Abstract
The aim of our study was to conduct a meta-analysis of reports published on hepatic resection for colorectal liver metastases (CRLM) and determine if a negative margin ≥1 cm confers a disease free survival (DFS) advantage over sub-centimeter negative margins. The 357 initially selected articles were screened to identify 90 articles of interest of which eleven were finally included in the meta-analysis. Patients with positive margins were excluded from the meta-analysis. Meta-analysis was performed using STATA 9.2 statistical software. A total of 1,989 patients with negative margins from the eleven studies were included in the meta-analysis. The 5-year DFS for all patients was 27.9 % (95 % CI 23.5 % to 32.2 %). The 5-year DFS for ≥1 cm negative margin subgroup was 34.4 % (95 % CI 27.97 % to 40.7 %) when compared to 21.0 % (95 % CI 17.4 % to 24.7 %) for <1 cm negative margin subgroup. The odds ratio for ≥1 cm negative margins was found to be 0.552 (95 % CI 0.408 to 0.747, p < 0.001) when compared to <1 cm negative margins. The results of this meta-analysis demonstrate that in patients undergoing hepatic resection for CRLM, a negative margin ≥ 1 cm may confer a better DFS compared with a sub-centimeter negative margin.
Keywords: Colorectal liver metastases, Meta-analysis, Margins of resection
Introduction
Hepatic resection remains a well accepted modality in the treatment of patients with colorectal liver metastases (CRLM) with 5 year overall survival (OS) ranging from 37 % to 58 % [1–4] and 5 year disease free survival (DFS) ranging from 11 % to 50 % [3, 5–14]. Recurrence of the disease after resection still remains a challenge and adversely affects the long term outcomes. Most of the factors associated with recurrence are related to the biology of the disease including primary tumor stage, disease free interval of less than 12 months, preoperative carcinoembryonic antigen (CEA) levels, number & size of metastatic tumors and presence of extra-hepatic disease [2, 15].
Surgical resection margin is also considered to be an additional prognostic factor. Although there is a consensus that positive margins portend a worse DFS as compared to negative margins, the extent of negative margins remains controversial. Several studies have documented a DFS advantage of ≥1 cm negative margins over <1 cm negative margins of resection [5, 8, 10, 13]. Whereas others have concluded that the width of negative margin has no influence on DFS [3, 6, 7, 9–11, 14]. Many authors have argued that tumor biology rather than the extent of surgical margin determines outcomes in these patients and the debate continues. Most of the data is derived either from single institutional studies or from select few multi-institutional studies. In such a scenario a meta-analysis may help provide useful clinical data.
It has been recently shown that in patients undergoing hepatic resection for CRLM a negative margin ≥1 cm confers an OS advantage when compared to sub-centimeter negative margins [16]. The aim of the current study was to perform a meta-analysis of studies published on CRLM (using surgical resection as a primary mode of treatment) to determine if negative resection margin of ≥1 cm confers a DFS advantage over negative resection margin of <1 cm.
Materials and Methods
Search Strategy and Inclusion/Exclusion Criteria
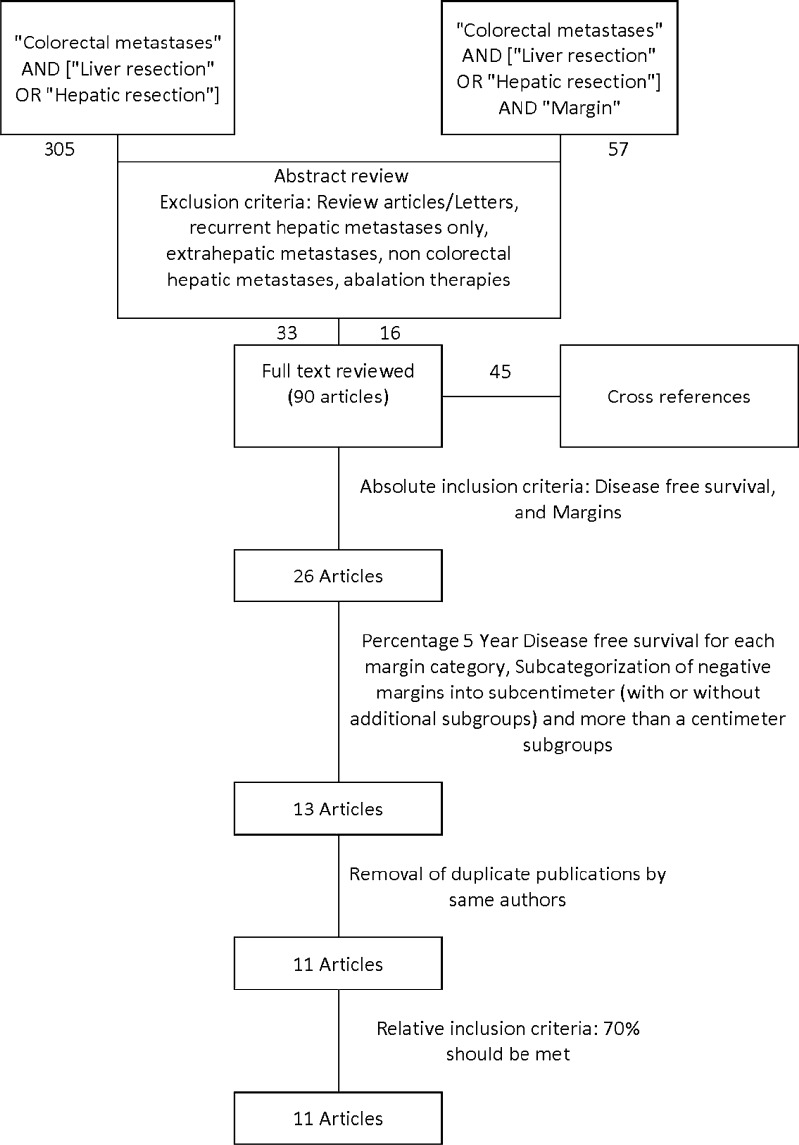
Articles in PubMed database from 1966 to the present were searched using the key words 1) “Colorectal metastases” AND [“Liver resection” OR “Hepatic resection”] 2) Search 1 AND “margins”. The search was restricted to articles published in English language. We identified 357 articles with some overlap between the two categories. A backward search was also performed using cross references from the bibliographies of relevant articles and review articles to ensure a comprehensive search. Figure 1 depicts the search strategy in detail.
Fig. 1.
Meta-analysis flow diagram depicting selection methodology for the eligible studies
The following articles were excluded from the analysis
Review articles/Letters
Articles analyzing patients with recurrent hepatic metastases only
Articles including patients with non colorectal hepatic metastases
Articles including patients mainly treated with ablation therapies such as cryotherapy, radiofrequency ablation or chemoembolization
Articles comparing patients who underwent resection, to patients who underwent no treatment or non-resectional treatment
In order to select relevant and high quality articles we further sub-stratified our inclusion criteria into absolute and relative inclusion criteria.
Absolute criteria for inclusion were as follows:
Inclusion of margin of resection as a variable in outcome analysis
Calculation of percentage 5-year DFS with margin of resection as a variable
Sub-categorization of negative margins into sub-centimeter (with or without additional sub-groups) and more than a centimeter sub-groups
Studies should meet at least 70 % (5 out of 7) of the relative criteria which were defined as:
Clear statement of study hypothesis
Clear statement of the main outcome of the study
Description of the demographic characteristics of the study population
Description of operative mortality
Description of length of follow up
Description of percentage of patients who were lost to follow up
Full texts were reviewed for 90 articles. Duplicate publications by the authors on the same dataset i.e. patients operated during the same duration, were excluded [17, 18]. In such cases the most recent publication of the authors was included in the analysis [7, 11].
Data Extraction and Definitions
Estimates of 5-year DFS and the number of patients (including patients in each margin sub-category) were extracted from the studies (texts or tables). Patients with positive margins were excluded from the current meta-analysis and only patients with negative margins were included. Many studies sub-stratified sub-centimeter negative margins into different sub-categories [4, 8, 10]. For these studies, a weighted mean was calculated using the number of patients in each sub-category and the corresponding percentage survival, to compute percentage survival for the whole <1 cm negative margin sub-group. Some studies [3, 5–7] categorized negative margins as “≤1 cm and >1 cm” while others [4, 8–14] used “<1 cm and ≥1 cm” for negative margins. For the purpose of meta-analysis ≤1 cm and <1 cm were treated as “<1 cm” and > 1 cm or ≥1 cm were treated as “≥1 cm”. Such sub-categorization was performed due to lack of individual patient data. This will lead to misclassification of some patients with exactly 1 cm margins into “<1 cm subcategory” and will bias our meta-analysis towards good outcome in <1 cm margins. However, it appeared to be the more conservative approach. This approach was chosen as it will increase the power and precision of our meta-analysis by allowing us to include more studies. Meta-analysis was performed using the number of patients with evidence of recurrent disease vs. patients who were disease free at 5-years in the “<1 cm” and “≥ 1 cm” subgroups.
Statistical Methods
Meta-analysis was performed using STATA 9.2 statistical software (StataCorp, 4905 Lakeway drive, College Station, Texas). All data were treated as binary (Disease free vs. evidence of recurrent disease at 5 years, <1 cm vs. ≥1 cm). An estimate of the number of patients who were disease free at 5 years was calculated by multiplying the total number of patients in <1 cm and ≥ 1 cm sub-categories included in the study by the corresponding 5 year DFS estimate. Odds ratios and 95 % confidence intervals computed from the binary data were used for the final meta-analysis. Random effects model was used due to our suspicion of heterogeneity amongst different studies. Heterogeneity was explored using the chi-squared test with a significance level of p = 0.10. I2 was calculated to further quantify heterogeneity. Publication bias was explored using funnel plots and symmetry of the funnel plot was analyzed using objective tests such as Egger and Begg tests.
Results
After the application of the absolute inclusion criteria, relative inclusion criteria and removal of duplicate publications, 11 studies [3, 5–14] were included in the meta-analysis. Table 1 summarizes the study characteristics of the final 11 studies included in the meta-analysis. All studies were retrospective analyses. A total of 1,989 patients with negative margins from the eleven studies were included in the meta-analysis with individual studies contributing 58 to 259 patients. Median or mean age for most of the studies was in 50’s or 60’s with majority reporting a mean or median follow up period of at least 2 years. Table 2 summarizes the different studies, number of patients with data on negative margins and estimates of disease free survival in each individual study. Estimates of the 5-year DFS ranged from 11 % to 28 % for the <1 cm negative margin subgroup and 20 % to 42 % for the ≥1 cm subgroup.
Table 1.
Important characteristics of studies included in the meta-analysis
| First author | Year | Type of study | Number of patients | Number of patients with data on negative margins | Age | Subastratification of margins | Duration of follow up |
|---|---|---|---|---|---|---|---|
| Muratore et al. | 2009 | Retrosepective, January 1999 to December 2007 | 314 | 259 | Mean age 62.8 years (95 % CI 61.7–63.9) | Negative <=1 cm, Negative >1 cm | Mean- 39.8 months |
| Vandeweyer et al. | 2009 | Retrospective, February 1992 to December 2007 | 261 | 194 | Median age 64 years | Positive, 0-1 mm, >1 mm-<4, 4-<10 mm, >=10 mm | Median - 4.7 years |
| Nuzzo et. al | 2008 | Retrospective study from a Prospective database, January 1992–December 2005 | 185 | 174 | Mean age 61 years (range 32–81 years) | Positive, Negative( <=2 mm, 3-5 mm, 6-9 mm, >=10 mm) | Mean - 39 months |
| Wakai et. al | 2008 | Retrospective, January 1989–December 2004 | 90 | 80 | Median age 64 years (range 32–80 years) | 0 (Positive), <1 cm, >=1 cm | Median - 127 months |
| Hamady ZZR et al. | 2006 | Retrospective, January 1993 and December 2001 | 293 | 187 | Mean age 61 years (range 38–80 years) | Positive, Negative (=1 mm vs >1 mm, 1–2 mm vs >2 mm, 1–5 mm vs >5 mm, 1–10 mm vs. >10 mm | Minimum −29 months |
| Kokudo et al. | 2002 | Retrospective, January1, 1980 to December 31, 2000 | 194 | 183 | Mean age 59 years (range 35 to 82 years) | Negative <2 mm, 2–4 mm, 5–9 mm and >=10 mm) | Median −29.1 months |
| Minagawa et. al. | 2000 | Retrospective 1980 to 1997 | 235 | 145 | Median age 59.2 years (range 30–80 years) | Unknown, <1 cm, >=1 cm | Median −28 months, mean- 43 months |
| Iwatsuki et al. | 1999 | Retrospective, Year 1981 to 1996 | 305 | 277 | Mean ± S.E. (60 ± 0.6 years) range 26 to 82 years | Positive (involved), >1 cm, <=1 cm | Median - 32 months |
| Scheele et al. | 1995 | Retrospective, 1960 to 1992 | 434 | 350 | Median age 59 years (range 26–91 years) | 1–9 mm, >=10 mm | All patients followed up to Nov of 1993. Median duration of follow up not specified |
| Cady et al. | 1992 | 142 | 58 | Median 61 years (range 31–80 years) | Positive, Negative >=1 cm, Negative < 1 cm, unknown | Minimum - 12 months | |
| van Ooijen et al. | 1991 | Retrospective, 1979 to 1989 | 118 | 82 | Median age 57 (range 28–83 years) | Positive, <1 cm, >1 cm | Median - 18 months |
Table 2.
Summary of data on disease free survival for studies included in the meta-analysis
| < 1 cm | ≥ 1 cm | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| First author | Patients with data on negative margins | n | One-year DFS (%) | Three-year DFS (%) | Five-year DFS (%) | n | One-year DFS (%) | Three-year DFS (%) | Five-year DFS (%) |
| Muratore et al. | 259 | 175 | – | 30.2 | 23.5 | 84 | – | 37.3 | 28.2 |
| Vandeweyer et al. | 194 | 107 | 58 | 28 | 20 | 87 | 64 | 41 | 29 |
| Nuzzo et. ala | 174 | 64 | – | – | 11.4 | 110 | – | – | 41.6 |
| Wakai et. al | 80 | 51 | – | – | 19 | 29 | – | – | 40 |
| Hamady ZZR et al. | 187 | 129 | – | – | 28 | 58 | – | – | 31 |
| Kokudo et al.a | 183 | 134 | 62 | 34.2 | 24.3 | 49 | 78.3 | 50.1 | 50.1 |
| Minagawa et. al. | 145 | 118 | – | 30 | 24 | 27 | – | 27 | 27 |
| Iwatsuki et al. | 277 | 147 | – | 30.2 | 22.5 | 130 | – | 38.5 | 28.9 |
| Scheele et al. | 350 | 204 | – | 38 | 28 | 146 | – | 46 | 33 |
| Cady et al. | 58 | 33 | – | – | 18 | 25 | – | – | 50 |
| van Ooijen et al. | 82 | 38 | – | – | 13 | 44 | – | – | 20 |
a For these studies different sub-centimeter sub-categories were pooled to compute percentage survival for “< 1 cm subgroup”
The 5-year DFS for all patients was 27.9 % (95 % CI 23.5 % to 32.2 %). The 5-year DFS for ≥1 cm negative margin subgroup was 34.4 % (95 % CI 27.97 % to 40.7 %) when compared to 21.0 % for <1 cm negative margin subgroup (95 % CI 17.4 % to 24.7 %).
Meta-Analysis and Publication Bias
All Studies
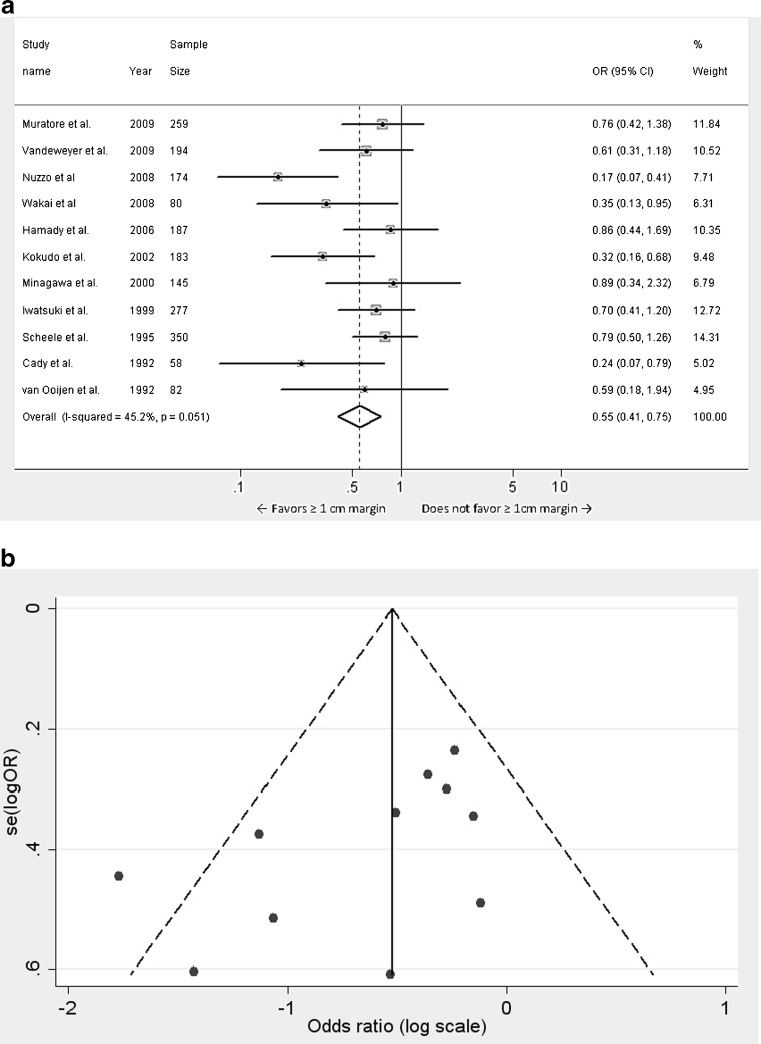
The odds ratio for ≥1 cm negative margins was found to be 0.552 (95 % CI 0.408 to 0.747, p < 0.001) when compared to <1 cm negative margins (Fig. 2a). A statistically significant improvement in 5 year DFS was seen in patients with negative margins ≥1 cm compared to patient with <1 cm negative margins. There was statistically significant heterogeneity amongst the studies (p = 0.051) and the contribution of heterogeneity to variation in odds ratio was 45.2 %. Publication bias was tested using Funnel plots (Fig. 2b) and multiple tests including egger test (Coef. = −2.538, Std. Err. = 1.211, t = −2.1, p = 0.066) and begg test (adj. Kendall’s score = −23, Std dev. = 12.85, z = −1.79, p = 0.073). Although these tests do not rule out publication bias in its entirety, it appears that publication bias in the selected studies is less prominent.
Fig. 2.
a Meta-analysis of influence of surgical margin on disease free survival after hepatic resection of colorectal metastases: all 11studies. (Odds ratios were calculated using the random effects model. Large diamond at the bottom represents the overall effect of negative margins on the disease free survival. Small diamonds embedded in squares represent the individual study effects with 95 % confidence intervals represented by the horizontal bars). b Funnel plot for meta-analysis of 11 studies depicting one notably outlier study
Studies with Sample Size of More Than 100 Patients
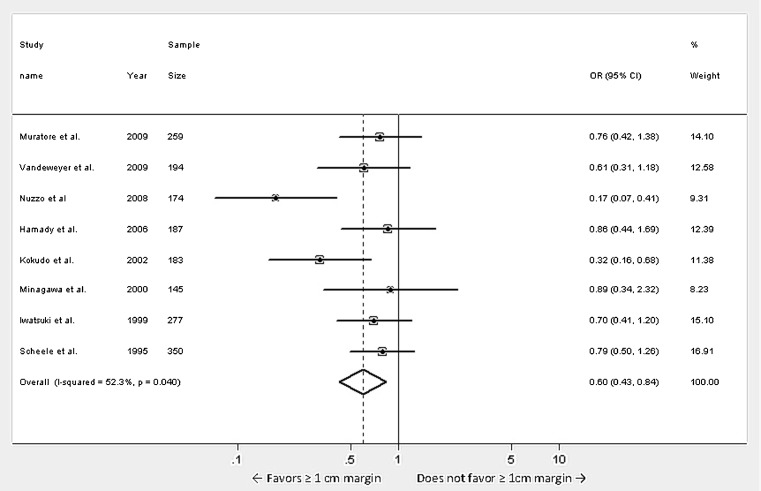
A subset analyses for the 8 studies with sample size more than 100 patients was performed (Fig. 3). Again a statistically significant improvement in 5 year DFS was seen in patients with ≥1 cm negative margin compared to <1 cm negative margin (Odds ratio 0.597, 95 % CI 0.427 to 0.837, p = 0.003). There was a statistically significant heterogeneity amongst the studies (p = 0.040) and the contribution of heterogeneity to the variation in odds ratio was 52.3 %. Publication bias was tested using egger test (Coef. = −3.183, Std. Err. = 2.144, t = −1.48, p = 0.188) and begg test (adj. Kendall’s score = −10, Std dev. = 8.08, z = −1.24, p = 0.216) suggesting that the chances of publication bias were minimal.
Fig. 3.
Meta-analysis of influence of surgical margin on disease free survival after hepatic resection of colorectal metastases: studies with sample size of more than 100 patients. (Odds ratios were calculated using the random effects model. Large diamond at the bottom represents the overall effect of negative margins on the disease free survival. Small diamonds embedded in squares represent the individual study effects with 95 % confidence intervals represented by the horizontal bars)
Recent Studies Published After Year 2000
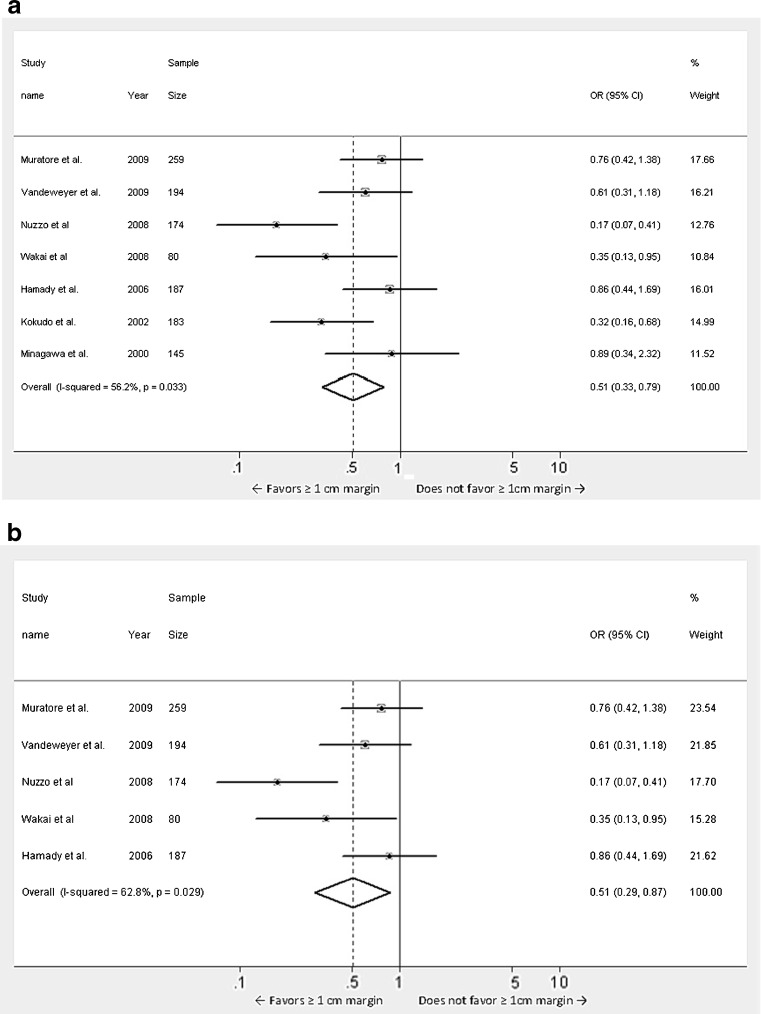
Since the meta-analysis included studies spanning over a period of 20 years we elected to perform a subset analyses of studies published in the last 10 years and 5 years respectively, as most of the advances in the surgical techniques and adjuvant therapies have occurred recently (Fig. 4a and b). There were 7 studies [3, 6, 8–10, 12, 13] published in the last 10 years and 5 studies [3, 6, 10, 12, 13] published in the last 5 years. These analyses also revealed a better DFS in patients with ≥1 cm margin compared to <1 cm negative margin and this was found to be statistically significant (Studies in the last 10 years: Odds ratio 0.509, 95 % CI 0.329 to 0.787, p = 0.002, heterogeneity 56.2 %, heterogeneity p = 0.033; Studies in the last 5 years: Odds ratio 0.506, 95 % CI 0.294 to 0.872, p = 0.014, heterogeneity 62.8 %, heterogeneity p = 0.029).
Fig. 4.
a Meta-analysis of influence of surgical margin on disease free survival after hepatic resection of colorectal metastases: studies published after the year 2000. b Meta-analysis of influence of surgical margin on disease free survival after hepatic resection of colorectal metastases: studies published after the year 2005. (Odds ratios were calculated using the random effects model. Large diamond at the bottom represents the overall effect of negative margins on the disease free survival. Small diamonds embedded in squares represent the individual study effects with 95 % confidence intervals represented by the horizontal bars)
Discussion
Recurrence is an important deterrent to the long term survival of patients with CRLM who undergo hepatic resection. Several clinic-pathologic factors have been shown to affect DFS but the surgical margins remains one of the unique prognostic factors as it could be influenced by the surgeon and the surgical technique. Some studies have found a DFS advantage of 1 cm margin [5, 8, 10, 13] whereas other studies have refuted the notion concluding that width of the negative surgical margin does not significantly influence the DFS [3, 6, 7, 9, 11, 12, 14]. However, most of the studies are either small or are from single institutions and therefore an informative conclusion is difficult to derive. Meta-analysis is a statistical tool which synthesizes the effects of smaller studies to estimate the true effect of a clinical intervention and provide the best available clinical evidence [19]. The aim of the current meta-analysis was to investigate if negative resection margins of ≥1 cm confer any DFS advantage over negative resection margins of <1 cm. To our knowledge this is the earliest meta-analysis on the subject with data on a large number of patients.
Our results show that a negative margin of ≥ 1 cm may have advantage of disease free survival over a sub-centimeter margin of resection. This was further tested using various subgroups such as studies with sample size of > 100, studies from last 10 years and 5 years. The uniformity of this survival benefit across the spectrum of analyses demonstrates robustness of our meta-analysis. A recent study has shown the OS advantage of ≥1 cm negative margins over sub-centimeter negative margins [16]. However, the exact reasons for the beneficial effect of margin status on DFS remain unclear. Wakai et al. have previously shown that 95 % of the intra-hepatic micro-metastases occur within 1 cm of the tumor deposits and there are a median of 4 micro-metastases per patient [13]. Therefore a 1 cm margin should help remove 95 % of the micro-metastases. Their results were different from Kokudo et al. who also analyzed the presence of the micro-metastases in the surrounding liver parenchyma using genetic and histologic methods and found micro-metastases to be rare (2 %) [8]. However micro-metastases through the Glisson pedicle were more common (14.3 %) and were located within 5 mm of the tumor edge. Based on these findings and other data the authors recommended a minimum margin of 2 mm (subcentimeter margin) as an acceptable margin. Although different tumor biology may account for the differences in the findings of Wakai et al. and Kokudo et al. but since both the studies are from the same region a different methodology may better explain the differences in the incidence and location of micrometastases as well as the recommended margins by the authors.
Some authors have hypothesized that if margins truly affect the DFS, most of the recurrences should occur locally at the site of resected tumor. Studies by Muratore et al., Pawlik et al. and Nuzzo et al. have shown that most of the recurrences regardless of the margin width are either extra-hepatic or at other intra-hepatic sites away from the resection end [3, 4, 10]. Interestingly, in the study by Kokudo et al. resection end recurrences although most common in patients with <2 mm surgical margin, were only seen in patients with up to 9 mm surgical margin and no such recurrences were seen in patient with ≥1 cm margin. In spite of these elegant studies, biological reasons for the beneficial effect of ≥1 cm margin on DFS still remains unclear and are beyond the purview of this analytical article.
Our study has several strengths. The current meta-analysis reflects good methodology. An extensive pubmed search using different relevant terms was performed along with backward search using references from the relevant articles, with well defined inclusion and exclusion criteria to include high quality and relevant studies, elimination of overlapping or duplicate studies, and extensive description of summary measures and results. Additionally, random effects model was used to account for heterogeneity of different studies and several subset analyses were performed to verify the results and rule out publication bias.
In spite of these strengths there are several limitations some of which have been discussed previously [16]. Meta-analysis is only as good as the studies included in it and there is a chance that inspite of our rigorous efforts some relevant studies might have been missed. The meta-analysis approach based on odds ratio is not as accurate as the one based on individual patient data and hazards ratio [20]. Procurement of individual patient data is very difficult and time consuming. Only 4 studies [6, 10, 13, 17] calculated hazard ratios for the margins and only one study had hazard ratios for sub-centimeter and ≥1 cm negative margins [13], making this approach difficult to use. Therefore, odds ratio based approach was found to be the most feasible and used in the current study. This approach although not as accurate as the individual patient data and hazard ratio based approach, has been used previously [21, 22].
The included studies in the current meta-analyses span over a period of 20 years during which many advances have been made in chemotherapy and resection techniques. We tried to minimize these effects by performing a subset analyses of studies over the last 10 and 5 years however a direct analysis of the effect of these factors have not been performed due to small number of studies and different chemotherapy regimens used. The influence of modern chemotherapy and newer resection techniques will require further studies. In the absence of any randomized controlled trial our meta-analysis may represent a comprehensive analysis of the impact of margins on DFS following hepatic resection for CRLM. The results of our work are reinforced by the simultaneous report by Cucchetti et al. [23]. Although performed independently, the similarity of our results to those of Cucchetti et al. demonstrates the beneficial influence of margins on DFS following hepatic resection for CRLM.
In conclusion, results of our meta-analysis demonstrate that there may be a DFS advantage of ≥1 cm negative margins over subcentimeter negative margins in patients undergoing hepatic resection for CRLM. Striving to achieve ≥1 cm margin should not preclude resection or compromise the safety of operation, as subcentimeter margins are also associated with a favorable disease fee survival. Since randomized controlled trial may not be feasible, this meta-analysis may represent a comprehensive analysis of the influence of margins on disease free survival following hepatic resection for CRLM.
Acknowledgments
Grant support
None.
References
- 1.Are C, Gonen M, Zazzali K, Dematteo RP, Jarnagin WR, Fong Y, Blumgart LH, D’Angelica M. The impact of margins on outcome after hepatic resection for colorectal metastasis. Ann Surg. 2007;246(2):295–300. doi: 10.1097/SLA.0b013e31811ea962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muratore A, Ribero D, Zimmitti G, Mellano A, Langella S, Capussotti L. Resection margin and recurrence-free survival after liver resection of colorectal metastases. Ann Surg Oncol. 2010;17(5):1324–1329. doi: 10.1245/s10434-009-0770-4. [DOI] [PubMed] [Google Scholar]
- 4.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, Curley SA, Loyer EM, Muratore A, Mentha G, Capussotti L, Vauthey JN. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241(5):715–722. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cady B, Jenkins RL, Steele GD, Jr, Lewis WD, Stone MD, McDermott WV, Jessup JM, Bothe A, Lalor P, Lovett EJ, Lavin P, Linehan DC. Surgical margin in hepatic resection for colorectal metastasis: a critical and improvable determinant of outcome. Ann Surg. 1998;227(4):566–571. doi: 10.1097/00000658-199804000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamady ZZ, Cameron IC, Wyatt J, Prasad RK, Toogood GJ, Lodge JP. Resection margin in patients undergoing hepatectomy for colorectal liver metastasis: a critical appraisal of the 1 cm rule. Eur J Surg Oncol. 2006;32(5):557–563. doi: 10.1016/j.ejso.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC, Geller DA, Gayowski TJ, Fung JJ, Starzl TE. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999;189(3):291–299. doi: 10.1016/S1072-7515(99)00089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kokudo N, Miki Y, Sugai S, Yanagisawa A, Kato Y, Sakamoto Y, Yamamoto J, Yamaguchi T, Muto T, Makuuchi M. Genetic and histological assessment of surgical margins in resected liver metastases from colorectal carcinoma: minimum surgical margins for successful resection. Arch Surg. 2002;137(7):833–840. doi: 10.1001/archsurg.137.7.833. [DOI] [PubMed] [Google Scholar]
- 9.Minagawa M, Makuuchi M, Torzilli G, Takayama T, Kawasaki S, Kosuge T, Yamamoto J, Imamura H. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg. 2000;231(4):487–499. doi: 10.1097/00000658-200004000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nuzzo G, Giuliante F, Ardito F, Vellone M, Giovannini I, Federico B, Vecchio FM. Influence of surgical margin on type of recurrence after liver resection for colorectal metastases: a single-center experience. Surgery. 2008;143(3):384–393. doi: 10.1016/j.surg.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 11.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19(1):59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 12.Vandeweyer D, Neo EL, Chen JW, Maddern GJ, Wilson TG, Padbury RT. Influence of resection margin on survival in hepatic resections for colorectal liver metastases. HPB (Oxford) 2009;11(6):499–504. doi: 10.1111/j.1477-2574.2009.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wakai T, Shirai Y, Sakata J, Valera VA, Korita PV, Akazawa K, Ajioka Y, Hatakeyama K. Appraisal of 1 cm hepatectomy margins for intrahepatic micrometastases in patients with colorectal carcinoma liver metastasis. Ann Surg Oncol. 2008;15(9):2472–2481. doi: 10.1245/s10434-008-0023-y. [DOI] [PubMed] [Google Scholar]
- 14.Ooijen B, Wiggers T, Meijer S, Heijde MN, Slooff MJ, Velde CJ, Obertop H, Gouma DJ, Bruggink ED, Lange JF, et al. Hepatic resections for colorectal metastases in The Netherlands. A multiinstitutional 10-year study. Cancer. 1992;70(1):28–34. doi: 10.1002/1097-0142(19920701)70:1<28::AID-CNCR2820700105>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, Jaeck D. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77(7):1254–1262. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1254::AID-CNCR5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 16.Dhir M, Lyden ER, Wang A, Smith LM, Ullrich F, Are C. Influence of margins on overall survival following hepatic resection for colorectal liver metastasis: a meta-analysis. Ann Surg. 2011;254(2):234–242. doi: 10.1097/SLA.0b013e318223c609. [DOI] [PubMed] [Google Scholar]
- 17.Gayowski TJ, Iwatsuki S, Madariaga JR, Selby R, Todo S, Irish W, Starzl TE. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery. 1994;116(4):703–710. [PMC free article] [PubMed] [Google Scholar]
- 18.Scheele J, Stangl R, Altendorf-Hofmann A, Gall FP. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery. 1991;110(1):13–29. [PubMed] [Google Scholar]
- 19.Mosteller F, Colditz GA. Understanding research synthesis (meta-analysis) Annu Rev Public Health. 1996;17:1–23. doi: 10.1146/annurev.pu.17.050196.000245. [DOI] [PubMed] [Google Scholar]
- 20.Michiels S, Piedbois P, Burdett S, Syz N, Stewart L, Pignon JP. Meta-analysis when only the median survival times are known: a comparison with individual patient data results. Int J Technol Assess Health Care. 2005;21(1):119–125. doi: 10.1017/S0266462305050154. [DOI] [PubMed] [Google Scholar]
- 21.Gurusamy KS, Imber C, Davidson BR. Management of the hepatic lymph nodes during resection of liver metastases from colorectal cancer: a systematic review. HPB Surg. 2008;2008:684150. doi: 10.1155/2008/684150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y, Zhao Y, Li B, Xu D, Yin Z, Xie F, Yang J. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol. 2010;10(1):78. doi: 10.1186/1471-230X-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cucchetti A, Ercolani G, Cescon M, Bigonzi E, Peri E, Ravaioli M, Pinna AD (2012) Impact of subcentimeter margin on outcome after hepatic resection for colorectal metastases: a meta-regression approach. Surgery. doi:10.1016/j.surg.2011.12.009 [DOI] [PubMed]