Abstract
In order to achieve early detection and specific cancer treatment we propose the use of multivalent interactions in which a series of binding events leads to increased affinity and consequently to selectivity. Using melanotropin (MSH) ligands, our aim is to target melanoma cells, which overexpress melanocortin receptors. In this study, we report the design and efficient synthesis of new trivalent ligands bearing MSH ligands. Evaluation of these multimers on a cell model engineered to overexpress melanocortin 4 receptors (MC4R) showed up to a 350-fold increase in binding compared to the monomer, resulting in a trivalent construct with nanomolar affinity starting from a micromolar affinity ligand. Cyclic adenosine monophosphate (cAMP) production was also investigated leading to more insights into the effects of multivalent compounds on transduction mechanisms.
Keywords: Binding, multiple interactions, multimeric ligand design, targeted therapy, cancer, melanoma
Introduction
Targeted therapy consists of drugs that will target specific patterns (e. g. proteins) involved in tumor spread and growth. Targeting tumor characteristics has the advantage of leading to specific action on the cancer cells, leaving normal cells intact1, 2. G protein coupled receptors (GPCRs) are intensively studied as targets for many diseases since they are involved in cell signaling pathways and have been shown to be up-regulated in many cancers3. Melanoma is the most threatening skin cancer: once the metastatic stage is reached the prognosis is poor since the tumor is resistant to most cures. Therefore, early diagnosis and specific treatment could increase the chances of survival. The melanocortin 1 receptor (MC1R) is known to be overexpressed4–6 in most forms of melanoma, which made an important target7–10 since it can be targeted using a variety of ligands including well-studied melanotropin ligands. The cooperative multivalency effect is involved in many biological pathways11, 12, and consists of the multiple and simultaneous binding of ligands to multiple targets leading to an increased affinity. Increases in specificity can also be provided by multivalency by a complex interaction between ligand affinity and receptor number13, 14. Our strategy consists in seeking cooperative effects using multimers to bind with a high affinity and selectivity to cancer cells targets. Previously, our group and others have described the use of different templates to construct multimers15–24 resulting in relative enhancement, up to about 50-fold. Herein, we describe the synthesis of new dendritic based multimers that can bear up to three melanotropin ligands and the study of their affinity and activity towards a cancer cell model readily available overexpressing MC4R.
Results
Design
In order to seek multivalent interactions, different criteria have to be considered such as the target, the nature of the multimer backbone, the linker length, the valency and the ligands to be used. Different kinds of scaffold have been used in the literature such as peptides23, 24, polymers19, 20 and dendrimers21, 22. Our aim is to use a tetravalent scaffold that will be able to bear three peptides to seek a cooperative effect and one tag that could be used for imaging or therapeutic purposes. In 2006, Chassaing et al., described the synthesis of a small tetravalent template25, which appeared readily modifiable and hence fit our criteria. However, the distance between ligands is important,15–24 and the described template was too small to “adjust” concomitantly three ligands in the binding sites of three GPCRs. Thus, we modified the backbone of our scaffold on the N-terminal with a Boc-Gly, so that every added peptide of a trivalent construct would be equidistant from the scaffold quaternary carbon. An additional β-Ala spacer was placed on the C-terminal of the scaffold as a small spacer to the potential tag moiety. Previous studies have described the optimal length to achieve crosslinking of GPCRs and a potential increase in affinity as between 25 to 50 Å23, 24, 26. The cooperative effect being a thermodynamic process, it is known that a compromise between linker properties such as flexibility and length are necessary. We have previously described PEGO-(Pro-Gly)3–6-PEGO as a more functional semi-flexible spacer, compared to fully flexible PEGO, moderately flexible β-Ala and the more rigid (Pro-Gly) as linkers21, 22.
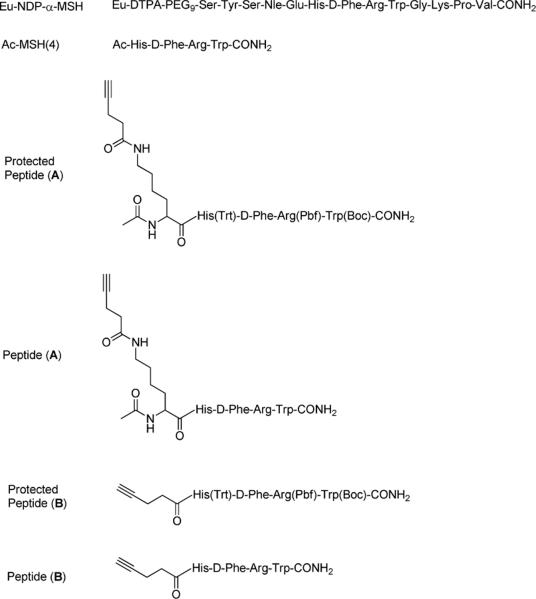
In the current work, we explore the multivalent cooperative effect of a low affinity melanotropin ligand pharmacophore, MSH(4): His-DPhe-Arg-Trp-CONH2 (Figure 1). This peptide was chosen as a model since it binds with micromolar affinity to both receptors MC1R and the MC4R. It can also be modified on its N-terminal without affecting the binding affinity and activity. Furthermore, the relatively low affinity provided a larger potential dynamic range with which to measure affinity enhancements13. We designed two different ligands (Figure 1): one with on its N-terminal a lysine bearing a triple bond on the side chain (peptide A) and one directly acylated on the N-terminal by a triple bond containing moiety (peptide B). To be able to have a strategic scheme that can be used for any other applications, the lysine modification was appropriate when modification within the peptide sequence is possible but not at the N- or C-terminus.
Figure 1.
Sequences of Eu-NDP-α-MSH, Ac-MSH(4), protected peptides (A) and (B) and peptides (A) and (B).
Scaffold synthesis
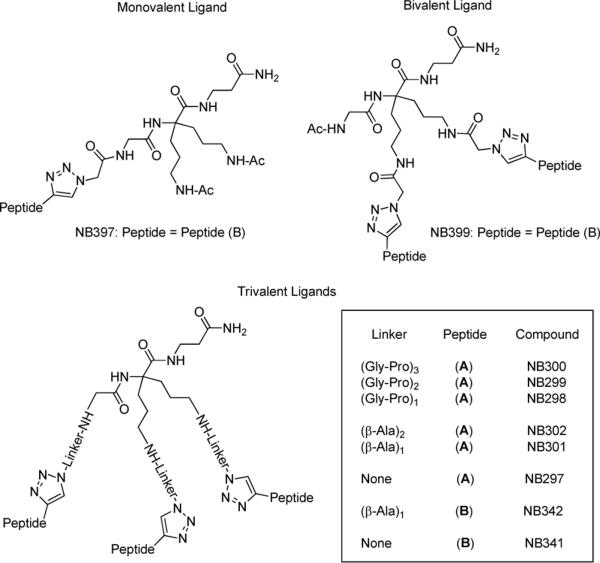
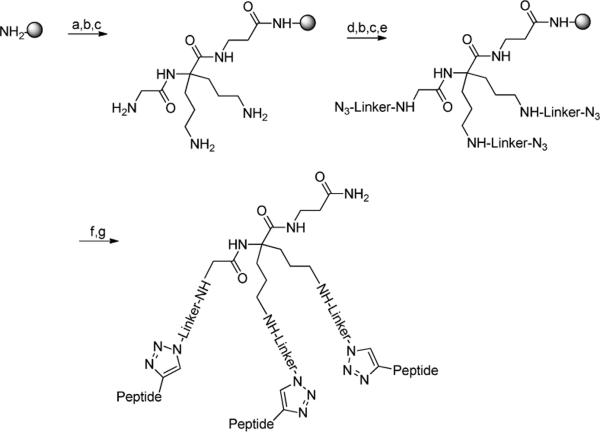
The synthesis of scaffold (7) is reported in Scheme 1. Compound (1) was synthesized as described in the literature by Chassaing et al.25. Coupling of (1) with Boc-Glycine was achieved using O-(Benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HCTU) in dimethylformamide (DMF) in the presence of diisopropylamine (DIEA) to afford the coupling product, which was then hydrolyzed into (3) with a 2N solution of lithium hydroxide in tetrahydrofuran (THF). Compound (3) was coupled to β-Alanine methyl ester (β-Ala-OMe) using (Benzotriazol-1-yloxy)-tris-dimethylaminophosphonium hexafluorophosphate (BOP) and DIEA in DMF and the resulting product (4) was saponified to yield (5), following the procedure described above, but at 0°C. Reduction of cyano groups into amines was done in a Parr apparatus under 50 psi of hydrogen with PtO2 in a mixture of methanol and chloroform to afford (6), that was further treated with di-tert-butyl dicarbonate (Boc2O) in a mixture of water and acetonitrile in the presence of triethylamine to generate (7). This scaffold (7) was used for the synthesis of the various trivalent ligands (Figure 2). Another scaffold with orthogonal protections was synthesized starting from compound (6), in order to selectively produce either the monovalent ligand or the divalent one (Figure 2). This orthogonally protected scaffold (8) was obtained after Fmoc protection of (6) using Fmoc-OSu in a mixture of water and acetonitrile in the presence of NaHCO3 (Scheme 1).
Scheme 1. Scaffold Synthesis.
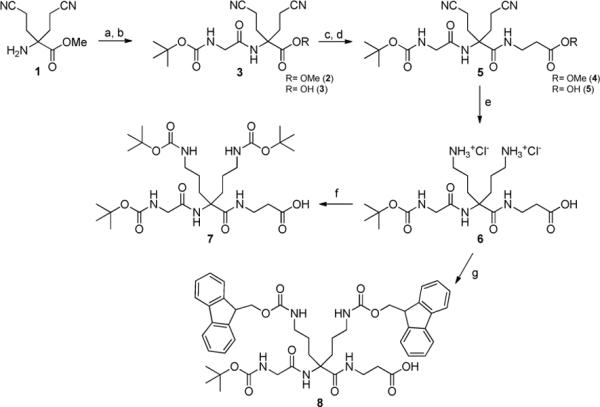
Reagents and conditions: (a) Boc-Gly-OH, HCTU, DIEA, DMF, rt, 92 %; (b) LiOH (2N), THF, rt, 95 %; (c) β-Ala-OMe, BOP, DMF, DIEA, rt, 85 %; (d) LiOH (2N), THF:MeOH, 0°C, 90 %; (e) PtO2, H2, 50 psi, MeOH:CHCl3, rt, 80%; (f) Boc2O, DIEA, H2O:MeCN, rt, 80%; (g) Fmoc-OSu, H2O:MeCN, NaHCO3 0°C to rt, 65 %.
Figure 2.
Structures of monovalent, bivalent and trivalent ligands.
Azide moiety and protected peptide synthesis
The azido acetic acid was prepared with good yields by substitution of sodium azide on the corresponding bromoacetic acid in water. Adequately protected MSH(4) analogs were synthesized on solid support using a Sieber amide resin following the standard Fmoc strategy synthesis procedure. These peptides were functionalized on the N-terminal with a triple bond either by acylation with pentynoic acid, or by coupling a lysine modified on its side chain by pentynoic acid. Cleavage of the resins, a very critical to keep the protections on the side chains was successfully processed using 1%Trifluoroacetic acid (TFA) in dichloromethane (DCM) to give the protected peptides (A) and (B) (Figure 1), with 85% yields. These crude protected peptides (A) and (B) were used without purification for the coupling to the different scaffolds.
Assembly of multivalent constructs
The synthesis of the final trivalent constructs, described in Scheme 2, was achieved on MBHA resin using regular Boc strategy. The scaffold (7) plus the amino acids composing the linkers were successively attached on the resin, until the desired sequence was reached. The azidoacetic acid was finally coupled with equal amounts of coupling agent and DIEA. The resin was dried and the protected peptides (A) or (B) were then attached to the template via reaction of click chemistry27, which was achieved in the presence of copper iodide, ascorbic acid and DIEA in a mixture of DMF and 2,6-lutidine either at room temperature or using microwave irradiation to accelerate the reaction rate. The monovalent and bivalent constructs NB 397 and NB 399, respectively, were synthesized, using the same strategy as above, starting from the scaffold (8) and by removing first the proper orthogonal protecting group, depending on the desired compound, i.e. monomeric or dimeric ligands (Figure 2). Analytical data is reported in Table 1.
Scheme 2. Assembly of the trivalent construct on solid support.
Reagents and conditions: (a) 7, HCTU, DIEA, DMF; (b) 100% TFA; (c) 20% DIEA in DCM; (d) Boc-linker-OH, HCTU, DIEA, DMF; (e) N3CH2CO2H, HBTU, DIEA, DMF; (f) CuI, Ascorbic acid, DIEA, peptide (A) or (B), (Cf. Figure 2 for the sequences), DMF:2,6-Lutidine; (g) HF.
Table 1.
Analytical data of synthesized multimers.
| Compounda | Linker | Mass Spectrumb | HPLCc, tR (min) | ||
|---|---|---|---|---|---|
| Ion | Calc. | Obsd. | |||
| Ac-MSH(4) | - | (M+1)+ | 686.3482 | 686.6389 | 9.75 |
| NB297 | No linker | (M+5)5+ | 650.1385 | 650.1376 | 12.37 |
| NB298 | (Gly-Pro)1 | (M+6)6+ | 647.4978 | 647.4982 | 12.97 |
| NB299 | (Gly-Pro)2 | (M+6)6+ | 724.5349 | 724.5359 | 12.90 |
| NB300 | (Gly-Pro)3 | (M+6)6+ | 801.5720 | 801.5709 | 12.86 |
| NB301 | β-Ala | (M+6)6+ | 577.4685 | 577.4683 | 12.90 |
| NB302 | (β-Ala)2 | (M+6)6+ | 612.9871 | 612.9861 | 12.83 |
| NB341 | No linker | (M+5)5+ | 548.0752 | 548.0752 | 12.94 |
| NB342 | β-Ala | (M+5)5+ | 590.6974 | 590.6969 | 12.90 |
| NB397 | No linker | (M+2)2+ | 689.3679 | 689.3666 | 11.58 |
| NB399 | No linker | (M+4)4+ | 578.8050 | 578.8053 | 12.62 |
NB297 to NB302 were synthesized using peptide (A) and NB341 to NB399 were synthesized using peptide (B). NB397 is the monovalent ligand and NB399 is the bivalent ligand.
Maldi-TOF or ESI-MS.
Peptides were eluted with a linear gradient from 0 to 100% acetonitrile containing 0.1%TFA in 30 min at 1mL/min. The purity of all peptides determinec by HPLC was ≥ 95%.
Binding Assays
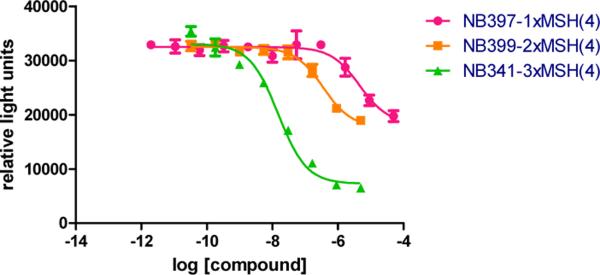
Ac-MSH(4) (Figure 1) has poor binding affinity for the MC4R (IC50 4900 nM, Table 3). The linker length effect on potency compared to Ac-MSH(4) was studied using two kinds of linker within the range of 25 to 60 Å (Table 2). A general trend can be observed: the shorter the linker, the better the affinity. Also, we can observe a difference depending on the type of linker used. When the semi-flexible Gly-Pro linker is used, the affinity was only increased from 8- to 20-fold compared to the monomer (IC50 600 to 200 nM). However, the use of the more flexible β-Ala linker showed an affinity enhancement from 40- to 70-fold depending on the length used. It is notable that the trivalent compounds, NB297 and NB342, are made of ligands (A) and (B), respectively, but the length from ligand to ligand is the same. These two trivalent ligands show the same IC50 of about 72 nM, which is consistent with the importance of the length and the N-terminal modifiability of MSH(4). Although analogues containing different linkers were synthesized, the construct that showed the best affinity was the shortest one, NB341, (24 ± 5 Å), which does not have any linker besides the scaffold bonds.
Table 3.
Influence of valency on affinity.
| Compound | IC50 (nM) | Relative potency to NB397 |
|---|---|---|
| NB397-1×MSH(4) | 4900 ± 760 | - |
| NB399-2×MSH(4) | 310 ± 73 | 16 |
| NB341-3×MSH(4) | 14 ± 1.5 | 350 |
Competition experiments were carried out using time resolved fluorescence31. Multimers were competed against Eu-NDP-α-MSH. The IC50 values are the average of 4 experiments each done in quadruplicate.
Table 2.
Influence of linker length on affinity.
| Compound | Linker | Estimated length between ligands (Å)a | IC50 (nM) | Relative potency to Ac-MSH(4) |
|---|---|---|---|---|
| Ac-MSH(4) | - | - | 4600 ± 790 | - |
| NB300 | (Gly-Pro)3 | 60±20 | 620 ± 51 | 7 |
| NB299 | (Gly-Pro)2 | 52±20 | 410 ± 70 | 11 |
| NB298 | (Gly-Pro)1 | 45±20 | 220 ± 59 | 21 |
| NB302 | (β-Ala)2 | 48±10 | 120 ± 10 | 38 |
| NB301 | β-Ala | 43±10 | 88 ± 12 | 52 |
| NB297 | No linker | 35±10 | 71 ± 5.3 | 64 |
| NB342 | β-Ala | 35±5 | 73 ± 3.5 | 63 |
| NB341 | No linker | 24±5 | 14 ± 1.5 | 330 |
Competition experiments were carried out using time resolved fluorescence31. Multimers were competed against Eu-NDP-α-MSH. The IC50 values are the average of 4 experiments each done in quadruplicate.
Linker length was estimated from an energy-minimized structure using MacroModel.
To get more insights on the valence effect, we synthesized and investigated monovalent and bivalent ligands of MSH(4), NB397 and NB399, respectively (Figure 2) and compared their affinities to the corresponding trivalent ligand (Table 3 and Figure 3). As predicted, the affinity of the monovalent ligand NB397, i.e. the ligand being directly attached to the scaffold was found similar to the affinity of acetylated MSH, Ac-MSH(4) (IC50 about 4700 nM ± 200 nM). Surprisingly, the affinity of the bivalent ligand, NB399, (IC50 310 nM) showed an order of magnitude improvement in binding compared to the monovalent ligand and with the trivalent ligand, NB341, another additional order of magnitude increase in the affinity was observed, leading to an IC50 of 14 nM. The fact that an order of magnitude is reached per additional ligand provides evidence that three receptors are involved in binding, which suggests a cooperative mechanism of binding rather than a statistic effect26.
Figure 3.
Time resolved fluorescence assay31. Competitive binding assay was performed by competing 10nM of Eu-NDP–α–MSH with various concentrations of multimers.
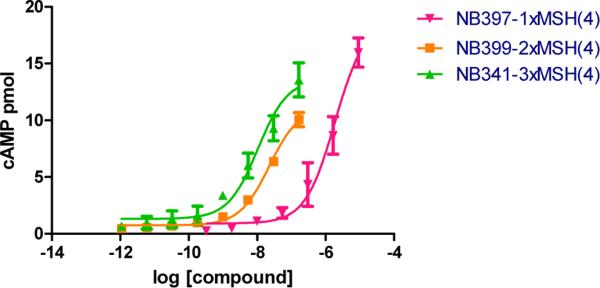
cAMP Assays
Melanotropin ligands are known to stimulate cAMP when bound to melanocortin receptors such as the human MC4R. cAMP measurements were made using the mono-, bi- and trivalent constructs (NB397, NB399 and NB341, respectively) to investigate how these ligands combinations affect cell signaling. cAMP production was activated by each of the constructs demonstrating that they all are agonists for the MC4R receptor. The sensitivity for activation of cAMP production was found to increase with the valency (Figure 4). In order to determine if the efficacy of signaling was similar between the ligands, the relationship between the number of ligands bound and the amount of cAMP released was evaluated. Receptor occupancy was calculated at different percentages of the maximal cAMP response, which was measured as the amount of cAMP produced (above control) compared to that observed in response of 10 μM of forskolin. As shown in Table 4, the number of receptors occupied by each construct was within the same range, suggesting that the bivalent and trivalent constructs activate the transduction mechanisms as a monovalent construct. It is also important to remember that the bivalent NB399 and trivalent NB341 molecules showed orders of magnitude higher affinity, which supports the fact that two and three receptors are bound. However, since the occupancy was similar at the same concentration of cAMP for every construct, it provides evidence that the bivalent and trivalent ligands activate the transduction mechanisms with effectiveness similar to a monovalent construct.
Figure 4.
cAMP accumulation in hMC4R cells in response to multivalent ligands. cAMP was quantified using a chemiluminescent immunoassay described on the Experimental section. cAMP is expressed as the amount released from treated cells above that observed for control cells.
Table 4.
Receptor occupancy.
| Compound | [RL] at 5% of forskolin response | [RL] at 10% of forskolin response | [RL] at 25% of forskolin response |
|---|---|---|---|
| NB397-1×MSH(4) | 13000 ± 1000 | 60000 ± 4900 | 420000 ± 34000 |
| NB399-2×MSH(4) | 12000 ± 1300 | 45000 ± 4100 | 400000 ± 22000 |
| NB341-3×MSH(4) | 14000 ± 1000 | 75000 ± 5400 | 450000 ± 33000 |
Receptor occupancy was calculated for the concentration of each ligand required to activate cAMP increase at 5%, 10% and 25% of the maximum observed with forskolin (10 μM) treatment using the following established equation: [RL] = Bmax ×x [L] / [L] + Kd. The Kd values used for this calculation were estimated from the competition binding assays (Figure 3). The numbers of receptors was assumed to be constant at 7.5 × 105 receptors per cell, as previously measured for this cell line32.
Discussion
We have been engaged in a program to design ligands that might selectively target human cancers for diagnosis and therapeutic purposes. Multivalent interactions are part of numerous biological systems and high-affinity highly selective complexes may be formed starting from low-affinity ligands. In this context, our aim was to: i) design new tools for diagnosis and treatment of melanoma cells using novel multivalent melanotropin trivalent molecules; and ii) investigate their binding and activity properties. In the multivalent strategy, particular care should be taken in the choice/design of the scaffold and the linker, which should have the “right size” in order to avoid a too large cost in entropy11, 12. Two types of linkers have been investigated within the proposed ideal range for spanning ligands for simultaneous binding (25–50 Å) 23, 26. “Bis-ornithine” represented a powerful scaffold to be explored for grafting ligands, since the χ1 and χ2 rotamers of this α,α'-amino acid side chains are locked, with a minimal to maximal distance between the two NH2 around 5 to 9 Å. The third amino function involved in the grafting of the MSH(4) ligand is on a more flexible position, as the three rotamers (Φ, ψ, θ) of β-Ala might theoretically be accessible. Altogether this construct appeared as the best compromise for offering four anchoring points with a minimal mobility, a critical point in this strategy. The synthetic scheme was developed so diverse multivalent compounds can be easily prepared with high yields. Competitive binding experiments were performed by evaluating displacement of a potent Eu-NDP-α-MSH ligand (Figure 1). Although β-Ala linkers showed a 40- to 70-fold increase in affinity the best results were reached for multimers without any linker. The monovalent compound attached to the β-Ala- “bis-ornithine” scaffold led to a similar micromolar affinity as Ac-MSH(4), but increasing the ligand valency increased the affinity from the monovalent to the divalent and the trivalent constructs, as expected for successful concomitant binding to multiple receptors. Selectivity of this trivalent construct, which displayed a 350 times-fold increase in affinity when compared to the monovalent ligand, will be ensured when a high density of MSH receptors is reached as shown for many melanoma cell lines.
Bivalent and trivalent ligands showed orders of magnitude increases in affinity, which supports the premise that when bound, these ligands occupy two and three receptors, respectively. Activity experiments established that starting from AcMSH(4), which is a weak agonist of MSH receptors, cAMP production increased with affinity of the multivalent ligand, the trivalent construct exhibiting an EC50 value in the nanomolar range close to its IC50 value. However, the occupancy is similar at the same concentration of cAMP for every construct, even though two or three receptors are involved in binding. This suggests that the bivalent and trivalent ligands activate the transduction mechanisms much as a monovalent ligand. G protein coupled receptors are known to dimerize, either in the presence of the ligand and/or at the basal level28, 29. Dimers of receptors first identified on cells overexpressing receptors, probably as a consequence of the high density of receptors, have been shown to exist in tissues. However, very recently28, 29 it has been demonstrated that the dimers of GPCRs were in fact asymmetric and that only one G protein in the complex presented the expected orientation in the complex to be activated. Thus, we can speculate that even though MSH divalent and trivalent constructs must recruit two to three receptors, respectively as ascertained by the gain in affinity, in the final ligand-receptor complex formed with G protein only one G protein can be activated.
Conclusion
New multimeric ligands were designed for multivalent interactions with MSH receptors. The optimal construct showed a 350-fold increase in the affinity vs. the corresponding micromolar affinity of the monovalent construct and Ac-MSH(4). Activity studies confirmed that starting from a weak agonist the final trivalent construct yielded an agonist with nanomolar potency. The pharmacokinetic properties of this highly potent melanotropin trivalent ligand will be explored to ascertain the sensitivity and selectivity to detect and target melanoma cells overexpressing MC1R. From a more fundamental point of view, these multimeric ligands should constitute powerful tools to explore the transduction mechanism of G protein coupled receptors.
Experimental Section
Chemicals and Materials
Reagents and solvents used were reagent grade quality from commercial sources and used without further purification unless noted otherwise. Purifications by flash chromatography were performed on EMD chemicals silica gel 60 (0.04–0.063mm). p-Methylbenzhydrylamine-resin (MBHA resin), (0.7 mmol/g) and Sieber amide resin (0.5mmol/g) were purchased from ChemPep, and amino acids were purchased from Aapptec and Iris Biotech GmbH. Other starting materials and chemicals were mostly purchased from Sigma Aldrich and from Acros for platinium dioxide. NMR spectra were recorded on Bruker ARX 250 or 500 MHz and are referenced in ppm (δ), and coupling constants (J) are expressed in Hz. Melting points were measured using a Kofler apparatus and are uncorrected. Mass spectra were obtained with an ESI-mass spectrometer (Finnigan, thermoelectron, lcq classic), and high-resolution fast atom bombardment spectrometer (JEOL HX110 sector instrument) or by MALDI-TOF (Voyager DE-Pro). HPLC was performed on a Hewlett-Packard 1100 series liquid chromatograph (Agilent technologies) with a C8 column (SymmetryPrep, 0.78 × 30cm) or C18 column (Vydac, 1.0 × 25cm), separations were monitored at 230 and 280 nm and final purity was determined as ≥95% using a C18 Vydac column (0.46 × 25 cm).
Methyl 2-(2-(tert-butoxycarbonylamino-acetamido)-4-cyano-2-(2-cyanoethyl-butanoate (2)
To compound (1) (5.85 g, 30 mmol, 1eq.) dissolved in 50 mL of dry DMF, were added Boc-Gly (37 mmol, 1.2 eq.), HCTU (30 mmol, 1eq.) and DIEA (33 mmol, 1.1 eq.). After overnight stirring at room temperature under argon atmosphere, the mixture was poured into a saturated solution of ammonium hydrochloride. After extraction with EtOAc, the organic phase was washed with 5% citric acid, sat. NaCl and 10% NaHCO3, dried over MgSO4 and concentrated in vacuo leading to (2), which was purified by silica gel chromatography EtOAc:Cy (6:4), crystallization from EtOAc afforded (2) as a white solid, (9.8 g, 28 mmoles, 92% yield); Rf 0.52 EtOAc:Cy (8:2); mp 102–103°C; 1H NMR (CDCl3) δ (ppm): 3.91 (s, 3H), 3.75 (m, 2H), 2.95 (m, 2H), 2.32 (m, 4H), 2.17 (m, 2H), 1.48 (s, 9H); 13C NMR (CDCl3) δ (ppm):12.1 (CH2), 28.2 (CH2), 30.2 (CH3), 45.2 (CH2), 53.9 (CH3), 62.5 (C), 80.8 (C), 118.4 (CN), 156.2 (CO), 165.6 (CO), 171.7 (CO); MS (HRMS) m/z calc. for C16H24N4O5Na 375.1644, found 375.1638.
2-(2-(tert-Butoxycarbonylamino-acetamido)-4-cyano-2-(2-cyanoethyl) butanoic acid (3)
A solution of (2) (8.8 g, 25 mmol, 1 eq.) in THF (100 mL) was treated by slow addition of 25 mL of LiOH 2N (51 mmol, 2 eq.). The solution was stirred for 1 hr and a solution of NaHSO4 (1N) was added until pH 3. The product was extracted using EtOAc and the organic phase was dried over MgSO4 and concentrated in vacuo to afford (3) as a white solid (8g, 24 mmoles, 95 % yield). Rf 0.38 DCM:MeOH:CH3COOH (8:2:0.1); mp 74–75°C; 1H NMR (CDCl3) δ (ppm): 3.88 (m, 2H), 2.96 (m, 2H), 2.37 (t, J 8Hz, 4H), 2.23 (m, 2H), 1.46 (s, 9H); 13C NMR (CDCl3) δ (ppm):12.2 (CH2), 28.4 (CH2), 30.1 (CH3), 45.3 (CH2), 53.9 (CH3), 64.5 (C), 80.8 (C), 118.4 (CN), 156.2 (CO), 158.2 (CO), 171.2 (CO); MS (HRMS) m/z calc. for C15H22N4O5Na 361.1488, found 361.1482.
Methyl 9,9-bis(2-cyanoethyl)-2,2-dimethyl-4,7,10-trioxo-3-oxa-5,8,11-triazatetradecan-14-oate (4)
To a solution of (3) (4g, 12 mmol, 1eq.), β-Ala-OMe (14 mmol, 1.2 eq.) and BOP (12 mmol, 1eq.) in 24 mL of dry DMF was added DIEA (24 mmol, 2 eq.). After overnight stirring at room temperature under argon atmosphere, the mixture was poured into a saturated solution of ammonium hydrochloride. After extraction with EtOAc, the organic phase was washed with 5% citric acid, sat. NaCl and 10% NaHCO3. The organic phase was dried over MgSO4 and concentrated under vacuum to yield compound (3) as an oil, which was purified by silica gel chromatography, EtOAc:Cy (6:4), leading to an oil, which was crystallized in EtOAc to afford (4) as white crystals (4.3g, 10 mmoles, 85% yield); Rf 0.56 EtOAc:Cy (8:2); mp 138–139°C; 1H NMR (CDCl3) δ (ppm): 3.75 (m, 2H), 3.71 (s, 3H), 3.57 (q, 2H), 3.01 (m, 2H), 2.61 (t, 2H), 2.27 (m, 4H), 1.92 (m, 2H), 1.46 (s, 9H); 13C NMR (CDCl3) δ (ppm):12.1 (CH2), 28.3 (CH2), 30.9 (CH3), 32.85 (CH2), 36.05 (CH2), 52.2 (CH2), 52.2 (CH3), 61.9 (C), 80.7 (C), 118.9 (CN), 160.63 (CO), 169.63 (CO), 169.76 (CO), 173.34 (CO); MS (HRMS) m/z calc. for C19H29N5O6Na 446.2010, found 446.2017.
9,9-bis(2-Cyanoethyl)-2,2-dimethyl-4,7,10-trioxo-3-oxa-5,8,11-triazatetradecan-14-oic acid (5)
To a solution of (4) (6.38g, 16 mmol, 1eq.) in THF:MeOH (5:1) cooled at 4° C, LiOH (2N) (20 mL, 40 mmol, 2.5 eq.) was added dropwise. After stirring 25 min at 4°C, the reaction was quenched by addition of water and decreasing the pH to 3 with a 1N NaHSO4 solution. After extraction with EtOAc (5x), the organic phases were assembled, dried over MgSO4 and concentrated in vacuo to yield the pure compound (5) as a white solid (5.9 g, 14 mmoles, 90% yield). Rf 0.45 DCM:MeOH:CH3COOH (8:2:0.1); mp 124–125°C; 1H NMR (MeOD) δ (ppm): 3.69 (s, 2H), 3.52 (t, J 3.5 Hz, 2H), 2.77 (m, 2H), 2.61 (t, J 2.6 Hz, 2H), 2.39 (m, 4H), 2.17 (m, 2H), 1.49 (s, 9H); 13C NMR (CDCl3) δ (ppm):12.1 (CH2), 28.3 (CH2), 30.4 (CH3), 33.6 (CH2), 36.6 (CH2), 54.5 (CH2), 57.4 (CH3), 62.22 (C), 80.9 (C), 119.7 (CN), 156.68 (CO), 170.3 (CO), 173.4 (CO), 176.57 (CO); MS (HRMS) m/z calc. for C18H28N5O6 410.2034, found 410.2040.
4-(2-(tert-Butoxycarbonylamino-acetamido)-4-((2-carboxyethylcarbamoyl) heptane-1,7-diaminium chloride (6)
Compound (5) (2g, 4.8 mmol, 1 eq.) in 15 mL of MeOH:CHCl3 (5:0.15) was reduced in the presence of PtO2 (0.228 g, 0.77mmol, 0.16 eq.) for 2 hours in a Parr apparatus under 4 bars of H2. The suspension was filtered through celite and evaporated under vacuum to give (6) as a white powder (1.6 g, 3.9 mmoles, 80 % yield), which was used in the next step without further purification since the NMR showed no detectable impurities. mp 116–117°C; 1H NMR (D2O) δ (ppm): 3.68 (s, 2H), 3.39 (m, 2H), 2.88 (m, 4H), 2.42 (m, 2H), 2.06 (m, 2H), 1.81 (m, 2H), 1.44 (m, 4H), 1.36 (s, 9H); 13C NMR (D2O) δ (ppm): 21.5 (CH2), 28.1 (CH3), 31.3 (CH2), 36.4 (CH2), 39.2 (CH2), 44.1 (CH2), 52.6 (CH2), 62.3 (C), 81.3 (C), 158.5 (CO), 171.2 (CO), 173.7 (CO), 175.8 (CO); MS (HRMS) m/z calc. for C18H36N5O6 418.2660, found 418.2664.
9,9-bis(3-(tert-Butoxycarbonylamino-propyl)-2,2-dimethyl-4,7,10-trioxo-3-oxa-5,8,11-triazatetradecan-14-oic acid (7)
To a solution of (6) (10.18 g, 16.5 mmol, 1eq.) in 80 mL H2O:MeCN, (1:1) Boc2O (50 mmol, 3 eq.) and triethylamine (6 eq.) were added. The solution was stirred at room temperature for 24 hours. The pH was then decreased to 3 with 1N NaHSO4. The solution was extracted with CH2Cl2. The organic phase was dried over MgSO4 and then concentrated in vacuo to afford an oil, which was purified by silica gel chromatography in EtOAc:Cy:MeOH:acetic acid (50:50:2.5:1) to afford (7) as a white solid (8.2g, 13.2 mmol, 80% yield). Rf 0.27 EtOAc:Cy:MeOH:acetic acid (50:46:3:1); mp 140–141°C; 1H NMR (CDCl3) δ (ppm): 3.72 (m, 2H), 3.51 (m, 2H), 2.99 (m, 4H), 2.57 (m, 2H), 2.39 (m, 2H), 1.65 (m, 2H), 1.42 (s, 9H), 1.39 (s, 18H), 1.38 (m, 2H); 13C NMR (CDCl3) δ (ppm): 21.6 (CH2), 24.6 (CH2), 28.4 (CH3), 32.5 (CH2), 34.1 (CH2), 40.4 (CH2), 44.9 (CH2), 63.7 (C), 79.6 (C), 156.7 (CO), 169.1 (CO), 172.7 (CO), 173.4 (CO); MS (HRMS) m/z calc. for C28H51N5O10Na 640.3534, found 640.3528.
9,9-bis(3-(9-Fluorenylmethoxy-carbonyl-aminopropyl)-2,2-dimethyl-4,7,10-trioxo-3-oxa-5,8,11-triazatetradecan-14-oic acid (8)
To a solution, at 0°C, of (6) (1.7 g, 4 mmol, 1eq.) in 20 mL H2O:MeCN (1:1) is added NaHCO3 (1.36g, 16 mmol, 4eq.) and dropwise Fmoc-OSu (3 g, 9 mmol, 2.2eq.) dissolved in 6mL of MeCN. The temperature was then raised at room temperature and the solution was stirred at room temperature for 24 hours. The pH was then decreased to 3 with 1N NaHSO4 and the solution was extracted with DCM. The organic phase was dried over MgSO4 and then concentrated in vacuo to afford an oil, which was purified by silica gel chromatography in EtOAc:Cy:MeOH:acetic acid (50:50:2.5:1) to afford (8) as a white solid (2.2 g, 2.6 mmol, 65% yield). Rf 0.35 EtOAc:Cy:MeOH:acetic acid (50:46:3:1); mp 159–160°C. 1H NMR (CDCl3) δ (ppm): 7.75 (m, 4H), 7.58 (m, 4H), 7.39 (m, 4H), 7.31 (m, 4H), 4.35 (d, J 8 Hz, 4H), 4.19 (m, 2H), 3.75 (m, 2H), 3.53 (m, 2H), 3.13 (m, 4H), 2.59 (m, 2H), 2.47 (m, 2H), 1.64 (m, 2H), 1.44 (s, 9H), 1.40 (m, 2H), 1.27 (m, 2H); 13C NMR (CDCl3) δ (ppm): 24.4 (CH2), 28.6 (CH3), 32.8 (CH2), 33.7 (CH2), 36.21 (CH2), 40.86 (CH2), 44.85 (CH), 63.59 (CH2), 80.6 (C), 120.1 (CH-Ar), 125.2 (CH-Ar), 127.34 (CH-Ar), 129.15 (CH-Ar), 141.4 (C-Ar), 143.8 (C-Ar), 157.2 (CO), 172.5 (CO), 172.86 (CO), 174.76 (CO); MS (HRMS) m/z calc. for C48H56N5O10 862.4022, found 862.4036.
Azidoacetic acid
To a solution of sodium azide (8.2 g, 126 mmol, 2 eq.) in 42 mL of water bromoacetic acid (10 g, 63 mmol, 1 eq.) was slowly added. The solution was stirred at room temperature overnight. The reaction was then quenched by dropwise addition of 35 mL conc. HCl (37%). After extraction with Et2O (4×50 mL), the organic phase was dried over MgSO4 and concentrated in vacuo to afford azidoacetic acid, as an oil (59 mmol, 95% yield). 1H NMR (CDCl3) δ (ppm): 10.16 (s, 1H), 3.97 (s, 2H). IR: 2108.45cm−1. MS (GC) m/z calc. for C2H3N3O2 101.02, found 101.02.
Synthesis of the protected peptides (A), (B)
The protected peptides (A) and (B) were synthesized starting from 1 g of Sieber amide resin (0.5 mmol/g) using Fmoc chemistry in Torviq fritted syringes. The resin was swollen in DMF:DCM (1:1) for 1 hour. The resin was first deprotected using 25 % piperidine in DMF (1×5 min and 1×20 min). The amino acids were coupled to the resin with an elongation from the C-terminal to the N-terminal with Fmoc-Trp(Boc)-OH, Fmoc-Arg(Pbf)-OH, Fmoc-D-Phe-OH, Fmoc-His(Trt) and either the modified lysine bearing a triple bond or pentynoic acid. Coupling was achieved for one hour using 3 equivalents (relative to resin loading) of HBTU and HOBt and 6 equivalents of DIEA. Coupling completion was confirmed using Kaiser test (negative test: yellow beads). Fmoc deprotections were performed as described above for the first deprotection step. After each step (coupling and deprotection) the resin was washed using DMF (3 × 2 min) and DCM (3 × 2 min) washings. Once the sequence was achieved the peptidyl-resin was thoroughly washed with DMF, DCM and finally MeOH before drying under vacuum. The resin was swollen in dry DCM and cleavage was performed by incubating (15 × 2 min) 1g of resin with a solution of distilled DCM (10 mL) containing 1%TFA and then pouring into 10% pyridine in methanol (30mL). The solution was then evaporated under reduced pressure and the resulting oil was dissolved in DCM (10 mL), which was washed with water to remove the trifluoroacetate pyridinium salt. The DCM solution was then transferred into a centrifuge tube and the protected peptide was precipitated with cold diethyl ether. The precipitate was isolated by centrifugation. The peptide was then triturated with ether and the pellet dried in the air to afford 85% yield of crude peptide, which was used for the next step without purification.
Assembly of the multivalent constructs
Multivalent constructs were synthesized on MBHA resin (20 mg, 0.7 mmol/g) using Boc chemistry in Torviq fritted syringes. The resin was swollen in DCM and then treated with 100% TFA (3 × 1 min), then rinsed with DCM following another rinse with 20 % DIEA in DCM. The amino acids were coupled to the resin with an elongation from C-ter to N-ter.
Trivalent constructs: NB297, NB298, NB299, NB300, NB301, NB302, NB341, NB342. General procedure
The scaffold (7) was first coupled to the resin followed by the linkers either Boc-βAla-OH, Boc-Pro-OH or Boc-Gly-OH. Couplings were done using 3 equivalents of HCTU and 6 equivalents of DIEA. Boc removal was achieved using 100% TFA (3×1min) following by DCM wash and 20% DIEA in DCM wash. Completion was monitored using Kaiser test. Azidoacetic acid was finally coupled using HBTU (3 eq) and DIEA (3 eq). The reaction was monitored using Kaiser test and a modified version of Kaiser test containing 5%PPh3 to unveil the presence of the azido group, (regular Kaiser test gives yellow beads and the Kaiser test containing the phenyl phosphine gives blue beads30). The resin was finally washed with DCM and MeOH and dried under vacuum for click chemistry. The resin was used unswollen, the “click reaction” was performed using per arm of template a 2-fold excess of protected peptides in the presence of CuI (5-fold excess), ascorbic acid (5-fold excess) and DIEA (7-fold excess) in a mixture of DMF:2,6-lutidine (7:3), either at room temperature for 4 days or with microwave activation at 250W, T max = 50°C for 60 × 5 sec with liquid nitrogen cooling between each cycle. The resin was then rinsed using DMF (5×2 min), 0.5% of diethylthiocarbamate in DMF (2 × 10 min) and finally DMF, DCM and MeOH. The resin was dried under vacuum then transferred into a Teflon reactor, for 20 mg of resin, 30 μL of anisole was added and HF cleavage was processed for 2 hours at 0°C. The resin was first filtered then washed with ether. The multivalent constructs were washed off from the resin using 10% acetic acid in H2O, transferred into a centrifuge tube for lyophilization to afford the crude multivalent ligands, which were purified by HPLC. Final purity was determined as ≥95% by analytical HPLC (0–100% acetonitrile in 30 min) with a C18 column Vydac (0.46 × 25 cm).
Bivalent construct: NB399
The scaffold (8) was coupled to the resin, followed by Boc group removal and acetylation of the resulting free amine. Fmoc groups were then removed and the azido acetic acid was coupled onto the two remaining amines to afford a bivalent construct with two azides. The azido group was coupled to protected peptide (B) via a click reaction. After HF cleavage the bivalent construct NB399 was obtained and purified by HPLC with a final purity ≥95% by analytical HPLC (0–100% acetonitrile in 30 min). All reactions were performed with the same protocols as described for the synthesis of the trivalent constructs.
Monovalent construct: NB397
The scaffold (8) was coupled to the resin, followed by Boc group removal and coupling of the azido acetic acid on the free amine. The azido group was then coupled to protected peptide (B) via a click reaction. The Fmoc groups were finally removed and the resulting free amines were acetylated. After HF cleavage the monovalent construct NB397 was purified by HPLC with a final purity ≥95% by analytical HPLC (0–100% acetonitrile in 30 min). All reactions were performed with the same protocols as described for the synthesis of the trivalent constructs.
Cell lines
HEK293 cells overexpressing the human MC4R were used to assess the affinity and the activity at the hMC4R. The coding region of the human MC4R gene was expressed in pcDNA3.1 (Invitrogen, V790-20). Hek293/hMC4R cells were grown in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% SCS and 1% Penicillin-Streptomycin and were kept under standard conditions (37°C and 5% CO2).
Time resolved fluorescence binding assay
The HEK293 cells were seeded at 20000 cells per well into 96 well Costar 3603 plates 3 days before the experiment. Competition assays were performed in quadruplicate unless noted otherwise using a fixed concentration of Eu-NDP-α-MSH (10 nM, 50 μL per well) and 8 to 10 different concentrations of ligand. The day of the experiment, media was aspirated. Ligands were diluted in binding medium (DMEM, 1mM 1,10-phenantroline, 200 mg/L bacitracin, 0.5 mg/L leupeptin, and 0.2% bovine albumin serum (BSA)) and added to the cells that were incubated for 2 h at 37°C. Following the incubation, cells were washed three times with wash buffer (DMEM, 20 μM EDTA, 0.2% BSA, 0.01% Tween 20), enhancement solution (Delfia, Perkin Elmer) was added to the plates (100 uL per well) and incubated for 30 min at 37°C. The plates were read on a Wallac Victor instrument using the standard Eu(III) TRL measurement (340 nm excitation, 400s delay and emission collection for 400s at 615 nm). Competitive binding data were analyzed with GraphPad Prism software using nonlinear regression analysis and fitted to a classic one site binding competition equation.
cAMP biological response immunoassay
The HEK293 cells were seeded at 20000 cells per well into 96 well Costar 3598 plates 3 days before the experiment and incubated at 37°C in 5% CO2. Assays were performed in triplicate in 3 different experiments. Media was aspirated and cells were pre-incubated with 1mM 3-isobutyl-1-methylxanthine (IBMX) in DMEM at 37°C for 10 min. 30 μL of compounds and standards were added to the cells and 8 concentrations of ligand were tested (10−7 to 10−12) and plates were incubated for 15 min at 37°C. cAMP production was measured using a chemiluminescent immunoassay kit as described in detail in the user manual (Invitrogen C10558). Briefly, cells were incubated in 60 μL of lysis buffer (provided with the cAMP assay kit) for 25 min at 37°C then transferred to precoated 96 well plates. To these plates were added 30 μL of cAMP-AP (alkaline phosphatase conjugate) and 60 μL of anti-cAMP antibody. The plates were incubated at room temperature for 1 hr and washed 5 times with wash buffer. Enhancement solution (CSPD substrate / Saphire II enhencer) was added to the plates following by incubation at room temperature in the dark for 30 min. Intensity of the signal was measured using Wallac Victor instrument and the concentrations of cAMP were calculated after the average value of the basal condition was subtracted and based on a standard curve that was acquired during the same assay.
Acknowledgment
This research was supported in parts by grants from CNRS, U-S. Public Health Service, National Institutes of Health and NCI. N. Brabez is a BDI recipient from CNRS. Authors are thankful to James P. Cain and Yeon Sun Lee for their helpful discussions and critical review of the manuscript and to Craig Weber, B.M.Thanuja Jayasundera and Kamya Ananthakrishnan for assistance and discussions on the bioassays.
Abbreviations
- cAMP
cyclic adenosine monophosphate
- β-Ala
β-alanine, NH2-CH2-CH2-COOH
- Boc2O
di-ter-butyl-carbonate BOP, (Benzotriazol-1-yloxy)-tris-dimethylaminophosphonium hexafluorophosphate
- BSA
bovine serum albumin
- Cy
cyclohexane
- DCM
dichloromethane
- DIEA
diisopropylethylamine
- DMEM
Dulbecco's Modified Eagle Medium
- DMF
dimethyformamide
- EDTA
Ethylenediaminetetraacetic acid
- Fmoc-OSu
N-(9-Fluorenylmethoxycarbonyloxy) succinimide
- GPCRs
G protein coupled receptors
- HBTU
O-(Benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate
- HCTU
O-(6-Chlorobenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate
- MC1R
melanocortin 1 receptor
- MC4R
melanocortin 4 receptor
- MSH
melanotropin, melanocyte stimulating hormone
- MSH(4)
His-DPhe-Arg-Trp-CONH2
- TFA
trifluoroacetic acid
- THF
tetrahydrofuran
References
- (1).Finley RS. Overview of targeted therapies for cancer. Am. J. Health-Syst Pharm. 2003;60:s5–s10. doi: 10.1093/ajhp/60.suppl_9.S4. [DOI] [PubMed] [Google Scholar]
- (2).Wu H, De-Kuan C, Chia-Ting H. Targeted therapy for cancer. Journal of Cancer Molecules. 2006;2:57–66. [Google Scholar]
- (3).Dorsam RT, Gutkind RJ. G-protein-coupled receptors and cancer. Nature Reviews. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- (4).Tatro JB, Atkins M, Mier JM, Hardason S, Wolfe H, Smith T, Entwistle ML, Reichlin S. Melanotropin receptors demonstrated in situ in human melanoma. J. Clin. Invest. 1990;85:1825–1832. doi: 10.1172/JCI114642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Carlson AJ, Linette GP, Aplin A, Ng B, Slominski A. Melanocyte receptors: clinical implications and therapeutic relevance. Dermatol. Clin. 2007;25:541–557. doi: 10.1016/j.det.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Jiang H, Wortsman J, Matsuoka L, Granese J, Carlson AJ, Mihm M, Slominski A. Molecular spectrum of pigmented skin lesions; from nevus to melanoma. Expert Rev. Dermatol. 2006;1:679–700. [Google Scholar]
- (7).Ren G, Pan Y, Cheng Z. Molecular probes for malignant melanoma imaging. Current Pharmaceutical Biotechnology. 2010;11:590–602. doi: 10.2174/138920110792246465. [DOI] [PubMed] [Google Scholar]
- (8).Sharma SD, Granberry ME, Jiang J, Leong SPL, Hadley ME, Hruby VJ. Multivalent melanotropic peptide and fluorescent macromolecular conjugates. Bioconjugate Chem. 1994;5:591–601. doi: 10.1021/bc00030a015. [DOI] [PubMed] [Google Scholar]
- (9).Sharma SD, Jiang J, Hadley ME, Bentley DL, Hruby VJ. Melanotropic peptide-conjugated beads for microscopic visualization and characterization. Proc. Nat. Acad. Sci. 1996;93:13715–13720. doi: 10.1073/pnas.93.24.13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Raposinho PD, Correia JDG, Alves S, Botelho MF, Santos AC, Santos IA. 99mTc(CO)3-labeled pyrazolyl-a-melanocyte-stimulating hormone analog conjugate for melanoma targetting. Nuclear Med. and Biol. 2008;35:91–99. doi: 10.1016/j.nucmedbio.2007.08.001. [DOI] [PubMed] [Google Scholar]
- (11).Mammen M, Choi S. Whitesides G. M. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew.Chem.Int.Ed. 1998;37:2754–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- (12).Handl HL, Vagner J, Han H, Mash E, Hruby VJ, Gillies RJ. Hitting multiple targets with multimeric ligands. Expert Opin. Ther. Targets. 2004;8:565–586. doi: 10.1517/14728222.8.6.565. [DOI] [PubMed] [Google Scholar]
- (13).Caplan MR, Rosca EV. Targeting drugs to combinations of receptors: A modeling analysis of potential specificity. Ann. Biomed. Eng. 2005;33:1113–1124. doi: 10.1007/s10439-005-5779-1. [DOI] [PubMed] [Google Scholar]
- (14).Rosca EV, Stukel JM, Gillies R. J.; Vagner J, Caplan MR. Specificity and mobility of biomacromolecular, multivalent constructs for cellular targeting. Biomacromolecules. 2007;8:3830–3835. doi: 10.1021/bm700791a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Vagner J, Handl HL, Monguchi Y, Jana U, Begay LJ, Mash EA, Hruby VJ, Gillies RJ. Rigid linkers for bioactive peptides. Bioconjugate Chem. 2006;17:1545–1550. doi: 10.1021/bc060154p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Lee Y, Sampson NS. Romping the cellular landscape : linear scaffolds for molecular recognition. Current Opin. In Structural Biology. 2006;16:544–550. doi: 10.1016/j.sbi.2006.05.015. [DOI] [PubMed] [Google Scholar]
- (17).Arosio D, Vrasidas I, Valentini P, Liskamp R. M. J; Pieters, R. J., Bernardi A. Synthesis and cholera toxin binding properties of multivalent GM1 mimics. Org. Biomol. Chem. 2004;2:2113–2124. doi: 10.1039/b405344c. [DOI] [PubMed] [Google Scholar]
- (18).Vadas O, Harthley O, Rose K. Characterization of new multimeric Erythropoietin receptors agonists. Peptide Science. 2008;90:496–502. doi: 10.1002/bip.20959. [DOI] [PubMed] [Google Scholar]
- (19).Kramer RH, Karpen JW. Spanning binding sites on allosteric proteins with polymer-linked ligand dimers. Letters to Nature. 1998;395:710–713. doi: 10.1038/27227. [DOI] [PubMed] [Google Scholar]
- (20).Kanai M, Mortell KH, Kiessling LH. Varying the size of multivalent ligands: the dependence of concanavalin a binding on neoglycopolymer length. J. Am. Chem. Soc. 1997;119:9931–9932. [Google Scholar]
- (21).Fan E, Zhang Z, Minke WE, Hou Z, Verlinde CLMJ, Hol WGH. High-affinity pentavalent ligands of E.coli heat-labile enterotoxin by modular structure-based design. J. Am. Chem. Soc. 2000;122:2663–2664. [Google Scholar]
- (22).Andre S, Pieters R, Vrasidas I, Kaltner H, Kuwabara I, Liu F, Liskamp RMJ, Gabius H. Wedgelike glycodendrimers as inhibitors of binding of mammalian galectins to glycoproteins, lactose maxiclusters, and cell surface glycoconjugate. Chem. Bio. Chem. 2001;2:822–830. doi: 10.1002/1439-7633(20011105)2:11<822::AID-CBIC822>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- (23).Handl HL, Sankaranarayanan R, Josan JS, Vagner J, Mash EA, Gillies RJ, Hruby VJ. Synthesis and evaluation of bivalent NDP-α-MSH peptide ligands for binding to the human melanocortin receptor 4 (hMC4R) Bioconjugate Chem. 2007;18:1101–1109. doi: 10.1021/bc0603642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Vagner J, Xu L, Handl HL, Josan JS, Morse DL, Mash EA, Gillies RJ, Hruby VJ. Heterobivalent ligands crosslink multiple cell-surface receptors: the human melanocortin-4 and Δ-opioid receptors. Angew. Chem. Int. Ed. 2008;47:1685–1688. doi: 10.1002/anie.200702770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Aussedat B, Chassaing G, Lavielle G, Burlina F. Bis-ornithine (2,2-bis(aminopropyl)glycine): a new tetravalent template for assembling different functional peptides. Tetrahedron Letters. 2006;47:3723–3726. [Google Scholar]
- (26).Josan JS, Handl HL, Sanbararyanan R, Xu L, Lynch RM, Vagner J, Mash EA, Hruby VJ, Gillies RJ. Specific targeting by heterovalent ligands. Bioconjugate Chem. 2011;22:1270–1278. doi: 10.1021/bc1004284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Zhang Z, Fan E. Solid phase synthesis of peptidotriazoles with multiple cycles of triazole formation. Tetrahedron Letters. 2006;47:665–669. [Google Scholar]
- (28).Bulenger S, Marullo S, Bouvier M. Emerging role of homo- and heteromerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol. Sci. 2005;26:131–137. doi: 10.1016/j.tips.2005.01.004. [DOI] [PubMed] [Google Scholar]
- (29).Pellisier LP, Barthet G, Gaven F, Cassier E, Trinquet E, Pin J, Marin P, Dumuis A, Bockaert J, Baneres J, Claeysen J. G protein activation by serotonin type 4 receptor dimers. J. Biol. Chem. 2011;286:9985–9996. doi: 10.1074/jbc.M110.201939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Punna S, Finn MG. A convenient colorimetric test for aliphatic azides. Synlett. 2004;1:99–100. [Google Scholar]
- (31).Handl HL, Vagner J, Yamamura H. I.; Hruby VJ, Gillies RJ. Anal. Biochem. 2004;330:242–250. doi: 10.1016/j.ab.2004.04.012. [DOI] [PubMed] [Google Scholar]
- (32).Xu L, Vagner J, Josan JS, Lynch RM, Morse DL, Baggett B, Han H, Mash EA, Hruby VJ, Gillies RJ. Enhanced targeting with heterobivalent ligands. Mol. Cancer Ther. 2009;8:2356–2365. doi: 10.1158/1535-7163.MCT-08-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]