIUE.
References
- ATSDR (Agency for Toxic Substances and Disease Registry) Toxicological Profile for Polycyclic Aromatic Hydrocarbons (PAHs) U.S. Department of Health and Human Services; 1995. p. 487. [PubMed] [Google Scholar]
- ATSDR . Health Consultation: Glover Site. Division of Health Assessment and Consultation; Atlanta, GA: 2006. [Google Scholar]
- Chun-The L, Yuan-Chung L, Wen-Jhy L, Perng-Jy T. Emission of Polycyclic Aromatic Hydrocarbons and Their Carcinogenic Potencies from Cooking Sources to the Urban Atmosphere. Environmental Health Perspectives. 2003;111:483–487. doi: 10.1289/ehp.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHHS (U.S. Department of Health and Human Services) The 12th Report on Carcinogens. National Institutes of Health, National Toxicology Program; 2011. p. 507. [Google Scholar]
- Doick K, Burauel P, Johnes K, Semple K. Distribution of Aged 14C-PCB and 14C-PAH Residues in Particle-Size and Humic Fractions of an Agricultural Soil. Environmental Science and Technology. 2005;39:6575–6583. doi: 10.1021/es050523c. [DOI] [PubMed] [Google Scholar]
- Dynamac Corporation . Field Investigation Report VIII work assignment No. r04019 Sampling and Analysis of Chattanooga Creek Chattanooga, Hamilton County, TN. ENFORCEMENT CONFIDENTAL. Chattanooga, Tn: 1991. pp. 1–60. [Google Scholar]
- EPA Carcinogenic Polycyclic Aromatic Hydrocarbons. Provisional Guidance for Quantitative Risk Assessment of Polycyclic Aromatic Hydrocarbons. 1993. p. 28.
- Fouchecourt MO, Arnold M, Berny P, Videmann B, Rether B, Riviere JL. Assessment of the Bioavailability of PAHs in Rats Exposed to a Polluted Soil by Natural Routes: Induction of EROD Activity and DNA Adducts and PAH Burden in Both Liver and Lung. Environmental Research Section A. 1999;80:330–339. doi: 10.1006/enrs.1998.3932. [DOI] [PubMed] [Google Scholar]
- Górecki T. Chromatography. In: Nollet LML, editor. Chromatographic analysis of the environment. CRC Press and Taylor and Francis; New York: 2006. pp. 133–176. [Google Scholar]
- Hensley AR, Scott A, Rosenfeld PE, Clark JJ. Attic dust and human blood samples collected near a former wood treatment facility. Environmental Research. 2007;105:194–9. doi: 10.1016/j.envres.2007.03.011. [DOI] [PubMed] [Google Scholar]
- HazDat . Database. Agency for Toxic Substances and Disease Registry (ATSDR); Atlanta, GA: [Accessed on: July 20, 2007]. 2007. < http://www2.atsdr.cdc.gov/gsql/sitecontam.script>. [Google Scholar]
- International Agency for Research on Cancer [Accessed on Oct 10, 2006];List of All Agents Evaluated to Date. 2006 < http://monographs.iarc.fr/ENG/Classification/index.php>.
- Krauss M, Wilcke W. Polychlorinated naphthalenes in urban soils: analysis, concentrations, and relation to other persistent organic pollutants. Environmental Pollution. 2003;122:75–89. doi: 10.1016/s0269-7491(02)00285-3. [DOI] [PubMed] [Google Scholar]
- Means JC, Wood SG, Hassett JJ, Banwart WL. Sorption of Polynuclear Aromatic Hydrocarbons by Sediments and Soils. Environmental Science and Technology. 1980;14:1524–1528. doi: 10.1021/es60172a005. [DOI] [PubMed] [Google Scholar]
- Menzie CA, Potocki BB, Santodonato J. Exposure to Carcinogenic PAHs in the environment. Environmental Science and Technology. 1992;26:1278–1284. [Google Scholar]
- Mielke H, Wang G, Gonzales C, Le B, Quach V, Mielke P. PAH and metal mixtures in New Orleans soils and sediments. The Science of the Total Environment. 2001;281:217–227. doi: 10.1016/s0048-9697(01)00848-8. [DOI] [PubMed] [Google Scholar]
- Nisbet IC, LaGoy PK. Toxic Equivalency Factors (TEFs) for Polycyclic Aromatic Hydrocarbons (PAHs) Regulatory Toxicology and Pharmacology. 1992;16:290–300. doi: 10.1016/0273-2300(92)90009-x. [DOI] [PubMed] [Google Scholar]
- O’Halloran K. Toxicological Considerations of Contaminants in the Terrestrial Environment for Ecological Risk Assessment. Human and Ecological Risk Assessment. 2006;12:74–83. [Google Scholar]
- Presley SM, Rainwater TR, Austin GP, Platt SG, Zak JC, Cobb G.P, Marsland, E.J., Tian K, Zhang B, Anderson TA, Cox SB, Abel MT, Leftwich BD, Huddleston JR, Jeter RM, Kendall RJ. Assessment of pathogens and toxicants in New Orleans, LA following Hurricane Katrina. Environmental Science and Technology. 2006;40:468–474. doi: 10.1021/es052219p. [DOI] [PubMed] [Google Scholar]
- Saltiene Z, Brukstiene D, Ruzgyte A. Contamination of Soil by Polycyclic Aromatic Hydrocarbons in Some Urban Areas. Polycyclic Aromatic Compounds. 2002;22:23–35. [Google Scholar]
- Tang L, Tang X, Zhu Y, Zheng M, Miao Q. Contamination of polycyclic aromatic hydrocarbons (PAHs) in urban soils in Beijing, China. Environment International. 2005;31:822–828. doi: 10.1016/j.envint.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Trust for Public Land Tennessee Products Superfund Redevelopment Initiative: Reuse Plans for the Tennessee Products Superfund Site & the Chattanooga Coke State Superfund Site. 2002. pp. 1–35. SRI.
- USEPA . Provisional Guidance for the Quantitative Risk Assessment of Polycyclic Aromatic Hydrocarbons. Office of Research and Development; Washington, D.C.: 1993. EPA/600/R-93/089. [Google Scholar]
- USEPA [Accessed: May 15, 2007. Last updated: July 30, 2007];Tennessee NPL/NPL Caliber Cleanup Site Summaries. 2007 http://www.epa.gov/region04/waste/npl/npltn/tennprtn.htm.
- Weinstein JE, Crawford KD, Garner TR, Flemming AJ. Screening-level ecological and human health risk assessment of polycyclic aromatic hydrocarbons in stormwater detention pond sediments of Coastal South Carolina, USA. Journal of Hazardous Materials. 178:906–16. doi: 10.1016/j.jhazmat.2010.02.024. [DOI] [PubMed] [Google Scholar]
- Weston, Inc. Ecological Risk Assessment for the Tennessee Products Site Chattanooga, Tennessee. Norcross, GA: 1999. [Google Scholar]
- Witt G, Siegel H. The Consequences of the Oder Flood in 1997 on the Distribution of Polycyclic Aromatic Hydrocarbons (PAHs) in the Oder River Estuary. Marine Pollution Bulletin. 2000;10:1124–1131. [Google Scholar]
- Witter B, Winkler M, Friese K. Depth Distribution of Chlorinated and Polycyclic Aromatic Hydrocarbons in Floodplain Soils of the River Elbe. Acta Hydrochmica et Hydrobiologica. 2003;31:411–422. [Google Scholar]