Abstract
Neurons receive signals through dendrites that vary widely in number and organization, ranging from one primary dendrite to multiple complex dendritic trees. For example, retinal amacrine cells (ACs) project primary dendrites into a discrete synaptic layer called the inner plexiform layer (IPL) and only rarely extend processes into other retinal layers. Here, we show that the atypical cadherin Fat3 ensures that ACs develop this unipolar morphology. AC precursors are initially multipolar, but lose neurites as they migrate through the neuroblastic layer. In fat3 mutants, pruning is unreliable and ACs elaborate two dendritic trees: one in the IPL and a second projecting away from the IPL that stratifies to form an additional synaptic layer. Since complex nervous systems are characterized by the addition of layers, these results demonstrate that mutations in a single gene can cause fundamental changes in circuit organization that may drive nervous system evolution.
Introduction
A defining characteristic of all neurons is the number and arrangement of primary dendrites. For instance, GABAergic cortical interneurons elaborate multiple primary dendrites, while Purkinje neurons extend a single dendritic tree. Dendrites develop from multipotential neurites that emerge from the cell body of developing neurons (Barnes and Polleux, 2009). One neurite is specified to become an axon while the remainder are either lost or become primary dendrites, each of which arborizes to form a dendritic tree. Although pathways establishing axonal versus dendritic identity are being elucidated, the steps that determine how many neurites are retained to become primary dendrites are poorly understood (Jan and Jan, 2010).
Much of what is known about dendrite development has come from studies of neurons with highly stereotyped branching patterns. For instance, the dendritic arborization neurons innervating the Drosophila larval body wall fall into four distinct classes on the basis of arbor complexity (Grueber et al., 2002), and genetic screens for changes in this morphology have been productive (Parrish et al., 2006; Ye et al., 2007). Amacrine cells (ACs) of the vertebrate retina offer many of the same advantages. ACs are interneurons that modulate the activity of bipolar cells and retinal ganglion cells (RGCs) via synapses in the inner plexiform layer (IPL). Different types of ACs exhibit distinct functions that are determined in part by their dendritic patterning, with at least 22 morphologically-defined classes (MacNeil and Masland, 1998). Despite this diversity, AC connectivity is relatively easy to assess due to the stereotyped laminar organization of the retina, which hasthree distinct cellular layers separated by two synaptic plexiform layers (Figure 1A). ACs reside both in the inner nuclear layer (INL) and in the ganglion cell layer (GCL). However, regardless of their location, many ACs are unipolar and extend a single primary dendrite oriented into the IPL, which separates the INL from the GCL. For ACs in the INL, these dendrites point inwards to the IPL. For “displaced” ACs in the GCL, which represent a large fraction of mouse ACs, the dendrites extend outwards to the IPL (Jeon et al., 1998). Hence, dendrite number and orientation is robustly controlled and coordinated with the laminar organization of the retina. In addition, the segregation of AC bodies and their processes facilitates detection of changes in the formation or alignment of the dendritic tree. Finally, ACs lack classic axons so dendrite morphogenesis can be studied independent of effects on axon specification.
Figure 1.
Fat3 is enriched in AC dendrites from the earliest stages of IPL development. (A) ACs (green) are born in the neuroblast layer (NBL; left) and establish specific connections with retinal RGCs (black) and bipolar cells (red) in the inner plexiform layer (IPL) of the mature retina (right). (GCL) RGC layer; (INL) inner nuclear layer; (OLM) outer limiting membrane; (ONL) outer nuclear layer; (OPL) outer plexiform layer. (B) A montage of representative ACs in Ptf1a-cre;Z/EG retinas at P1, which transform from multipolar (4+) to unipolar (1) morphologies as they migrate through the NBL. A subset of cells with two neurites (2*) have contacted the IPL. Subsequently, trailing processes are retracted and cells become unipolar (1). (C) Quantification confirms that neurite number varies as a function of AC position. (D,E) At P3 and P12, fat3 in situ hybridization labels ACs at the inner boundary of the INL (asterisk) and cells in the GCL (arrowhead). (F–I) Fat3 protein (red) is present in the IPL at E17.5 and increases as more ACs are added (green; GFP reporter from Ptf1a-cre;Z/EG) until the mature pattern emerges at P11. In contrast, migrating cells do not exhibit enhanced Fat3 immunolabeling (arrowhead, F, F′). See also Figure S1D. Note that double labeling of GFP and Fat3 requires a chicken anti-GFP antibody that introduces some background in the GCL. See Figure S1C. Scale bars 10 μm (B); 50 μm (D,E); 20 μm (F–I).
ACs acquire their final dendritic morphology through a series of events involving multiple signaling systems. The earliest changes occur as ACs migrate through the neuroblast layer (NBL). Live imaging in zebrafish and histological studies in chicks and rodents suggest that AC precursors are initially multipolar, migrate to their final position, and then form polarized dendritic trees projecting into the IPL (Godinho et al., 2005; Hinds and Hinds, 1978; Prada et al., 1987). Although the RGCs are born first, ACs appear to play a dominant role in the initial development of the IPL. For instance, live imaging studies indicate that ACs extend their projections directly to specific sublaminae in the IPL, followed by remodelling of RGCs arbors (Godinho et al., 2005; Mumm et al., 2006). Stratification of dendrites into distinct sublaminae is regulated in part by a repulsive Semaphorin signaling event (Matsuoka et al., 2011). Within each sublamina, dendrites from specific pairs of ACs and RGCs may recognize each other via homophilic cell adhesion molecules such as Sidekicks and Dscam proteins (Yamagata and Sanes, 2008), though whether the same mechanisms operate in chick and mouse is unclear (Fuerst et al., 2009). Final dendritic morphology is further influenced by self-avoidance that is also mediated by Dscam and ensures that individual classes of ACs spread their arbors evenly across the retina (Fuerst et al., 2009; Fuerst et al., 2008). In contrast to these advances regarding IPL stratification, little is known about earlier mechanisms that mediate the initial extension of primary dendrites.
One receptor with a potential role in AC development is the atypical cadherin Fat3, which is localized to processes in the IPL (Nagae et al., 2007). Fats are transmembrane proteins that typically have 34 cadherin repeats as well as laminin A–G domains and EGF repeats in a huge extracellular domain (Sopko and McNeill, 2009; Tanoue and Takeichi, 2005). Fat function is best understood in flies, which have two fat genes: fat and fat-like (or fat2). Mutations in fat cause overgrowth of larval imaginal discs and long-range planar polarity defects, such as misalignment of ommatidia in the eye and bristles in the wing and abdomen (Goodrich and Strutt, 2011). How Fat signaling influences planar polarity is not well understood, especially with respect to possible interactions with the core planar cell polarity pathway (Bayly and Axelrod, 2011; Lawrence et al., 2007). Fat signaling events are initiated by another large cadherin, Dachsous (Ds), and the strength of Fat-Ds binding is modulated by phosphorylation of both the ligand and receptor by the Golgi kinase Four-jointed (Fj) (Brittle et al., 2010; Ishikawa et al., 2008; Simon et al., 2010). Fat-like is less studied, but is required for polarization of follicle cells surrounding the egg chamber (Viktorinova et al., 2009). This effect seems to be independent of Ds and of core planar cell polarity components such as Van Gogh, suggesting that Fat-like signals through a novel polarization pathway. Fat3 is one of four Fat-related proteins in mammals (Tanoue and Takeichi, 2005). The closest ortholog to Drosophila Fat is Fat4, which plays a conserved role in planar polarity (Saburi et al., 2008). Fat1 and Fat3 are more closely related to Drosophila Fat-like at the amino acid level (Castillejo-Lopez et al., 2004). Fat1 is not required for classic planar polarity events (Ciani et al., 2003), but is implicated in regulation of the actin cytoskeleton, perhaps acting via the Ena/Vasp proteins (Moeller et al., 2004; Schreiner et al., 2006; Tanoue and Takeichi, 2004). Although Fat2 and Fat3 are present in the nervous system (Mitsui et al., 2002; Nagae et al., 2007; Nakayama et al., 2002), no function for either family member has been described.
Based on its localization during IPL development, we hypothesized a role for Fat3 in AC dendrite morphogenesis. Here, we show that Fat3 acts in ACs to restrict dendrite number. In addition, Fat3 controls the distribution of ACs between the INL and the GCL. In mice lacking fat3, ACs develop an extra dendritic arbor and migrate into the GCL in excess numbers, where they recruit contacts with surrounding cells. As a consequence, the fat3 mutant retina contains two new plexiform layers. Hence, our data establish Fat3 as a novel regulator of dendrite morphogenesis and retinal circuit assembly.
Results
Amacrine cells lose neurites as they migrate into the INL
Dendrite morphogenesis begins with the selection of a specific number of dendrites, each of which branch and elaborate to form mature arbors appropriate for that neuron’s function (Jan and Jan, 2010). In many AC classes, the number of dendrites is highly stereotyped and cells develop single primary dendrites oriented towards the IPL, regardless of whether the cell is located in the INL or GCL. Electron microscope studies showed developing ACs are bipolar when they initially reach the IPL, followed by elaboration of the dendritic arbor (Hinds and Hinds, 1978), suggesting a link between the end of migration and the beginning of dendrite morphogenesis. We extended these studies using genetic labeling to distinguish migrating ACs from RGCs unambiguously and to quantify dendrite number with respect to cell position during migration. Since ACs but not RGCs derive from progenitors expressing Ptf1a, ACs were labeled by crossing Ptf1a-cre knock-in mice (Fujitani et al., 2006) to the Z/EG fluorescent indicator line (Novak et al., 2000) (Figure S1). Using this approach, cells expressing Cre-recombinase permanently express GFP and can be imaged at any stage of development, regardless of whether the Ptf1a promoter remains active. Although Ptf1a is also expressed in horizontal cells, we observed only a low frequency of Ptf1a-cre-mediated recombination in these cells; therefore, most labeled cells come from AC lineages (Figure S1A,B). Amacrine cells labeled using this method extended only a single primary dendrite, confirming that this population offers a useful entry point for studying regulation of dendrite number.
Genetically labelled cells were visualized in Ptf1a-cre;Z/EG mice at postnatal day 1 (P1), a time of active AC production and migration (Voinescu et al., 2009) (Figure 1B). This approach confirmed that ACs lose neurites as they migrate closer to the IPL (Figures 1B–C, F–I). We find that cells in the outer neuroblastic layer (NBL) are multipolar, with >4 neurites. In the middle of the NBL, cells reduce neurite number and assume a bipolar morphology, with a leading process directed toward the GCL and a trailing process pointing to the NBL. This bipolar morphology is retained as cells reach the IPL, but subsequently resolves into a unipolar morphology, with dendrites extending only into the IPL. These observations highlight the close relationship between the morphology of migrating and mature neurons and are consistent with live imaging in zebrafish and histological analysis in rodents and chicks (Godinho et al., 2005; Hinds and Hinds, 1978; Prada et al., 1987).
Fat3 protein is localized to dendrites from the earliest stages of IPL development
Our observations suggest that the transition to a unipolar morphology is controlled by cell-cell interactions in the nascent IPL. Specifically, we hypothesized that ACs receive signals through a cell-surface receptor present on dendrites that mediates changes in cell morphology. An excellent candidate is the atypical cadherin Fat3, which is localized to processes throughout the developing and mature IPL (Nagae et al., 2007). Fat3 is a large >500 kDa protein with thirty-four cadherin domains, a laminin A–G and four EGF repeats in its ectodomain (Figure S2A) (Tanoue and Takeichi, 2005). Although the functions of Fat3 are unknown, the closely related Fat1 can control cell-cell contacts (Ciani et al., 2003) and induce polarized changes in the actin cytoskeleton (Moeller et al., 2004; Schreiner et al., 2006; Tanoue and Takeichi, 2004).
In situ hybridization confirmed that fat3 is transcribed during IPL formation in cells at the bottom of the INL, where ACs reside, as well as in the GCL, which contains RGCs and displaced ACs (Figure 1D). Expression is maintained after the retina has acquired a mature morphology (Figure1E). To pinpoint the onset of Fat3 expression relative to dendrite morphogenesis, we generated an antibody to Fat3 and performed double immunolabeling of Fat3 and GFP on Ptf1a-cre; Z/EG retinas at times spanning the initial production of ACs to stratification of the IPL. This allowed correlation of Fat3 localization with specific changes in AC morphology. During early stages of AC development (E17.5), Fat3 is present in the GCL, with no obvious enrichment in migrating ACs (Figure 1F). At P0 a discrete band of Fat3 protein emerges in the nascent IPL, which now contains more AC processes (Figure 1G). Although many ACs retain trailing processes at this stage, Fat3 is restricted to the IPL, suggesting enrichment in the early primary dendrite. By P5, there are more ACs with extensive arbors and Fat3 immunolabeling increases accordingly (Figure 1H). This expression is maintained at P11 and extends across the entire width of the IPL. Fat3-positive processes stratify in the IPL and are present in all sublaminae (Figure 1I). Hence, Fat3 is localized to dendrites after ACs reach their final destination and is then maintained throughout dendrite morphogenesis and maturation.
Neurite number is increased in fat3 mutant amacrine cells
The enhancement of Fat3 protein in the IPL upon arrival of ACs suggested that Fat3 might play a role during the earliest stages of dendrite development. To test this idea, we generated fat3 mutant mice by flanking the exon encoding the Fat3 transmembrane domain with LoxP sites (fat3floxed) (Figures S2B–G); a null allele (fat3KO) was generated by deleting this exon using a global Cre driver. No full-length Fat3 protein can be detected in fat3KO tissue by Western blot using two different antibodies against the cytoplasmic domain (Figure S2A,H). Since this domain is critical for Fat signaling in flies and vertebrates (Matakatsu and Blair, 2006; Tanoue and Takeichi, 2004), the fat3KO mutation is likely a complete loss of function. In addition, no differences were identified between fat3 heterozygotes and WTco ntrols (Figure 5K and data not shown).
Figure 5.
Fat3 restricts migration of GABAergic AC classes into the GCL. (A–F)Cross-sections of P11 WT (A,C,E,G,I) and fat3KO (B,D,F,H,J) retinas labeled with DAPI (A,B) or cell-specific markers. (C,D) Pan-Brn3 antibody staining shows no change in RGC number or distribution. (E,F) Instead, there is a change in the distribution of ACs between the INL and GCL, as demonstrated by non-selective labeling for GFP-positive ACs in Ptf1a-cre;Z/EG retinas. (G–J) Class-specific markers reveal a selective redistribution of GABAergic types of ACs. Bhlhb5-positive cells (green,G,H) are more frequent in the mutant GCL and IPL while the distribution of EBF-positive cells (red, G,H) and ChAT-positive starburst ACs (I,J) is unaltered. (K) Quantification of the number and distribution of cells in each AC class. Absolute bar length reports the total number of cells of each class and the shaded divisions indicate how many are in the INL (light blue) or in the IPL/GCL (dark blue). Overall, the IPL and GCL contain more GFP-labeled and Bhlhb5-positive ACs in fat3KOthan controls, with no change in overall cell number (K). (* denotes P<0.0005 and ** denotes P<10−11 by a two-tailed Student’s t-test). Scale bars: 50μm.
Analysis of fat3KO retinas at P3 revealed striking changes in AC morphology. In WT retina, calretinin immunolabeling marks several classes of unipolar ACs that extend processes into the nascent IPL (Figure 2A). Yet in fat3KOs, 23% (+/−8%) of these cells have two neurites: a normal process projecting into the IPL and a second process extending into the INL (Figure 2B–C). Unaffected cells are likely starburst cells which also express ChAT (Gabriel and Witkovsky, 1998) and appear normal in the fat3KO retina (Figure 5I–J). Changes in neurite number are also evident in thy1::YFP-H transgenic mice (Feng et al., 2000), which express YFP in subsets of isolated retinal cells at these early postnatal stages, allowing independent confirmation that ACs extend ectopic neurites towards the outer retina in fat3KOs (Figures 2D–E).
Figure 2.
Extra neurites extend from ACs in fat3KOs.(A–E) At P3, calretinin-positive (A) and Thy1:YFP labeled (D) ACs extend one dendritic arbor into the IPL in WT mice, but develop extra processes that point away from the IPL in fat3KOs (B,E arrowheads). (C) 23% of calretinin expressing ACs extend a second neurite towards the outer retina compared to WT controls (** P<10−6 by unpaired two-tailed t-test). (F) A montage of representative Ptf1a-cre;Z/EG labeled cells in P1 fat3KO mice. Neurite length was quantified for cells at positions (a) and (b) in WTs and fat3KOs. (G) Migrating cells in the middle of the NBL (a) have trailing processes of equal lengths in WTs and mutants. In contrast, fat3KO cells near the IPL (b) have longer trailing processes than WT cells in the same location (*** P<0.0001 by unpaired two-tailed t-test). Mean is represented by a straight line. Scale bars: 20μm (A–B); 10μm (D–F). See Figure S2 for fat3 gene targeting strategy.
To determine the origin of the extra neurites, we examined Ptf1a-cre;Z/EG labeled ACs during migration in fat3KO retinas. Initially, mutant ACs transform from multipolar to bipolar with clear leading and trailing processes, similar to controls (Figures 1B, 2F). In contrast, mutant neurons frequently maintain two processes after reaching the IPL (Figure 2G). Most WTAC s are unipolar at this stage, although 14% extend a short trailing process that is likely in the process of retraction (mean=5.5 ± 0.5 μm; n=38 cells). In contrast, 35% of mutant neurons retain a trailing process. The length of these extra neurites is much longer than in controls (mean=24.5 ± 0.5 μm; n=58 cells) and is similar to the length of the trailing process in migrating WT neurons at this stage (mean=35.3 ± 1.7 μm; n=50 cells). The simplest explanation for these observations is that fat3KOAC s develop abnormal shapes due to a failure to retract the trailing process upon reaching the IPL.
Fat3 mutant amacrine cells create two novel plexiform layers
During normal development, most ACs retain a single neurite that develops as a primary dendrite and arborizes in the IPL. However, it is not known if this neurite becomes a dendrite by default or if additional cues are involved. Therefore, we asked whether retention of an extra neurite in fat3KOs is sufficient to promote dendrite development by examining different classes of ACs in the mature fat3KO retina. We found that multiple types of ACs develop extra dendrites that project away from the IPL and stratify in a single layer dividing the INL, as visualized by staining for calretinin (Figure 3B). Furthermore, while GFP-positive ACs in Ptf1a-cre;Z/EG retinas are unipolar (Figure 3C), extra dendrites extend away from mutant cells and into the INL (arrow, Figure 3D). Ectopic arborization is easiest to appreciate in the dopaminergic (TH-positive) ACs, which extend multiple processes away from the cell soma, through the INL and towards the OPL. Although WT dopaminergic ACs also send processes towards the OPL, these are usually dendritic branches originating from the IPL rather than the cell soma (arrows, Figure 3E) (Gustincich et al., 1997). Thus, the ectopic dendrites in fat3KO mice may be directed toward appropriate synaptic targets (arrows, Figure 3F). AII amacrine cells also have ectopic processes in the INL (Figure 3G,H); however these processes divide the AII population so ectopic processes extending to the outer retina cannot be distinguished from normal dendrites directed at the IPL. AII cells located in the outer half of the mutant INL also commonly send long dendrites into the OPL (arrows, Figure 3H). Like dopaminergic ACs, these extra dendrites appear to be recruited by natural targets as rare misplaced AII cells make similar projections in WT retina (Lee et al., 2006). In addition, some AII amacrine cell dendrites were detected in the vicinity of the nerve fiber layer (NFL) (Figure 3H), a region that is devoid of processes in the WT retina (Figure 3G). Analysis with additional markers (described below) confirms the presence of a second layer of processes here, likely arising from displaced ACs in the GCL. Thus, mutantAC s can develop remarkably extensive dendritic arbors from extra neurites located outside of the IPL.
Figure 3.
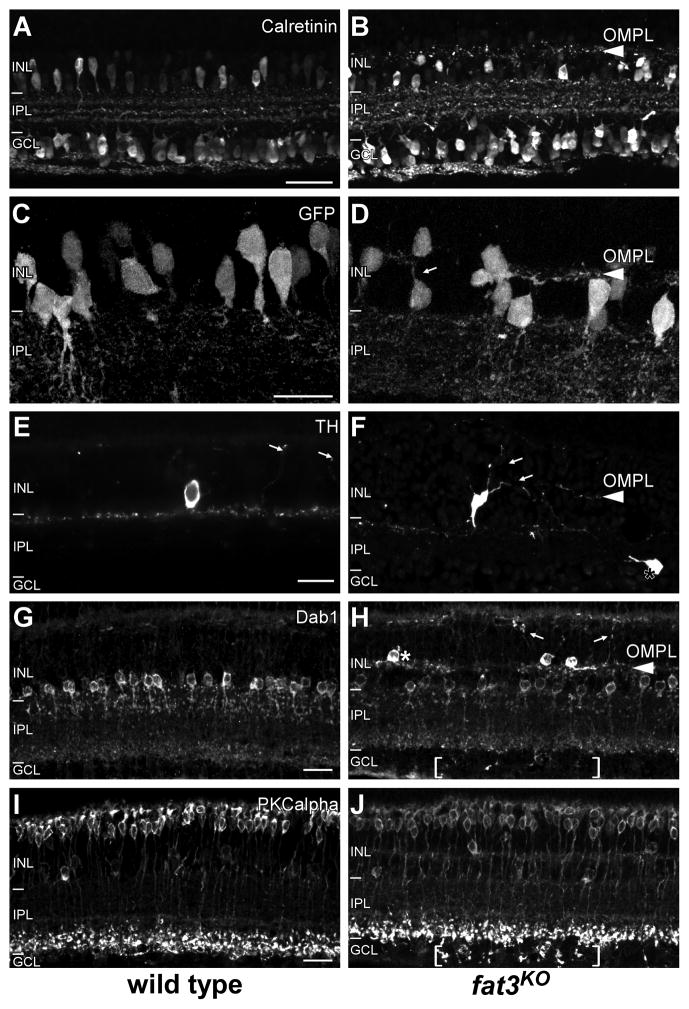
ACs develop a second dendritic arbor in fat3KOretina. (A,B) At P11, calretinin labels a subset of ACs and RGCs that form three bands in the WT IPL (A). This persists in the fat3KOretina (B) but labeled dendrites are also present in a new layer in the INL. We refer to this layer as the outer misplaced plexiform layer (OMPL) due to its position and composition of ectopic dendrites. (C,D) At P11, the arbors of GFP-positive ACs in Ptf1a-Cre;Z/EG mice are restricted to the IPL, while fat3KO ACs develop a second arbor (arrow) extending into the OMPL (arrowhead). (E) Dendrites from TH-positive ACs stratify at the outermost IPL layer and occasionally extend branches to the OPL (arrows). (F) In fat3KOs, ectopic dendrites project from the scleral side of TH-positive ACs into the OPL or stratify in the OMPL (arrows). Some TH-positive ACs are misplaced in the GCL (*). (G,H) Dab1-positive AII cells form a single layer at the boundary between the INL and the IPL. In fat3KOs (H), AIIs are divided into two groups by the OMPL; an example of an AII trapped in the middle of the INL is marked (*). In fat3KOs, AII cells also extend dendrite branches to the OPL, and a smaller number of dendrites project into GCL (brackets). (I,J) The morphology of rod bipolar cells is also altered in the fat3KO retina, with endings frequently mislocalized in the IMPL (brackets). Scale bars: 50 μm (A–B); 20 μm (C–J).
Since dendrites serve as synaptic targets, we investigated whether the extra dendritic arbors in fat3KO retina can recruit contacts from surrounding neurons. To distinguish between secondary effects and fat3-dependent changes in morphology, we focused on rod bipolar cells (RBCs) because fat3 mRNA is not expressed in the vicinity of developing or mature RBCs (Figure 1D–E). In the WT retina, RBCs extend dendrites to the inner boundary of the IPL, where they contact post-synaptic AC dendrites, including AII cells (Figure 3I). In fat3KO retina, RBCs frequently overshoot the IPL and form ectopic endings in the NFL (bracketed region, Figure 3J). This is the same region that contains ectopic AII cell processes (bracketed region, Figure 3H), suggesting that ectopic AC dendrites can attract normal pre-synaptic partners, evidently via a Fat3-independent mechanism.
Since both pre- and post-synaptic processes are present in ectopic locations in the fat3KO retina, we asked whether synaptogenesis can occur in such unusual conditions. We examined two synaptic vesicle markers: VGAT, a vesicular glutamate transporter present at GABAergic synapses, and SV2, which is a general synaptic vesicle marker. Indeed, VGAT staining reveals extensive ramification of GABAergic dendrites in the INL and in the NFL (Figure 4A,B). Moreover, SV2 staining confirmed the presence of synaptic proteins in both ectopic locations (Figure 4C,D). Most strikingly, electron micrographs from adult fat3KO retina reveal the presence of ectopic synaptic contacts in the INL, where they are separated from the IPL by AC cell bodies. These contacts were characterized by a cluster of synaptic vesicles adjacent to the presynaptic membrane, a narrow synaptic cleft and a thinner postsynaptic density, as typically seen in GABAergic and glycinergic AC synapses (Figure 4E). Within the NFL, we identified dyad synapses, which are characterized by glutamatergic bipolar cell endings onto AC and RGC dendrites. These contacts were characterized by a presynaptic ribbon surrounded by synaptic vesicles in the bipolar ending, an enlarged synaptic cleft, and prominent postsynaptic densities in both members of the dyad (Figure 4F). Thus in fat3KOs, ACs form stable synapses in ectopic locations that are maintained into adulthood. Altogether, the ultrastructural evidence, presence of synaptic proteins, and recruitment of bipolar cell endings indicate that ectopic AC dendrites produce bona fide plexiform layers in fat3KOs. Therefore, we refer to the new layer in the INL as the outer misplaced plexiform layer (OMPL), and the layer inside of the GCL the inner misplaced plexiform layer (IMPL).
Figure 4.
Additional plexiform layers form in fat3KO retina.(A–D)VGAT (A,B) and SV2 (C,D) immunolabeling of AC dendrites and synaptic vesicles in the WT (A,C) and fat3KO (B,D) OPL and IPL at P11. Staining also highlights the IMPL (arrow) and OMPL (arrowhead) in fat3KOs. Retinas in A and B were counterstained with DAPI (blue). (E, E′) Transmission electron micrograph of adult fat3KOretina in the region of the OMPL. E′ corresponds to the boxed region in E. Synaptic vesicles are present in the dendrites here and are frequently in the vicinity of post-synaptic densities (E′, arrowhead). Pseudocoloring in E highlights the AC bodies (green), Muller glia (blue), and neuropil in the IPL (red) and OMPL (orange). (F) Similarly, electron micrographs of the IMPL reveal ribbon synapses that are characteristic of synapses formed between bipolar cells (rbc) and AC (ac) targets (F′, arrow).F′ corresponds to the boxed region in F. Pseudocoloring in F marks RGC bodies (yellow), rod bipolar ending (red), AC dendrites (green), and Muller glia end feet (blue). Scale bars: 50 μm (A–D); 2 μm (E,F); 0.2 μm (E′, F′).
Subsets of amacrine cells are mislocalized in the fat3 mutant GCL
The addition of two new plexiform layers is accompanied by a striking re-organization of the cellular layers in fat3KO retinas. First, the OMPL creates a break at the level of the Muller glia cell bodies that separates the majority of ACs from the remainder of the INL (Figures 5A–B). Second, and more unexpectedly, the GCL is thicker than in control retinas, with a ~45% increase in total cell number (Figure 5K). The additional cells are not RGCs, as demonstrated by expression of the RGC marker Brn3 (Figures 5C–D, 5K). Instead, there is a significant increase in the number of displaced ACs in the GCL of fat3KOs compared to littermate controls (Figures 5E–F). Since there is no change in total AC number between genotypes (Figure 5K), we conclude that the increase in GCL content reflects changes in AC distribution rather than proliferation. Consistent with this finding, we also observed a ~50% reduction in the frequency of calretinin-positive ACs in the mutant INL (Figure 3A–B).
These changes in retinal lamination could reflect an additional function for Fat3 in migration or could be secondary to the presence of the IMPL and OMPL. To distinguish between these possibilities, we asked if specific classes of ACs are affected using two general markers: the transcription factor Bhlhb5, which is present in populations of GABAergic ACs and off-cone bipolars (Feng et al., 2006), and EBF, which is expressed by glycinergic ACs with the exception of the AIIs (Voinescu et al., 2009). AII cells were marked by Dab1 (Rice and Curran, 2000)and the cholinergic starburst ACs by ChAT.
We found that GABAergic AC distribution is specifically disrupted by loss of fat3, with a significant proportion of Bhlhb5-positive cells mislocalized in the GCL or trapped within the IPL (Figure 5G–H,K). In contrast, glycinergic ACs and the starburst cells, which are equally divided between the INL and GCL in WT retina, are properly distributed in fat3KOs (Figure 5G–K). Although the different classes of ACs are born sequentially (Voinescu et al., 2009), the migration phenotype does not correlate with birth order, as neither early born starburst cells nor the late born AII amacrine or EBF-positive cells are mislocalized to the GCL. However, AII amacrine cells are frequently mispositioned within the INL, where they are located outside of the OMPL (Figure 3H). This distribution likely occurs due to formation of an OMPL before all AIIs have migrated away from the NBL. In contrast, only the GABAergic classes marked by Bhlhb5 and born during intermediate stages of retinal development were mislocalized. The highly specific effect on GABAergic AC distribution suggests that Fat3 signaling actively restricts this cell population to the INL. Despite these changes, the overall organization of the mature retina is surprisingly intact, with clearly defined nuclear and synaptic layers and a persistent stratification of the IPL into sublaminae.
Fat3 acts independently to regulate dendrite number and cell migration
Our data suggest that Fat3 influences multiple aspects of AC development, with effects on dendrite number and cell migration combining to create a novel pattern of retinal lamination in fat3KO mice. Given the tight temporal relationship between the end of migration and the beginning of dendrite development, one attractive interpretation is that Fat3 functions as a receptor to induce changes in the cytoskeleton that are critical for both cellular events. However, fat3 is also expressed by RGCs and could play an independent role in GCL development. To separate the functions of Fat3 in these two cell populations, we selectively deleted fat3 from ACs by crossing the fat3floxedmice to Ptf1a-cre mice to create AC conditional knock-outs (fat3CKO).Ptf1a-cre is well suited for this experiment because Cre expression occurs early during AC differentiation (Fujitani et al., 2006) and drives recombination in ACs prior to Fat3 expression and before migration and dendrite extension (Figures 1, S1). Ptf1a-cre mediated recombination of fat3floxedproved highly efficient, and in the fat3CKO retina, fat3 mRNA is severely diminished in the INL but is maintained in the GCL (Figures 6A–B).
Figure 6.
Fat3 independently regulates AC morphology and position. (A,B) In situ hybridization for fat3 in control (A) and Ptf1a-cre;fat3 floxed/KO (B) retinas indicates decreased mRNA in the INL (arrowhead) but not the GCL (arrow). (C,D) OMPL formation is evident in DAPI-stained fat3CKOs (D, arrowhead). In contrast, the GCL is normally organized in fat3CKOs (D) as compared to controls (C). (E,F) Immunostaining for calretinin (red) and calbindin (green) reveals the overall organization of the retina in control (E) and fat3CKO (F) retinas. Calretinin-positive dendrites extend into the OMPL in mutants, but the distribution of calretinin-positive ACs is unchanged. (G,H) SV2 (green) and PKCalpha (red) labeling confirms the presence of the OMPL in fat3CKOs(H, green); however, no IMPL is apparent. (I) Quantification of DAPI-stained cells in the GCL reveals no significant difference between fat3CKOs and littermate controls (n=4 CKO, 4 control). Furthermore, cell numbers are the same as in WT retina (data reprinted from Fig. 5 for comparison). (J) Math5KOretinas do not form ectopic plexiform layers as revealed both by DAPI staining (gray) and VGAT labeling (green), further indicating that control of dendrite number is an AC autonomous event. (K) However, in fat3;math5 DKOs, VGAT-positive OMPL and IMPL formation is prominent similar to fat3KOs, confirming that Fat3 activity is responsible for the development of normal AC morphologies in the math5KO. (L) Fat3 immunolabeling (red) persists in math5KO, confirming that Fat3 protein is localized to AC dendrites and does not require a signal from RGCs to maintain this localization. All specimens were collected at P11. Scale bars: 200 μm (A,B); 50 μm (C–K).
Contrary to our hypothesis, analysis of fat3CKOs revealed that dendrite number and cell migration defects do not appear to share a common origin. As predicted, the OMPL is present in all fat3CKOs examined (n=4) based upon the organization of nuclei in the INL (Figures 6C–D) and the distribution of calretinin-positive dendrites (Figures 6E–F). Thus, Fat3 signaling is required in ACs to ensure the polarized extension of dendrites into the IPL. However, no IMPL was detected, as revealed both by SV2 immunolabeling and the distribution of RBC endings (Figures 6G–H). Moreover, fat3CKO mice lack the migration defect apparent in fat3KOs, with no significant change in the number of DAPI-stained nuclei in the GCL of fat3CKO vs. Cre-positive controls (Figures 6C–D,I). These observations suggest that the OMPL found in both types of mutant is created by ACs that are properly positioned but develop bipolar morphologies, while the IMPL, which is found only in fat3KOs, is created by ACs that migrate inappropriately into the GCL and then extend ectopic dendrites into the NFL. These phenotypic differences imply that Fat3 acts independently to control AC morphology and migration. The clear separation of effects on morphology versus migration indicates that the persistence of the trailing process does not a priori cause a migration phenotype and conversely, that the presence of this process is not secondary to abnormal migration.
The fat3CKO phenotype demonstrates that the Fat3 receptor is required in ACs to control dendrite number and raises the question of which cells might provide the relevant ligand. Fat3 protein is enriched in the developing IPL (Figure 1), suggesting that Fat3 localization and signaling occurs in response to cell-cell interactions within the IPL. Two types of interactions can be envisioned: AC-AC interactions and AC-RGC interactions. We distinguished between these possibilities by investigating plexiform layer development in math5KOmice. Math5 is a basic helix-loop helix transcription factor required for RGC differentiation and in math5KOs the majority of RGCs are absent because their precursors become ACs (Feng et al., 2010; Wang et al., 2001). However, despite the dramatic reduction of RGCs, neither an OMPL nor an IMPL formed, as evidenced by the absence of VGAT-labeled processes outside of the IPL (Figure 6J). In contrast, simultaneous loss of math5 and fat3 recapitulates the fat3KO phenotype, with formation of an extensive OMPL and IMPL (Figure 6K). This strongly suggests that Fat3 in ACs receives signals from other ACs to govern dendrite morphogenesis. In support of this idea, the distribution of Fat3 in the IPL of the math5KOis largely unaltered, which is consistent with the argument that only AC-AC contacts are necessary for Fat3 localization (Figure 6L). Together, these results show that regulation of dendrite number in ACs does not require RGCs, nor are RGCs necessary for the maintenance of ectopic AC dendrites in the fat3KO retina. Unfortunately, the role of RGCs in regulating AC migration remains unclear because the changes in overall AC number precluded an informative analysis of AC distribution (Feng et al., 2006).
Fat3 genetically interacts with fjx1 to restrict plexiform layer formation
In Drosophila Fat is activated by another atypical cadherin, Dachsous (Ds), and the strength of this interaction is modulated by the Golgi kinase Four-jointed (fj) (Brittle et al., 2010; Ishikawa et al., 2008; Simon et al., 2010). Fj plays a central role in this system by enhancing Fat activity while simultaneously reducing Ds’s ability to bind to Fat (Simon, 2004; Simon et al., 2010). As a consequence, Fat activity is proposed to become asymmetric within individual cells. Although subsequent signaling events may vary depending on the context, the Fat-Ds-Fj cassette is used in multiple developmental events (Sopko and McNeill, 2009). Similarly in mammals, fat4 genetically interacts with the sole fj ortholog fjx1 to control oriented cell divisions in the kidney (Saburi et al., 2008). Intriguingly, dendrite phenotypes have been described in the hippocampus of fjx1 mutant mice (Probst et al., 2007) raising the possibility that fjx1 might interact with fat3 during AC development. To test this idea, we asked whether fat3 and fjx1 are co-expressed in the developing and mature retina. At P3, both fat3 and fjx1 are present in regions containing ACs and RGCs, with additional fjx1 expression in the top of the developing INL, where bipolar cells will reside (Figure 7A). This expression fits with predictions, since fjx1 expression should overlap with fat3 if an interaction is conserved. By P11, fjx1 expression is much more restricted, but continues to be expressed with fat3 in the GCL (Figure 7B).
Figure 7. Fat3 genetically interacts with fjx1 to regulate plexiform layer formation.
(A,B) In situ hybridization for fjx1 at P3 (A) and P11 (B) shows a dynamic pattern of fjx1 expression in presumptive bipolar cells (asterisk) and the GCL at P3, and prominent expression in the GCL at P11, similar to fat3 in this layer (see Figure 1). (C–F) VGAT-labeling (green) at P11 reveals formation of an IMPL in fat3+/−;fjx1−/− retinas (E, arrows). This ectopic layer is not present in fat3+/−;fjx1+/− (C) or fjx1−/− (D) control retinas. IMPL formation in fat3+/−;fjx1−/− mice is restricted to the periphery of the ventral retina, a region that is enriched for SOP expressing blue cones (blue). No IMPL develops in dorsal regions (F). Scale bars: 100 μm (A, B); 50 μm (C–F).
We tested whether Fjx1 enhances Fat3 signaling by intercrossing fjx1 and fat3KO lines. Of note, Fat phosphorylation is not an obligatory modification, as Fat-Ds interactions can occur in the absence of fj, and fj mutant flies do not exhibit striking planar polarity defects on their own (Zeidler et al., 2000). Hence, Fj function is best revealed through genetic interactions. Similar to the situation in flies, there are no fat3-like phenotypes in the retina of fjx1 mutant mice (Figure 7C–D). Moreover, fat3;fjx1 DKOs do not show enhanced OMPL or IMPL formation or changes in Bhlhb5-positive AC distribution in the central retina (data not shown). This fits with predictions, as Fjx1 is modelled to act upstream of Fat3 and should therefore have no effect in the absence of Fat3. However, a mild fat3-like phenotype does emerge in fat3+/−;fjx1−/− retinas, as revealed by the presence of a thin, VGAT-positive IMPL (Figure 7C–E). This ectopic layer only forms in the periphery of the ventral retina as identified by the presence of blue cones in the ONL. In contrast, an IMPL cannot be detected in the dorsal periphery of fat3+/−;fjx1−/− mice (Figure 7F). Hence, loss of fjx1 enhances the fat3 heterozygous phenotype, a genetic interaction that is consistent with results from Drosophila, where Fj normally promotes Fat signaling.
Discussion
During development neurons acquire specific morphologies, with one axon and a variable number of dendrites branching in complex, yet stereotyped, patterns. Previous studies have focused on the initial axon specification or final arborization events, with little known about the mechanisms that define dendrite number. Here, we provide new insights into the cellular and molecular events that coordinate dendrite number and orientation during development in vivo and report evidence that this process depends on cell-cell interactions mediated by Fat3. As well as revealing a novel mechanism for the control of dendrite number, these studies provide new insights into the diverse functions of Fat cadherins, which are best known for their role in planar polarity.
Most studies of neuronal morphogenesis have focused on intrinsic mechanisms (Barnes and Polleux, 2009), leading to the identification of pathways that establish apical-basal polarity and cytoplasmic effectors that sculpt the cytoskeleton (Barnes et al., 2007; Witte and Bradke, 2008) or plasma membrane (Guerrier et al., 2009). Similarly, formation of the primary dendrite may depend on the atypical protein kinase C (aPKC), which restricts dendrite number in Purkinje neurons by localizing the Golgi apparatus (Tanabe et al., 2010). However, little is known about how these intracellular events are triggered or controlled in the developing brain. Indeed, recent evidence suggests that there are fundamental differences in how axon specification occurs in vitro, where extracellular signals are presumably uniform, and in vivo, where the specification and orientation of axons depends on environmental cues (Randlett et al., 2011; Zolessi et al., 2006). Our results underscore the influence of the extracellular environment not only for axons, but also for dendrites. While RGC axons are oriented by a laminin-1 based cue in the basal lamina, our data suggest that amacrine dendrites rely on the cell surface receptor Fat3 to respond to signals localized in the IPL. As a result, RGCs reliably extend a single axon basally and towards the optic nerve head, while ACs direct a single primary dendrite towards the IPL, regardless of cell body location. This offers an attractive mechanism for linking the final number of dendrites with the overall organization of the tissue.
An outstanding question is how Fat3 signaling in the dendrite leads to retraction of the trailing process. Most evidence points to direct regulation of the actin cytoskeleton. Indeed Fat3 is closely related to Fat1, which can affect cell morphology in vitro, likely via an EVH domain that binds Ena/Vasp family cytoskeletal regulators as well as the Homer scaffold protein (Moeller et al., 2004; Schreiner et al., 2006). A similar domain is present in Fat3, suggesting that Fat3 might induce neurite retraction by direct regulation of the actin cytoskeleton. Since Ena/Vasp proteins have a well-established role in neurite formation (Kwiatkowski et al., 2007), an attractive idea is that Fat3 transforms the leading process into a dendrite by controlling the local distribution of Ena/Vasp. One effector might be aPKC, based on its role in Purkinje neuron dendrite development (Tanabe et al., 2010). However since many proteins and organelles are influenced by gross changes to the actin cytoskeleton, a major research effort combining biochemistry, cell biology, and mouse models will be needed to determine which are directly linked to Fat3 signaling.
Given the known role of Fat proteins in PCP (Sopko and McNeill, 2009) it is possible that Fat3 ensures development of unipolar morphologies by coupling planar polarity cues and cytoskeletal regulators. Drosophila Fat has a well-established role in planar polarity in the eye, wing and abdomen, and a link to the cytoskeleton is likely, as Fat’s effects on planar cell polarity in the developing wing involve the asymmetric growth of microtubules (Harumoto et al., 2010). In comparison less is known about the function of fat-like. However, recent evidence showed that Fat-like is also a polarity protein that is asymmetrically distributed within ovarian follicle cells where it functions to align actin filaments (Viktorinova et al., 2009). Notably, neither Ds nor members of the core PCP complex are required for follicle cell polarization, suggesting that Fat-like signaling diverges from what has been shown for Fat. A role for Fj has not been investigated in this system.
Our evidence from the vertebrate retina suggests that Fat3 acts more like Fat-like than Fat. Consistent with this, Fat3 is more closely related to Fat-like at the amino acid level, due largely to similarities between the intracellular domains, and both proteins exhibit asymmetric subcellular distributions (Figure 1) (Viktorinova et al., 2009). In contrast, the intracellular domains of Fat3 and Fat4 are highly divergent. Moreover, unlike fat4 mutants, fat3KOs do not exhibit obvious PCP defects in the inner ear (Figure S3), nor are new polarity phenotypes revealed in fat3;fat4 double mutants (Saburi et al., submitted). Instead, fat3 and fat4 appear to have distinct and sometimes opposing functions in many tissues, apart from the vertebral arches where fat3 and fat4 may synergize (Saburi et al., submitted). Nevertheless, both Fat3 and Fat4 appear to be subject to modulation by Fjx1, with loss of fjx1 enhancing both fat3 and fat4 phenotypes (Saburi et al., 2008). While such an interaction is known to be part of the Fat system (Simon et al., 2010), our results provide the first evidence that the Fat-like cadherins may also be modulated by Fj/Fjx1.
If Fat3 is indeed analogous to Fat-like, then a Ds ligand may not be required for AC development. An alternative possibility is that Fat3 mediates homophilic interactions between AC dendrites, consistent with the report that mammalian Fat2 proteins can bind homophilically (Nakayama et al., 2002). This model fits with our observation that RGCs are not required for Fat3 protein localization or for proper development of unipolar morphologies. Whether this is a general mechanism for AC polarization is unclear, though this may offer a molecular explanation for the proposal that AC-AC interactions direct IPL development in the absence of RGCs in zebrafish (Kay et al., 2004). Further, our studies suggest a prominent role for Fat3 in some GABAergic ACs, but Fat3 is broadly expressed and other types are also affected. Indeed ACs are a morphologically and functionally diverse population of neurons, so it is not surprising that not all classes are equally affected by the loss of Fat3. Similarly, studies of axon specification suggest that multiple cues are involved in neuronal morphogenesis in vivo (Barnes and Polleux, 2009). Future studies are needed to decipher how subtypes of ACs respond differently to Fat3 and whether additional Fat molecules or pathways participate.
Fat cadherins are emerging as highly versatile molecules that act through multiple pathways to regulate diverse aspects of cell behavior (Sopko and McNeill, 2009). Our results reveal still more functions for Fat cadherins, establishing independent roles in neuronal morphogenesis and cell migration. While the effects on AC morphology likely involve regulation of the cytoskeleton, how Fat3 signaling in RGCs ultimately cordons ACs in the INL is unclear. However, a non-autonomous function for Fat has been suggested in flies, where Fat may regulate transcription of secreted factors essential for PCP, possibly through the transcriptional repressor Atrophin (Fanto et al., 2003). Similarly, Fat3 might act in RGCs to control production of a chemorepellent that prevents ACs from migrating through the IPL.
The nature of the fat3KO phenotype provides an excellent example of how mutations in one gene can create new cellular layers that are associated with equally discrete synaptic layers, as likely occurred during the evolution of the nervous system. Indeed, even a relatively subtle change in neuronal morphology such as the retention of a trailing process is apparently sufficient to drive development of an entirely new plexiform layer. In addition, certain neurons seem to serve as master regulators of circuit assembly, and our findings support emerging models of retinal development in which maturation of the IPL is guided by ACs (Mumm et al., 2005). Thus, when AC development is disrupted, the overall structure of the retina is as well. Notably, despite the presence of two additional plexiform layers and extra cells in the GCL, the overall organization of the retina is not severely disrupted: the basic layers are present and the new layers are neatly organized. This suggests that the retina is quite plastic in its ability to accommodate changes in the organization and shapes of ACs. Thus while mutations in critical regulators of cell fate and proliferation lead to catastrophic failure of brain development, the fat3 phenotype demonstrates that single gene changes can also generate orderly changes in the structure of the nervous system. This provides a potential explanation for how expanded populations of neurons can be incorporated into pre-existing circuits without compromising animal viability.
Experimental Procedures
Mouse lines
Fat3 mutant mice were generated by homologous recombination in ES cells followed by breeding to Cre or FLPe transgenic mice. Additional details are provided in Supplemental Experimental Procedures. Fat3KO and fat3floxed lines were maintained by backcross to B6129PF1/J mice (Jackson Laboratory). Transgenic mice came from the following sources: ACTB-Cre, Thy1::YFP-H, Z/EG (Jackson Laboratory); ACTB-FLPe (S. Dymecki, Harvard Medical School); Fjx1 (A. Vortkamp, U. Duisburg-Essen); Ptf1a-cre (C. Wright, Vanderbilt U.); Math5Cre (L.Gan, U. Rochester). Experimental breeding strategies are described in Supplemental Experimental Procedures. All mice were maintained at Harvard Medical School or Johns Hopkins U. School of Medicine under the corresponding IACUC-approved guidelines.
Quantification
For migrating ACs, the number of neurites per Ptf1a-cre;Z/EG labeled cell was counted at P1. Trailing process length and cell position relative to the OLM was measured using ImageJ (NIH). Cells in group A had 2–3 neurites but had not yet reached the IPL, while cells in group B had elaborated a dendritic tuft in the IPL. For all cell quantifications: 14 μm cryosections were cut perpendicular to the retina and only fields containing intact, PKCalpha labeled bipolar cells were analyzed. For Brn3, Bhlhb5, Chat, EBF or GFP-positive AC, and GCL nuclei quantification images were collected by confocal microscopy with an optical thickness of 3.6 μm. Three to six sections were analyzed per retina separated by at least 50 μm. To control for eccentricity only cells within 600 μm of the optic nerve head were analyzed. In all cases, cells were counted using the Cell Counter plug-in (ImageJ). For AC morphology, cells were evaluated individually by high magnification epifluorescence microscopy. Only calretinin-positive cells in the INL located within 40 μm of the IPL and extending a dendrite into the IPL were scored. Processes greater than 10 μm in length were called dendrites.
In situ hybridization
In situ hybridizations were completed for fat3 using a probe specific for the mRNA encoded by exon 23 that is deleted in the fat3KO corresponding to nucleotides 12273–12925 of NM_001080814. The fjx1 probe corresponds to nucleotides 931–1563 of NM_010218. A detailed protocol is available online (http://goodrich.med.harvard.edu/resources/protocols/in_situ.pdf)
Antibodies
Polyclonal antibodies against the C-terminal 245 amino acids of Fat3 were prepared using a His-tagged antigen injected into mouse and rabbit (Primm Biotech, Cambridge, MA). Subsequent standard affinity purification was done on rabbit antisera using a GST-C-terminal Fat3 fusion protein produced in E. coli. The anti-Dab1 antibody was a gift from Brian Howell (Upstate Medical U.), and the anti-EBF antibody was a gift from Randall Reed (Johns Hopkins U. School of Medicine). All other antibodies are commercially available as listed in Supplemental Experimental Procedures.
Western Blots and Immunofluorescence
For Western blots, P7 olfactory bulbs were lysed in 20 mM Tris HCl, 2 mM EGTA, 1 mM MgCl2, 150 mM NaCl, and 1% Triton X-100. P5 retinas were lysed in 50 mM Hepes, 2mM EGTA, 2 mM MgCl2, 10% glycerol, and 1% NP40. Buffers contained 1 mM Pefabloc SC PLUS protease inhibitor (Roche). Protein was transferred onto Immobilon-P Membrane (Millipore) in 25 mM Tris, 192 mM glycine, 10–15% methanol and 0–0.05% SDS followed by standard Western blotting using antibodies to Fat3 or β-actin. Immunofluorescence was completed using standard protocols on 4% paraformaldehyde-fixed tissue. Images were collected on Nikon E600 and E800 fluorescent microscopes or Olympus Fluoview and Zeiss LSM510 confocal microscopes.
Supplementary Material
Acknowledgments
This work was funded by NIDCD RO1 DC007195, the Genise Goldenson Research Fund, the Mathers Charitable Foundation, and a Basil O’Connor Starter Scholar Research Award (L.V.G.). M.R.D. was funded by NEI R01 EY021146 and NINDS T32 NS07484. A.K. was supported by the NSF Graduate Research Fellowship Program (DGE 0644491,0946799). We thank D. Corey for sharing equipment, N. Pogue for genotyping assistance, L. Hu for affinity purification of Fat3 antisera, and E. Raviola for assistance with electron microscopy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnes AP, Lilley BN, Pan YA, Plummer LJ, Powell AW, Raines AN, Sanes JR, Polleux F. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129:549–563. doi: 10.1016/j.cell.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Barnes AP, Polleux F. Establishment of axon-dendrite polarity in developing neurons. Annu Rev Neurosci. 2009;32:347–381. doi: 10.1146/annurev.neuro.31.060407.125536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayly R, Axelrod JD. Pointing in the right direction: new developments in the field of planar cell polarity. Nat Rev Genet. 2011 doi: 10.1038/nrg2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittle AL, Repiso A, Casal J, Lawrence PA, Strutt D. Four-jointed modulates growth and planar polarity by reducing the affinity of dachsous for fat. Curr Biol. 2010;20:803–810. doi: 10.1016/j.cub.2010.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillejo-Lopez C, Arias WM, Baumgartner S. The fat-like gene of Drosophila is the true orthologue of vertebrate fat cadherins and is involved in the formation of tubular organs. J Biol Chem. 2004;279:24034–24043. doi: 10.1074/jbc.M313878200. [DOI] [PubMed] [Google Scholar]
- Ciani L, Patel A, Allen ND, ffrench-Constant C. Mice lacking the giant protocadherin mFAT1 exhibit renal slit junction abnormalities and a partially penetrant cyclopia and anophthalmia phenotype. Mol Cell Biol. 2003;23:3575–3582. doi: 10.1128/MCB.23.10.3575-3582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanto M, Clayton L, Meredith J, Hardiman K, Charroux B, Kerridge S, McNeill H. The tumor-suppressor and cell adhesion molecule Fat controls planar polarity via physical interactions with Atrophin, a transcriptional co-repressor. Development. 2003;130:763–774. doi: 10.1242/dev.00304. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Feng L, Xie X, Joshi PS, Yang Z, Shibasaki K, Chow RL, Gan L. Requirement for Bhlhb5 in the specification of amacrine and cone bipolar subtypes in mouse retina. Development. 2006;133:4815–4825. doi: 10.1242/dev.02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Xie ZH, Ding Q, Xie X, Libby RT, Gan L. MATH5 controls the acquisition of multiple retinal cell fates. Mol Brain. 2010;3:36. doi: 10.1186/1756-6606-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst PG, Bruce F, Tian M, Wei W, Elstrott J, Feller MB, Erskine L, Singer JH, Burgess RW. DSCAM and DSCAML1 function in self-avoidance in multiple cell types in the developing mouse retina. Neuron. 2009;64:484–497. doi: 10.1016/j.neuron.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst PG, Koizumi A, Masland RH, Burgess RW. Neurite arborization and mosaic spacing in the mouse retina require DSCAM. Nature. 2008;451:470–474. doi: 10.1038/nature06514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujitani Y, Fujitani S, Luo H, Qiu F, Burlison J, Long Q, Kawaguchi Y, Edlund H, MacDonald RJ, Furukawa T, et al. Ptf1a determines horizontal and amacrine cell fates during mouse retinal development. Development. 2006;133:4439–4450. doi: 10.1242/dev.02598. [DOI] [PubMed] [Google Scholar]
- Gabriel R, Witkovsky P. Cholinergic, but not the rod pathway-related glycinergic (All), amacrine cells contain calretinin in the rat retina. Neurosci Lett. 1998;247:179–182. doi: 10.1016/s0304-3940(98)00323-1. [DOI] [PubMed] [Google Scholar]
- Godinho L, Mumm JS, Williams PR, Schroeter EH, Koerber A, Park SW, Leach SD, Wong RO. Targeting of amacrine cell neurites to appropriate synaptic laminae in the developing zebrafish retina. Development. 2005;132:5069–5079. doi: 10.1242/dev.02075. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- Guerrier S, Coutinho-Budd J, Sassa T, Gresset A, Jordan NV, Chen K, Jin WL, Frost A, Polleux F. The F-BAR domain of srGAP2 induces membrane protrusions required for neuronal migration and morphogenesis. Cell. 2009;138:990–1004. doi: 10.1016/j.cell.2009.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustincich S, Feigenspan A, Wu DK, Koopman LJ, Raviola E. Control of dopamine release in the retina: a transgenic approach to neural networks. Neuron. 1997;18:723–736. doi: 10.1016/s0896-6273(00)80313-x. [DOI] [PubMed] [Google Scholar]
- Harumoto T, Ito M, Shimada Y, Kobayashi TJ, Ueda HR, Lu B, Uemura T. Atypical cadherins Dachsous and Fat control dynamics of noncentrosomal microtubules in planar cell polarity. Dev Cell. 2010;19:389–401. doi: 10.1016/j.devcel.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds JW, Hinds PL. Early development of amacrine cells in the mouse retina: an electron microscopic, serial section analysis. J Comp Neurol. 1978;179:277–300. doi: 10.1002/cne.901790204. [DOI] [PubMed] [Google Scholar]
- Ishikawa HO, Takeuchi H, Haltiwanger RS, Irvine KD. Four-jointed is a Golgi kinase that phosphorylates a subset of cadherin domains. Science. 2008;321:401–404. doi: 10.1126/science.1158159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan YN, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay JN, Roeser T, Mumm JS, Godinho L, Mrejeru A, Wong RO, Baier H. Transient requirement for ganglion cells during assembly of retinal synaptic layers. Development. 2004;131:1331–1342. doi: 10.1242/dev.01040. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski AV, Rubinson DA, Dent EW, Edward van Veen J, Leslie JD, Zhang J, Mebane LM, Philippar U, Pinheiro EM, Burds AA, et al. Ena/VASP Is Required for neuritogenesis in the developing cortex. Neuron. 2007;56:441–455. doi: 10.1016/j.neuron.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Struhl G, Casal J. Planar cell polarity: one or two pathways? Nat Rev Genet. 2007;8:555–563. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Mann LB, Rickman DW, Lim EJ, Chun MH, Grzywacz NM. AII amacrine cells in the distal inner nuclear layer of the mouse retina. J Comp Neurol. 2006;494:651–662. doi: 10.1002/cne.20838. [DOI] [PubMed] [Google Scholar]
- MacNeil MA, Masland RH. Extreme diversity among amacrine cells: implications for function. Neuron. 1998;20:971–982. doi: 10.1016/s0896-6273(00)80478-x. [DOI] [PubMed] [Google Scholar]
- Matakatsu H, Blair SS. Separating the adhesive and signaling functions of the Fat and Dachsous protocadherins. Development. 2006;133:2315–2324. doi: 10.1242/dev.02401. [DOI] [PubMed] [Google Scholar]
- Matsuoka RL, Nguyen-Ba-Charvet KT, Parray A, Badea TC, Chedotal A, Kolodkin AL. Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature. 2011;470:259–263. doi: 10.1038/nature09675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui K, Nakajima D, Ohara O, Nakayama M. Mammalian fat3: a large protein that contains multiple cadherin and EGF-like motifs. Biochem Biophys Res Commun. 2002;290:1260–1266. doi: 10.1006/bbrc.2002.6338. [DOI] [PubMed] [Google Scholar]
- Moeller MJ, Soofi A, Braun GS, Li X, Watzl C, Kriz W, Holzman LB. Protocadherin FAT1 binds Ena/VASP proteins and is necessary for actin dynamics and cell polarization. Embo J. 2004;23:3769–3779. doi: 10.1038/sj.emboj.7600380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm JS, Godinho L, Morgan JL, Oakley DM, Schroeter EH, Wong RO. Laminar circuit formation in the vertebrate retina. Prog Brain Res. 2005;147:155–169. doi: 10.1016/S0079-6123(04)47012-5. [DOI] [PubMed] [Google Scholar]
- Mumm JS, Williams PR, Godinho L, Koerber A, Pittman AJ, Roeser T, Chien CB, Baier H, Wong RO. In vivo imaging reveals dendritic targeting of laminated afferents by zebrafish retinal ganglion cells. Neuron. 2006;52:609–621. doi: 10.1016/j.neuron.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagae S, Tanoue T, Takeichi M. Temporal and spatial expression profiles of the Fat3 protein, a giant cadherin molecule, during mouse development. Dev Dyn. 2007;236:534–543. doi: 10.1002/dvdy.21030. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Nakajima D, Yoshimura R, Endo Y, Ohara O. MEGF1/fat2 proteins containing extraordinarily large extracellular domains are localized to thin parallel fibers of cerebellar granule cells. Mol Cell Neurosci. 2002;20:563–578. doi: 10.1006/mcne.2002.1146. [DOI] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- Parrish JZ, Kim MD, Jan LY, Jan YN. Genome-wide analyses identify transcription factors required for proper morphogenesis of Drosophila sensory neuron dendrites. Genes Dev. 2006;20:820–835. doi: 10.1101/gad.1391006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada C, Puelles L, Genis-Galvez JM, Ramirez G. Two modes of free migration of amacrine cell neuroblasts in the chick retina. Anat Embryol (Berl) 1987;175:281–287. doi: 10.1007/BF00309842. [DOI] [PubMed] [Google Scholar]
- Probst B, Rock R, Gessler M, Vortkamp A, Puschel AW. The rodent Four-jointed ortholog Fjx1 regulates dendrite extension. Dev Biol. 2007;312:461–470. doi: 10.1016/j.ydbio.2007.09.054. [DOI] [PubMed] [Google Scholar]
- Randlett O, Poggi L, Zolessi FR, Harris WA. The oriented emergence of axons from retinal ganglion cells is directed by laminin contact in vivo. Neuron. 2011;70:266–280. doi: 10.1016/j.neuron.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice DS, Curran T. Disabled-1 is expressed in type AII amacrine cells in the mouse retina. J Comp Neurol. 2000;424:327–338. doi: 10.1002/1096-9861(20000821)424:2<327::aid-cne10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet. 2008 doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- Schreiner D, Muller K, Hofer HW. The intracellular domain of the human protocadherin hFat1 interacts with Homer signalling scaffolding proteins. FEBS Lett. 2006;580:5295–5300. doi: 10.1016/j.febslet.2006.08.079. [DOI] [PubMed] [Google Scholar]
- Simon MA. Planar cell polarity in the Drosophila eye is directed by graded Four-jointed and Dachsous expression. Development. 2004;131:6175–6184. doi: 10.1242/dev.01550. [DOI] [PubMed] [Google Scholar]
- Simon MA, Xu A, Ishikawa HO, Irvine KD. Modulation of fat:dachsous binding by the cadherin domain kinase four-jointed. Curr Biol. 2010;20:811–817. doi: 10.1016/j.cub.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopko R, McNeill H. The skinny on Fat: an enormous cadherin that regulates cell adhesion, tissue growth, and planar cell polarity. Curr Opin Cell Biol. 2009;21:717–723. doi: 10.1016/j.ceb.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Kani S, Shimizu T, Bae YK, Abe T, Hibi M. Atypical protein kinase C regulates primary dendrite specification of cerebellar Purkinje cells by localizing Golgi apparatus. J Neurosci. 2010;30:16983–16992. doi: 10.1523/JNEUROSCI.3352-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoue T, Takeichi M. Mammalian Fat1 cadherin regulates actin dynamics and cell-cell contact. J Cell Biol. 2004;165:517–528. doi: 10.1083/jcb.200403006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoue T, Takeichi M. New insights into Fat cadherins. J Cell Sci. 2005;118:2347–2353. doi: 10.1242/jcs.02398. [DOI] [PubMed] [Google Scholar]
- Viktorinova I, Konig T, Schlichting K, Dahmann C. The cadherin Fat2 is required for planar cell polarity in the Drosophila ovary. Development. 2009;136:4123–4132. doi: 10.1242/dev.039099. [DOI] [PubMed] [Google Scholar]
- Voinescu PE, Kay JN, Sanes JR. Birthdays of retinal amacrine cell subtypes are systematically related to their molecular identity and soma position. J Comp Neurol. 2009;517:737–750. doi: 10.1002/cne.22200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SW, Kim BS, Ding K, Wang H, Sun D, Johnson RL, Klein WH, Gan L. Requirement for math5 in the development of retinal ganglion cells. Genes Dev. 2001;15:24–29. doi: 10.1101/gad.855301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte H, Bradke F. The role of the cytoskeleton during neuronal polarization. Curr Opin Neurobiol. 2008;18:479–487. doi: 10.1016/j.conb.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–469. doi: 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]
- Ye B, Zhang Y, Song W, Younger SH, Jan LY, Jan YN. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell. 2007;130:717–729. doi: 10.1016/j.cell.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler MP, Perrimon N, Strutt DI. Multiple roles for four-jointed in planar polarity and limb patterning. Dev Biol. 2000;228:181–196. doi: 10.1006/dbio.2000.9940. [DOI] [PubMed] [Google Scholar]
- Zolessi FR, Poggi L, Wilkinson CJ, Chien CB, Harris WA. Polarization and orientation of retinal ganglion cells in vivo. Neural Dev. 2006;1:2. doi: 10.1186/1749-8104-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.