Abstract
We tested the hypothesis that astrocytic activity modulates neuronal uptake and signaling of leptin in the adult-onset obese agouti viable yellow (Avy) mouse. In the immunohistochemical study, Avy mice were pretreated with the astrocyte metabolic inhibitor fluorocitrate or phosphate-buffered saline (PBS) vehicle intracerebroventricularly (icv) followed 1 h later by Alexa568-leptin. Confocal microscopy showed that fluorocitrate pretreatment reduced astrocytic uptake of Alexa568-leptin 30 min after icv while increasing neuronal uptake in the arcuate nucleus and dorsomedial hypothalamus. Fluorocitrate also induced mild astrogliosis and moderately increased pSTAT3 immunopositive neurons in response to Alexa568-leptin in the dorsomedial hypothalamus. In the Western blotting study, Avy mice were pretreated with either PBS or fluorocitrate, and received PBS or leptin 1 h later followed by determination of pSTAT3 and GFAP expression an additional 30 min afterward. The results show that fluorocitrate induced a mild pSTAT3 activation but attenuated leptin-induced pSTAT3 activation and decreased GFAP expression independently of leptin treatment. We conclude that inhibition of astrocytic activity resulted in enhanced neuronal leptin uptake and signaling. This suggests opposite roles of astrocytes and neurons in leptin’s actions in the Avy mouse with adult-onset obesity.
Keywords: Leptin, Astrocytes, Neuron, Hypothalamus, ObR, pSTAT3, Obesity
Introduction
We tested the hypothesis that astrocytes control the fate and signaling patterns of leptin after intracerebroventricular (icv) injection. Adult-onset obesity is associated with increased astrocytic leptin receptor (ObR) in selective brain regions. This is seen in agouti viable yellow (Avy) mice, particularly in the arcuate nucleus and dorsomedial hypothalamus (Pan et al. 2008), as well as in normal C57 mice with diet-induced obesity (DIO) (Hsuchou et al. 2009). In both models, neuronal ObR expression did not show a parallel increase, but rather decreased or remained unchanged. Microglial ObR was not significantly changed, and the extrahypothalamic region also did not show apparent ObR regulation at the protein level measured by immunohistochemistry (Pan et al., unpublished observations).
Central administration of leptin results in reduction of food intake although obesity is associated with leptin resistance at multiple levels, including saturation of its transport and signaling pathways (Pan and Kastin 2007a). While neurons have been the focus of study for most neuroscientists in the ever expanding leptin field, Avy and DIO mice show a reduction of ObR-positive neurons and a corresponding increase of ObR-positive astrocytes in the hypothalamus (Pan et al. 2008;Hsuchou et al. 2009). These regulatory changes suggest that astrocytes play an essential role in leptin distribution and signaling. Using a metabolic inhibitor against astrocytes in Avy mice, we determined whether astrocytic activity affects the distribution and pSTAT3 signaling induced by leptin after its icv administration into Avy mice with adult-onset obesity.
Materials and Methods
The animal protocol was approved by the Institutional Animal Care and Use Committee. Avy mice were originally obtained from Jackson Laboratory (Bar Harbor, ME) and maintained in the animal care facility for several generations. The mice were housed with a 12/12 h light–dark cycle with free access to water and food (Lab rodent diet 5001). Obesity is evident after 2 months and correlates with increased fat composition (Pan and Kastin 2007b). Obese mice with a solid yellow coat (resulting from the MC1R mutation) were used at body weights of 43.1–48.8 g. We used only male mice to avoid interference from the estrous cycle, and only Avy mice to address the effects of fluorocitrate on astrogliosis since age-matched normal B6 controls do not show hypothalamic astrogliosis (Pan et al. 2008). Carrier-free recombinant murine leptin (R & D Systems, Minneapolis, MN) was conjugated with Alexa568 with a protein labeling kit from Invitrogen (Molecular Probes, Eugene, Oregon) and purified on a BioGel P10 column (Bio-Rad Laboratories, Hercules, CA, USA) as described previously (Tu et al. 2010).
For immunohistochemistry (IHC), 4-month-old male Avy mice were used. After anesthesia, the experimental group received stereotaxically guided injection of fluorocitrate (FC), 1 nmole in 1 μl of phosphate-buffered saline (PBS; n=3/group) through a 30 gauge needle into the left lateral ventricle whereas the control group received PBS only. The coordinates in relation to the bregma were: 1 mm lateral, 0.2 mm posterior, and 2.5 mm below the skull. The injection was driven by a syringe pump connected to PE10 tubing and delivered at a speed of 0.5 μl/min. The needle remained for an additional 2 min before being withdrawn. The mouse was maintained on a 37°C heating pad. One hour later, Alexa568-leptin (1 μg in 1 μl for each mouse) was delivered through the opposite (right) lateral ventricle with the corresponding coordinates and same delivery speed. The preparation of FC solution was described by Paulsen et al. (Paulsen et al. 1987), and the conjugation and purification of Alexa568-leptin was described by us previously (Tu et al. 2010). Thirty minutes after Alexa568-leptin injection, the mice were perfused intracardially with PBS followed by 2% paraformaldehyde (PFA). The brain was post-fixed in 2% PFA at 4°C for 1 h and cryoprotected in sucrose. Coronal sections of 30 μm thickness were permeabilized with 0.3% Triton X-100, blocked with 10% normal donkey serum, and incubated with anti-pSTAT3 (Tyr 705) (1:100; Cell Signaling, Danvers, MA) or anti-GFAP (1:500; Sigma) at 4°C for 48 h. After thorough washes with Tris-buffered saline, the sections were incubated with Alexa488-conjugated secondary antibody. An Olympus FV1000 laser confocal microscope was used to acquire images with ×20 and ×60 oil objectives.
For Western blotting (WB), 7-month-old Avy mice were used (n=2/group) in four study groups: (1) pretreatment with PBS icv in the left lateral ventricle, treatment with PBS icv in the right lateral ventricle 1 h later, and decapitation an additional 30 min later; (2) pretreatment with PBS and treatment with leptin (1 μg in 1 μl); (3) pretreatment with FC (1 nmole in 1 μl) and treatment with PBS; and (4) pretreatment with FC (1 nmole in 1 μl) and treatment with leptin. The hypothalamus was dissected and processed for WB for pSTAT3, GFAP, and the housekeeping gene β-actin as described previously (He et al. 2009; Pan et al. 2009; 2010).
Results
Effects of FC on the Distribution of Alexa568-leptin in the Arcuate Nucleus
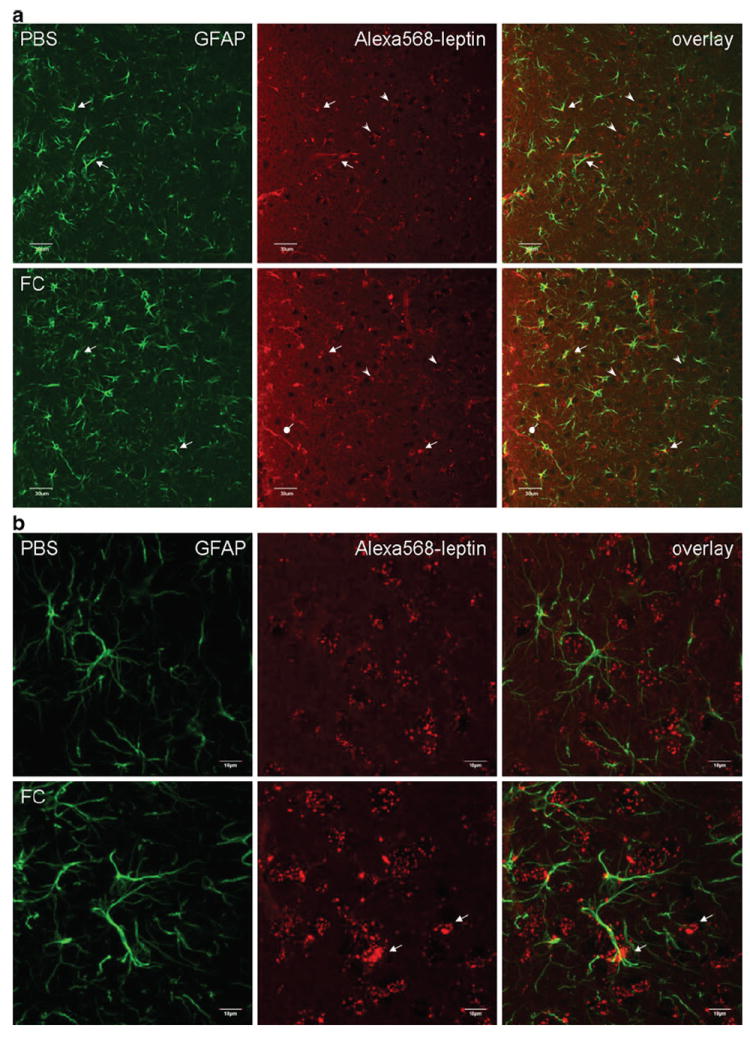
In the control group, Alexa568-leptin was seen both in astrocytes and neurons. In the astrocytes, Alexa568 showed colocalization with GFAP (+) processes. In the neurons, Alexa568 was mainly present in vesicular form whereas fewer neurons showed diffuse cytoplasmic staining. In the FC group, the fluorescent intensity of Alexa568 in astrocytes was reduced but that in neurons was increased. In the dorsal part of the arcuate nucleus, there was an apparent increase of neurons with diffuse cytoplasmic Alexa568-leptin (Fig. 1a, lower panel, yellow arrowheads). In the ventromedial part along the third ventricular recess bordering the median eminence, there was an increase of Alexa568 fluorescence in β1 tanycytes. The vesicular pattern of Alexa568-leptin in neurons was also increased by FC treatment. The astrocytic endocytosis of Alexa568-leptin seen in the PBS treated group was scarcely present in the FC-treated mice (Fig. 1b–c). Furthermore, the GFAP (+) processes showed asymmetry and partial retraction in FC-treated mice, indicating mild astrogliosis. Overall, FC treatment suppressed astrocytic uptake of Alexa568-leptin and induced a shift of its cellular distribution from astrocytes to neurons. An exception, however, was seen in the specialized β1 tanycytes that appeared to accumulate more Alexa568-leptin.
Fig. 1.
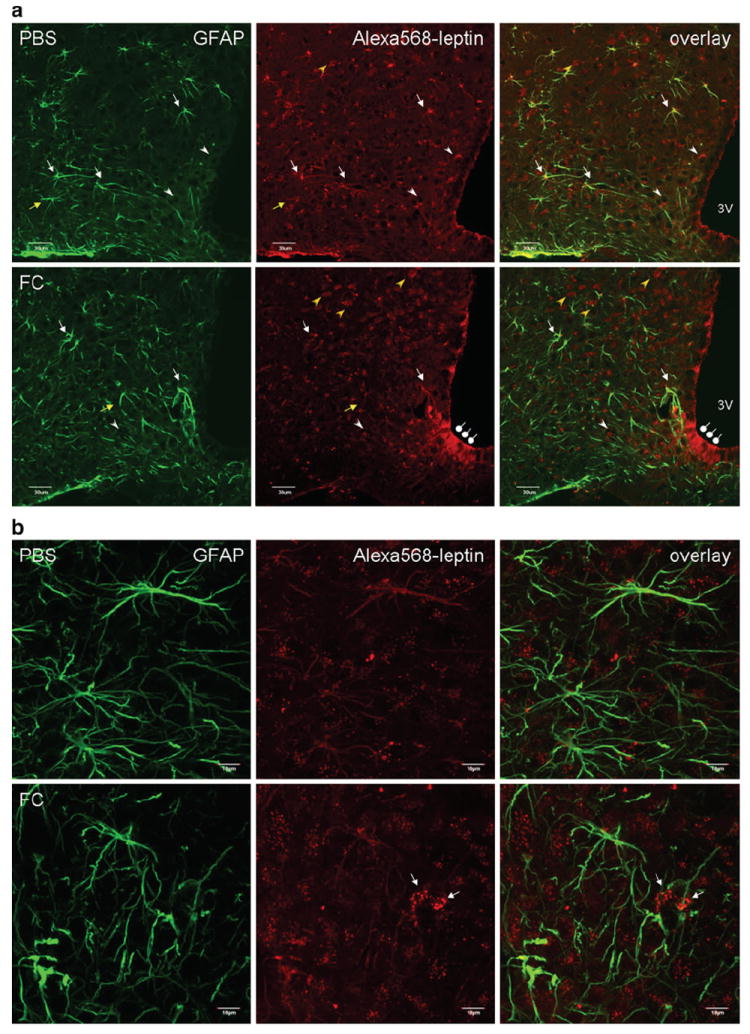
Distribution of Alexa568-leptin (red) in the arcuate nucleus 30 min after icv administration, and partial colocalization with GFAP immunopositive cells (green). FC treatment increased the neuronal distribution of Alexa568-leptin. a In a representative brain section from a mouse receiving the control PBS, Alexa568-leptin is present in GFAP-positive cells (white arrows) and GFAP-negative cells that show neuronal morphology (yellow arrows). The Alexa dye shows either a vesicular distribution (white arrowheads) or a homogeneous pattern in the cytoplasm (yellow arrowheads). In a representative brain section from an FC pretreated mouse, there is a reduction of Alexa568 intensity in the GFAP-positive cells but an increase of Alexa568 (+) neurons that show a diffuse cytoplasmic staining pattern. FC pretreatment also induced subtle gliotic morphology in the astrocytes and increased the permeation of Alexa568-leptin along β1 tanycytes (oval arrows). 3V ventral third ventricle. Scale bars 30 μm. b From higher magnification confocal z-stacking sections in an enlarged area in the arcuate nucleus, Alexa568-leptin is seen both in astrocytes (homogeneous, corresponding to GFAP immmoreactivity) and neurons (vesicular pattern of cytoplasmic fluorescence). In the FC pretreated group, there is a reduction of Alexa568-leptin intensity in GFAP (+) cells, an increase in neurons adjacent to the GFAP (+) cells (arrows), and a ruffled morphology of GFAP (+) processes in contrast to the more extended filaments in the PBS pretreated group. c Overlay images with z-lines showing the presence of Alexa568-leptin in GFAP (+) cells (arrowheads) in the PBS pretreated group and lack of colocalization in the FC pretreated group. Scale bars 10 μm
Effects of FC on the Distribution of Alexa568-leptin and pSTAT3 Activation in the Dorsal Medial Hypothalamus
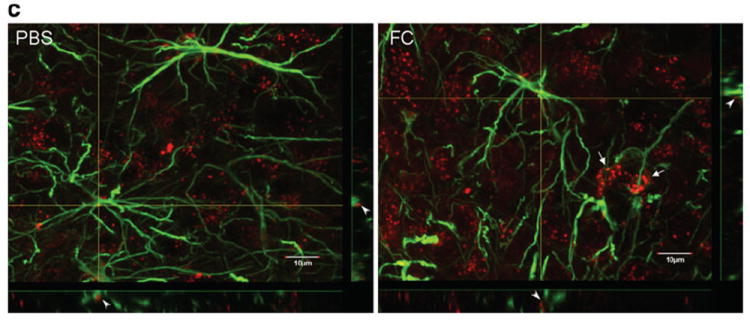
Although astrocytes are abundant in the dorsal medial hypothalamus (DMH), only very few endocytosed a substantial amount of Alexa568-leptin 30 min after its icv delivery. Most of the Alexa568 (+) cells resembled neuronal morphology and showed a punctate distribution of the fluorescence. The α1 and α2 tanycytes also endocytosed Alexa568-leptin. In mice receiving FC pre-treatment, there was an increase in the fluorescent intensity of Alexa568-leptin, mainly in neurons (Fig. 2a). This was better seen in the higher magnification images. FC also induced mild astrogliosis, shown by the higher fluorescent intensity of GFAP and more tortuous and condensed processes (Fig. 2b).
Fig. 2.
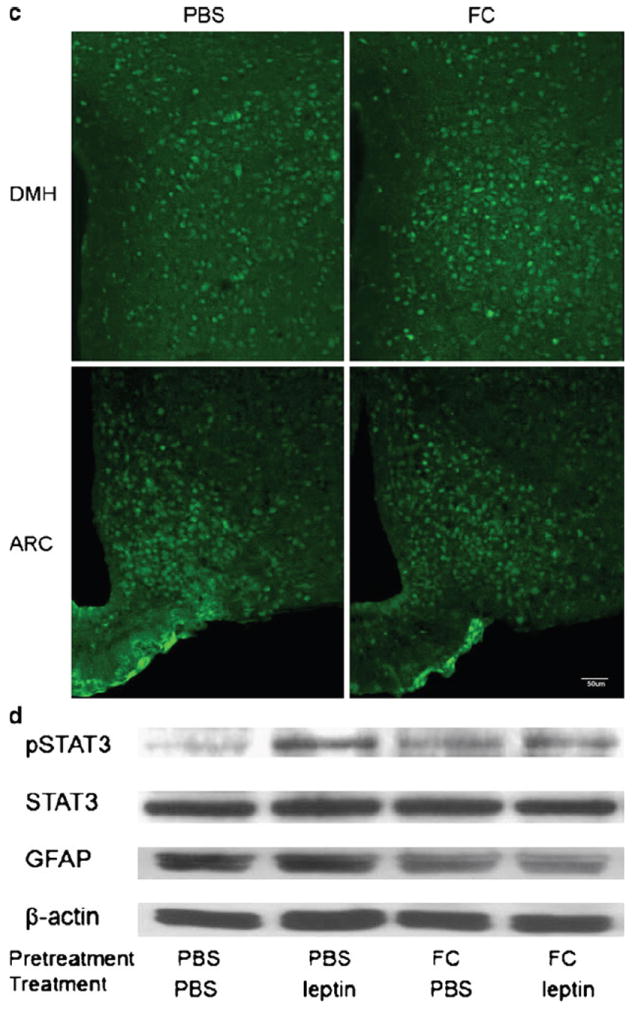
Distribution of Alexa568-leptin (red) in the DMH 30 min after icv administration, its partial colocalization with GFAP immunopositive astrocytic cells (green), and activation of STAT3. FC treatment increased the neuronal distribution of Alexa568-leptin and the number of pSTAT3 immunopositive cells. a In the PBS pretreated group, there are fewer GFAP cells that internalized Alexa568-leptin (arrows) in the DMH than those in the arcuate nucleus. In neurons, the endocytosed Alexa568-leptin is present in perinuclear regions (arrowheads). In the FC pretreated group, tanycytes at the left border of the image are also Alexa568-leptin positive (oval arrow). The third ventricle is at the left border of each image. Scale bars 30 μm. b In higher magnification images with z-stacking, Alexa568-leptin has a vesicular distribution in the cytoplasm of neurons. There is an increase of Alexa568-leptin fluorescent intensity in neurons adjacent to the GFAP astrocytes in the FC pretreatment group (arrows). FC also induced an altered morphology, as the GFAP (+) processes appear more compact. Scale bars 10 μm. c Regional difference and effects of FC on pSTAT3 distribution in mice 30 min after Alexa568-leptin icv. There are more pSTAT3 (+) cells in the arcuate nucleus (ARC) than DMH. FC treatment increased the amount of pSTAT3 (+) cells in the DMH whereas that in the ARC did not show apparent change. Scale bars 50 μm. d pSTAT3 activation by leptin was seen by WB and reduced by FC pretreatment. In mice receiving FC, leptin did not show an additional effect on the level of pSTAT3 activation but GFAP expression was reduced in comparison with the internal control β-actin
In mice pretreated with PBS vehicle before icv injection of Alexa568-leptin, there were more pSTAT3 (+) cells in the arcuate nucleus than in the DMH. Most of the cells showed neuronal morphology, although there were sparse astrocytic processes in the arcuate nucleus adjacent to the median eminence that were also pSTAT3 (+). In mice receiving FC pretreatment, there were increased numbers of pSTAT3 (+) cells, more apparent in the DMH than in the arcuate nucleus (Fig. 2c).
In hypothalamic homogenates from the Avy mice, the basal level of pSTAT3 signal was low though GFAP expression was high. Leptin treatment induced a robust increase of pSTAT3 at 30 min. FC pretreatment also induced a mild increase of pSTAT3 at 1.5 h after pretreatment, but the GFAP signal was reduced. Pretreatment of mice with FC and later leptin caused a reduction of pSTAT3. The reduction of GFAP in the FC+leptin treated mice was similar to that in the mice without leptin treatment (Fig. 2d).
Discussion
Avy mice provide a unique model of adult-onset obesity as a result of a gain-of-function mutation resulting from constitutive activation of the promoter driving the expression of agouti signaling peptide. This leads to ectopic expression of agouti-related protein that serves as a reverse antagonist against melanocortin receptors, resulting in an agouti coat color by MC1R blockade, defective hypothalamic MC3R and MC4R signaling, and eventually an obesity phenotype (Wolff 1965). In Avy mice, we have shown a greater reduction of influx rate of leptin across the blood–brain barrier (BBB) in middle-aged adult mice than older adult mice, mainly explained by changes in blood concentrations of leptin (Pan et al. 2008). Although the BBB transport systems for leptin (Banks et al. 1996) and mahogany peptide (Kastin and Akerstrom 2000;Pan and Kastin 2007b) persist in the Avy mouse, leptin processing within the brain is greatly modulated by astrocytic activity. This suggests that astrocytes provide a second barrier and probably contribute to leptin resistance in obesity. This is strongly supported by the present observation that FC altered the distribution and signaling of Alexa568-leptin. Mild astrogliosis is not seen in lean controls but only in mice with adult-onset obesity, as we have shown in previous studies (Pan et al. 2008;Hsuchou et al. 2009). Therefore, the present study used Avy mice to test whether inhibition of astrocytic activity altered the distribution pattern in neurons while PBS vehicle treated mice served as the negative controls.
FC is an established chemical inhibitor of astrocyte activity without affecting neuronal function (Paulsen et al. 1987;Clarke 1991;Swanson and Graham 1994;Nakanishi et al. 1996). Fluoroacetate disrupts the citric acid (Krebs) cycle by combining with coenzyme A to form fluoroacetyl CoA, which reacts with citrate synthase to produce FC. A metabolite of FC binds very tightly to aconitase, thereby halting the citric acid cycle. The specificity of astrocytic inhibition by low doses of FC is related to biochemical differences between astrocytes and neurons. Dose–response experiments have shown that the dose we used provides selective inhibition of astrocytic metabolism without affecting neuronal function. Use of the icv route to deliver Alexa568-leptin avoids the variable levels of serum leptin, while use of Alexa dye-conjugated leptin allows differentiation from the endogenous leptin that can be transported from the periphery as well as the minute intrathecal production in the pituitary and cerebral cortex detected by RT-PCR (Morash et al. 1999).
The transmigration of Alexa568-leptin from CSF to medial hypothalamus probably involves both volume transmission along the extracellular space (Johanson 1995) and specific transport by tanycytes along the walls of the third ventricle (Blazquez and Rodriguez 2010). The α1 tanycytes adjacent to the DMH and the α2 tanycytes near the arcuate nucleus do not form as tight a junction as do the β1 tanycytes at the junction of the arcuate nucleus and median eminence recess, yet transport along tanycyte processes was seen. It has been clearly established that the arcuate nucleus lies within the BBB and is separated from the median eminence by well-formed junctional structures (Blazquez and Rodriguez 2010). The β1 tanycytes provided a barrier to Alexa-leptin diffusion, but this seems to be weakened in the FC-treated group since stronger fluorescence was seen along the intracellular space as well as within the tanycytes. The increased accumulation of Alexa568-leptin in β1 tanycytes probably represents a decrease of the transport process. The distribution of Alexa568-leptin along the border of the median eminence and arcuate nucleus resembles that of Cdk5/P35, signaling proteins that can be activated by leptin and in turn modulate the actions of leptin (He et al. 2009).
In the Avy mice, more than half the neurons in the arcuate nucleus and some in the DMH showed robust pSTAT3 immunoreactivity. FC treatment further increased the number of pSTAT3 (+) neurons. The results indicate that inhibition of astrocytic metabolic activity enhances neuronal leptin signaling. This is consistent with the finding of increased Alexa568-leptin distribution in neurons in FC-treated mice. Although we did not detect strong ObR immunoreactivity in DMH neurons (Hsuchou et al. 2009), it remains possible that leptin directly activates these neurons despite their low level of ObR expression. Nonetheless, WB analysis of hypothalamic homogenates of the Avy mice showed that the overall pSTAT3 signal was reduced by FC in comparison with the group receiving PBS pretreatment and leptin treatment, although the sample size was too small for statistical analysis. When both groups with FC treatment were compared, leptin did not induce a significant increase of pSTAT3 in the total hypothalamus. This indicates a dilutional effect as the increase of pSTAT3 in IHC was seen mainly in the DMH, an effector region of the hypothalamus.
In summary, Avy mice showed region-specific distribution of icv Alexa568-leptin and pSTAT3 signaling. In these mice we showed for the first time that inhibition of astrocytic activity alters the fate of leptin after icv delivery. The increase of neuronal leptin uptake and enhanced pSTAT3 signaling in the DMH suggest that the astrocytic leptin system provides a counter-regulatory role, opposite to that seen in neuronal leptin signaling. This important principle may be a missing link in leptin resistance.
Acknowledgments
This study was supported by NIH (DK54880 to AJK and NS62291 to WP).
Footnotes
Disclosures/Conflicts of Interest The authors have no conflict of interest and nothing to disclose.
Contributor Information
Weihong Pan, Email: weihong.pan@pbrc.edu, Blood–Brain Barrier Group, Pennington Biomedical Research Center, 6400 Perkins Road, Baton Rouge, LA 70808, USA.
Hung Hsuchou, Blood–Brain Barrier Group, Pennington Biomedical Research Center, 6400 Perkins Road, Baton Rouge, LA 70808, USA.
Changlei Xu, Blood–Brain Barrier Group, Pennington Biomedical Research Center, 6400 Perkins Road, Baton Rouge, LA 70808, USA.
Xiaojun Wu, Blood–Brain Barrier Group, Pennington Biomedical Research Center, 6400 Perkins Road, Baton Rouge, LA 70808, USA.
Sebastien G. Bouret, Children’s Hospital, University of Southern California, Los Angeles, CA, USA
Abba J. Kastin, Blood–Brain Barrier Group, Pennington Biomedical Research Center, 6400 Perkins Road, Baton Rouge, LA 70808, USA
References
- Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- Blazquez JL, Rodriguez EM. The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: the former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides. 2010;31:757–776. doi: 10.1016/j.peptides.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Clarke DD. Fluoroacetate and fluorocitrate: mechanism of action. Neurochem Res. 1991;16:1055–1058. doi: 10.1007/BF00965850. [DOI] [PubMed] [Google Scholar]
- He Y, Kastin AJ, Hsuchou H, Pan W. The Cdk5/p35 kinases modulate leptin-induced STAT3 signaling. J Mol Neurosci. 2009;39:49–58. doi: 10.1007/s12031-008-9174-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuchou H, He Y, Kastin AJ, Tu H, Markadakis EN, Rogers RC, Fossier PB, Pan W. Obesity induces functional astrocytic leptin receptors in hypothalamus. Brain. 2009;132:889–902. doi: 10.1093/brain/awp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE. Ventricles and cerebrospinal fluid. In: Conn PM, editor. Neuroscience in Medicine. Lippincott; Philadelphia: 1995. pp. 171–196. [Google Scholar]
- Kastin AJ, Akerstrom V. Mahogany (1377–1428) enters brain by a saturable transport system. J Pharmacol Exp Ther. 2000;294:633–636. [PubMed] [Google Scholar]
- Morash B, Li A, Murphy PR, Wilkinson M, Ur E. Leptin gene expression in the brain and pituitary gland. Endocrinology. 1999;140:5995–5998. doi: 10.1210/endo.140.12.7288. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Kawachi A, Okada M, Fujiwara M, Yamamoto K. Protective effect of MK-801 on the anoxia-aglycemia induced damage in the fluorocitrate-treated hippocampal slice of the rat. Brain Res. 1996;732:232–236. doi: 10.1016/0006-8993(96)00689-0. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Adipokines and the blood-brain barrier. Peptides. 2007a;28:1317–1330. doi: 10.1016/j.peptides.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Mahogany, blood-brain barrier, and fat mass surge in Avy mice. Intl J Obes. 2007b;31:1030–1032. doi: 10.1038/sj.ijo.0803536. [DOI] [PubMed] [Google Scholar]
- Pan W, Hsuchou H, He Y, Sakharkar A, Cain C, Yu C, Kastin AJ. Astrocyte leptin receptor (ObR) and leptin transport in adult-onset obese mice. Endocrinology. 2008;149:2798–2806. doi: 10.1210/en.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Yu C, Hsuhou H, Khan RS, Kastin AJ. Cerebral microvascular IL15 is a novel mediator of TNF action. J Neurochem. 2009;111:819–827. doi: 10.1111/j.1471-4159.2009.06371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Wu X, Kastin AJ, Zhang Y, Hsuchou H, Halberg F, Chatu F, Khan RS, Robert B, Kastin AJ, Cornelissen-Guillaume GG. Potential protective role of IL15Rα during inflammation. J Mol Neurosci. 2010 doi: 10.1007/s12031-010-9459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen RE, Contestabile A, Villani L, Fonnum F. An in vivo model for studying function of brain tissue temporarily devoid of glial cell metabolism: the use of fluorocitrate. J Neurochem. 1987;48:1377–1385. doi: 10.1111/j.1471-4159.1987.tb05674.x. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Graham SH. Fluorocitrate and fluoroacetate effects on astrocyte metabolism in vitro. Brain Res. 1994;664:94–100. doi: 10.1016/0006-8993(94)91958-5. [DOI] [PubMed] [Google Scholar]
- Tu H, Hsuchou H, Kastin AJ, Wu X, Pan W. Unique leptin trafficking by a tailless receptor. FASEB J. 2010;24:2281–2291. doi: 10.1096/fj.09-143487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff GL. Body composition and coat color correlation in different phenotypes of “viable yellow” mice. Science. 1965;147:1145–1147. doi: 10.1126/science.147.3662.1145. [DOI] [PubMed] [Google Scholar]


