Abstract
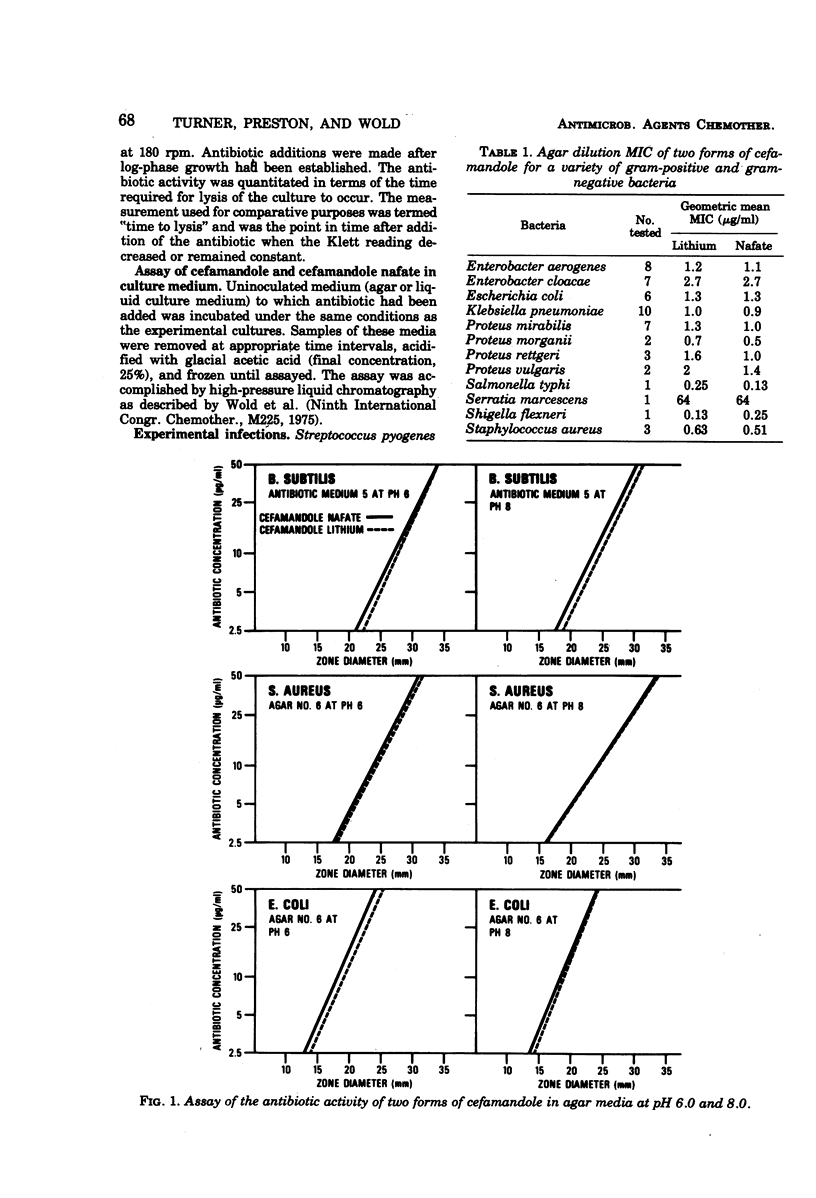
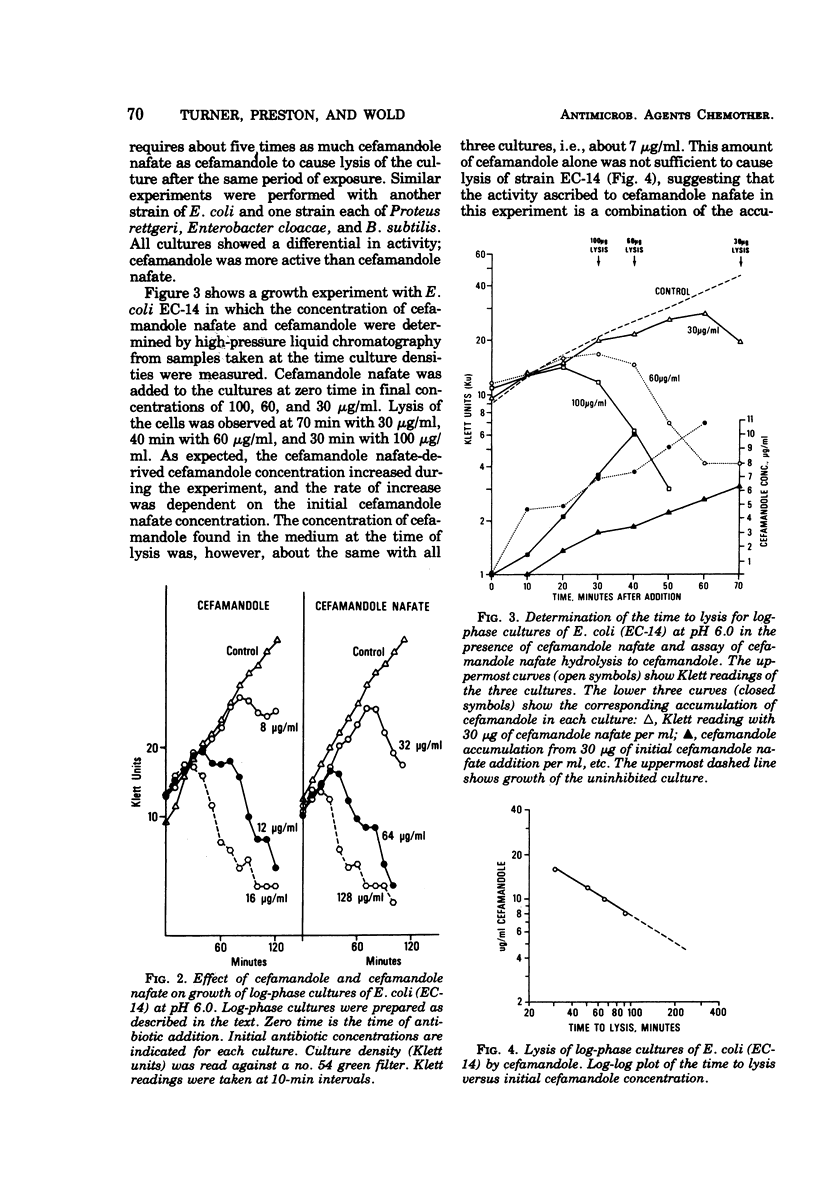
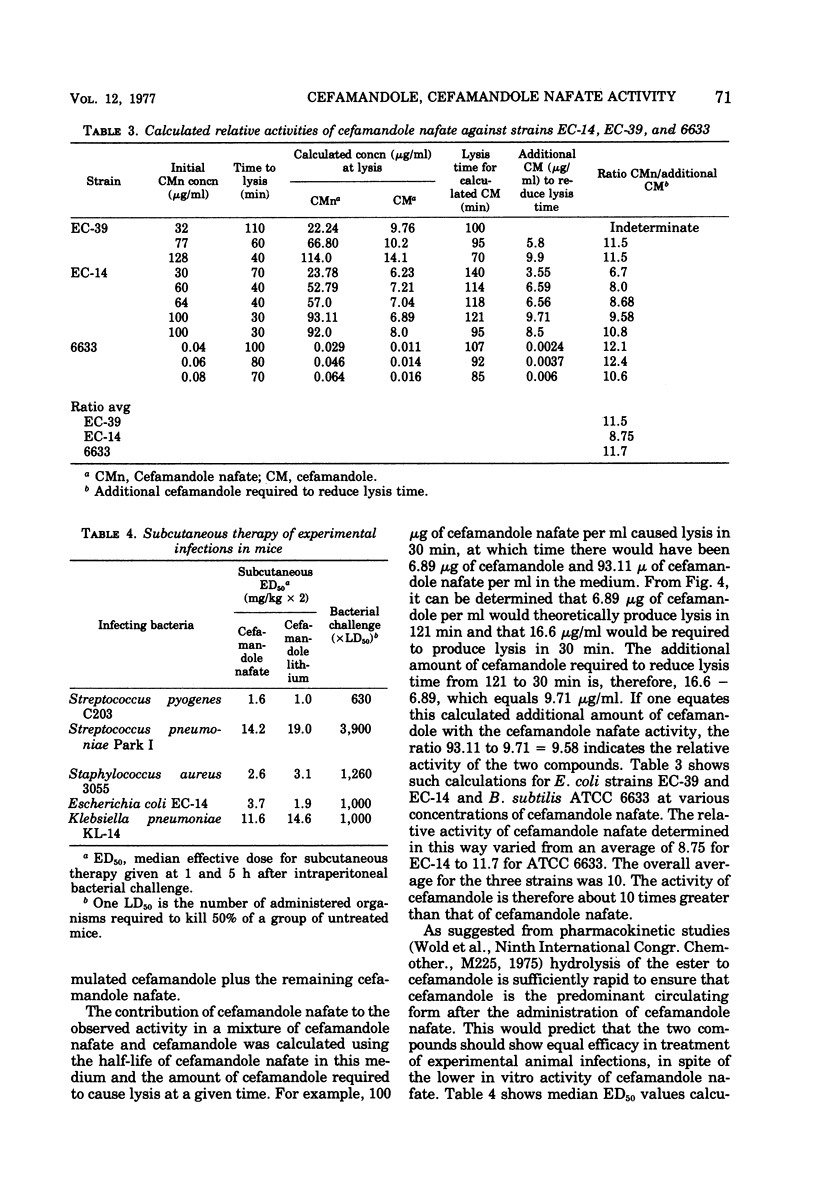
By conventional laboratory evaluation procedures, the in vitro antibacterial activities of cefamandole and its O-formyl ester, cefamandole nafate, appear virtually identical. When the activities of these two compounds were examined for their ability to lyse log-phase cultures of susceptible bacteria, however, cefamandole was found to be about 10 times more active than cefamandole nafate. Cefamandole nafate was shown to be rapidly converted to cefamandole in bacteriological media, with a half-life of less than 1 h at a pH of 7.0 or above. At pH 6.0, in log-phase inhibition experiments, however, cefamandole nafate is more stable, allowing delineation of the activity between cefamandole and cefamandole nafate. The efficacy of cefamandole was identical to that of cefamandole nafate in treating experimental animal infections, indicating that rapid conversion of cefamandole nafate to cefamandole occurs in vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ericsson H. M., Sherris J. C. Antibiotic sensitivity testing. Report of an international collaborative study. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;217(Suppl):1+–1+. [PubMed] [Google Scholar]
- Greenwood D., O'Grady F. Comparison of the responses of Escherichia coli and proteus mirabilis to seven beta-lactam antibodies. J Infect Dis. 1973 Aug;128(2):211–222. doi: 10.1093/infdis/128.2.211. [DOI] [PubMed] [Google Scholar]
- Indelicato J. M., Wilham W. L., Cerimele B. J. Conversion of cefamandole nafate to cefamandole sodium. J Pharm Sci. 1976 Aug;65(8):1175–1178. doi: 10.1002/jps.2600650811. [DOI] [PubMed] [Google Scholar]
- Pfeiffer R. R., Engel G. L., Coleman D. Stable antibiotic sensitivity disks. Antimicrob Agents Chemother. 1976 May;9(5):848–851. doi: 10.1128/aac.9.5.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick W. E., Preston D. A. Biological properties of three 3-heterocyclic-thiomethyl cephalosporin antibiotics. Antimicrob Agents Chemother. 1972 Mar;1(3):221–234. doi: 10.1128/aac.1.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]



