Abstract
The successful translation of the scientific principles of targeting the breast tumour oestrogen receptor (ER) with the nonsteroidal anti-oestrogen tamoxifen and using extended durations (at least 5-years) of adjuvant therapy, dramatically increased patient survivorship and significantly enhanced a drop in national mortality rates from breast cancer. The principles are the same for the validation of aromatase inhibitors to treat post-menopausal patients but tamoxifen remains a cheap, life-saving medicine for the pre-menopausal patient. Results from the Oxford Overview Analysis illustrate the scientific principle of “longer is better” for adjuvant therapy in pre-menopausal patients. One-year of adjuvant therapy is ineffective at preventing disease recurrence or reducing mortality, whereas five-years of adjuvant tamoxifen reduces recurrence by 50% which is maintained for a further ten-years after treatment stops. Mortality is reduced but the magnitude continues to increase to 30% over a 15-year period. With this clinical database, it is now possible to implement simple solutions to enhance survivorship. Compliance with long-term anti-hormone adjuvant therapy is critical. In this regard, the use of selective serotonin reuptake inhibitors (SSRIs) to reduce severe menopausal side effects may be inappropriate. It is known that SSRIs block the CYP2D6 enzyme that metabolically activates tamoxifen to its potent anti-oestrogenic metabolite, endoxifen. The selective nor-epinephrine reuptake inhibitor, venlafaxine, does not block CYP2D6, and may be a better choice. Nevertheless, even with perfect compliance, the relentless drive of the breast cancer cell to acquire resistance to therapy persists. The clinical application of long-term anti-hormonal therapy for the early treatment and prevention of breast cancer, focused laboratory research on the discovery of mechanisms involved in acquired anti-hormone resistance. Decades of laboratory study to reproduce clinical experience described not only the unique mechanism of SERM-stimulated breast cancer growth, but also a new apoptotic biology of oestradiol action in breast cancer, following 5-years of anti-hormonal treatment. Oestradiol-induced apoptotic therapy is currently shown to be successful for the short-term treatment of metastatic ER positive breast cancer following exhaustive treatment with anti-hormones. The “oestrogen purge” concept is now being integrated into trials of long-term adjuvant anti-hormone therapy. The Study of Letrazole Extension (SOLE) trial employs “anti-hormonal drug holidays” so that a woman’s own oestrogen may periodically purge and kill the nascent sensitized breast cancer cells that are developing. This is the translation of an idea first proposed at the 1992 St. Gallen Conference. Although tamoxifen is the first successful targeted therapy in cancer, the pioneering medicine is more than that. A study of the pharmacology of tamoxifen opened the door for a pioneering application in cancer chemoprevention and created a new drug group: the Selective ER Modulators (SERMs) with group members (raloxifene and lasofoxifene) approved for the treatment and prevention of osteoporosis with a simultaneous reduction of breast cancer risk. Thus, the combined strategies of long-term anti-hormone adjuvant therapy, targeted to the breast tumour ER, coupled with the expanding use of SERMs to prevent osteoporosis and prevent breast cancer as a beneficial side effect have advanced patient survivorship significantly and promises to reduce breast cancer incidence.
Keywords: tamoxifen, selective oestrogen receptor modulators (SERMs), raloxifene, apoptosis, oestrogen, acquired drug resistance, chemoprevention
INTRODUCTION
Professor Hans-Joerg Senn asked me to cast light on future opportunities for improving adjuvant anti-hormone therapy that can be implemented or tested in clinical trial. This I will do, but first I will preface my remarks with a quote from Patrick Henry, the first elected Governor of Virginia, who said it best: “I have but one lamp by which my feet are guided, and that is the lamp of experience. I know no way of judging of the future, but by the past.” In 1969, when I started my research on the pharmacology of non-steroidal anti-oestrogen, there was no tamoxifen (Fig 1), only ICI 46,474, an effective anti-fertility agent in rats1. The compound had anti-oestrogenic properties, so I proposed2 to enhance its clinical application from an orphaned drug, with modest efficacy in metastatic breast cancer, to a targeted anti-cancer agent for adjuvant therapy and chemoprevention. Tamoxifen became my lamp, and subsequent laboratory research results shed light on the future of successful and safe adjuvant anti-hormone therapy, a new drug group of selected estrogen receptor modulation (SERMs)3, a lead compound in the SERMs raloxifene for clinical applications, the promise of multi-functional medicines, the unique qualities of acquired anti-hormone drug resistance and a new apoptotic biology of oestrogen in breast cancer (Fig 1, Table 1)4. Tamoxifen, a failed contraceptive in women, is now a pioneering medicine in oncology 1 and is listed as an essential medicine by the World Health Organization.
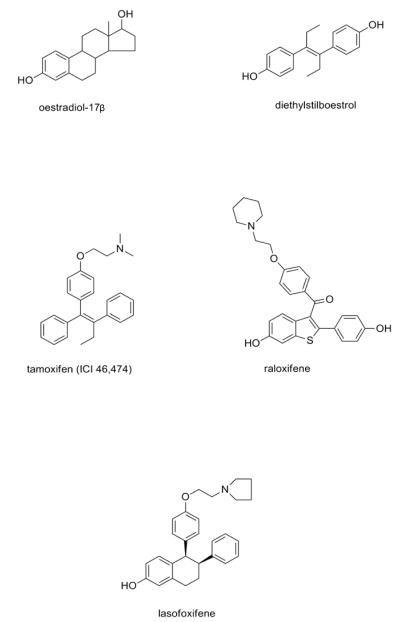
Fig. 1.
The structure of medicines and compounds mentioned in the text. Oestradiol and diethylstilboestrol are oestrogens, whereas all others are selective oestrogen receptor modulators (SERMs) used in medicine for the treatment and chemoprevention of breast cancer (tamoxifen), treatment and prevention of osteoporosis and the chemoprevention of breast cancer (raloxifene). The new SERM, lasofoxifene, is approved for the treatment and prevention of osteoporosis in the European Union.
Table 1.
Decades of discovery. The development of scientific principles in the laboratory were translated to clinical trials ten years later and subsequently became the standards for clinic care for the treatment or chemoprevention of breast cancer, or in the case of the SERM, raloxifene, a treatment option for the treatment and prevention of osteoporosis with the prevention of breast carcinogenesis as a beneficial side effect.
| Decades of Translational Discovery | ||
|---|---|---|
| DECADE | SCIENTIFIC PRINCIPLE | CLINICAL BENEFIT |
| 1970s | Long-term adjuvant tamoxifen therapy targeted to ER |
--------------------- |
| Foundation of chemoprevention with tamoxifen |
--------------------- | |
|
| ||
| 1980s | Selective ER modulation | Survival benefits for long-term adjuvant tamoxifen |
| 1990s | Evolution of drug resistance to hormones |
Chemoprevention with SERMs, tamoxifen and raloxifene |
| Anti-tumour actions of physiologic oestrogens |
||
|
| ||
| 2000s | Oestrogen-induced apoptosis | Clinical translation of oestrogen-induced apoptosis |
The clinical validation5, 6 of the laboratory principles of targeting the breast tumour oestrogen-receptor (ER)7 with long-term adjuvant antihormonal therapy (tamoxifen and oestrogen withdrawal)8, 9 using a long acting anti-oestrogen, metabolically activated to potent hydroxylated metabolites9-12, established a treatment strategy that continues to enhance the survivorship of millions of women world-wide. The key to success was the application of the first effective medicine to target the tumour through blocking oestrogen-stimulated growth at the ER, but coupled with the application of the counter-intuitive laboratory finding, that long-term adjuvant therapy would be superior to short-term therapy to control recurrence. The strategy succeeded, despite initial clinical findings that the tumour response to tamoxifen was not strongly correlated to ER status13, 14 and the legitimate concern that long-term therapy would precipitate early drug resistance. This concern was based on the fact that tamoxifen was only an effective treatment in unselected metastatic disease for about a year or two15, so why would extended or indefinite adjuvant tamoxifen treatment be effective at preventing recurrence in the adjuvant setting?
Clinical trials finally demonstrated that the laboratory principle of “longer was more effective at controlling recurrence” was correct5, 6. The subsequent development of the aromatase (AIs)16 expanded post-menopausal patient treatment options and reduced “oestrogen-like” side effects associated with tamoxifen, such as endometrial cancer and thromboembolic disorders17. There was also a modest improvement of disease-free survival compared with tamoxifen. The widespread acceptance of long-term antihormonal therapy as the standard of care and the intense and exhaustive examination of patient population databases, now permit questions to be addressed to improve patient survivorship. At a time of shrinking resources for biomedical research but expanding menus of purported targeted drugs to close one pathway or another, it is time to apply simple, basic rules that will make an impact immediately on enhancing survivorship. Only then, is it prudent to fine tune the results from a position of strength, by interrogating the tumour biology with blockers of survival pathways.
SIMPLE SOLUTIONS TO ENHANCE SURVIVAL
It seems obvious but it must be stated. The past 30-years of successful translational research is without value if an infrastructure does not exist to ensure that a patient’s treatment is maintained when the medicine has proven value to aid survival from breast cancer. A medical team is available to support a patient’s needs but there must be a refocus of the team to relearn basic principles: chronic therapy that requires years to provide benefit is worthless if the patients will not follow the regimen. This act will dramatically reduce their potential for survival. The fashion over the past four decades, for evidence based medicine, requires effective delivery. Significantly, delivery is a minor commitment compared to the effort behind discovering and proving the efficiency of a medicine in prospective clinical trials.
Based on the published evidence, several general principles are emerging about compliance. A recent analysis of anti-hormone therapy conducted in patients enrolled in the Kaiser Permanente of Northern California health system18, revealed that approximately 30% of all patients discontinued either AI or tamoxifen early but of those who did continue, 70% were fully adherent for up to 5-years. Thus, only 49% overall are adherent for the full course of adjuvant anti-hormonal therapy. Predictors of non-adherence were African-American race, lumpectomy, unknown tumour site, lymph node involvement and other co-morbidities. Adherence was associated with Asian/Pacific Island ethnicity, married, earlier years of diagnosis (tamoxifen era), prior chemotherapy, radiation therapy and longer prescription refills. These and similar findings19, 20 describe the extent of the problem but noncompliance with effective therapeutic agents also increases recurrence and mortality21-23.
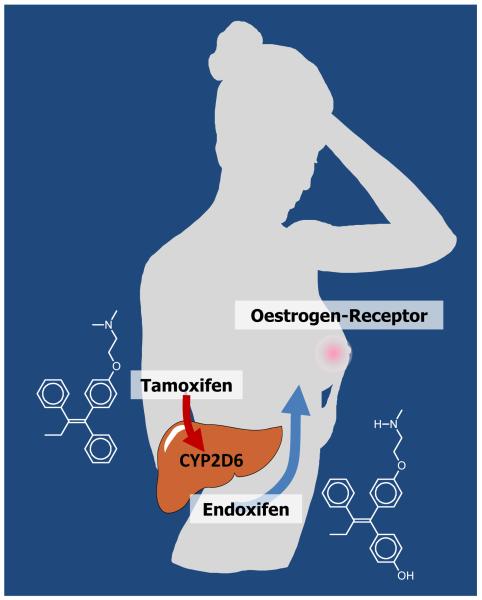
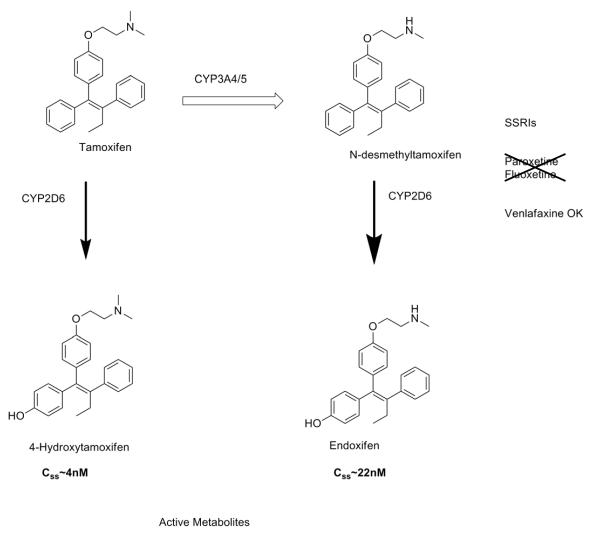
Another significant finding of the Hershman study18 was that young women under 40-years old were more likely to discontinue anti-hormone therapy. This group would be prescribed tamoxifen but reasons for stopping could be because the women chose to start a family or the menopausal side effects were too severe. In regard to the latter, many women have been routinely prescribed selective serotonin reuptake inhibitors (SSRIs) over the past decade to reduce menopausal side effects. Members of this drug group block the CYP2D6 enzyme that metabolically activates tamoxifen to the potent anti-oestrogen endoxifen thereby (Fig. 2) impairing full drug benefit (Fig. 3)24. However, it must be stressed that not all SSRIs have the same ability to block tamoxifen metabolism and as a result, studies that group all SSRIs together are not uniformly consistent with the hypothesis25, 26. Nevertheless, the recent Canadian study of co-prescription of various SSRIs and the selective nor-epinephrine reuptake inhibitor (SNRI) venlafaxine does implicate paroxitene as increasing mortality during tamoxifen treatment and venlafaxine decreases mortality27. Overall, enhancing compliance and avoiding SSRIs that block CYP26D will significantly increase the chances of patient survival. That being said, the next issue to address is anti-hormone drug resistance.
Fig. 2.
The metabolic activation of tamoxifen with a low affinity to the tumour oestrogen receptor by the P450 enzyme CYP2D6 enzyme to endoxifen with a high affinity for the tumour oestrogen receptor.
Fig. 3.
The metabolism of tamoxifen to 4-hydroxytamoxifen, a metabolite with a high affinity for the oestrogen receptor. Tamoxifen’s major metabolite is N-desmethyltamoxifen that has a similar binding affinity to the oestrogen receptors as tamoxifen. However, N-desmethyltamoxifen is metabolically activated to endoxifen, with a high binding affinity for the oestrogen receptor. The selective serotonin re-uptake inhibitors (SSRIs), paroxetine and fluoxetine block the metabolic activation of tamoxifen by blocking CYP2D6. Venlafaxine, a selective norepinephrine re-uptake inhibitor (SNRI), does not affect tamoxifen’s metabolic activation, and therefore is the preferred choice to treat menopausal symptoms experienced with tamoxifen.
Anti-hormonal drug resistance can be manifest in two forms for the ER positive tumour: intrinsic resistance where the tumour does not respond at all to anti-hormone therapy, despite being ER positive and acquired anti-hormone therapy where the tumour initially responds to anti-hormone therapy but then grows despite the continuing treatment. Much effort has focused on an understanding of the molecular mechanism of intrinsic anti-hormone resistance and it seems that cross-talk between growth factor receptors and the low levels of ER have essentially made the ER irrelevant for cell survival. No scientific advance has yet reversed intrinsic resistance and aided patients. In contrast, there have been significant advances in understanding acquired anti-hormone resistance in the laboratory and these emerging data have been translated to clinical practice.
THE CHALLENGE: ACQUIRED DRUG RESISTANCE
Clinical experience with the successful application of long-term tamoxifen as an adjuvant therapy produced a clear survival advantage for patients28. Unselected patients treated for 5-years with adjuvant tamoxifen lived longer than patients in the non-treatment (placebo) arm but who were treated with tamoxifen at first recurrence as they had metastatic breast cancer. The clinical results with successful adjuvant tamoxifen therapy demonstrated28 that our understanding of the development of drug resistance to tamoxifen treatment in ER positive disease were incorrect on July 25, 1987 (the publication date of the Scottish MRC trial), but supported the principle of early treatment of micrometastatic disease. Also, it highlights the fact that resistance to tamoxifen for the treatment of metastatic disease occurs rapidly within 2-years, and this biology did not apply to an adjuvant application of tamoxifen. Despite the fact that the rat mammary carcinoma model demonstrates that earlier, longer treatment with an anti-oestrogen was a suitable clinical strategy8, there was no model of human diseases to test this hypothesis. However, in the mid-1980s, this was about to change. The ER positive breast cancer cell line MCF-729 exhibits oestradiol-stimulated tumor growth when transplanted into ovariectomized athymic mice. Tamoxifen blocks oestradiol-stimulated tumor growth but cannot maintain growth inhibition as ER positive tumors eventually grow despite tamoxifen treatment30. However, it seems that SERM and antihormonal resistance in breast cancer evolves and exposes a vulnerability in breast cancer that can be exploited in the clinic31.
The first transplantable model of tamoxifen resistance in breast cancer demonstrated that drug resistance to tamoxifen was unique32. Although tamoxifen can initially block oestradiol-stimulated growth of MCF-7 cells, resistant ER positive tumors can use either oestradiol or tamoxifen to stimulate tumor growth (Fig. 4). Tumours do not grow unless treated with tamoxifen or oestradiol so in the ovariectomized mouse, this is equivalent to the “non-oestrogen state” created by aromatase inhibitors. Tumours also do not grow if treated with the pure anti-oestrogen fulvestrant that destroys the ER33, 34. This laboratory model replicates clinical experience with drug resistance to tamoxifen in metastatic breast cancer and explains why aromatase inhibitors or fulvestrant are effective second line treatments35, 36. So, how does a study of the drug resistance to tamoxifen in the laboratory explain the effectiveness of 5-years of adjuvant tamoxifen to reduce recurrence rates in ER positive breast cancer to tamoxifen by fifty-percent and continue to reduce mortality a decade after tamoxifen treatment is stopped? The answer is the evolution and reconfiguration of cell survival pathways that occurs in micrometastatic breast cancer during years of treatment.
Fig. 4.
The development of acquired antihormone resistance to selective oestrogen receptor modulators (SERMs) (tamoxifen or raloxifene). The unique feature of Phase I antihormone resistance is that oestrogen receptor positive breast tumours grow in response to either physiological oestradiol or the SERM. In the clinical setting (and laboratory models), an aromatase inhibitor (no oestrogen) or the pure anti-oestrogen, fulvestrant, that destroys the oestrogen receptor, stops the growth of Phase I resistant tumours to tamoxifen.31
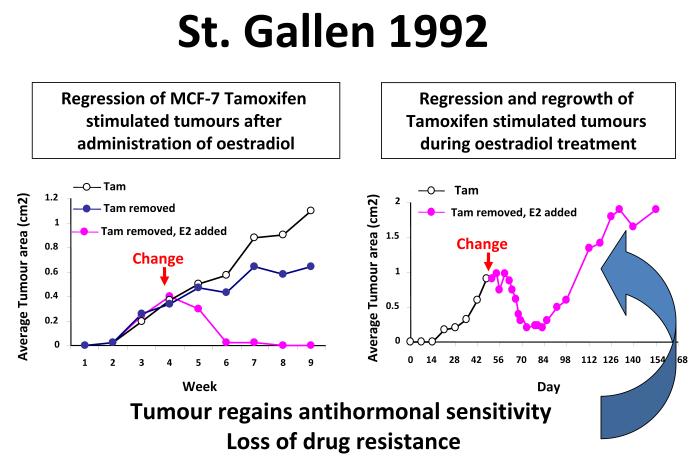
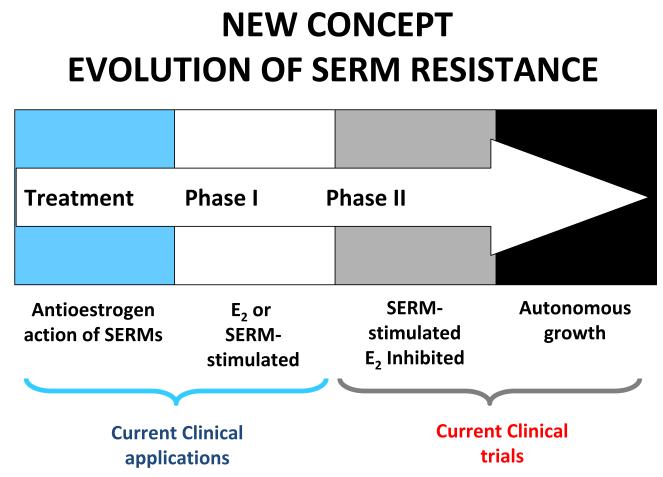
Continuous retransplantation of successive generations of tamoxifen-stimulated MCF-7 tumor lines into athymic mice for more than 5-years results in a derived tumor line that does not respond to physiologic oestradiol with growth but rapid tumor regression through apoptotic cell death (Fig. 5)37, 38. These data were first presented at the St. Gallen meeting in 199237. The concept offered at the time was that the ultimate and long lasting value of adjuvant tamoxifen therapy derives from stopping adjuvant tamoxifen when the woman’s own oestrogen can now destroy the micrometastases that have been sensitized to oestrogen-induced apoptosis. The initial laboratory observations on low dose oestradiol-induced tumour regression were subsequently confirmed38, expanded39-42 and translated successfully to clinical trial43, 44. As a result, it is now possible to define the evolution of acquired anti-hormone therapy into a Treatment Phase where the anti-hormone blocks oestradiol stimulated tumour growth, Phase I when a SERM or oestradiol stimulates growth (or an aromatase inhibitor creates oestrogens independent growth) and Phase II when a SERM stimulates growth but physiological oestrogen provokes apoptosis either after stopping a SERM or after stopping an aromatase inhibitor (Fig. 6).
Fig. 5.
Diagrammatic representation of the actions of physiologic oestradiol (E2) on the growth of small phase II MCF-7 tamoxifen resistant tumors in ovariectomized athymic mice. A larger tumour will regress with oestradiol treatment but will eventually display oestrogen-stimulated growth. If tumours are re-transplanted into a new generation of ovariectomized athymic mice and treated with oestradiol, tamoxifen will block oestrogen-stimulated tumour growth.38 First presented in St. Gallen, 1993.37
Fig. 6.
The evolution of drug resistance to SERMs. Acquired resistance occurs during long-term treatment with a SERM and is evidenced by SERM-stimulated breast tumour growth. Tumours also continue to exploit oestrogen for growth when the SERM is stopped, so a dual signal transduction process develops. The aromatase inhibitors prevent tumour growth in SERM-resistant disease and fulvestrant that destroys the ER is also effective. This phase of drug resistance is referred to as Phase I resistance. Continued exposure to a SERM results in continued SERM-stimulated growth (Phase II), but eventually autonomous growth occurs that is unresponsive to fulvestrant or aromatase inhibitors. The event that distinguishes Phase I from Phase II acquired resistance is a remarkable switching mechanism that now causes apoptosis, rather than growth, with physiologic levels of oestrogen. A similar evolution occurs with aromatase inhibitor resistance from oestrogen independent growth with a transition to oestrogen-induced apoptosis. These distinct phases of laboratory drug resistance have their clinical parallels and this new knowledge is being integrated into the treatment plan.
Thus, over the past four decades, general scientific principles have emerged and translated to clinical care for patients. The application of these principles of endocrine adjuvant therapy have benefited, and continued to benefit, millions of women worldwide, through a simple and cheap therapeutic intervention. We will now consider how emerging laboratory knowledge may reverse or at least hold Phase II resistance to enhance the longevity of the patient. We will, however then, revisit the clinical reality that increased tumour burden is a poor indicator of patient survival, so that the founding principles of our initial work, i.e. early treatment targeting the ER with long-term therapy2 must be embraced by the clinical community.
Oestradiol-Induced Apoptosis under Laboratory Conditions
The administrations of physiologic oestradiol to athymic mice implanted with phase II SERM (tamoxifen or raloxifene) resistant ER positive MCF-7 tumours38, 40, 41, 45 causes tumours to stop growing and/or rapidly regress. Similarly, the long-term oestrogen deprived clinical cell line MCF-7:5C42, 46 rapidly undergoes oestrogen-induced apoptosis both in vitro and in vivo. These laboratory observations are reminiscent of the pioneering studies of Sir Alexander Haddow FRS with his application of the first Chemical Therapy to successfully treat any cancer – high dose synthetic oestrogens to treat metastatic breast cancer47, 48. He observed a 25% response rate but these were short-lasting47. The observation was made that no responses were observed close to menopausal but often dramatic responses occurred in women in their late 60s and 70s. By 1970, during the presentation of the Inaugural Karnofsky Award Lecture at the American Society of Clinical Oncology (ASCO)48 (incidentally, when I was starting my PhD in Pharmacology at Leeds University) he stated: “…the extraordinary extent of tumour regression observed in perhaps 1% of post-menopausal cases (with oestrogen) has always been regarded as of major theoretical importance, and it is a matter for some disappointment that so much of the underlying mechanisms continues to elude us…”
Now we know that the responses Haddow observed occur because of oestrogen deprivation following the menopause. Longer oestrogen withdrawal after menopause was more effective at creating Phase II resistance in select patients, but high dose oestrogen therapy was necessary. Based on laboratory studies and clinical correlations, anti-hormone therapy does a better job in driving the rapid evolution to Phase II resistance and as a result, only physiological oestrogen is necessary to trigger apoptosis. Haddow’s paradox that stood for 40-years now has clarity and we can start to offer treatment options to exploit the concept further.
Cell culture models provide a vehicle to examine, over time, oestrogen-induced apoptosis with the aim of pharmacologic modulation and the discovery of mechanisms that may have relevance for patient care. Through a knowledge of mechanisms, the elegant oestrogen trigger for naturally initiating tumour cell death may subsequently be exploited to other treatment scenarios. If we can decipher the process of ER-induced apoptosis from its current obscurity, this knowledge could be applied with the discovery of new drugs to trigger the mechanism without the involvement of ER. The ER is our current guide and light to find a new drug group.
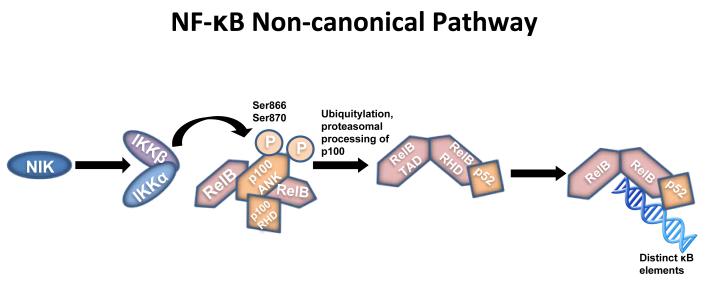
We have undertaken an extensive examination of the actions of oestradiol on the growth (MCF-7), immediate apoptosis (MCF-7:5C) and delayed apoptosis (MCF-7:2A)49 of our model cells using a 2-week time course of gene activity documented through mRNA analysis, creation of cDNA libraries and competitive hybridization with a cDNA library from no treatment controls using Agilent Gene Arrays. These studies were conducted in collaboration with Dr. Eric Ariazi and Dr. Heather Cunliffe. We extensively analyzed the gene time course, and completed gene segregation based on hierarchical pathway analysis. We found that MCF-7 and MCF-7:2A, our control cells remained quiescent during the initial few days of oestradiol treatment (1nM) whereas the pre-apoptotic MCF-7:5C cells responded with a massive rise in the activation of inflammatory genes. Analysis of the sequence of events during the first few days of gene activation, we propose that apoptosis occurs in MCF-7:5C cells by the exploitation of the non-canonical pathway for NF-κB signal transduction (Fig. 7). Furthermore, we have mapped out the time-course activation of each caspase (except caspase 3 that is absent in MCF-7) and determined that caspase 4 is the first and controlling executioner to provoke programmed cell death. We have interrogated the apoptotic process with purported inhibitors of individual activated caspases to confirm our conclusion of the role of caspase 4. Blockade of caspase 4, blocks oestrogen-induced apoptosis.
Fig. 7.
The non-canonical pathway results in the activation of IKKα by NIK and phosphorylation of the NF-κB subunit. This process results in the conversion of p100 to p52. It is the p52-RelB heterodimers that target distinct κB elements on DNA. ANK (ankyrin-repeat motifs). NIK (NF-κB kinase). RelB (NF-κB family member). RHD (Rel-homology domain). TAD (transcriptional activation domain).
Most importantly, the activation of inflammatory genes suggests that oestradiol-induced apoptosis could be inhibited or at least modulated by glucocorticoids. We have subsequently established that dexamethazone inhibits oestrogen-induced apoptosis in a concentration related manner. This novel observation may have important implications for the application of oestradiol-induced apoptosis for individualized patient care. It is possible that the inadvertent administration of glucocorticoids during patient care could block oestrogen-induced apoptosis or that a patient’s own glucocorticoids may also inhibit apoptosis, if patients are challenged with oestrogen following exhaustive anti-hormone therapy? The anti-glucocorticoid mifipristone (RU486) could potentially be used with oestrogen to block glucocorticoid action temporarily for a few weeks during low dose oestrogen administration to enhance apoptosis.
Examination of the Agilent gene array data confirmed our previous work49 that elevated synthesis of glutathione, is protecting MCF-7:2A cells from immediate apoptosis in response to oestrogen. Apoptosis appears to be retarded in MCF-7:2A cells but an activation of autophagy heralds an enhanced transcription of caspase 4 and then triggers oestrogen-induced apoptosis during the second week of oestradiol treatment. We have previously successfully used pharmacological inhibitors to test our hypothesis. Buthionine sulfoximine (BSO), an inhibitor of glutathione synthesis49 enhances oestradiol-induced apoptosis from a slow event lasting 2-weeks to an immediate event. Unfortunately, BSO, though used extensively in clinical trial a decade or more ago, is no longer available to examine whether it is possible to enhance oestrogen-induced apoptosis in patients with select tumours.
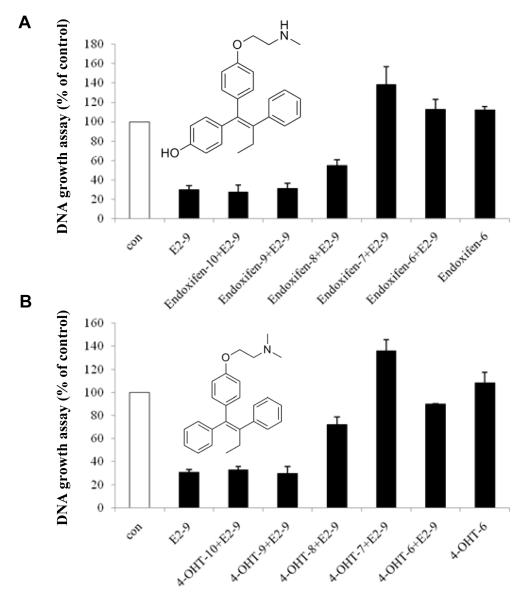
Thus far, our studies have described what happens, but the real question is how does the oestradiol/ER complex triggers apoptosis? Are there clues about the actual shape or structure of the oestrogen ER complex that can be modulated and investigated further? The MCF-7:5C cells depend on a functioning ER for oestradiol-induced apoptosis. The pure anti-oestrogen fulvestrant binds to the ER and causes the rapid destruction of the protein complex. As a result, fulvestrant blocks oestradiol-induced apoptosis in a concentration related manner. Interestingly enough, the tamoxifen metabolites 4-hydroxytamoxifen (4OHTam) and endoxifen do not block or affect the autonomous growth of MCF-7:5C cells but do block the initiation of oestradiol-induced apoptosis. Herein lies a clue to the mechanism that triggers oestradiol-induced apoptosis (Fig. 8). X-Ray crystallographic studies of the ER ligand binding domain and the oestrogens, oestradiol and diethylstilboestrol (DES) and the SERMs 4OHTam50 and raloxifene51 provide a fascinating insight into oestrogen and anti-oestrogen action. The solution of the crystal structures demonstrate that the planar oestrogens are sealed within the ligand binding domain by helix 12 which then allows co-activators to bind to the activating function (AF)-2 site on the complex. This event amplifies oestrogen action through gene transcription. In contrast, the bulky side chain of the triphenylethylene 4OHTam and the benzothiophene raloxifene prevent helix 12 from sealing the hydrophobic ligand binding domain which prevents coactivator binding to AF-2. The promiscuous oestrogen-like activity of 4OHTam is explained by the inability of the anti-oestrogenic side chain to neutralize and shield the exposed aspartate at position 351 at the surface of the ligand binding domain. This exposed carboxylic acid communicates with AF-1 to induced oestrogen-like actions. Raloxifene completely blocks and neutralizes the aspartate at 351 and the raloxifene ER complex does not activate AF-1. This hypothesis has been successfully interrogated with changes in the ligand and the aspartate at 351 to modulate the activation of a model oestrogen target gene Transforming Growth Factor α52-55. Overall, we concluded that activation of AF-1 by an exposed surface aspartate 351 confirms that helix 12 is not sealing the ligand binding domain so it can, therefore, communicate a signal to AFI to induce oestrogen-like gene activation. If aspartate 351 is masked under helix 12 with a planar oestrogen than AF-2 is activated and the communication between AF-1 and aspartate 351 is mute. These data and conclusions subsequently resulted in a reclassification of oestrogens into class 1 (planar) and class 2 (non-planar)56 using a simple assay to determine whether helix 12 was locking the ligand into the hydrophobic ligand binding domain or not. However, the biological significance of this molecular insight was not apparent until recently.
Fig. 8.
The reversal of oestradiol-induced apoptosis (1 nM) by increasing concentrations of 4-hydroxytamoxifen or endoxifen. This nonsteroidal antioestrogen effect highlights the ER dependence for oestradiol-induced apoptosis.
Based on the fact that 4OHTam blocks oestradiol induced apoptosis at the ER and the statement that the “bulky side chain” of 4OHTam altered the conformation of the ER preventing helix 12 from sealing the ligand binding domain50, we advanced the hypothesis that the “bulky side chain” of 4OHTam was the phenyl ring of the oestrogenic triphenylbut-l-ene not just the para-dimethylaminoethoxy group traditionally associated with anti-oestrogen action. Perhaps the phenyl ring of the triphenylbut-l-ene anti-oestrogen was stopping helix 12 from sealing the binding site? A series of triphenylethylenes (TPEs), previously known to be classified exclusively as oestrogens in rodent uterine weight and vagina cornification assays, was used to establish oestrogenic activity in MCF-7 breast cancer cells. All compounds were found to be full oestrogens in growth assays compared with oestradiol and DES and fully-activate an ERE luciferase report ER gene system in MCF-7 cells57. In contrast, while oestradiol and DES will trigger apoptosis and cell death in MCF-7:5C cells within a week, the synthetic TPE “oestrogens” do not provoke massive apoptosis and indeed block oestradiol-induced apoptosis. Studies using the CHIP assay at the ERE site in the promoter region of the oestrogen responsive pS2 gene demonstrate that whereas oestradiol E2ER complex is recruited with the co-activator SRC3 in AF-2 neither 4OHTam nor the TPE ER complexes are recruited to the promoter58.
Overall, these data demonstrate that oestrogen-induced apoptosis is governed and programmed by the shape of the ER complex. As a consequence, shape governs coactivator binding at AF-2 and these events subsequently trigger apoptosis. A recent study by59 advances our initial oestrogen reclassification paper56 and confirmed, using a phage display library, that the shape of the ligand programs the external shaped of the ER complex. A precise evaluation of the immediate early genes involved in the apoptotic response will describe the mechanism of the oestrogenic trigger for cell death. Exploitation of this knowledge may find applications in other disease states.
OESTROGEN TREATMENT: CURRENT CLINICAL FINDINGS AND TRANSLATION TO ADJUVANT THERAPY
The laboratory finding37-39 that acquired resistance to anti-hormone therapy evolves and exposes a vulnerability of breast cancer cells to the apoptotic actions of physiological oestrogen, provides an important insight into potential therapeutic applications. As previously noted in this paper, the anti-tumour effect of physiological oestrogen is reminiscent of the early therapeutic use of high dose oestrogen therapy for the treatment of metastatic breast cancer in post-menopausal women47. It was noted that the further from menopause patients were, the more likely there was to be a tumour response, but these responses never exceeded 30% in any given population.
It is now clear that the acute oestrogen deprivation caused by anti-hormones speeds up the molecular adaptation and reconfiguration of vulnerable survival pathways. The surviving populations of susceptible breast cancer cells also have increased sensitivity to oestrogen-induced apoptosis. Low dose oestrogen therapy now becomes a clinically viable strategy with the prospect of reducing oestrogen associated side effects.
The laboratory data generated and published in the 1990s proposed the clinical strategy of using low-dose oestrogen therapy to “purge” breast cancer cells with Phase II–acquired anti-hormone resistance, but then the re-introduction of anti-hormone therapy would control oestradiol-stimulated tumour growth37, 38. Nevertheless, a European trial lead by Dr. Per Lonning43 recruited patients with metastatic breast cancer following exhaustive anti-hormone therapy were treated with standard high-dose DES (5mg tid). Results are summarized in Table 2a. Select patients responded well with one patient subsequently reported60 being disease-free more than 10-years after first initiating a high dose oestrogen “purge” therapy. “One of the patients (AO) who achieved a complete response of a 16 × 16 mm cytological confirmed chest wall relapse, received DES treatment for five years, where after she been subject to regular follow-up without active treatment. To this day, she remains disease-free 10 years and six months after commencing DES treatment.”
Table 2.
The proof of principle for a) high dose oestrogen (DES, 15 mg daily) triggering tumour responses in patients with metastatic breast cancer following exhaustive antihormone therapy43 or b) a comparison of high dose oestrogen (oestradiol, 30 mg daily) or low dose oestrogen (oestradiol, 6 mg daily), producing similar clinical benefit rates following the failure of therapy with an aromatase inhibitor.44
| A | Response | |
|---|---|---|
| COMPLETE | PARTIAL | SD |
| 4a/32 | 6/32 | 2/32 |
| B | |||
|---|---|---|---|
| Dose | # patients | Response | Clinical benefit |
| 6 mg | 34 | 10/34 | 29% |
| 30 mg | 32 | 9/32 | 28% |
One patient remains disease-free 10 years and six months after commencing DES treatment.”
In a follow-up study, Ellis44 compared and contrasted high dose (10mg tid) and low dose (1mg tid) oestradiol therapy in patients who relapsed during adjuvant aromatase inhibitor therapy. Results are summarized in Table 2b. Results were not as impressive as the Lonning study probably because patients did not receive “exhaustive” endocrine therapy prior to an oestrogen “purge”. Nevertheless, the clinical trial confirms that low dose oestrogen can produce similar clinical benefit when compared with high-dose oestrogen treatment but with fewer, serious side effects.
Finally, there is further clinical evidence from the Women’s Health Initiative (WHI) that oestrogen replacement therapy (ERT) alone causes a decrease rather than increase in the incidence of breast cancer61 and a recent report from the Million Women Study in the UK demonstrates that oestrogen alone increases breast cancer incidence immediately following the menopause but if ERT is used more than 5-years after oestrogen exposure, oestrogen replacement therapy does not cause a rise in breast cancer incidences62. An overarching explanation for these apparently confusing clinical observations is clarified by our evolving molecular model to exploiting the role of oestradiol in the life and death of breast cancer cells63, 64. We interpret these clinical findings based on the evolution of anti-hormone resistance as follows: breast cancer cells in an environment of oestrogen only grow in response to exogenous oestrogen, but following long-term oestrogen depriving surviving breast cancer cells either die or at least do not develop into tumours.
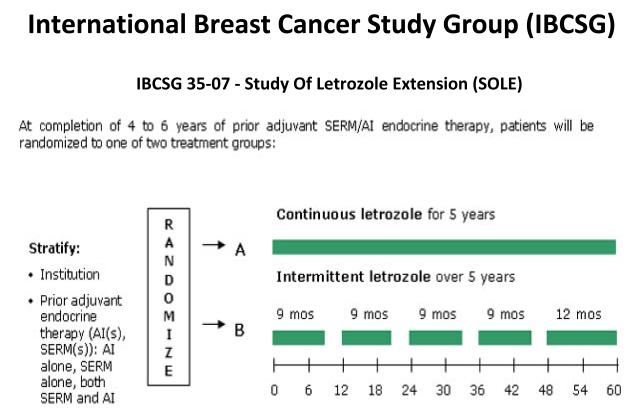
The clinical and laboratory database also provides continuing support for the ongoing adjuvant Study of Letrozole Extension (SOLE) trial (Fig. 9). Patients who have completed 5-years of adjuvant therapy with tamoxifen, an AI or any sequence are then randomized to an AI continuously for 5-years or an AI with a drug holiday for 3-months a year. The trial seeks to exploit the hypothesis, advanced at the 1992 St. Gallen Meeting, that a woman’s own oestrogen may act as an anti-tumour agent after adjuvant anti-hormone therapy is stopped. The SOLE trial proposes a rigorous test of the hypothesis under controlled conditions that promises to create a practical advantage for patients following drug holidays. Results from this trial coupled with the expanding molecular database concerning the modulation of oestrogen-induced apoptosis may result in the proposition of regularly purging patients for a week or two with ERT if decades of anti-hormone therapy are to become common place in order that the disease is held in check and prevented from recurring. The question is now – at what point is oestrogen intervention too late?
Fig. 9.
The Schema for the Study of Letrozole Extension (SOLE) conducted by the International Breast Cancer Study Group (IBCSG 35-07). Patients randomized following five years of adjuvant antihormone therapy to letrozole continuously or intermittent letrozole (3 month drug holidays per year for 5 years). The rationale is that the woman’s own oestrogen in the intermittent arm will trigger apoptosis in aromatase inhibitor resistant cells and reduce recurrence rates.
FIGHTING OVERWHELMING CANCER CELL FLEXIBILITY
The enemy is us, Haddow48 in the Inaugural Karnofsky Lecture reasoned that it would not be possible to develop a cancer specific therapy in the same way Ehrlich had for syphilis, as cancer was our own cells. What he did not know was that the situation is worse than that. The replicative fidelity of normal cells replace exactly what is lost, but in its own special place. Cytotoxic chemotherapy kills the patient by indiscriminately killing normal differentiated cells, and perhaps stem cells, so life saving repopulation for the host organism is impossible or too late. In contrast, human populations eventually adapt to external destructive forces such as fatal infectious diseases (plague, small pox, etc.) but individuals only survive through their preprogrammed nimble immunology. The survivors repopulate. And so it is with cancer at the cellular level within the body. However, immunology has not yet been proven to be of significance for breast cancer prevention. Haddow was right there – the enemy is us. The tumour at diagnosis has hundreds of mutations compared to the (purportedly) normal human genome65, 66. This and activated oncogenes, or loss of tumour suppression genes, provides the random survival flexibility within the cancer cell population to adapt and eventually thrive in a hostile (cytotoxic) environment within a few months. The principle is a microscopic adaptation of simple Darwinian evolution that has played out over the millennia by animals on earth. Random mutations create a preferred trait that permits survival, while the non-adaptive species or population dies out. The situation with cancer only becomes worse through adaptive survival responses preprogrammed in the cancer stem cell. These cellular “spores” seek to expand and prepare for massive repopulation in an enforced anoxic environment. The clinician is confronted with a perverted microcosm of the struggle for life by cancer cells programmed to create infinite candidates in the quest for survival. The patient is overwhelmed by sheer numbers in the wrong places. This is the challenge of targeted molecular therapeutics but how to build rationally on the advances in survivorship achieved over the past 40-years in breast cancer?
The path to progress in drug development has not changed significantly during this time, despite our new knowledge of the disease. The administrative plan for drug evaluation is in place to protect citizens and provide safe and therapeutically proven medicines for clinical care. To market a new drug to treat breast cancer, a precise system must be followed to obtain government approval. Phase I clinical trials must offer the hope of potentially effective treatment to patients who have received all possible therapeutic options. The goal is to document dose limiting toxicities and at this stage of the disease, responses are a major bonus. Phase II trials focus on a cancer type of interest based on reasonable data from preclinical studies or an unanticipated response in Phase I trials. If a candidate is successful in Phase II trials, the drug is evaluated against or with the current standard of care. It should be emphasized that therapeutic results from Phase II trials with tamoxifen were not very dramatic, but Phase I data on toxicity for the patient was excellent compared with other therapies available. Only by targeting the ER in the tumour and applying long-term adjuvant therapy did patient survivorship increase. A discarded contraceptive became the “gold standard” for breast cancer therapy over a 30-year journey1.
With this background, how do we build on success? Today there are dozens of good potential targets and dozens of plausible candidates for each target. However, unlike the ER which was, it is turned out, the principle messenger to stimulate breast tumour growth in about 30% of tumours, other candidate targets are proving to be not the star but part of the chorus. In late stage disease, one pathway is blocked but others now compensate. Pathways to preserve cellular life can be essential in all cells, but a cancer cell specific pathway is the only key to success in cancer therapeutics.
Based on our current work investigating oestradiol-induced apoptosis of breast cancer cells with long-term acquired resistance, we purposed a hypothesis: can we block breast cancer cell survival mechanisms and enhance the chances that the cell must undergo apoptosis in response to oestradiol?
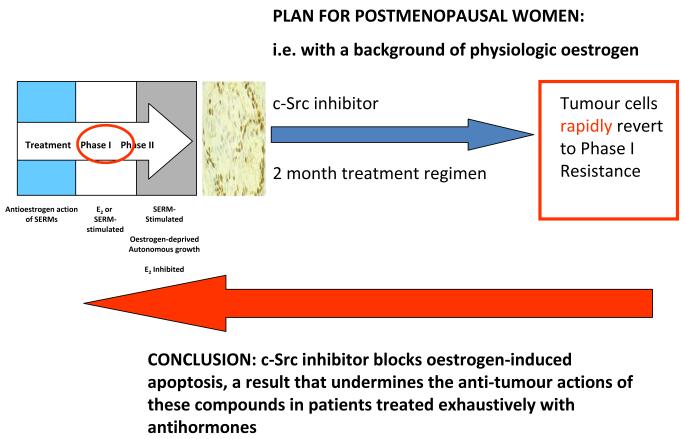
c-Src was the first identified oncogene in cancer and is said to be present in more than 70% of breast cancer67. It controls AKT and MAPK phosphorylation cascades as the intermediary from growth factor receptor activation. It would appear to be an ideal target to subvert cell survival; almost as good as the ER! We posed the question, that if we blocked c-Src in breast cancer cells resistant to aromatase inhibitors would we then enhance apoptosis? In other words, would we generate value for the cancer patient by increasing cell kill as we have previously found that c-Src inhibitors were completely ineffective in affecting growth of oestrogen stimulated MCF-7 cells, but had significant efficiency in blocking the growth of ER-negative MDA-MB-231 and oestrogen stimulated ER-positive T47D cells. More importantly, long-term oestrogen-deprived MCF-7 cells have elevated pSrc. As most ER-positive cancers are exhaustively treated with antihormones before Phase I/II testing and we were building on a known efficacy of estrogen therapy, the proposition appeared sound. Our model cell, MCF-7:5C, had elevated phospho c-Src and are targeted inhibitor PP2 completely blocked phosphorylation. However, a 2 month course of treatment of MCF-7:5C cells with physiological oestrogen levels (1nM) that would be present in a postmenopausal patient plus the c-Src inhibitor (5 microMolar), resulted in the blockade of oestrogen-induced apoptosis and the reversion of the cell population to Phase I drug resistance (Fig. 10), i.e. estrogen or SERM-stimulated for growth. Within 2 months, the flexibility of cell populations had created no real advance that could realistically aid the patient.
Fig. 10.
The evolution of drug resistance and rapid alterations in cell populations if a c-Src inhibitor PP2 (5 μM) is incubated with MCF-7:5C cells in the presence of 1 nM oestradiol for two months to mimic a clinical scenario of a postmenopausal woman who fails an aromatase inhibitor to block growth. Apoptosis from oestrogen is blocked and the cells revert to Phase I resistance, i.e. oestrogen and SERM-stimulated growth.
Thus, as an illustration of the challenge, we face for the application of logical targeted therapy, one could conclude the following: an expanding menu of targeted medicines is available for testing, but only select populations will respond. Testing a c-Src inhibitor in the incorrect stage of antihormone resistance or patient populations cannot be successful. This is the problem: the testing populations for registration may be inappropriate for a drug candidate that is magnificent in a neoadjuvant therapy naïve disease study. However, does this enhance registration? Unfortunately not.
We need practical strategies to aid communities to hold the development and death from breast cancer while we attempt to decipher the enormous complexity of pathways and permutations of targeted therapies. This conclusion brings us back to the second piece of translational research started in our laboratory in the 1970’s – chemoprevention. Remarkably, the lamp of tamoxifen shed light on an alternative strategy to reduce cancer incidence and preempt the aforementioned Gordian Knot. Unfortunately, the initial strategy for the clinical application of chemoprevention requires the identification of high-risk populations to be treated with the pioneer tamoxifen. This approach is flawed. However, a public health strategy for an aging population that creates wellness for as long as possible is a laudable goal now within our grasp.
TAMOXIFEN IS ALSO ABOUT PROGRESS IN CHEMOPREVENTION
An extensive study of the pharmacology of tamoxifen68 identified its ability to modulate oestrogen target tissues around the body; tamoxifen is anti-oestrogenic in the breast and oestrogen-like in bone and lowers circulating cholesterol69-72. Translational research also first identified the potential of tamoxifen to increase the risk of developing endometrial cancer during extended treatment schedules73-75. Tamoxifen blocks breast tumour growth and development but enhances endometrial cancer growth. As a result, new procedures were introduced for the gynecological monitoring of post-menopausal patients receiving long-term tamoxifen therapy. New agents, without endometrial problems, were needed for investigation. Knowledge of selective ER modulation by tamoxifen and also the pharmacology of the structurally-related failed breast cancer drug, raloxifene, led to the creation of a new drug group, the Selective ER Modulators (SERMs)76, with the potential to treat and prevent multiple diseases in women and prevent breast cancer at the same time. The fact that raloxifene was less oestrogen-like than tamoxifen in the rodent uterus and less likely to increase the incidence of the endometrial cancer in patients77, 78 meant that safer compounds could be identified as chemopreventives for breast cancer but a new strategy to achieve the goal was essential. Benefits for a tiny, unidentifiable minority is unacceptable if the vast majority of women in a high risk population have side effects, some life-threatening. The road map for the pharmaceutical industry was clearly stated in 199079. Is this the end of the possible applications for anti-oestrogens? Certainly not! We have obtained valuable clinical information about this group of drugs that can be applied in other disease states. Research does not travel in straight lines and observations in one field of science often become major discoveries in another. Important clues have been garnered about the effects of tamoxifen on bone and lipids so it is possible that derivatives could find targeted applications to retard osteoporosis or atherosclerosis. The ubiquitous application of novel compounds to prevent diseases associated with the progressive changes after menopause may, as a side effect, significantly retard the development of breast cancer. The target population would be postmenopausal women in general, thereby avoiding the requirement to select a high risk group to prevent breast cancer.
Raloxifene pioneered the concept in the clinic confirming the prediction that the prevention of breast cancer would occur during the treatment and prevention of osteoporosis in high risk post-menopausal women80 with no increase in endometrial cancer. Today, the prediction that SERMs could control multiple diseases in women following the menopause is poised to become a reality. Lasofoxifene (Fig. 1) is approved in the European Union for the prevention and treatment of osteoporosis which simultaneously decreases the incidence of breast cancer, strokes and myocardial infarction, but without increasing endometrial cancer risk81. Lasofoxifene is more than one hundred times more potent than raloxifene and the aforementioned strategy79 to improve women’s health in aging populations is the new face of chemoprevention in breast cancer - treat the majority of women for major diseases like osteoporosis and coronary heart disease and prevent breast cancer as a beneficial side effect. The saving in health care costs by not paying for the treatment of breast cancer in tens of thousands of women without breast cancer will be considerable, but admittedly hard to quantitate.
Raloxifene is not only available in the United States of America for the treatment and prevention of osteoporosis but also for reduction of the incidence for breast cancer in post-menopausal, high-risk women82. However, the SERM must be given indefinitely to remain effective in both diseases83. In contrast, tamoxifen remains effective for decades after the limited treatment period of 5-years is stopped84, 85. As mentioned previously, the key to understanding this fact probably resides in the laboratory study of drug resistance to SERMs and aromatase inhibitors and the development of a cellular susceptibility to oestrogen-induced apoptosis. The fact that the same tumour responsiveness to raloxifene appears to be retarded in clinical practice suggests that the known poor pharmacokinetics and bioavailability of raloxifene is not able to rapidly produce an “anti-oestrogenic” state around the nascent tumour like tamoxifen. This may explain the reduced performance of raloxifene against tamoxifen in the STAR trial, following the cessation of 5-years of treatment83.
SUMMARY AND CLOSING THOUGHTS
Over the past 40-years, we have witnessed, a dramatic improvement in the survivorship of the majority of patients with a diagnosis of ER positive breast cancer. The SERM tamoxifen pioneered the process. Translational research has added further cheap and effective targeted anti-hormonal therapies to the physician’s armamentarium that are proven to be of benefit in randomized adjuvant clinical trials world-wide. Not only has therapy been improved substantially over the past 40-years, from the time in the early 1970s when there was stated to be little prospect of successful survival advances with “endocrine therapy”, but also the parallel path of chemoprevention has been pioneered successfully with the same SERM tamoxifen. This SERM heralded a new era of general medicine where a family of SERMs would allow women to expect to reduce their risk of fractures but prevent breast cancer at the same time. This was only a laboratory concept 20-years ago79, 86 but it seems obvious that with an aging population that seeks to remain active for as long as possible, that the SERMs will play their part in reducing the incidence of breast cancer if used wisely in the post-menopausal population.
The lessons learned with the lamp-light of the pioneer tamoxifen are now established principles in cancer therapeutics. The principles are: aim at the target (ER), start therapy as early as possible (i.e. as few lymph nodes as possible involved), long therapy is preferable to shorter therapy, compliance with the medicine is essential and drug interaction with SSRIs to stop menopausal side effect in a few should be avoided. Conforming to these principles aids patients’ survival. The light from the lamp also taught us what we did not know. Firstly tamoxifen is a selective modulator of ER action around a woman’s body and heralded a new drug group (the SERMs) that prevent osteoporosis and prevent breast cancer as a beneficial side effect. This avoids the need to find the exact women who would benefit from chemoprevention using the Gail Model87. Secondly, drug resistance evolves so that oestradiol becomes an apoptotic trigger. Further studies solved the concern expressed by Haddow in 1970 that the mechanism of oestrogen-induced apoptosis was a mystery. Now oestrogen therapy has a niche application in patient care.
During the past 40-years, the mosaic of endocrine adjuvant therapy and chemoprevention with SERMs has been clarified by effective translational research4, 88. The next challenge for a generation of “omic” scientists is to prioritize the opportunities in molecular therapeutics based on this solid start, so as to advance and individualize treatment.
ACKNOWLEDGEMENTS
We would like to thank Julia Jessup Tijerina and Russell E. McDaniel for their administrative support in preparing this manuscript.
Funding: This work (VCJ) was supported by the Department of Defense Breast Program under Award number BC050277 Center of Excellence; subcontract under the SU2C (AACR) Grant number SU2C-AACR-DT0409; the Susan G Komen for the Cure Foundation under Award number SAC100009 and the Lombardi Comprehensive Cancer Center Support Grant (CCSG) Core Grant NIH P30 CA051008. The project described was supported by Award Number P30 CA051008 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The views and opinions of the author(s) do not reflect those of the US Army or the Department of Defense.
Footnotes
Competing interests: None.
REFERENCES
- 1.Jordan VC. Tamoxifen: a most unlikely pioneering medicine. Nat Rev Drug Discov. 2003;2:205–13. doi: 10.1038/nrd1031. [DOI] [PubMed] [Google Scholar]
- 2.Jordan VC. Tamoxifen: catalyst for the change to targeted therapy. Eur J Cancer. 2008;44:30–8. doi: 10.1016/j.ejca.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan VC. Selective estrogen receptor modulation: a personal perspective. Cancer Res. 2001;61:5683–7. [PubMed] [Google Scholar]
- 4.Jordan VC. The 38th David A. Karnofsky lecture: the paradoxical actions of estrogen in breast cancer--survival or death? J Clin Oncol. 2008;26:3073–82. doi: 10.1200/JCO.2008.17.5190. [DOI] [PubMed] [Google Scholar]
- 5.EBCTCG Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351:1451–67. [PubMed] [Google Scholar]
- 6.EBCTCG Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 7.Jordan VC, Koerner S. Tamoxifen (ICI 46,474) and the human carcinoma 8S oestrogen receptor. Eur J Cancer. 1975;11:205–6. doi: 10.1016/0014-2964(75)90119-x. [DOI] [PubMed] [Google Scholar]
- 8.Jordan V, Dix CJ, Allen KE. The effectiveness of long term tamoxifen treatment in a laboratory model for adjuvant hormone therapy of breast cancer. Adjuvant Ther Cancer. 1979;2:19–26. [Google Scholar]
- 9.Jordan VC, Allen KE. Evaluation of the antitumour activity of the non-steroidal antioestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma model. Eur J Cancer. 1980;16:239–51. doi: 10.1016/0014-2964(80)90156-5. [DOI] [PubMed] [Google Scholar]
- 10.Jordan VC, Collins MM, Rowsby L, Prestwich G. A monohydroxylated metabolite of tamoxifen with potent antioestrogenic activity. J Endocrinol. 1977;75:305–16. doi: 10.1677/joe.0.0750305. [DOI] [PubMed] [Google Scholar]
- 11.Jordan VC, Dix CJ, Naylor KE, Prestwich G, Rowsby L. Nonsteroidal antiestrogens: their biological effects and potential mechanisms of action. J Toxicol Environ Health. 1978;4:363–90. doi: 10.1080/15287397809529666. [DOI] [PubMed] [Google Scholar]
- 12.Allen KE, Clark ER, Jordan VC. Evidence for the metabolic activation of non-steroidal antioestrogens: a study of structure-activity relationships. Br J Pharmacol. 1980;71:83–91. doi: 10.1111/j.1476-5381.1980.tb10912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NATO Controlled trial of tamoxifen as adjuvant agent in management of early breast cancer. Interim analysis at four years by Nolvadex Adjuvant Trial Organisation. Lancet. 1983;1:257–61. [PubMed] [Google Scholar]
- 14.NATO Controlled trial of tamoxifen as single adjuvant agent in management of early breast cancer. Analysis at six years by Nolvadex Adjuvant Trial Organisation. Lancet. 1985;1:836–40. [PubMed] [Google Scholar]
- 15.Cole MP, Jones CT, Todd ID. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br J Cancer. 1971;25:270–5. doi: 10.1038/bjc.1971.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan VC, Brodie AM. Development and evolution of therapies targeted to the estrogen receptor for the treatment and prevention of breast cancer. Steroids. 2007;72:7–25. doi: 10.1016/j.steroids.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–2. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 18.Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai WY, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–8. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26:556–62. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 20.Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat. 2006;99:215–20. doi: 10.1007/s10549-006-9193-0. [DOI] [PubMed] [Google Scholar]
- 21.McCowan C, Shearer J, Donnan PT, Dewar JA, Crilly M, Thompson AM, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer. 2008;99:1763–8. doi: 10.1038/sj.bjc.6604758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geiger AM, Thwin SS, Lash TL, Buist DS, Prout MN, Wei F, et al. Recurrences and second primary breast cancers in older women with initial early-stage disease. Cancer. 2007;109:966–74. doi: 10.1002/cncr.22472. [DOI] [PubMed] [Google Scholar]
- 23.Yood MU, Owusu C, Buist DS, Geiger AM, Field TS, Thwin SS, et al. Mortality impact of less-than-standard therapy in older breast cancer patients. J Am Coll Surg. 2008;206:66–75. doi: 10.1016/j.jamcollsurg.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Brauch H, Jordan VC. Targeting of tamoxifen to enhance antitumour action for the treatment and prevention of breast cancer: the ‘personalised’ approach? Eur J Cancer. 2009;45:2274–83. doi: 10.1016/j.ejca.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 25.Dezentje VO, van Blijderveen NJ, Gelderblom H, Putter H, van Herk-Sukel MP, Casparie MK, et al. Effect of concomitant CYP2D6 inhibitor use and tamoxifen adherence on breast cancer recurrence in early-stage breast cancer. J Clin Oncol. 2010;28:2423–9. doi: 10.1200/JCO.2009.25.0894. [DOI] [PubMed] [Google Scholar]
- 26.Lash TL, Cronin-Fenton D, Ahern TP, Rosenberg CL, Lunetta KL, Silliman RA, et al. Breast cancer recurrence risk related to concurrent use of SSRI antidepressants and tamoxifen. Acta Oncol. 2010;49:305–12. doi: 10.3109/02841860903575273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly CM, Juurlink DN, Gomes T, Duong-Hua M, Pritchard KI, Austin PC, et al. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population based cohort study. BMJ. 2010;340:c693. doi: 10.1136/bmj.c693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.SCTO Adjuvant tamoxifen in the management of operable breast cancer: the Scottish Trial. Report from the Breast Cancer Trials Committee, Scottish Cancer Trials Office (MRC), Edinburgh. Lancet. 1987;2:171–5. [PubMed] [Google Scholar]
- 29.Levenson AS, Jordan VC. MCF-7: the first hormone-responsive breast cancer cell line. Cancer Res. 1997;57:3071–8. [PubMed] [Google Scholar]
- 30.Osborne CK, Coronado EB, Robinson JP. Human breast cancer in the athymic nude mouse: cytostatic effects of long-term antiestrogen therapy. Eur J Cancer Clin Oncol. 1987;23:1189–96. doi: 10.1016/0277-5379(87)90154-4. [DOI] [PubMed] [Google Scholar]
- 31.Jordan VC. Selective estrogen receptor modulation: concept and consequences in cancer. Cancer Cell. 2004;5:207–13. doi: 10.1016/s1535-6108(04)00059-5. [DOI] [PubMed] [Google Scholar]
- 32.Gottardis MM, Jordan VC. Development of tamoxifen-stimulated growth of MCF-7 tumors in athymic mice after long-term antiestrogen administration. Cancer Res. 1988;48:5183–7. [PubMed] [Google Scholar]
- 33.Gottardis MM, Jiang SY, Jeng MH, Jordan VC. Inhibition of tamoxifen-stimulated growth of an MCF-7 tumor variant in athymic mice by novel steroidal antiestrogens. Cancer Res. 1989;49:4090–3. [PubMed] [Google Scholar]
- 34.Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51:3867–73. [PubMed] [Google Scholar]
- 35.Osborne CK, Pippen J, Jones SE, Parker LM, Ellis M, Come S, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002;20:3386–95. doi: 10.1200/JCO.2002.10.058. [DOI] [PubMed] [Google Scholar]
- 36.Howell A, Robertson JF, Quaresma Albano J, Aschermannova A, Mauriac L, Kleeberg UR, et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20:3396–403. doi: 10.1200/JCO.2002.10.057. [DOI] [PubMed] [Google Scholar]
- 37.Wolf DM, Jordan VC. A laboratory model to explain the survival advantage observed in patients taking adjuvant tamoxifen therapy. Recent Results Cancer Res. 1993;127:23–33. doi: 10.1007/978-3-642-84745-5_4. [DOI] [PubMed] [Google Scholar]
- 38.Yao K, Lee ES, Bentrem DJ, England G, Schafer JI, O’Regan RM, et al. Antitumor action of physiological estradiol on tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res. 2000;6:2028–36. [PubMed] [Google Scholar]
- 39.Song RX, Mor G, Naftolin F, McPherson RA, Song J, Zhang Z, et al. Effect of long-term estrogen deprivation on apoptotic responses of breast cancer cells to 17beta-estradiol. J Natl Cancer Inst. 2001;93:1714–23. doi: 10.1093/jnci/93.22.1714. [DOI] [PubMed] [Google Scholar]
- 40.Liu H, Lee ES, Gajdos C, Pearce ST, Chen B, Osipo C, et al. Apoptotic action of 17beta-estradiol in raloxifene-resistant MCF-7 cells in vitro and in vivo. J Natl Cancer Inst. 2003;95:1586–97. doi: 10.1093/jnci/djg080. [DOI] [PubMed] [Google Scholar]
- 41.Osipo C, Gajdos C, Liu H, Chen B, Jordan VC. Paradoxical action of fulvestrant in estradiol-induced regression of tamoxifen-stimulated breast cancer. J Natl Cancer Inst. 2003;95:1597–608. doi: 10.1093/jnci/djg079. [DOI] [PubMed] [Google Scholar]
- 42.Lewis JS, Meeke K, Osipo C, Ross EA, Kidawi N, Li T, et al. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation. J Natl Cancer Inst. 2005;97:1746–59. doi: 10.1093/jnci/dji400. [DOI] [PubMed] [Google Scholar]
- 43.Lonning PE, Taylor PD, Anker G, Iddon J, Wie L, Jorgensen LM, et al. High-dose estrogen treatment in postmenopausal breast cancer patients heavily exposed to endocrine therapy. Breast Cancer Res Treat. 2001;67:111–6. doi: 10.1023/a:1010619225209. [DOI] [PubMed] [Google Scholar]
- 44.Ellis MJ, Gao F, Dehdashti F, Jeffe DB, Marcom PK, Carey LA, et al. Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. JAMA. 2009;302:774–80. doi: 10.1001/jama.2009.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balaburski GM, Dardes RC, Johnson M, Haddad B, Zhu F, Ross EA, et al. Raloxifene-stimulated experimental breast cancer with the paradoxical actions of estrogen to promote or prevent tumor growth: a unifying concept in anti-hormone resistance. Int J Oncol. 2010;37:387–98. doi: 10.3892/ijo_00000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis JS, Osipo C, Meeke K, Jordan VC. Estrogen-induced apoptosis in a breast cancer model resistant to long-term estrogen withdrawal. J Steroid Biochem Mol Biol. 2005;94:131–41. doi: 10.1016/j.jsbmb.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 47.Haddow A, Watkinson JM, Paterson E, Koller PC. Influence of Synthetic Oestrogens on Advanced Malignant Disease. Br Med J. 1944;2:393–8. doi: 10.1136/bmj.2.4368.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haddow A, David A. Karnofsky memorial lecture. Thoughts on chemical therapy. Cancer. 1970;26:737–54. doi: 10.1002/1097-0142(197010)26:4<737::aid-cncr2820260402>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 49.Lewis-Wambi JS, Kim HR, Wambi C, Patel R, Pyle JR, Klein-Szanto AJ, et al. Buthionine sulfoximine sensitizes antihormone-resistant human breast cancer cells to estrogen-induced apoptosis. Breast Cancer Res. 2008;10:R104. doi: 10.1186/bcr2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, et al. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95:927–37. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- 51.Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O, et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–8. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- 52.Levenson AS, Jordan VC. The key to the antiestrogenic mechanism of raloxifene is amino acid 351 (aspartate) in the estrogen receptor. Cancer Res. 1998;58:1872–5. [PubMed] [Google Scholar]
- 53.MacGregor Schafer J, 2Liu H, Bentrem DJ, Zapf JW, Jordan VC. Allosteric silencing of activating function 1 in the 4-hydroxytamoxifen estrogen receptor complex is induced by substituting glycine for aspartate at amino acid 351. Cancer Res. 2000;60:5097–105. [PubMed] [Google Scholar]
- 54.Liu H, Lee ES, Deb Los, Reyes A, Zapf JW, Jordan VC. Silencing and reactivation of the selective estrogen receptor modulator-estrogen receptor alpha complex. Cancer Res. 2001;61:3632–9. [PubMed] [Google Scholar]
- 55.Liu H, Park WC, Bentrem DJ, McKian KP, Reyes Ade L, Loweth JA, et al. Structure-function relationships of the raloxifene-estrogen receptor-alpha complex for regulating transforming growth factor-alpha expression in breast cancer cells. J Biol Chem. 2002;277:9189–98. doi: 10.1074/jbc.M108335200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jordan VC, Schafer JM, Levenson AS, Liu H, Pease KM, Simons LA, et al. Molecular classification of estrogens. Cancer Res. 2001;61:6619–23. [PubMed] [Google Scholar]
- 57.Maximov PY, Myers CB, Curpan RF, Lewis-Wambi JS, Jordan VC. Structure-function relationships of estrogenic triphenylethylenes related to endoxifen and 4-hydroxytamoxifen. J Med Chem. 2010;53:3273–83. doi: 10.1021/jm901907u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sengupta S, Sharma CG, Jordan VC. Estrogen regulation of X-box binding protein-1 and its role in estrogen induced growth of breast and endometrial cancer cells. Horm Mol Biol Clin Investig. 2010;2:235–43. doi: 10.1515/HMBCI.2010.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bourgoin-Voillard S, Gallo D, Laios I, Cleeren A, Bali LE, Jacquot Y, et al. Capacity of type I and II ligands to confer to estrogen receptor alpha an appropriate conformation for the recruitment of coactivators containing a LxxLL motif-Relationship with the regulation of receptor level and ERE-dependent transcription in MCF-7 cells. Biochem Pharmacol. 2010;79:746–57. doi: 10.1016/j.bcp.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 60.Lonning PE. Additive endocrine therapy for advanced breast cancer - back to the future. Acta Oncol. 2009;48:1092–101. doi: 10.3109/02841860903117816. [DOI] [PubMed] [Google Scholar]
- 61.Stefanick ML, Anderson GL, Margolis KL, Hendrix SL, Rodabough RJ, Paskett ED, et al. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. JAMA. 2006;295:1647–57. doi: 10.1001/jama.295.14.1647. [DOI] [PubMed] [Google Scholar]
- 62.Beral V, Reeves G, Bull D, Green J. Breast Cancer Risk in Relation to the Interval Between Menopause and Starting Hormone Therapy. J Natl Cancer Inst. 2011;103:296–305. doi: 10.1093/jnci/djq527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lewis-Wambi JS, Jordan VC. Estrogen regulation of apoptosis: how can one hormone stimulate and inhibit? Breast Cancer Res. 2009;11:206. doi: 10.1186/bcr2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maximov PY, Lewis-Wambi JS, Jordan VC. The Paradox of Oestradiol-Induced Breast Cancer Cell Growth and Apoptosis. Curr Signal Transduct Ther. 2009;4:88–102. doi: 10.2174/157436209788167484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berger MF, Lawrence MS, Demichelis F, Drier Y, Cibulskis K, Sivachenko AY, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–20. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pfeifer GP, Hainaut P. Next-generation sequencing: emerging lessons on the origins of human cancer. Curr Opin Oncol. 2011;23:62–8. doi: 10.1097/CCO.0b013e3283414d00. [DOI] [PubMed] [Google Scholar]
- 67.Ottenhoff-Kalff AE, Rijksen G, van Beurden EA, Hennipman A, Michels AA, Staal GE. Characterization of protein tyrosine kinases from human breast cancer: involvement of the c-src oncogene product. Cancer Res. 1992;52:4773–8. [PubMed] [Google Scholar]
- 68.Jordan VC. Tamoxifen (ICI46,474) as a targeted therapy to treat and prevent breast cancer. Br J Pharmacol. 2006;147(Suppl 1):S269–76. doi: 10.1038/sj.bjp.0706399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jordan VC, Robinson SP. Species-specific pharmacology of antiestrogens: role of metabolism. Fed Proc. 1987;46:1870–4. [PubMed] [Google Scholar]
- 70.Jordan VC, Phelps E, Lindgren JU. Effects of anti-estrogens on bone in castrated and intact female rats. Breast Cancer Res Treat. 1987;10:31–5. doi: 10.1007/BF01806132. [DOI] [PubMed] [Google Scholar]
- 71.Love RR, Mazess RB, Barden HS, Epstein S, Newcomb PA, Jordan VC, et al. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med. 1992;326:852–6. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- 72.Love RR, Wiebe DA, Newcomb PA, Cameron L, Leventhal H, Jordan VC, et al. Effects of tamoxifen on cardiovascular risk factors in postmenopausal women. Ann Intern Med. 1991;115:860–4. doi: 10.7326/0003-4819-115-11-860. [DOI] [PubMed] [Google Scholar]
- 73.Gottardis MM, Robinson SP, Satyaswaroop PG, Jordan VC. Contrasting actions of tamoxifen on endometrial and breast tumor growth in the athymic mouse. Cancer Res. 1988;48:812–5. [PubMed] [Google Scholar]
- 74.Fornander T, Cedermark B, Mattsson A, Skoog L, Theve T, Askergren J, et al. Adjuvant Tamoxifen in Early Breast-Cancer - Occurrence of New Primary Cancers. Lancet. 1989;1:117–20. doi: 10.1016/s0140-6736(89)91141-0. [DOI] [PubMed] [Google Scholar]
- 75.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 76.Smith CL, O’Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev. 2004;25:45–71. doi: 10.1210/er.2003-0023. [DOI] [PubMed] [Google Scholar]
- 77.Black LJ, Jones CD, Falcone JF. Antagonism of estrogen action with a new benzothiophene derived antiestrogen. Life Sci. 1983;32:1031–6. doi: 10.1016/0024-3205(83)90935-9. [DOI] [PubMed] [Google Scholar]
- 78.Gottardis MM, Ricchio ME, Satyaswaroop PG, Jordan VC. Effect of steroidal and nonsteroidal antiestrogens on the growth of a tamoxifen-stimulated human endometrial carcinoma (EnCa101) in athymic mice. Cancer Res. 1990;50:3189–92. [PubMed] [Google Scholar]
- 79.Lerner LJ, Jordan VC. Development of antiestrogens and their use in breast cancer: eighth Cain memorial award lecture. Cancer Res. 1990;50:4177–89. [PubMed] [Google Scholar]
- 80.Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189–97. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- 81.Cummings SR, Ensrud K, Delmas PD, LaCroix AZ, Vukicevic S, Reid DM, et al. Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med. 2010;362:686–96. doi: 10.1056/NEJMoa0808692. [DOI] [PubMed] [Google Scholar]
- 82.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–41. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 83.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res (Phila) 2010;3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–62. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 85.Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99:283–90. doi: 10.1093/jnci/djk050. [DOI] [PubMed] [Google Scholar]
- 86.Jordan VC. Chemosuppression of breast cancer with tamoxifen: laboratory evidence and future clinical investigations. Cancer Invest. 1988;6:589–95. doi: 10.3109/07357908809082124. [DOI] [PubMed] [Google Scholar]
- 87.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–86. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 88.Jordan VC. A century of deciphering the control mechanisms of sex steroid action in breast and prostate cancer: the origins of targeted therapy and chemoprevention. Cancer Res. 2009;69:1243–54. doi: 10.1158/0008-5472.CAN-09-0029. [DOI] [PubMed] [Google Scholar]