Abstract
In order to obtain a metabolically more stable analgesic peptide derivative, O-β-glycosylated serine (Ser(Glc)) was introduced into TY027 (Tyr-D-Ala-Gly-Phe-Met-Pro-Leu-Trp-NH-3’,5’-Bzl(CF3)2) which was a previously reported bifunctional compound with delta/mu opioid agonist and neurokinin-1 receptor antagonist activities, and with a half life of 4.8 h in rat plasma. Incorporation of Ser(Glc) into various positions of TY027 gave analogues with variable bioactivities. Analogue 6 (Tyr-D-Ala-Gly-Phe-Nle-Pro-Leu-Ser(Glc)-Trp-NH-3’,5’-Bzl(CF3)2) was found to have effective bifunctional activities with a well-defined conformation with two β-turns based on the NMR conformational analysis in the presence of DPC micelles. In addition, 6 showed significant improvement in its metabolic stability (70 ± 9 % of 6 was intact after 24 h incubation in rat plasma). This improved metabolic stability, along with its effective and delta selective bifunctional activities, suggests that 6 could be an interesting research tool and possibly a promising candidate as a novel analgesic drug.
Keywords: bifunctional peptide derivatives, glycopeptides, analgesics, opioid induced tolerance, opioid receptor agonist, neurokinin-1 receptor antagonist, conformation-activity relationships, NMR structure, DPC micelles
Introduction
Endogenous peptides play important roles in the maintenance of homeostasis as hormones and neurotransmitters in both the periphery and the central nerve system (CNS). Indeed, the biochemical degradation of endogenous peptides appears to be an aspect in their roles as signal-transmitters since, in many cases, protease degradation changes them into an inactive form after the signal has been appropriately transferred. However, the rapid degradation of peptides is undesirable for analgesic drugs, since generally it is desirable that the effects should last for a longer period in order to maintain effective therapeutic potency. Thus, despite their effective potentials, as well as their low toxicities, endogenous peptides have been rarely used in clinical treatment of pain, primarily due to their poor metabolic stabilities, which limits the delivery of periphery administered peptides into the site of action. Moreover, the blood-brain barrier (BBB), which is a protective barrier of the CNS, possesses membrane-bound oxidative enzymes and peptidases which degraded peripherally administered peptide drugs before they reach the CNS.1 Therefore, a peptide drug possessing good metabolic stability together with an effective therapeutic potential should be a promising candidate for novel types of drugs.
To date, several studies have shown that the glycosylation of a peptide can provide a significant increase in stability and other biological properties,2-5 although in some cases the introduction of hydrophilicity into these molecules can result in a decrease or even loss of bioactivities.6, 7 Other reports have shown that the glycosylation of short peptides can change tissue distribution patterns8-10 and enhance peptide-membrane interactions11 compared to the original non-glycosylated sequences. In fact, we have shown that the introduction of a glycosylated residue into enkephalin derivatives leads to the enhanced biodistribution of a peptide into the brain which show better analgesic efficacies compared to the non-glycosylated enkephalins.5, 12-15 Glycosylation can also modify the molecular structure of a synthetic peptide in both the aqueous phase and membrane-like environments,16, 17 to a change in the molecular structure which has significant effects on the pharmacological and metabolic properties of bioactive peptides.18
In the present study, we report the design, synthesis and biological evaluation for a series of glycosylated peptide derivatives, to examine the effect of glycosylation on metabolic stability, bioactivity and pseudo-membrane-bound conformation for targeting analgesic peptides in comparison with the non-glycosylated derivatives. The peptide design was based on our developing bifunctional concept, in which the molecule has both an opioid agonist pharmacophore at the N-terminal portion and a NK1 antagonist pharmacophore at the C-terminus (Figure 1).19-21 This concept was based on the important observations that the simultaneous administration of opioid agonist and NK1 antagonist provided significant benefit of enhanced antinociceptive potency in acute pain states and in preventing opioid-induced tolerance in chronic preclinical trials.22-26 Therefore, the developing bifunctional compounds should be expected to show enhanced analgesic effects without showing the undesirable adverse effects and thus be a drug candidate for pain control.19-21 For the best bioactivity profile for the δ/μ opioid receptor ligands, a compound that is a δ opioid receptor preferring agonist may possess potential clinical benefits compared to the ones for a μ opioid receptor agonist in terms of the greater relief of neuropathic pain,27 reduced respiratory depression,28 and constipation29 as well as a minimal potential for the development of physical dependence.30 Thus, a new generation of opioid agonist with selectivity and efficacy for δ-opioid receptors over μ-opioid receptors, should display reduced toxicity and abuse potential coincident with therapeutic use. In fact, our lead bifunctional compounds, TY005 (Tyr1-D-Ala2-Gly3-Phe4-Met5-Pro6-Leu7-Trp8-O-3’,5’-Bzl(CF3)2)31 and TY027 (1: Tyr1-D-Ala2-Gly3-Phe4-Met5-Pro6-Leu7-Trp8-NH-3’,5’-Bzl(CF3)2),20, 32 have been shown to reverse neuropathic pain without producing opioid-induce tolerance, which validates our hypothesis that opioid agonist/NK1 antagonist bifunctional ligands would be as effective in treating neuropathic pain.
Figure 1.
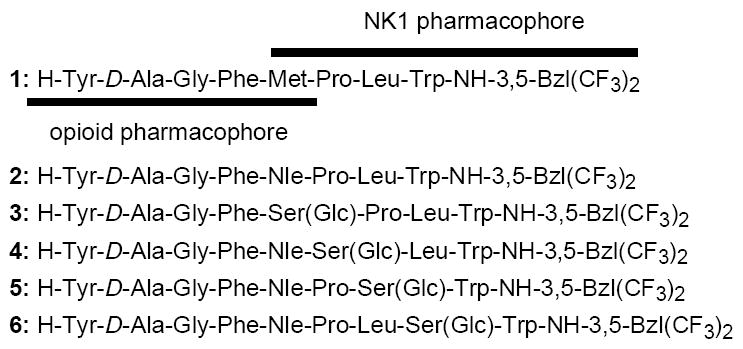
Sequences of opioid and NK1 receptor peptides.
In our present drug design, Nle, which is a bioisoster of Met with high resistance to oxidation,33-35 was first introduced into the fifth position of 1 to provide 2, which could be considered as a biological and physicochemical isoster of 1 with improved metabolic stability (Figure 1). Further glycosylation was made on 2 with O-β-glucosylated serine (Ser(Glc)) which was incorporated or inserted into residues 5, 6, 7 or 8 of 2 to form four novel carbohydrate derivatives 3-6 (Figure 1). The biological activities of the synthesized bifunctional glycopeptides (3-6) were extensively evaluated on our well-established radioligand binding assays, GTPγS binding assays, and isolated tissue-based functional assays using guinea pig ileum (GPI) and mouse isolated vas deferens (MVD) tissues.19-21 The metabolic stability of the synthesized derivatives were tested by incubation in rat plasma at 37 °C. Since understanding membrane-bound structures of ligands and ligand-membrane interactions is indispensable for further insight into their diverse biological behaviors,20 the NMR structures of synthesized glycopeptides 3-6 were obtained using distance and φ dihedral angle constraint information in membrane-mimicking DPC micelles,20, 36, 37 to evaluate the biological and conformational effect of site-specific glycosylation. Further NMR studies using the paramagnetic agent Mn2+ were conducted to clarify the specific interactions of the glycopeptides with model cell membranes.15, 20, 38
Results and Discussion
Peptide Synthesis
The glycopeptide derivatives 3-6 were synthesized using the reductive amination technique on a 4-(4-Formyl-3-methoxyphenoxy)butyryl AM (FMPB-AM) resin, which is a common solid support for carboxamides,39 using the Nα-Fmoc solid-phase peptide synthesis (SPPS) strategy. First, an excess amount of 3’,5’-bis-trifluoromethylbenzyl amine was introduced on the resin in the presence of NaBH(OAc)3 and trimethylorthoformate (TMOF) for converting the aldehyde moiety of FMPB-AM resin to the corresponding secondary amine. The obtained resin-bound 3’,5’-bistrifluoromethylbenzyl amine was reacted with Nα-Fmoc-Trp(Nin-Boc)-OH using HCTU in the presence of 2,6-lutidine, followed by treatment with 20% piperidine to remove the Nα-Fmoc protecting group. Couplings of the following protected amino acids were carried out with standard in situ activating reagents HCTU, in the presence of DIEA, to generate Cl-HOBt esters. Fmoc-Ser(O-β-D-Glc(OAc)4)-OH,40 Fmoc-Leu-OH, Fmoc-Pro-OH, Fmoc-Nle-OH, Fmoc-Phe-OH, Fmoc-Gly-OH and Boc-Tyr(tBu)-OH were used for respective coupling as protected amino acids. The removal of the acetyl protecting groups on glucose was accomplished with 80% H2NNH2·H2O in methanol,15 and then the resin was treated with TFA/TIPS-OTf/thioanisole (9 : 2 : 1; v/v) to obtain the corresponding crude glycopeptide derivative which was treated with 2 N NH4F solution followed by triethylamine. The obtained glycopeptide derivative was purified on a C-18 reversed phase silica gel column followed by RP-HPLC purification, (> 98 %). The final purified peptides were characterized by analytical HPLC, 1H-NMR, HRMS and TLC. 1H-NMR studies showed cis/trans isomerization at the Pro6 residue in some peptides. The ratios of the cis/trans isomers and their assignments are available in the Supporting Information. Compound 2 was synthesized as previously described.20
Biological activities
The initial biological evaluations were performed on the bifunctional peptide 2, in which Met5 of 1 was substituted by Nle, to confirm their bioisosterism. As expected, almost all the bioactivities of 2 were within or close to the experimental error range of 1 (Tables 1-3). Although the only major difference was found in the Emax values at δ-opioid receptors in the GTPγS binding assays (60 and 121 % for 1 and 2, respectively), these two compounds had very similar bioactivities. Thus, the biologically active conformation of 2 was expected to be comparable to that of 1. As also expected, 2 degraded more slowly in rat plasma than 1 did (Figure 2). Because of this improvement in terms of metabolic stability, carbohydrate residues were incorporated into the sequence of 2.
Table 1.
Binding affinities of bifunctional peptide derivatives at δ/μ opioid receptors and NK1 receptors
| no | hDORa, [3H]DPDPE b | rMORa, [3H]DAMGO c | Ki(μ) /Ki(δ) | hNK1d, [3H]Substance Pe | rNK1d, [3H]Substance Pf | Ki(hNK1) /Ki(rNK1) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| LogIC50g | Ki (nM) | LogIC50g | Ki (nM) | LogIC50g | Ki (nM) | LogIC50g | Ki (nM) | |||
| 1h | −8.84 ± 0.07 | 0.66 | −7.44 ± 0.05 | 16 | 24 | −10.91 ± 0.10 | 0.0065 | −7.61 ± 0.03 | 7.3 | 1100 |
| 2 | −8.67 ± 0.05 | 1.0 | −7.17 ± 0.07 | 32 | 32 | −11.57 ± 0.59 | 0.0028 | −7.68 ± 0.03 | 6.8 | 2400 |
| 3 | −6.91 ± 0.09 | 59 | −6.12 ± 0.15 | 260 | 4.4 | −12.21 ± 0.61 | 0.0011 | −8.35 ± 0.02 | 1.5 | 5600 |
| 4 | −7.12 ± 0.09 | 36 | −5.15 ± 0.20 | 3400 | 94 | −8.38 ± 0.07 | 1.8 | −7.14 ± 0.03 | 23 | 13 |
| 5 | −8.10 ± 0.05 | 3.7 | −7.77 ± 0.07 | 8.0 | 2.2 | −9.75 ± 0.30 | 0.077 | −7.30 ± 0.03 | 14 | 180 |
| 6 | −8.83 ± 0.06 | 0.77 | −7.22 ± 0.09 | 30 | 39 | −9.93 ± 0.05 | 0.052 | −7.02 ± 0.05 | 34 | 650 |
| Biphalini | 2.6 | 1.4 | 0.54 | |||||||
| DPDPEj | 1.6 | 610 | 380 | |||||||
| DPDPEk | 10 | 3700 | 370 | |||||||
| L-732,138 | −8.83 ± 0.02 | 0.73 | −6.40 ± 0.03 | 130 | 180 | |||||
Competition analyses were carried out using membrane preparations from transfected HN9.10 cells that constitutively expressed the δ and μ opioid receptors, respectively.
Kd = 0.45 ± 0.1 nM.
Kd = 0.50 ± 0.1 nM.
Competition analyses were carried out using membrane preparations from transfected CHO cells that constitutively expressed rat or human NK1 receptors.
Kd = 0.16 ± 0.03 nM.
Kd = 0.40 ± 0.17 nM.
The logIC50 ± standard error are expressed as logarithmic values determined from the non linear regression analysis of data collected from at least two independent experiments performed in duplicate. The Ki values are calculated using the Cheng and Prusoff equation to correct for the concentration of the radioligand used in the assay.
Reference.20
Reference.61
Reference.63
Table 3.
Functional assay results for bifunctional peptide derivative ligands at opioid and Substance P receptors
| No | Opioid agonist | Substance P antagonist | |||
|---|---|---|---|---|---|
|
| |||||
| MVD (δ) | GPI (μ) | GPI | |||
|
| |||||
| IC50 (nM)a | Emax (%)b | IC50 (nM)a | Emax (%)b | Ke (nM)c | |
| 1d | 15 ± 2.0 | 100 ± 0 | 490 ± 29 | 92.5 ± 3.1 | 10 ± 2.1 |
| 2 | 14 ± 1.6 | 100 ± 0 | 460 ± 160 | 100 ± 0 | 10 ± 2.6 |
| 3 | 110 ± 21 | 100 ± 0 | 1900 ± 470 | 100 ± 0 | 2.8 ± 0.73 |
| 4 | 18 ± 4.9 | 100 ± 0 | 250 ± 48 | 100 ± 0 | 18 ± 6.0 |
| 5 | 13 ± 5.8 | 100 ± 0 | 520 ± 56 | 95.0 ± 2.9 | 1.8 ± 0.30 |
| 6 | 17 ± 4.3 | 100 ± 0 | 670 ± 134 | 93.7 ± 4.1 | 8.4 ± 1.0 |
| Biphalin | 2.7 ± 1.5 | 8.8 ± 0.3 | |||
| DPDPEe | 4.1 | 7300 | |||
| DPDPEf | 2.5 | 2720 | |||
| L-732,138 | 250 ± 87 | ||||
Concentration at 50% inhibition of muscle contraction at electrically stimulated isolated tissues (n = 4).
The δ and μ opioid agonist efficacies (Emax values) of tested compounds were calculated using DPDPE and PL-017 as standards (Emax = 100 %) for MVD and GPI assays, respectively.
Inhibitory activity against the Substance P induced muscle contraction in the presence of 1 μM naloxone, Ke: concentration of antagonist needed to inhibit Substance P to half its activity (n = 4).
Reference.20
Reference.62
Reference.63
Figure 2.
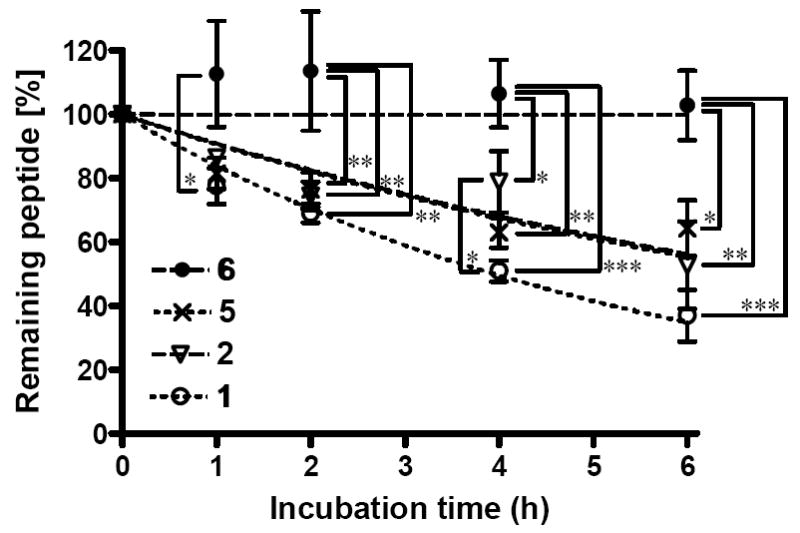
Comparison of the in vitro metabolic stability for 1 (open circle), 2 (open triangle), 5 (crossing) and 6 (filled circle) incubated in rat plasma at 37°C. Calculated half lives of peptide derivatives (T1/2) were 4.8 h for 1 and > 6 h for 2, 5 and 6. 70 ± 9 % of 6 was found intact after 24 h incubation. The samples were tested in three independent experiments (n = 3) and the mean values were used for the analysis with the SD. Statistical significance was determined by Kruskal-Wallis test followed by Tukey’s test. Asterisks denote significant differences (* p < 0.05; ** p < 0.01; *** p < 0.001).
The substitution of the fifth position in the sequence of 2 by Ser(Glc) (3) resulted in a large decrease in binding affinities at both δ and μ opioid receptors with only 4.4-fold δ-selectivity (Ki = 59 and 260 nM, respectively; Table 1). This trend in the opioid selectivity was maintained in the GTPγS binding assays, and also in the isolated tissue bioassays using the MVD and GPI, suggesting the importance of the fifth position for both μ and δ opioid agonist activities (Tables 2 and 3).19 Next, the Ser(Glc) was substituted for Pro at the sixth position (4), which was reported to be an important residue for affinity at the NK1 receptors,41 but also influenced the opioid receptors as well. The affinity of 4 at the δ opioid receptor was reduced (Ki = 36 nM) with very low affinity for the μ opioid receptor (Ki = 3400 nM). GTPγS binding assays also gave decreased activities at both the δ and μ opioid receptors (EC50 = 51 and 380 nM, respectively) with efficient Emax values (134 and 81 % for δ and μ opioid receptors, respectively). Interestingly, and different from the binding assay results for the receptors, the IC50 value of 4 in the MVD tissue assay showed almost the same potency as 1 and 2, with better activity in the GPI assay (18 and 250 nM for MVD and GPI assays, respectively). This might be due to the difference of efficacy requirement in the isolated tissue.
Table 2.
Opioid agonist functional activities in [35S]GTPγS binding assays
| No | hDORa | rMORa | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| LogEC50b | EC50 (nM)c | Emax (%)d | LogEC50b | EC50 (nM)c | Emax (%)d | |
| 1e | −8.07 ± 0.11 | 8.6 | 60 ± 2 | −8.16 ± 0.17 | 7.0 | 51 ± 3 |
| 2 | −8.30 ± 0.09 | 5.0 | 120 ± 3 | −7.74 ± 0.14 | 18 | 66 ± 3 |
| 3 | −7.29 ± 0.12 | 52 | 49 ± 2 | −6.76 ± 0.31 | 180 | 24 ± 3 |
| 4 | −7.56 ± 0.14 | 51 | 130 ± 3 | −6.42 ± 0.19 | 380 | 81 ± 6 |
| 5 | −8.10 ± 0.08 | 7.9 | 64 ± 2 | −7.75 ± 0.23 | 18 | 38 ± 3 |
| 6 | −7.56 ± 0.27 | 28 | 49 ± 3 | −8.21 ± 0.24 | 6.2 | 50 ± 3 |
| Biphalin | −8.95 ± 0.17 | 1.1 | 83 | |||
| DPDPE | −8.80 ± 0.25 | 1.6 | 69 | |||
| DAMGO | −7.44 ± 0.19 | 37 | 150 | |||
Expressed from HN9.10 cell.
The log EC50 ± standard error are logarithmic values determined from the non-linear regression analysis of data collected from at least two independent experiments performed in duplicate.
Anti-logarithmic value of the respective EC50.
[Total bound − Basal]/[Basal − Non-specific] × 100
Reference.20
The replacement with Ser(Glc) at the seventh position (compound 5) yielded a good result for radioligand binding assays at both the δ and μ opioid receptors (Ki = 3.7 and 8.0 nM, respectively, Table 1) relative to those of 3 and 4. The binding affinity of 5 at the μ opioid receptor was 3.8-fold higher than that of 2, whereas its affinity at the δ receptor was 3.7 times lower, leading to a ligand with only modest δ-selectivity (2.2-fold). This selectivity was maintained in the GTPγS binding assays, but its IC50 in the MVD tissue was 13 nM with 40-fold selectivity over the one in the GPI assay (Tables 2 and 3). As a result, the glycopeptide with Ser(Glc)7 (5) had the same levels of functional activities to those of 2 in the isolated tissues, and gave the best affinities and activities among the three glycosylated octapeptides 3-5. It should be noted that the direct modifications of 4 and 5 were made within the NK1 antagonist pharmacophores which is located in the C-terminal half of these peptides, and thus 2, 4 and 5 had the same sequence for the opioid pharmacophore. However, the binding affinities of 4 and 5 at the opioid receptors, especially the affinity of 4 at the μ opioid receptors, showed the changes from those of 2. This change may be due to the glycosylation-induced conformational change in the N-terminal halves where the opioid pharmacophore was incorporated, or a direct steric effect of the introduced sugar moiety could make some contribution in the binding. Finally, the insertion of Ser(Glc) between Leu7 and Trp8 in the sequence of 2 produced 6 which showed increased opioid affinities at δ receptors, resulting in the best affinity and δ-selectivity among the synthesized glycopeptides 3-6 (Ki = 0.77 and 30 nM for δ and μ opioid receptors, respectively). The Ki values of 6 for δ and μ opioid receptors were within or close to the error range of the corresponding values of 2, suggesting that the insertion of Ser(Glc)8 influenced the three-dimensional conformation of the opioid pharmacophore less than for 3-5. The compound 6 showed 49 % agonist efficacy at the δ opioid receptor compared to the standard compound (DPDPE) and the observed highly δ-selective binding affinity was consistent with the results in the isolated tissue-based assays (IC50 = 17 and 670 nM in the MVD and GPI assays, respectively). Thus 6 could be regarded as an effective opioid agonist with the highest δ-selectivity among 1-6.
The binding affinities (Ki values) at the hNK1 receptors of 5 and 6, whose residues next to Trp were glycosylated, were good (77 and 52 pM, respectively, Table 1), but decreased from those of 1 and 2. The affinities of 5 and 6 at the rNK1 receptor also were less than those of 1 and 2. These biological shifts were reasonable, since the sterically hindered glucose moiety could interact spatially with the neighboring Trp, which is a critical pharmacophore for binding to the NK1 receptors.41 However, the activities of 5 and 6 against substance P stimulation in the GPI tissue were higher than those of 1 and 2 (Ke = 1.8 and 8.4 nM for 5 and 6, respectively), while 3, which had the Ser(Glc) at the fifth position far from Trp8, showed 10-fold higher affinity for the hNK1 receptor (Ki = 1.1 pM) than 2. The affinity of 3 at the rNK1 receptor was 5600-fold lower than the Ki value at the hNK1 receptor (Ki = 1.5 nM) and its activity in the GPI was consistent with the binding affinity at the rNK1 receptors. The general explanation of such a difference in the NK1 antagonist activities between different species has been provided previously by the known homology differences between rat, human and guinea pig NK1 receptors: it is well known that the human NK1 receptor has higher homology to the guinea pig NK1 receptor than to the rat or mouse NK1, and some NK1 antagonists have large species differences consistent with the reported homology.42, 43 However, our results suggested that the known species difference does not give a sufficient explanation in the case of these bifunctional peptides, independent of the presence or absence of a glycosylated residue. These significant differences may suggest that these peptides have affinities for guinea pig NK1 receptor similar to those for the rNK1 receptor.
The Pro6 was reported to play an important role for hNK1 affinity,41, 44 and thus the replacement of Pro6 with Ser(Glc) resulted in a ligand with 640 times less affinity at the hNK1 receptor compared to that of 2 (4; Ki = 1.8 nM). According to a reported modeling study,42 the Trp-derived NK1 antagonist L-732,138 (Ac-Trp-O-3’,5’-Bzl(CF3)2) binds at the transmembrane domain of hNK1 receptor with a great deal of tolerance for substitution of its acetyl group which was directed toward the extracellular region. Although a large binding tolerance was expected, the Ser(Glc)6 incorporation in 4 might induce changes in the three-dimensional structure. 4 also had the lowest Ke value in the GPI tissue among the glycopeptides 3-6 (18 nM; Table 3). As for the species difference between the hNK1 and rNK1 receptors, all the synthesized glycopeptide derivatives 3-6 showed higher affinities at the hNK1 than at the rNK1 receptor (Table 1). It should be noted that 4 showed only a 13-fold difference between species, whereas the other glycopeptide derivatives (3, 5 and 6) showed at least 180 times better affinities for the hNK1 receptors than for the rNK1, suggesting the importance or Pro6 for the ligand at the hNK1 receptors. 4 also had the smallest species difference between human and guinea pig NK1 receptors, implying that the incorporation of Ser(Glc)6 or the removal of Pro6 has an important effect on the species differences in binding at the NK1 receptors.
Considering both the opioid agonist and NK1 antagonist activities, the glycosylation at the position next to the Trp showed the best results (5 and 6) with good opioid affinities and activities together with effective potency at the NK1 receptors. Thus, the metabolic stabilities of 5 and 6 were evaluated to estimate the effect of glycosylation and to compare with those of the peptides possessing no glycosylated residue (1 and 2), by incubation in rat plasma at 37°C (Figure 2). The degradation curve of 5 was improved from that of 1, but was almost equivalent to that of 2, implying that glucose introduction at Ser7 had a negligible effect on recognition by degrading enzymes (Figure 2). Interestingly, the Ser(Glc)8 insertion (6) resulted in a large improvement: 70 % of 6 was still found intact after 24 h incubation. Therefore, existence of the Ser(Glc)8 played an important role in the enzymatic degradation due to the masking of the cleavage site by the large carbohydrate portion. Another possibility is that the observed improved metabolic stability of compound 6 can attribute to the existence of Leu7 and Trp9 next to the sterically hindered Ser(Glc): the sequence of Leu7-Ser(Glc)8-Trp9 in compound 6 has three bulky side-chains which should repel each other to form a rather fixed conformation at the C-terminus. This effect may be due to the insertion of large sugar portion in the appropriate position of the peptide and this conformational change should have some contribution to metabolic stability as well. Because of this improved metabolic stability together with the excellent and δ-selective bifunctional activities, 6 could be considered as the best derivative among 3-6.
Conformational Analysis Based on 1H NMR Studies in Membrane-mimicking Circumstances
As discussed previously,20 the membrane-bound conformations of peptides has attracted interest recently, since the docking event of a ligand with its receptor, such as a GPCR, with its binding site in the transmembrane domain (such as opioid receptors and NK1 receptors), must take place near the membrane.45, 46 Hence, the transfer from solvent to cell membrane may be considered as an important step in a receptor-ligand binding event, and may be accompanied with a conformational change to a more biological relevant form, although it may differ from the binding conformation at the corresponding receptor.12, 15 Thus, the NMR structural analysis of 3-6 were performed to clarify the relationship between biological activity and conformation in membrane-mimicking environment in accord with the site-specific glycosylation in their peptide sequence.
Two-dimensional 1H NMR studies including TOCSY, DQF-COSY and ROESY (for 3, 4 and 5) or NOESY (for 6) in pH 4.5 buffer (45 mM CD3CO2Na/HCl, 1 mM NaN3, 90% H2O/10% D2O) with a 40-fold excess of perdeuterated DPC micelles were performed on all four glycopeptide derivatives 3-6. Nuclear Overhauser effects (NOEs) were measured using either 2D NOESY (optimal mixing time 450 ms) or 2D ROESY (150 ms), depending upon which method gave the larger number of cross-peaks. The NMR structure of 1 in the presence of DPC micelles was previously reported.20 DPC is a widely used lipid-like surfactant to determine the solution NMR structures of membrane-bound proteins and peptides,20, 36, 37 and forms micelles above the critical micelle concentration.47 All 1H chemical shift assignments of 3-6 are listed in the Supporting Information.
Conformational Calculations
A large number of non-redundant NOE restraints (101, 110, 92 and 240 for 3, 4, 5 and 6, respectively) were used for the conformational analysis. The interresidual NOE connectivities and the 3JHN-Hα coupling constants of all the peptide derivatives are illustrated in Figure 3.20 Only 3JHN-Hα values of more than 8 Hz or less than 6 Hz were used for φ dihedral angle constraints,36, 37 and thus the numbers of applied dihedral angle constraints were 0, 2, 1 and 3 for 3, 4, 5 and 6, respectively. Therefore, the total numbers of restraints were 101, 112, 93 and 243, corresponding to 10.1, 11.2, 9.3 and 22.1 restraints per residue (the C-terminal moiety and Glc were considered as residues). In the case of the observation of cis/trans isomers at the Pro6 residue, only the major isomer derived cross-peaks are considered in the structural calculation process, and all other amide bonds are fixed in the trans configuration (the detailed data are found in the Supporting Information).20
Figure 3.
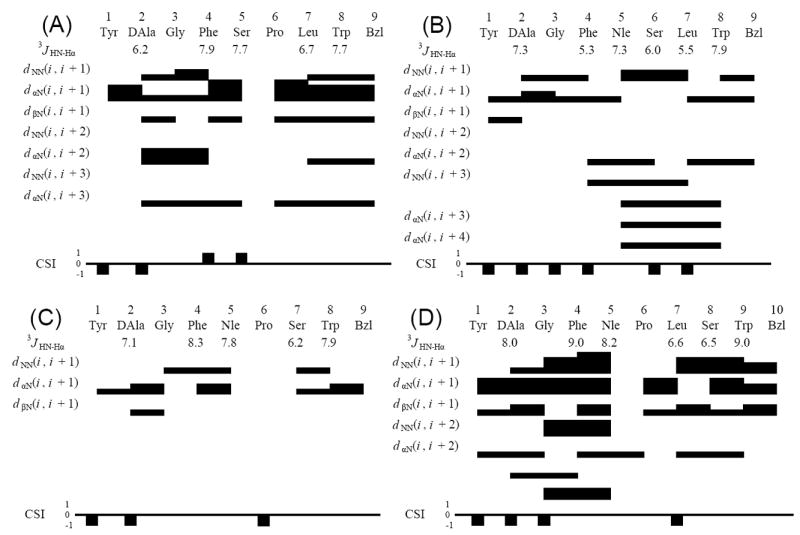
Diagram of HN-Hα coupling constants, NOE connectivities, and Hα chemical shift index (CSI) for the (A) 3, (B) 4 and (C) 5 and (D) 6. The Hα CSI was calculated using the random-coil values reported by Andersen et al.64, 65
The 20 lowest-energy structures from restrained molecular dynamics (rMD) calculations48, 49 were used for analysis of the glycopeptide derivatives 3-6 (Table 4). The superimposed images of the 20 structures are shown in Figure 4. The ensembles of structures for the glycopeptides derivatives 3-6 have small numbers of violations for total NOE restraints, maximum NOE distances and φ dihedral angles. The restraint energies derived from the Amber force field also were reasonably small (0.79, 0.68, 0.87 and 1.22 kcal mol-1for 3, 4, 5 and 6, respectively). Since 2 showed similar biological activities as 1, the sequence with Nle5 could be considered as equivalent to the one with Met5 in terms of the bioactive peptide conformation. Thus, the conformation of 1 will be used as the standard for comparison with the glycopeptide derivatives 3-6 throughout this discussion.
Table 4.
Atomic rmsd values (Å) for the final 19 conformers compared to the most stable conformer of bifunctional peptide derivatives.
| 1a | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|
| Backbone atoms (N, Cα, C’) | |||||
|
| |||||
| Calculated on whole molecule | 1.14 ± 0.43 | 2.04 ± 0.40 | 2.92 ± 0.67 | 0.93 ± 0.27 | 0.68 ± 0.30 |
| Calculated only on 1-4 res. | 1.05 ± 0.63 | 1.83 ± 0.43 | 1.38 ± 0.26 | 0.60 ± 0.33 | 0.13 ± 0.11 |
| Calculated only on 5-8 res. | 0.45 ± 0.38 | 0.48 ± 0.39 | 0.94 ± 0.22 | 0.56 ± 0.32 | 0.47 ± 0.23l |
|
| |||||
| all non-hydrogen atoms | |||||
|
| |||||
| Calculated on whole molecule | 2.09 ± 0.64 | 3.35 ± 0.86 | 4.44 ± 1.04 | 1.68 ± 0.44 | 2.01 ± 0.79 |
| Calculated only on 1-4 res. | 2.16 ± 0.98 | 2.41 ± 0.62 | 2.92 ± 0.54 | 0.78 ± 0.63 | 0.31 ± 0.40 |
| Calculated only on 5-8 res. and C-terminus | 1.02 ± 0.25 | 0.99 ± 0.60 | 1.56 ± 0.51 | 0.88 ± 0.35 | 0.57 ± 0.32b |
Reference.20
Calculated on 5-9 res. and C-terminus.
Figure 4.
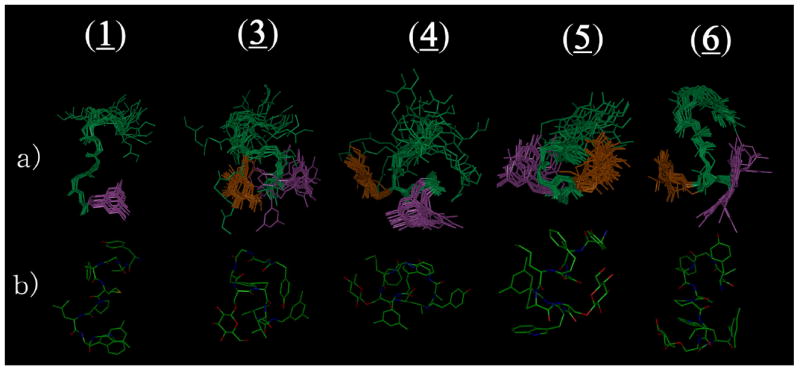
Ensembles of the best 20 calculated structures in 40-fold DPC micelle / pH 4.5 buffer for 1,20 3, 4, 5 and 6, respectively, with the lowest restraint energy, (a) aligned on backbone atoms of residues 5-8. The aligned structures are illustrated with the C-terminal benzyl moiety (purple) and glucose (orange). (b) The most stable conformers are shown with all heavy atoms (C, N and O).
The calculated structures of 3, which has a Ser(Glc) in the fifth position of the sequence in place of Met in 1, showed larger rmsd values, especially for alignment on residues 1-4 (1.83 for backbone atoms and 2.41 for all heavy atoms; Table 4), than that of 1. The rmsd values for the entire molecule also were increased from those of 1, mostly because of the poorly-defined opioid pharmacophore at the N-terminus. Its rmsd values with respect to residues 5-8 were small, however, and nearly the same to those of 1. In the original definition, a structure in which the Cα of the i th residue and the Cα in the i + 3 rd residues are located less than 7 Å apart is considered a β-turn.50 3 had two β-turns common to 1 at residues 2-5 and 6-9 (Table 5). It should be noted that the distance between the Cα of Ser5 and the Cα of Trp8 also were within 7 Å, suggesting a different alignment of the same turn at the C-terminus. As a result, glycosylation at the residue 5 induced conformational changes to give a relatively less structured N-terminus (opioid pharmacophore) and an ordered C-terminus with rather structured β-turn structures (NK1 pharmacophore) in the pseudo-membrane circumstances. Interestingly, 3 had decreased affinities for the opioid pharmacophore, whereas the NK1 binding affinities of 3 were improved from those of 1 and 2 (Table 1).
Table 5.
Number of β-turn structural elements and the distance between alpha carbons of i th and (i + 3)rd residues.a
| Residues | 1b | 3 | 4 | 5 | 6 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| number of β-turns | distance (Å) | number of β-turns | distance (Å) | number of β-turns | distance (Å) | number of β-turns | distance (Å) | number of β-turns | distance (Å) | |
| C1α-C4α | 3 | 7.86 ± 1.21 | 1 | 8.57 ± 0.62 | 3 | 8.08 ± 1.13 | 9 | 6.91 ± 0.41 | 0 | |
| C2α-C5α | 20c | 4.95 ± 0.71 | 11d | 6.99 ± 1.08 | 6 | 7.00 ± 1.05 | 0 | 20 | 4.98 ± 0.33 | |
| C3α-C6α | 0 | 8 | 7.39 ± 1.24 | 0e | 0 | 0 | ||||
| C4α-C7α | 0 | 0 | 17 | 5.37 ± 0.74 | 20f | 3.98 ± 0.23 | 0 | |||
| C5α-C8α | 0c | 20d | 5.79 ± 0.39 | 17 | 5.46 ± 0.60 | 0 | 5g | 7.05 ± 0.16 | ||
| C6α-Bzl | 19 | 6.32 ± 0.43 | 11 | 6.96 ± 0.57 | 17e | 5.50 ± 0.36 | 18 | 6.56 ± 0.56 | 20h | 5.21 ± 1.02 |
Out of the best 20 calculated structures. The distance is the mean distance between two alpha carbons ± standard deviation (SD). The sequences with less than 7 Å distance between alpha carbons of i th and (i + 3) rd residues without helical structure were considered as a β-turn.50 Bzl stands for the benzyl moiety at the C-terminus.
Reference.20
When the site of glycosylation was shifted to residue 6 (4), the number of β-turn elements in the N-terminal half got smaller, whereas the distances between two Cα atoms for residues 4-7, 5-8 and 6-9 were less than 7 Å, indicating an apparent turn structure around these regions (Table 5). Since the two Cα atoms for residues 4-7 and 5-8 in 1 were larger than 7 Å, these secondary structural elements in 4 clearly were induced by the substitution of Pro6 by Ser(Glc). Generally, Pro is known as a turn-inducing amino acid, thus it is interesting that the Ser(Glc), but not the Pro residue, could yield such a β-turn-rich structure near the substituted site, possibly due to the large steric effect of the introduced sugar moiety. Although 4 had a large number of secondary structural elements in the ensemble of NMR structures, its structural definition was further decreased from those of 3 (the rmsd values for the whole molecule of 4 were 2.92 for the backbone atoms and 4.44 for all heavy atoms; Table 4). These results imply that Pro6 in 1 had an important contribution to its conformational stability. In fact, 4 had the least structured conformation among 3-6 and showed the lowest binding affinities at almost all the receptors tested (Table 1).
On the other hand, the introduction of Ser(Glc) at the seventh position (5) led to a more defined structure whose rmsd values (0.93 and 1.68 for backbone and all heavy atoms, respectively) were smaller than those of 1, although the number of NOE restraints for 5 was the smallest among the tested peptides (Table 4). Interestingly, residues 1-4 had a rather extended and better-defined conformation than in 1, but residues 5-8 did not, suggesting that the Ser(Glc)7 might act as an address region for the opioid agonist pharmacophore. The rmsd values of 5 were smaller than for 3 and 4, with β-turn structural elements for residues 4-7 (Type I) and 6-9 (Type IV) (Table 5).
DPDPE (Tyr-c[D-Pen-Gly-Phe-D-Pen]OH) is a derivative of enkephalin and widely-used opioid agonist with high δ-selectivity over the μ receptor. In this compound, the structurally-fixed β-turn at residues 2-5 was found in its X-ray crystal structure,51 in the solution NMR structure52 and in the docking study at the δ opioid receptor.53 Based on these observations, we hypothesized that this β-turn at residues 2-5 could be a key structure to enhance the δ-selectivity of enkephalin analogues. In fact, 5, possessing an extended conformation in the opioid pharmacophore, has low δ-selectivity in terms of the binding affinities. Hydrogen bonds between glucose and Gly3 or Phe4 were observed in 5, implying a direct interaction between Ser(Glc)7 and the N-terminal half that results in a fixed turn structure about residues 4-7 (Table 5 and 6). This interaction might play a part in the induction of the ordered N-terminal half of 5 that was observed. Collectively, the single substitution of Ser(Glc) at the 5, 6 or 7 residue of 2 led to diverse structural differences in their NMR structures in the membrane-mimicking environment depending on the site of glycosylation, but all of these glycopeptides 3-5 showed tandem-β-turn structures in the NK1 pharmacophores where the sugar was introduced, with more extended opioid pharmacophores.
Table 6.
Hydrogen Bond Observed in the NMR structures of 3-6.a
| Molecule | No.b | Donor | Acceptor | Distance (Å)c | Angle (deg)d |
|---|---|---|---|---|---|
| 1e | 7 | Gly3 HN | Tyr1 O | 2.05 ± 0.11 | 137.8 ± 8.1 |
| 5 | Trp8 HN | Met5 O | 2.04 ± 0.02 | 132.3 ± 1.1 | |
| 9 | Bzl9 HN f | Pro6 O | 2.16 ± 0.11 | 158.5 ± 1.9 | |
|
| |||||
| 3 | 10 | Phe4 HN | DAla2 O | 2.06 ± 0.09 | 144.1 ± 5.4 |
| 7 | Gly3 HN | Tyr1 O | 2.03 ± 0.08 | 142.8 ± 7.2 | |
| 16 | Leu7 HN | Ser5 O | 1.96 ± 0.05 | 132.1 ± 6.5 | |
| 13 | Trp8 HN | Ser5 O | 2.19 ± 0.12 | 151.4 ± 7.6 | |
| 5 | Glc HO4 | Glc O6 | 2.11 ± 0.02 | 127.3 ± 3.1 | |
|
| |||||
| 4 | 8 | Nle5 HN | Gly3 O | 2.08 ± 0.16 | 138.3 ± 10.7 |
| 8 | Bzl9 HNe | Nle5 O | 1.97 ± 0.12 | 168.6 ± 9.5 | |
| 7 | Bzl9 HN | Ser6 O | 2.30 ± 0.12 | 132.8 ± 4.2 | |
| 11 | Glc HO2 | Bzl9 Ff | 2.33 ± 0.05 | 150.6 ± 10.9 | |
| 6 | Glc H6 | Glc O4 | 2.19 ± 0.01 | 130.1 ± 0.3 | |
| 7 | Glc HO4 | Glc O6 | 2.12 ± 0.03 | 129.8 ± 1.5 | |
|
| |||||
| 5 | 6 | Trp8 HN | Pro6 O | 2.15 ± 0.13 | 144.8 ± 4.1 |
| 6 | Glc HO3 | Gly3 O | 2.25 ± 0.13 | 133.7 ± 6.3 | |
| 7 | Glc H6 | Phe4 O | 1.96 ± 0.06 | 155.1 ± 9.3 | |
| 7 | Glc HO3 | Glc O1 | 2.28 ± 0.07 | 128.6 ± 4.5 | |
|
| |||||
| 6 | 13 | Tyr1 HN | Nle5 O | 1.99 ± 0.04 | 137.4 ± 3.0 |
| 20 | Phe4 HN | DAla2 O | 2.22 ± 0.11 | 146.6 ± 2.3 | |
| 5 | Glc HO4 | Glc O6 | 2.22 ± 0.06 | 129.3 ± 1.7 | |
The hydrogen bonds which were observed in more than five structures are listed.
The number of structures of the final 20 for which the listed hydrogen bond is observed.
The distance is the mean proton-acceptor atom distance (± SD) in the structures for which a hydrogen bond is observed.
The angle is the mean angle (± SD) in the structures for which a hydrogen bond is observed.
Reference.20
Amide proton of C-terminal benzyl moiety.f Fluorine atom of C-terminal benzyl moiety.
Compared to the glycopeptide 5 with Ser(Glc)7, the derivative possessing Ser(Glc)8 (6) showed a further decrease in rmsd values, especially for backbone atoms (the rmsd value was only 0.13 for residues 1-4; Table 4), mostly owing to its structured N-terminus with a well-defined β-turn around residues 2-5. The better-defined structure of the N-terminus, as well as the smaller rmsd values, in the glycopeptide derivative 6 compared to 1-5 was also confirmed by the φ and ψ angular order parameters shown in Figure 5E and 5F.54 It is worth mentioning that although the carbohydrate residue was inserted in the C-terminus, it led to a more structured N-terminus even though it is distant from the glycosylated position. Although the rmsd values of 6 decreased from those of 1, the secondary structural elements of 6 were the same as those of 1; both of these peptides had two β-turns, at residues 2-5 and at the C-terminus (Table 5). Interestingly, 6 had a well-defined β-turn at the residues 2-5 and showed good biological affinities for δ opioid receptors with the highest (39-fold) selectivity over the μ receptor among the tested peptides. Indeed, the insertion of a glycosylated moiety at the eighth residue gave rise to δ-selective opioid agonist and effective NK1 antagonist activities, presumably in relation to a structured conformation possessing two β-turns which were common to those in 1. It should be noted that the glucose moiety in 5 and 6 should have a similar effect of masking their backbones from proteolytic enzymes, but only 6 showed improved metabolic stability compared to 2. A possible explanation for this difference is that the ordered peptide conformation induced by the Ser(Glc)8 insertion could resist enzymatic cleavage of the peptide backbone. In fact, compound 6 has three bulky amino-acid residues in a row, Leu7-Ser(Glc)8-Trp9, for which a number of inter-residual NOE cross-peaks were found between Ser(Glc)8 and Leu7 (8 NOE cross-peaks) or Trp9 (21 NOE cross-peaks), suggesting a sequence with a rather fixed conformation at the C-terminus.
Figure 5.
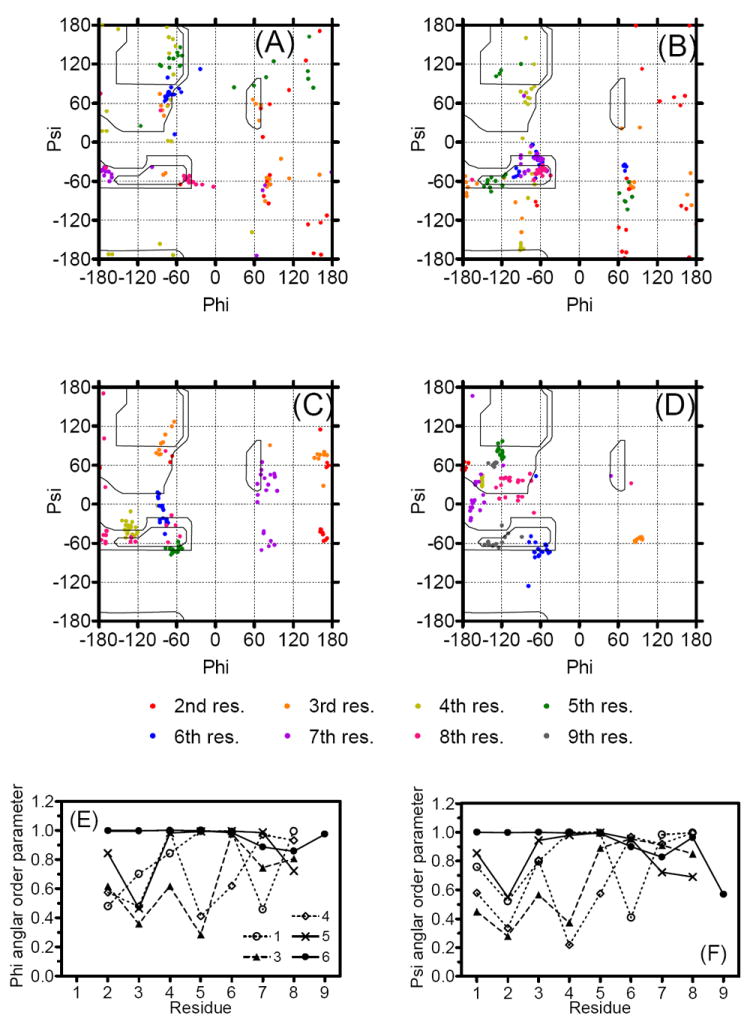
The Glycosylated Ser (crosses) and Gly3 (open circle) were indicated in the Ramachandran φ,ψ plots for (A) 3, (B) 4 and (C) 5 for residues 2-7 and (D) 6 for residues 2-8 of 20 final structures. Ser(OGlc) (crossing) and Gly3 (open diamond) were specified. The Nle5 with negative φ angle in 4 (circled) were illustrated in (B). Angular order parameters54 for φ (E) and ψ (F) angles calculated from the 20 final structures for 1 (open circle),20 3 (filled triangle), 4 (open diamond), 5 (crossing) and 6 (filled circle), respectively. For calculating the ψ angles of Trp8, the nitrogen atoms of C-terminal benzyl amide were used instead of N (i + 3), respectively.
As observed in the Ramachandran plot, 5 possesses a Ser residue with positive φ and ψ angles in 14 out of the 20 best structures including the most stable one (Figure 5C). These positive φ angles of Ser7 in 5 with a bulky glucose could help drive its structure to be a β-turn near the glycosylated site, thus leading to the fixed C-terminal structures (Table 5). Gly3 was the other residue with positive φ angles; 14, 9, 10 and 20 φ angles for the best 20 structures were found positive at Gly3 for 3, 4, 5 and 6, respectively (Figure 5A-D). It is interesting that 4 and 5, with smaller numbers of β-turn elements in the N-terminal halves, had fewer positive φ angles at Gly3, whereas 3 and 6, which had β-turns around residues 2-5 in which Gly3 was at the (i + 1) position, possessed a larger number of Gly3 with positive φ angles. Thus, these positive φ angles in the amino acid residues might be a good driving force to form secondary structural elements in the backbone, and could lead to a more ordered turn conformation, especially in 6.
Paramagnetic Broadening Studies on 1H NMR
In order to evaluate the mode of interaction of 6 with the model membrane, further NMR experiments using paramagnetic ions (Mn2+) were conducted in the presence of DPC micelles. The Mn2+ ions cause paramagnetic broadening and loss of resonance intensity of solvent-exposed protons observed as cross-peaks in the TOCSY spectra (Figure 6).20 As a result of Mn2+ addition, all the resonances belonging to the backbone NH protons in 6 were eliminated except for that of Nle5, indicating that the backbone of 6 is located at the micelle surface, with only Nle5 buried inside the micelles. Most of the cross-peaks derived from protons in side-chains as well as in the C-terminus were preserved. It is reasonable that mostly lipophilic side-chains and the C-terminus interact with the lipophilic core of the micelles. These results for 6 were very similar to the observations for 1 in the same lipid-like enviornments.20 Interestingly, cross-peaks related to the hydrophilic glucose were also preserved after Mn2+ addition, suggesting that the carbohydrate moiety is located inside the micelles. Thus, the introduction of a glycosylated residue at the eighth position did not disturb the strong interaction of the peptide with membrane-mimicking micelles as observed in 1, but provided a more structured conformation.20 Since both the binding site of opioid and NK1 receptors were found in or near their transmembrane domains,42, 55 the ligand-membrane interaction should make an important contribution to the docking of the ligand at these receptors.
Figure 6.
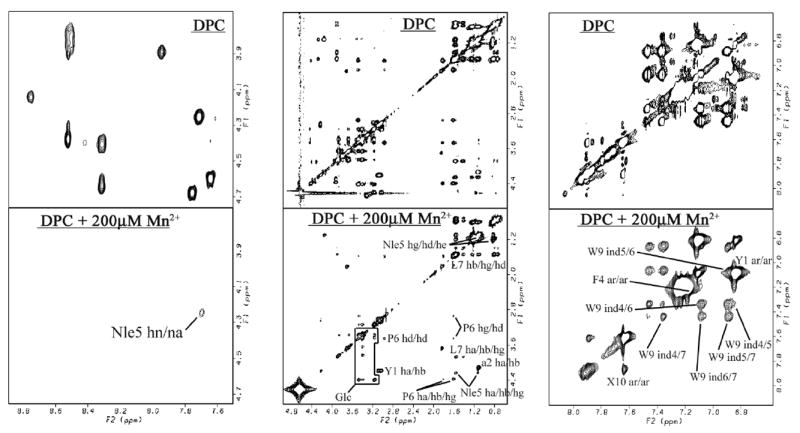
The paramagnetic effects on TOCSY Spectra of 6. 6 with DPC micelles (top row) and with 200 μM Mn2+ (bottom). Preserved resonances (labeled) are in a phase not missed by the phase-specific radical probe (Mn2+). Spectra were compared from the same noise level. X10 represents the cross-peaks derived from the corresponding aromatic protons of benzyl moiety.
Conclusion
In order to obtain a novel type of analgesic compound with good metabolic stability, Ser(Glc) was introduced into the 5, 6, 7 and 8 position in the sequence of 2. Among the synthesized derivatives, 6, in which Ser(Glc) was inserted in between two bulky residues Leu and Trp in the sequence of 2, showed improved metabolic stability compared to 2 in rat plasma. NMR structural analysis in the presence of DPC micelles indicated that this insertion of the sugar moiety into 6 led to a well-defined conformation with two β-turns at the residues 2-5 and the C-terminus, both of which were common to the previously observed conformation of 1. In fact, the glycopeptides derivative 6 showed strong interactions with the model-membrane as previously observed for the non-glycosylated derivative 1. The well-defined conformation and the existence of a large sugar portion in the sequence of 6 might play a part in its not being recognized by degrading enzymes. 6 also showed picomolar-level affinity for the hNK1 receptor and partial but effective agonist activity at the opioid receptors with improved δ-selectivity compared to that of 1. Because of the improved metabolic stability, along with the bifunctional activities, 6 could be considered as a valuable research tool and possibly a promising candidate for a novel analgesic drug. The importance of these studies is that the site of glycosylation and the introduced steric repulsion with the neighboring residues are likely critical factors for the conformational and secondary structural elements of the peptide, both of which should have a significant contribution to biological recognition at a variety of proteins, including opioid receptors, NK1 receptors and several peptide-degrading proteases.
Experimental Section
Materials
All amino acid derivatives, coupling reagents and resins were purchased from EMD Biosciences (Madison, WI), Bachem (Torrance, CA), SynPep (Dublin, CA) and Chem Impex International (Wood Dale, IL). The 4-(4-Formyl-3-methoxyphenoxy)butyryl aminomethyl (FMPB-AM) resin was acquired from EMD Biosciences. Perdeuterated DPC was purchased from C/D/N Isotopes (Quebec, Canada). ACS grade organic solvents were purchased from VWR Scientific (West Chester, PA), and other reagents were obtained from Sigma-Aldrich (St. Louis, MO) and used as obtained. The polypropylene reaction vessels (syringes with frits) were purchased from Torviq (Niles, MI). Myo-[2-3H(N)]-inositol; [Tyrosyl-3,5-3H(N)] D-Ala2-Mephe4-glyol5-enkephalin (DAMGO); [Tyrosyl-2,6-3H(N)]-(2-D-Penicillamine, 5-D-Penicillamine)enkephalin (DPDPE); [3H]-substance P; and [35S]-guanosine 5’-(γ-thio) triphosphate (GTPγS) were purchased from Perkin Elmer (Wellesley, MA). Bovine serum albumin (BSA), protease inhibitors, Tris and other buffer reagents were obtained from Sigma (St. Louis, MO). Culture medium, Penicillin/ Streptomycin and fetal calf serum (FCS) were purchased from Invitrogen (Carlsbad, CA).
Peptide Synthesis
The glycopeptides (3, 4, 5 and 6) were synthesized by using reductive amination of 3,5-bistrifluoromethylbenzyl amine on FMPB-AM resin followed by the Nα-Fmoc solid-phase methodology. The FMPB-AM resin (675 mg, 0.50 mmol/g) was placed into a 50 mL polypropylene syringe with the frit on the bottom and swollen in DMF (20 mL) for 1 h. The resin was washed with DMF (3 × 15 mL), and then the solution of 3’,5’-bistrifluoromethylbenzyl amine (650 mg, 2.67 mmol) in the mixture of DMF (5 mL) and TMOF (5 mL) with 4 N HCl in 1,4-dioxane (0.6 mL, 2.40 mmol) was introduced. After shaking for overnight, the solution was eluted off and a solution of NaBH(OAc)3 (508 mg, 2.40 mmol) in 5% AcOH in DMF (10 mL) was transferred into the reaction vehicle for 2 h. The resin was washed three times with DMF (15 mL) and three times with DCM (15 mL), and then with DMF (3 × 15 mL). The solution of 3’,5’-bistrifluoromethylbenzyl amine (650 mg, 2.67 mmol) in DMF : TMOF = 1 : 1 (10 mL) with 4 N HCl in 1,4-dioxane (0.6 mL, 2.40 mmol) was prepared again, then added to the reaction vehicle for 2 h. The solution was filtered off, and the resin was treated with NaBH(OAc)3 (508 mg, 2.40 mmol) in 5% AcOH in DMF (10 mL). After shaking for 1 h, the resin was washed with DMF (3 × 15 mL), DCM (3 × 15 mL) and DMF (3 × 15 mL). Fmoc-Trp(Boc)-OH (1.47 g, 2.79 mmol) and HCTU (1.15 g, 2.79 mmol) were dissolved in 15 mL of DMF, and then 2,6-lutidine (536 mg, 5.00 mmol) was added. The coupling mixture was transferred into the syringe with the resin and shaken overnight. The coupling was repeated for 2 h, and the unreacted amino groups were capped using acetic anhydride (2 mL) and pyridine (2 mL) in DCM (15 mL) for 30 min, then the resin was once again washed with DMF (3 × 15 mL), DCM (3 × 15 mL) and DMF (3 × 15 mL). The Nα-Fmoc protecting group was removed by 20% piperidine in DMF (20 mL, 1 × 2 min and 1 × 20 min). The deprotected resin was washed with DMF (3 × 15 mL), DCM (3 × 15 mL) and then with DMF (3 × 15 mL). The protected amino acid (3 equiv) and HCTU (2.9 equiv) were dissolved in 15 mL of DMF, then DIEA (6 equiv) was added. Fmoc-Ser(O-β-D-Glc(OAc)4)-OH,40 Fmoc-Leu-OH, Fmoc-Pro-OH, Fmoc-Nle-OH, Fmoc-Phe-OH, Fmoc-Gly-OH Fmoc-D-Ala-OH and Boc-Tyr(tBu)-OH were used for respective coupling as protective amino acids. The coupling mixture was transferred into the reaction vehicle, and then shaken for 1.5 h. All the other amino acids were sequentially coupled using the procedure described above, using the TNBS test or chloranil test to check the extent of coupling. In case of a positive test result, the coupling was repeated until a negative test result was obtained. The resulting batch of resin-bound protected glycopeptide was carefully washed with DMF (3 × 15 mL), DCM (3 × 15 mL), DMF (3 × 15 mL), and DCM (3 × 15 mL), and dried under reduced pressure. After the glycopeptide was assembled on resin, the acetyl protecting groups of glucose moiety ware removed with 80% H2NNH2·H2O in CH3OH (20 mL, 1 × 30 min and 1 × 1 h). The resin was washed three times with EtOAc (15 mL) and three times with DCM : CH3OH = 1 : 1 (15 mL), then with DCM (3 × 15 mL). The cleavage of glycopeptide from the solid support was performed with the 3 mL of TFA/TIPS-OTf/thioanisole (9 : 2 : 1; v/v) for 45 min. The obtained crude glycopeptide was treated with chilled dry ether (45 mL) to give a dark oil. After centrifuge, the supernatant was decanted off, and then the crude glycopeptide was precipitated out with chilled dry ether (45 mL). The obtained yellow solid was dried under reduced pressure. The CH3CN (5 mL) was added to the precipitated crude glycopeptide, and the solution was treated with 2 N NH4F (2 mL) for 30 min. The solution was adjusted to pH 8.0 with triethylamine, stirred for 30 min at 0 °C, and then H2O (20 mL) was added. CH3CN was carefully titrated until the crude glycopeptide solution became clear which were subsequently passed through a C-18 reversed-phase silica gel column (Sep-Pak C18 cartridge, 5 g, Waters, Milford, MA) and the column was carefully washed by H2O (40 mL). The glycopeptide absorbed on the column were eluted with CH3CN (40 mL). The obtained solution was evaporated, and the residue was dissolved into H2O : CH3CN = 1 : 1 (5 mL) for the purification on Waters Delta Prep 4000 RP-HPLC system equipped with Waters XTerra C-18 column (19 × 250 mm, 10 μm, a linear gradient of 33-53%, 33-53%, 35-52% and 32-52% CH3CN/0.1% TFA H2O in 35 min for 3, 4, 5 or 6, respectively at a flow rate of 15.0 mL/min) followed by lyophilizing. The final pure glycopeptides (>98 %) were characterized by analytical HPLC, 1H-NMR, HRMS and TLC as previously described (available in the Supporting Information).19-21 1H-NMR studies showed cis/trans isomerization about the X-Pro6. The ratios of two amide rotamers and their assignments also are available in the Supporting Information. The lyophilized product was a white amorphous solid.
In Vitro Stability of peptide derivatives in Rat Plasma56
Stock solution of compounds (50 mg/mL in DMSO) were diluted 1000-fold into rat plasma (Lot 24927, Pel-Freez Biologicals, Rogers, AK) to give an incubation concentration of 50 μg/mL. All samples were incubated at 37 °C and 200 μL of aliquots were withdrawn at 1 min, 10 min, 30 min, 1 h, 2 h, 4 h, 6 h and 24 h (only for 6). Then 300 μL of acetonitrile was added and the proteins were removed by centrifugation. The supernatant was analyzed for the amount of remaining parent compound by HPLC (Hewlett Packard 1090m with Vydac 218TP104 C-18 column; 4.6 × 250 mm, 10 μm, 300 Å). The samples were tested in three independent experiments (n = 3) and the mean values were used for the analysis with the SD.
NMR Spectroscopy in DPC amphipathic media and Conformational Structure Determination
All the conformational determinations were performed by the same methods as previously described, 20, 36, 37 based on the NMR spectra using a Bruker DRX600 600 MHz spectrometer.
Briefly, the samples were prepared by dissolving the peptide (4 to 6 mM) in 0.5 mL of 45 mM sodium acetate-d3 buffer (pH 4.5) containing 40 equivalents of dodecylphosphocholine-d38 and 1 mM sodium azide (90% H2O/10% D2O) followed by sonication for 5 min. Two-dimensional double quantum filtered correlation (DQF-COSY), nuclear Overhauser enhancement spectroscopy57 (NOESY, mixing time = 450 ms), Rotating frame Overhauser Effect Spectroscopy (ROESY, mixing time = 150 ms) and total correlation spectra58 (TOCSY, MLEV-17 mixing time = 62.2 ms, spin-lock field = 8.33 kHz) were acquired using standard pulse sequences at 310 K. Coupling constants (3JNH-Hα) were measured from 2D DQF-COSY spectra by analysis of the fingerprint region with a curve-fitting using 5-parameter Levenberg-Marquardt nonlinear least-squares protocol to a general antiphase doublet.
For conformational structure determination, the volumes of the assigned cross-peaks in the 2D NOESY spectrum were converted into upper distance bounds of 3.0, 3.8, 4.8, or 5.8 Å. For overlapping cross-peaks, the distance categories were increased by one or two levels, depending on the qualitative estimate of the extent of overlap. Pseudoatoms were created for nonstereospecifically assigned methylene protons with a correction of 1.0 Å applied to their upper bound distances.59 In addition to the distance constraints, φ dihedral angle constraints derived from 3JHN-Hα coupling constants were set to between -90 and 40° for 3JHN-Hα< 6 Hz and to between -150 and -90° for 3JHN-Hα> 8 Hz. Dihedral angle constraints of 180 ± 5° for peptide bonds (ω) were also used to maintain the planarity of these bonds. Simulated annealing molecular dynamics analysis was done for all the peptides to obtain an ensemble of NMR structures using NOE-derived distance constraints and dihedral angle (φ) constraints using the DGII60 program within the software package Insight II 2000 (Accelrys Inc., San Diego, CA). The final 20 structures with the lowest energies were used for the analysis. All calculations were performed on a Silicon Graphics Octane computer.
Radioligand Labeled Binding Assay, [35S]GTP-γ-S Binding Assay, GPI and MVD in Vitro Bioassay
The methods were carried out as previously described.19-21 Briefly, the evaluation of the binding affinities of the synthesized bifunctional peptide derivatives at the human δ-opioid receptors (hDOR) and rat μ-opioid receptors (rMOR) were performed on the cell (HN9.10) membranes from cells that stably express these corresponding receptors using [3H]-c[D-Pen2, D-Pen5]-enkephalin ([3H]DPDPE) and [3H]-[D-Ala2, NMePhe4, Gly5-ol]-enkephalin ([3H]DAMGO) as the radioligands, respectively. [35S]GTPγS binding assays were used to estimate the functional activities for δ and μ opioid agonist efficacies on the same cell membrane. The isolated tissue-based functional assays also were used to evaluate opioid agonist activities in the GPI (δ) and MVD (μ). For the affinity at the human NK1 (hNK1) receptors, binding assays utilized membranes from transfected CHO cells that stably express hNK1 receptors, using [3H]-substance P as the standard radioligand. The binding assay at the rat NK1 (rNK1) receptors also were performed using transfected CHO cells that stably express rNK1 receptors. To evaluate antagonistic activities against substance P stimulation, isolated tissue bioassays using GPI were performed in the presence of naloxone to block any opioid activities.
Supplementary Material
Acknowledgments
The work was supported by grants from the USDHS, National Institute on Drug Abuse, DA-13449 and DA-06284. We thank Dr. Guangxin Lin for kind assistance and advice with the NMR measurements, and Professor Robin Polt for discussions on glycosylated peptides, Ms. Magdalena Kaczmarska for culturing cells, the University of Arizona Mass Spectrometry Facility for the mass spectra measurements and Mr. Hajime Koganei for helpful scientific discussions. We express appreciation to Ms. Margie Colie and Ms. Brigid Blazek for assistance with the manuscript.
A List of Abbreviations
Abbreviations used for amino acids and designation of peptides follow the rules of the IUPAC-IUB Commission of Biochemical Nomenclature in J. Biol. Chem. 1972, 247, 977-83. The following additional abbreviations are used:
- BBB
blood brain barrier
- Boc
tert-butyloxycarbonyl
- BSA
bovine serum albumin
- Cl-HOBt
1-hydroxy-6-chlorobenzotriazole
- CHO
Chinese hamster ovary
- CNS
central nerve system
- DAMGO
[D-Ala2, NMePhe4, Gly5-ol]-enkephalin
- DCM
dichloromethane
- DIEA
diisopropylethylamine
- DOR
δ opioid receptor
- DPC
dodecylphosphocholine
- DPDPE
c[D-Pen2, D-Pen5]-enkephalin
- DQF-COSY
double quantum filtered correlation
- FMPB-AM
4-(4-formyl-3-methoxyphenoxy)butyryl aminomethyl
- Fmoc
fluorenylmethoxycarbonyl
- GPI
guinea pig ileum
- GTPγS
guanosine 5’-(γ-thio) triphosphate
- HCTU
1H-Benzotriazolium-1-[bis(dimethylamino)methylene]-5-chloro-hexafluorophosphate-(1-),3-oxide
- HRMS
high-resolution mass spectroscopy
- LMMP
longitudinal muscle with myenteric plexus
- MOR
μ opioid receptor
- MVD
mouse vas deferens
- NK1
neurokinin-1
- NOE
nuclear Overhauser enhancement
- NOESY
nuclear Overhauser enhancement spectroscopy
- rMD
restrained molecular dynamics
- rmsd
root mean square deviation
- ROESY
rotating frame Overhauser effect spectroscopy
- RP-HPLC
reverse phase high performance liquid chromatography
- Ser(Glc)
O-β-glucosylated serine
- SPPS
solid phase peptide synthesis
- TFA
trifluoroacetic acid
- TIPS-OTf
tirisopropylsilyl trifluoromethanesulfonate
- TMOF
trimethyl orthoformate
- TNBS
2,4,6-trinitrobenzene sulfonic acid
- TOCSY
total correlation spectra
- Trp-NH-3,5-Bzl(CF3)2
3’,5’-(bistrifluoromethyl)-benzyl amide of tryptophan
Footnotes
Supporting Information Available: HR-MS, TLC, HPLC and 1H-NMR data of the peptides 2-6. The metabolic stability of peptides 3 and 4 in rat plasma. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Witt KA, Davis TP. CNS drug delivery: opioid peptides and the blood-brain barrier. Aaps J. 2006;8:E76–88. doi: 10.1208/aapsj080109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert R, Marbach P, Bauer W, Briner U, Fricker G, Bruns C, Pless J. SDZ CO 611: a highly potent glycated analog of somatostatin with improved oral activity. Life Sci. 1993;53:517–525. doi: 10.1016/0024-3205(93)90703-6. [DOI] [PubMed] [Google Scholar]
- 3.Bilsky EJ, Egleton RD, Mitchell SA, Palian MM, Davis P, Huber JD, Jones H, Yamamura HI, Janders J, Davis TP, Porreca F, Hruby VJ, Polt R. Enkephalin glycopeptide analogues produce analgesia with reduced dependence liability. J Med Chem. 2000;43:2586–2590. doi: 10.1021/jm000077y. [DOI] [PubMed] [Google Scholar]
- 4.Negri L, Lattanzi R, Tabacco F, Scolaro B, Rocchi R. Glycodermorphins: opioid peptides with potent and prolonged analgesic activity and enhanced blood-brain barrier penetration. Br J Pharmacol. 1998;124:1516–1522. doi: 10.1038/sj.bjp.0701971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polt R, Porreca F, Szabo LZ, Bilsky EJ, Davis P, Abbruscato TJ, Davis TP, Harvath R, Yamamura HI, Hruby VJ. Glycopeptide enkephalin analogues produce analgesia in mice: evidence for penetration of the blood-brain barrier. Proc Natl Acad Sci U S A. 1994;91:7114–7118. doi: 10.1073/pnas.91.15.7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quartara L, Fabbri G, Ricci R, Patacchini R, Pestellini V, Maggi CA, Pavone V, Giachetti A, Arcamone F. Influence of lipophilicity on the biological activity of cyclic pseudopeptide NK-2 receptor antagonists. J Med Chem. 1994;37:3630–3638. doi: 10.1021/jm00047a020. [DOI] [PubMed] [Google Scholar]
- 7.Hansch C, Dunn WJ., III Linear relationships between lipophilic character and biological activity of drugs. J Pharm Sci. 1972;61:1–19. doi: 10.1002/jps.2600610102. [DOI] [PubMed] [Google Scholar]
- 8.Susaki H, Suzuki K, Yamada H, Okuno S, Watanabe HK. Renal targeting of arginine-vasopressin by modification with carbohydrates at the tyrosine side chain. Biol Pharm Bull. 1999;22:1094–1098. doi: 10.1248/bpb.22.1094. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki K, Susaki H, Okuno S, Sugiyama Y. Renal drug targeting using a vector “alkylglycoside”. J Pharmacol Exp Ther. 1999;288:57–64. [PubMed] [Google Scholar]
- 10.Suzuki K, Susaki H, Okuno S, Yamada H, Watanabe HK, Sugiyama Y. Specific renal delivery of sugar-modified low-molecular-weight peptides. J Pharmacol Exp Ther. 1999;288:888–897. [PubMed] [Google Scholar]
- 11.Sargent DF, Bean JW, Schwyzer R. Conformation and orientation of regulatory peptides on lipid membranes. Key to the molecular mechanism of receptor selection. Biophys Chem. 1988;31:183–193. doi: 10.1016/0301-4622(88)80024-3. [DOI] [PubMed] [Google Scholar]
- 12.Dhanasekaran M, Palian MM, Alves I, Yeomans L, Keyari CM, Davis P, Bilsky EJ, Egleton RD, Yamamura HI, Jacobsen NE, Tollin G, Hruby VJ, Porreca F, Polt R. Glycopeptides related to beta-endorphin adopt helical amphipathic conformations in the presence of lipid bilayers. J Am Chem Soc. 2005;127:5435–5448. doi: 10.1021/ja0432158. [DOI] [PubMed] [Google Scholar]
- 13.Egleton RD, Davis TP. Development of Neuropeptide Drugs that Cross the Blood-Brain Barrier. NeuroRx. 2005;2:44–53. doi: 10.1602/neurorx.2.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egleton RD, Mitchell SA, Huber JD, Janders J, Stropova D, Polt R, Yamamura HI, Hruby VJ, Davis TP. Improved bioavailability to the brain of glycosylated Met-enkephalin analogs. Brain Res. 2000;881:37–46. doi: 10.1016/s0006-8993(00)02794-3. [DOI] [PubMed] [Google Scholar]
- 15.Palian MM, Boguslavsky VI, O’Brien DF, Polt R. Glycopeptide-membrane interactions: glycosyl enkephalin analogues adopt turn conformations by NMR and CD in amphipathic media. J Am Chem Soc. 2003;125:5823–5831. doi: 10.1021/ja0268635. [DOI] [PubMed] [Google Scholar]
- 16.Live DH, Williams LJ, Kuduk SD, Schwarz JB, Glunz PW, Chen XT, Sames D, Kumar RA, Danishefsky SJ. Probing cell-surface architecture through synthesis: an NMR-determined structural motif for tumor-associated mucins. Proc Natl Acad Sci U S A. 1999;96:3489–3493. doi: 10.1073/pnas.96.7.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McManus AM, Otvos L, Jr, Hoffmann R, Craik DJ. Conformational studies by NMR of the antimicrobial peptide, drosocin, and its non-glycosylated derivative: effects of glycosylation on solution conformation. Biochemistry. 1999;38:705–714. doi: 10.1021/bi981956d. [DOI] [PubMed] [Google Scholar]
- 18.Lis H, Sharon N. Protein glycosylation. Structural and functional aspects. Eur J Biochem. 1993;218:1–27. doi: 10.1111/j.1432-1033.1993.tb18347.x. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto T, Nair P, Davis P, Ma SW, Navratilova E, Moye M, Tumati S, Vanderah TW, Lai J, Porreca F, Yamamura HI, Hruby VJ. Design, Synthesis and Biological Evaluation of Novel Bifunctional C-terminal Modified Peptides for δ/μ Opioid Receptor Agonists and Neurokinin-1 Receptor Antagonists. J Med Chem. 2007;50:2779–2786. doi: 10.1021/jm061369n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto T, Nair P, Jacobsen NE, Davis P, Ma SW, Navratilova E, Lai J, Yamamura HI, Vanderah TW, Porreca F, Hruby VJ. The Importance of Micelle-Bound States for the Bioactivities of Bifunctional Peptide Derivatives for δ/μ Opioid Receptor Agonists and Neurokinin 1 Receptor Antagonists. J Med Chem. 2008;51:6334–6347. doi: 10.1021/jm800389v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto T, Nair P, Vagner J, Davis P, Ma SW, Navratilova E, Moye M, Tumati S, Vanderah TW, Lai J, Porreca F, Yamamura HI, Hruby VJ. A Structure Activity Relationship Study and Combinatorial Synthetic Approach of C-Terminal Modified Bifunctional Peptides That Are δ/μ Opioid Receptor Agonists and Neurokinin 1 Receptor Antagonists. J Med Chem. 2008;51:1369–1376. doi: 10.1021/jm070332f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King T, Gardell LR, Wang R, Vardanyan A, Ossipov MH, Malan TP, Jr, Vanderah TW, Hunt SP, Hruby VJ, Lai J, Porreca F. Role of NK-1 neurotransmission in opioid-induced hyperalgesia. Pain. 2005;116:276–288. doi: 10.1016/j.pain.2005.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma W, Zheng WH, Kar S, Quirion R. Morphine treatment induced calcitonin gene-related peptide and substance P increases in cultured dorsal root ganglion neurons. Neuroscience. 2000;99:529–539. doi: 10.1016/s0306-4522(00)00226-8. [DOI] [PubMed] [Google Scholar]
- 24.Powell KJ, Quirion R, Jhamandas K. Inhibition of neurokinin-1-substance P receptor and prostanoid activity prevents and reverses the development of morphine tolerance in vivo and the morphine-induced increase in CGRP expression in cultured dorsal root ganglion neurons. Eur J Neurosci. 2003;18:1572–1583. doi: 10.1046/j.1460-9568.2003.02887.x. [DOI] [PubMed] [Google Scholar]
- 25.Misterek K, Maszczynska I, Dorociak A, Gumulka SW, Carr DB, Szyfelbein SK, Lipkowski AW. Spinal co-administration of peptide substance P antagonist increases antinociceptive effect of the opioid peptide biphalin. Life Sci. 1994;54:939–944. doi: 10.1016/0024-3205(94)00494-3. [DOI] [PubMed] [Google Scholar]
- 26.Gu G, Kondo I, Hua XY, Yaksh TL. Resting and evoked spinal substance P release during chronic intrathecal morphine infusion: parallels with tolerance and dependence. J Pharmacol Exp Ther. 2005;314:1362–1369. doi: 10.1124/jpet.105.087718. [DOI] [PubMed] [Google Scholar]
- 27.Dickenson AH. Plasticity: Implications for opioid and other pharmacological interventions in specific pain states. Behav Brain Sci. 1997;20:392–403. doi: 10.1017/s0140525x97241488. [DOI] [PubMed] [Google Scholar]
- 28.Chang KJ, Rigdon GC, Howard JL, McNutt RW. A novel potent and selective nonpeptidic delta opioid receptor agonist BW373U86. J Pharmacol Exp Ther. 1993;267:852–857. [PubMed] [Google Scholar]
- 29.Sheldon RJ, Riviere PJ, Malarchik ME, Mosberg HI, Burks TF, Porreca F. Opioid regulation of mucosal ion transport in the mouse isolated jejunum. J Pharmacol Exp Ther. 1990;253:144–151. [PubMed] [Google Scholar]
- 30.Cowan A, Zhu XZ, Mosberg HI, Omnaas JR, Porreca F. Direct dependence studies in rats with agents selective for different types of opioid receptor. J Pharmacol Exp Ther. 1988;246:950–955. [PubMed] [Google Scholar]
- 31.Largent-Milnes TM, Yamamoto T, Davis P, Ma SW, Hruby VJ, Yamamura HI, Lai J, Porreca F, Vanderah TW. Neuroscience. San Diego, CA: 2007. Dual acting opioid agonist/NK1 antagonist reverses neuropathic pain and does not produce tolerance. Poster 725. Visceral Pain: Transmitters and Receptors. [Google Scholar]
- 32.Largent-Milnes TM, Yamamoto T, Nair P, Navratrilova E, Davis P, Ma SW, Hruby VJ, Yamamura HI, Lai J, Porreca F, Vanderah TW. Dual acting opioid agonist/NK1 antagonist peptide reverses neuropathic pain in an animal model withoug demonstrating common opioid unwanted side effects. International Association for the Study of Pain / 12th World Congress on Pain; Glasgow, Scotland. 2008. [Google Scholar]
- 33.Cirino PC, Tang Y, Takahashi K, Tirrell DA, Arnold FH. Global incorporation of norleucine in place of methionine in cytochrome P450 BM-3 heme domain increases peroxygenase activity. Biotechnol Bioeng. 2003;83:729–734. doi: 10.1002/bit.10718. [DOI] [PubMed] [Google Scholar]
- 34.Gilles AM, Marliere P, Rose T, Sarfati R, Longin R, Meier A, Fermandjian S, Monnot M, Cohen GN, Barzu O. Conservative replacement of methionine by norleucine in Escherichia coli adenylate kinase. J Biol Chem. 1988;263:8204–8209. [PubMed] [Google Scholar]
- 35.Sawyer TK, Sanfilippo PJ, Hruby VJ, Engel MH, Heward CB, Burnett JB, Hadley ME. 4-Norleucine, 7-D-phenylalanine-alpha-melanocyte-stimulating hormone: a highly potent alpha-melanotropin with ultralong biological activity. Proc Natl Acad Sci U S A. 1980;77:5754–5758. doi: 10.1073/pnas.77.10.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobsen NE, Abadi N, Sliwkowski MX, Reilly D, Skelton NJ, Fairbrother WJ. High-resolution solution structure of the EGF-like domain of heregulin-alpha. Biochemistry. 1996;35:3402–3417. doi: 10.1021/bi952626l. [DOI] [PubMed] [Google Scholar]
- 37.Ying J, Ahn JM, Jacobsen NE, Brown MF, Hruby VJ. NMR solution structure of the glucagon antagonist [desHis1, desPhe6, Glu9]glucagon amide in the presence of perdeuterated dodecylphosphocholine micelles. Biochemistry. 2003;42:2825–2835. doi: 10.1021/bi026629r. [DOI] [PubMed] [Google Scholar]
- 38.Matsumori N, Houdai T, Murata M. Conformation and position of membrane-bound amphotericin B deduced from NMR in SDS micelles. J Org Chem. 2007;72:700–706. doi: 10.1021/jo061309p. [DOI] [PubMed] [Google Scholar]
- 39.Dörner B, White P. In: Peptides 1998, Proc 25th European Peptide Symposium. Bajusz S, H F, editors. Akadémiai Kiadó; Budapest: 1999. p. 90. [Google Scholar]
- 40.Seibel J, Hillringhaus L, Moraru R. Microwave-assisted glycosylation for the synthesis of glycopeptides. Carbohydr Res. 2005;340:507–511. doi: 10.1016/j.carres.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 41.Millet R, Goossens L, Bertrand-Caumont K, Chavatte P, Houssin R, Henichart JP. Synthesis and biological evaluation of tripeptide derivatives of Cbz-Gly-Leu-Trp-OBzl(CF3)2 as NK1/NK2 ligands. Lett Pep Sci. 1999;6:255–262. [Google Scholar]
- 42.Cascieri MA, Macleod AM, Underwood D, Shiao LL, Ber E, Sadowski S, Yu H, Merchant KJ, Swain CJ, Strader CD, Fong TM. Characterization of the interaction of N-acyl-L-tryptophan benzyl ester neurokinin antagonists with the human neurokinin-1 receptor. J Biol Chem. 1994;269:6587–6591. [PubMed] [Google Scholar]
- 43.Datar P, Srivastava S, Coutinho E, Govil G. Substance P: structure, function, and therapeutics. Curr Top Med Chem. 2004;4:75–103. doi: 10.2174/1568026043451636. [DOI] [PubMed] [Google Scholar]
- 44.Millet R, Domarkas J, Rigo B, Goossens L, Goossens JF, Houssin R, Henichart JP. Novel potent substance P and neurokinin A receptor antagonists. Conception, synthesis and biological evaluation of indolizine derivatives. Bioorg Med Chem. 2002;10:2905–2912. doi: 10.1016/s0968-0896(02)00144-x. [DOI] [PubMed] [Google Scholar]
- 45.D’Alagni M, Delfini M, Di Nola A, Eisenberg M, Paci M, Roda LG, Veglia G. Conformational study of [Met5]enkephalin-Arg-Phe in the presence of phosphatidylserine vesicles. Eur J Biochem. 1996;240:540–549. doi: 10.1111/j.1432-1033.1996.0540h.x. [DOI] [PubMed] [Google Scholar]
- 46.Deber CM, Behnam BA. Role of membrane lipids in peptide hormone function: binding of enkephalins to micelles. Proc Natl Acad Sci U S A. 1984;81:61–65. doi: 10.1073/pnas.81.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lazaridis T, Mallik B, Chen Y. Implicit solvent simulations of DPC micelle formation. J Phys Chem B. 2005;109:15098–15106. doi: 10.1021/jp0516801. [DOI] [PubMed] [Google Scholar]
- 48.Weiner SJ, Kollman PA, Case DA. An all atom force field for simulations of proteins and nucleic acids. J Comput Chem. 1986;7:230–252. doi: 10.1002/jcc.540070216. [DOI] [PubMed] [Google Scholar]
- 49.Weiner SJ, Kollman PA, Case DA, Singh UC, Ghio C, Alagona GS, Profeta J, Weiner P. A New Force Field for Molecular Mechanical Simulation of Nucleic Acids and Proteins. J Am Chem Soc. 1984;106:765–784. [Google Scholar]
- 50.Wagner G, Neuhaus D, Worgotter E, Vasak M, Kagi JH, Wuthrich K. Nuclear magnetic resonance identification of “half-turn” and 3(10)-helix secondary structure in rabbit liver metallothionein-2. J Mol Biol. 1986;187:131–135. doi: 10.1016/0022-2836(86)90413-4. [DOI] [PubMed] [Google Scholar]
- 51.Flippen-Anderson JL, Hruby VJ, Collins N, George C, Cudney B. X-ray Structure of [D-Pen2,D-Pen5]enkephain, a Highly Potent, delta Opioid Receptor-Selective Compound: Comparisons with Proposed Solution Conformations. J Am Chem Soc. 1994;116:7523–31. [Google Scholar]
- 52.Shenderovich MD, Kover KE, Nikiforovich GV, Jiao D, Hruby VJ. Conformational analysis of beta-methyl-para-nitrophenylalanine stereoisomers of cyclo[D-Pen2, D-Pen5]enkephalin by NMR spectroscopy and conformational energy calculations. Biopolymers. 1996;38:141–156. doi: 10.1002/(sici)1097-0282(199602)38:2<141::aid-bip2>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 53.2007 Feb; http://www-personal.umich.edu/~him/modeling.htm.
- 54.Hyberts SG, Goldberg MS, Havel TF, Wagner G. The solution structure of eglin c based on measurements of many NOEs and coupling constants and its comparison with X-ray structures. Protein Sci. 1992;1:736–751. doi: 10.1002/pro.5560010606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eguchi M. Recent advances in selective opioid receptor agonists and antagonists. Med Res Rev. 2004;24:182–212. doi: 10.1002/med.10059. [DOI] [PubMed] [Google Scholar]
- 56.Hu X, Nguyen KT, Jiang VC, Lofland D, Moser HE, Pei D. Macrocyclic inhibitors for peptide deformylase: a structure-activity relationship study of the ring size. J Med Chem. 2004;47:4941–4949. doi: 10.1021/jm049592c. [DOI] [PubMed] [Google Scholar]
- 57.Kumar A, Ernst RR, Wüthrich K. A two-dimensional nuclear Overhauser enhancement (2D NOE) experiment for the elucidation of complete proton-proton cross-relaxation networks in biological macromolecules. Biochem Biophys Res Commun. 1980;95:1–6. doi: 10.1016/0006-291x(80)90695-6. [DOI] [PubMed] [Google Scholar]
- 58.Davis DG, Bax A. Assignment of complex proton NMR spectra via two-dimensional homonuclear Hartmann-Hahn spectroscopy. J Am Chem Soc. 1985;107:2820–2821. [Google Scholar]
- 59.Wüthrich K, B M, Braun W. Pseudo-structures for the 20 common amino acids for use in studies of protein conformations by measurements of intramolecular proton-proton distance constraints with nuclear magnetic resonance. J Mol Biol. 1983;169:949–961. doi: 10.1016/s0022-2836(83)80144-2. [DOI] [PubMed] [Google Scholar]
- 60.Havel TF. An evaluation of computational strategies for use in the determination of protein structure from distance constraints obtained by nuclear magnetic resonance. Prog Biophys Mol Biol. 1991;56:43–78. doi: 10.1016/0079-6107(91)90007-f. [DOI] [PubMed] [Google Scholar]
- 61.Malicka J, G M, Czaplewski C, Kasprzykowska R, Liwo A, Lankiewicz L, Wiczk W. Lett Pept Sci. 1998;5:445–447. [Google Scholar]
- 62.Toth G, Russell KC, Landis G, Kramer TH, Fang L, Knapp R, Davis P, Burks TF, Yamamura HI, Hruby VJ. Ring substituted and other conformationally constrained tyrosine analogues of [D-Pen2,D-Pen5]enkephalin with delta opioid receptor selectivity. J Med Chem. 1992;35:2384–2391. doi: 10.1021/jm00091a006. [DOI] [PubMed] [Google Scholar]
- 63.Mosberg HI, Hurst R, Hruby VJ, Gee K, Yamamura HI, Galligan JJ, Burks TF. Bis-penicillamine enkephalins possess highly improved specificity toward delta opioid receptors. Proc Natl Acad Sci U S A. 1983;80:5871–5874. doi: 10.1073/pnas.80.19.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wishart DS, Sykes BD, Richards FM. The Chemical Shift Index: A Fast and Simple Method for the Assignment of Protein Secondary Structure through NMR Spectroscopy. Biochemistry. 1992;31:1647–1651. doi: 10.1021/bi00121a010. [DOI] [PubMed] [Google Scholar]
- 65.Andersen NH, Neidigh JW, Harris SW, Lee GM, Liu ZH, Tong H. Extracting Information from the Temperature Gradients of Polypeptide NH Chemical Shifts. 1. The Importance of Conformational Averaging. J Am Chem Soc. 1997;119:8547–8561. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


