Summary
Pregnant women with substance use disorders have multiple special needs, which might be best managed within a multiprofessional treatment setting involving medical, psychological and social care. Adequate treatment provision remains a challenge for healthcare professionals, who should undergo special training and education when working with this patient population. Careful assessment and screening is necessary to tailor interventions individually to the woman's needs in order to achieve beneficial clinical outcomes for mothers and newborns, whereas the choice of treatment options highly depends on the type of substance of abuse and evidence-based treatment interventions available. Economic considerations have shown that early multiprofessional treatment might yield better clinical outcomes and save healthcare costs over the lifespan.
Substance use disorders (SUDs) during pregnancy represent a challenging health issue for clinicians all over the world. The scope of the problem is wide-ranging; approximately a third of substance-dependent individuals are women of childbearing age [101]. In 2002 and 2003, a nationwide survey in the USA revealed that 4.3% of pregnant women in the age group of 15–44 years had used an illicit drug during the past month, 9.8% used alcohol and 18% were smoking cigarettes [102]. In Europe, there are an estimated number of 60,000 pregnant drug users each year, with half of them being opioid users [1]. The UK Home Office reported in 2003 that there were 200,000–300,000 children (i.e., 2–3% of children under the age of 16 years) living in England and Wales where one or both parents had drug problems [103]; the prevalence of mothers that were substance misusing during pregnancy was not separately indicated. For many countries, no prevalence data were available; however, given these figures from industrialized countries, we might get an idea of how many people are affected by this special constellation that impacts on the lives of all family members involved. Nevertheless, this article focuses only on pregnant substance-misusing women and their newborn children.
The pregnant substance-misusing woman is not only confronted with psychological problems such as feelings of guilt regarding her unborn child and fears regarding her future motherhood, but she usually faces stigmatization by society and often also by medical care-givers when she has entered treatment [2]. In addition, she might struggle with physiological problems due to pregnancy and her SUD, as well as with socioeconomic hardship, including problems such as unemployment, homelessness or legal problems. Frequently, substance-misusing women have relationships with substance-misusing men [3], which might complicate treatment issues, just as somatic and psychiatric comorbidities would, which are highly prevalent in this especially vulnerable patient population. Moreover, many patients are polysubstance users or show regular concomitant consumption of additional legal or illegal substances, which might also have an impact on the mother's health status and pregnancy, as well as on the unborn child.
The effects on the fetus are highly dependent on the type and amount of substances consumed throughout pregnancy, as well as on the nutritional and health status and the wellbeing of the mother. The type and amount of interventions offered and applied to the mother therefore depends on the individual patient, and seems to be provided most effectively in the frame of an individually tailored multidisciplinary treatment approach [4]. However, the particular need for treatment in this patient population is highly evident, and often also very costly, due to frequent pregnancy complications or postnatal morbidity of the newborns. The economic aspect is of high importance to us, because many decisions in health politics are based on cost evaluations of applied interventions and their respective ‘success’.
Unfortunately, there are no universally established treatment standards, neither for pregnant patients with SUDs or for the newborns. Frequently, authors give cautious recommendations for clinical practice or for individual interventions studied, but there are no official detailed treatment guidelines, neither for the pregnant patients nor for the newborns. However, in some specific cases, certain procedures have become ‘state-of-the-art’. This article gives an overview of general crucial aspects to consider in the treatment of pregnant substance users and, wherever available, information on the established state-of-the-art treatment interventions. Our management perspective focuses on the typical pregnant drug users attending addiction clinics in industrialized countries and the healthcare procedures offered to mothers and their newborns, taking into account current research findings. Additionally, we will include economical considerations and critically discuss them in the light of concrete examples. We would like to stress that we cite research results to underpin our statements or to provide support for our perspective without any claim to completeness.
Nonjudgmental, respectful attitude of caregivers & comprehensive view of the patient
Pregnant women with SUDs are often reluctant to seek help due to their fears of negative judgment or hostile reactions from caregivers [104]. A nonjudgmental, respectful attitude from caregivers is therefore essential to create an environment that allows a trustful relationship to be built with the patient. Morton and Konrad already pointed out the importance of healthcare professionals acquiring knowledge, skills and attitudes that promote the development of trustful relationships with addicted mothers that represent the basis for beneficial outcomes [5]. Moreover, it is important to recognize that pregnancy might be a strong motivation for patients to enter and stay in treatment [104] and an opportunity for professionals to foster this approach rather than adopting a punitive attitude. Thus, it is indispensable to provide a special training/education for healthcare professionals working with pregnant women with SUDs in regard to ethical questions that might arise during the course of treatment.
Treatment for pregnant substance users should be voluntary except in life-threatening situations for the mother or child, where treatment might become compulsory. Furthermore, it is important to comprehensively address the patients' needs in order to offer efficient treatment. Frequently, substance-misusing women have somatic and psychiatric comorbidities and the treatment of these disorders has to be integrated into the patient's therapy. Moreover, many patients have a poly-SUD that needs to be taken into account. Table 1 gives an overview of the most frequent comorbid conditions found in recent studies of pregnant women with SUDs. The cited studies were selected by the authors after a literature search on ‘PubMed’ to show the high comorbidity rates of pregnant women with SUDs in industrialized countries; studies were selected only if they were original research studies, the sample size was larger than 50 and the study had been conducted within the past 10 years. Moreover, we tried to incorporate prevalence data from Europe, Australia and North America, and to vary the primary substance of use.
Table 1.
Frequent comorbidities among pregnant patients with substance use disorders.
| Comorbidity | Study (year), country | Patient population | Prevalence (%) | Ref. |
|---|---|---|---|---|
|
| ||||
| Hepatitis C | Wachman et al. (2010), USA | 276 opioid-dependent pregnant women in maintenance treatment (buprenorphine/methadone) | 65 | [51] |
|
|
||||
| Dryden et al. (2009), UK | 450 opioid-dependent pregnant women in methadone treatment | 50.4 | [52] | |
|
|
||||
| Vucinovic et al. (2008), Croatia | 85 opioid-dependent pregnant women (heroin/methadone) | 49 | [53] | |
|
|
||||
| Kashiwagi et al. (2005), Switzerland | 84 opioid-dependent pregnant women in methadone treatment (n = 77 screened) | 54.5 | [54] | |
|
| ||||
| HIV | Ziegler et al. (2000), Germany | 109 opiate-dependent pregnant women (not in maintenance treatment/in methadone treatment) | 22.9/23.5 | [55] |
|
|
||||
| Rohrmeister et al. (2001), Austria | 88 opioid-dependent women in maintenance treatment | 5.7 | [56] | |
|
|
||||
| Kashiwagi et al. (2005), Switzerland | 84 opioid-dependent women in methadone treatment | 20.2 | [54] | |
|
| ||||
| Hepatitis B | Almario et al. (2009), USA | 258 opiate-dependent pregnant women in methadone treatment | 30.3 | [57] |
|
|
||||
| Kelly et al. (2000), Australia | 96 substance-dependent (methadone, heroin, amphetamines or other substances) women delivering in a hospital in Melbourne | 3.0 | [58] | |
|
| ||||
| Sexually transmitted diseases | Cavanaugh et al. (2010), USA | 76 pregnant heroin/cocaine-dependent women tested for chlamydia, gonorrhea, trichomoniasis, syphilis, genital warts and herpes | 18.4 | [59] |
|
|
||||
| Fiocchi and Kingree (2001), USA | 135 pregnant crack users enrolled in a comprehensive residential treatment program | Lifetime: 50 | [60] | |
|
| ||||
| Psychiatric comorbidity/psychiatric medications | Wachman et al. (2010), USA | 276 opioid-dependent women in maintenance treatment (buprenorphine/methadone); data on psychiatric diagnoses and medications were obtained from retrospective chart reviews of patients treated at Boston Medical Center (MA, USA) | Depression: 42Bipolar disorder: 17Anxiety disorder: 42SSRI: 20Benzodiazepine: 23 | [51] |
|
|
||||
| Dryden et al. (2009), UK | 450 opioid-dependent pregnant women in methadone treatment; data on depression and psychiatric medications were obtained from retrospective chart reviews of patients treated at Princess Royal Maternity Hospital (UK) | History of depression: 18.2SSRI: 7.1Tricyclic/related: 3.8Other antidepressant: 1.3 | [52] | |
|
|
||||
| Kissin et al. (2001), USA | 240 pregnant opioid/cocaine-dependent women; data on psychiatric diagnoses were obtained by using the Structured Clinical Interview for DSM-III-R [61] | Organic mood syndrome: 31.1Social phobia: 8.1Simple phobia: 7.1OCD: 6.2Organic anxiety disorder: 6.2Major depression: 3.3Alcohol dependence: 8.1Sedative dependence: 4.3Marijuana dependence: 11.4 | [62] | |
|
|
||||
| Goel et al. (2011), UK | 186 pregnant women with illicit substance use; data on alcohol abuse were collected retrospectively from hospital databases | Alcohol abuse: 48.3 | [63] | |
|
|
||||
| Almario et al. (2009), USA | 258 opiate-dependent pregnant women in methadone treatment; psychiatric data were obtained from retrospective chart reviews at the Family Center of Thomas Jefferson University Hospital (PA, USA) | SSRI: 12.0Antipsychotic: 5.8 | [57] | |
|
|
||||
| Benningfeld et al. (2010), USA | 174 opioid-dependent pregnant women in pharmacological maintenance therapy; assessment of psychiatric symptoms with Mini International Neuropsychiatric Interview [64] | Mood symptoms: 48.6Anxiety symptoms: 40.0Suicidal thinking: 12.6 | [65] | |
|
| ||||
| Polysubstance abuse (nicotine excluded) | Mayet et al. (2008), UK | 126 pregnant substance-using women attending a specialist perinatal addictions outreach service | 48.7 | [66] |
|
|
||||
| Dryden et al. (2009), UK | 450 opioid-dependent pregnant women in methadone treatment | 80 | [52] | |
|
|
||||
| Goel et al. (2011), UK | 186 pregnant women with illicit substance use | 61.3 | [63] | |
|
|
||||
| Liu et al. (2010), Australia | 228 opioid-dependent pregnant women in methadone treatment | 60 | [67] | |
|
| ||||
| Smoking (nicotine) | Little et al. (2003), USA | 55 pregnant women with substance abuse (cocaine, heroin or alcohol) in residential substance abuse treatment | Current smoker: 94.7 | [68] |
|
|
||||
| McCarthy et al. (2005), USA | 81 opioid-dependent pregnant women in methadone treatment | Current smoker: 77 | [69] | |
|
|
||||
| Jones et al. (2010), USA | 131 opioid-dependent pregnant women in maintenance treatment (buprenorphine/methadone) | Current smoker: 97 | [40] | |
|
|
||||
| Ho et al. (2001), Canada | 132 pregnant MDMA users contacting a local helpline | Current smoker: 53.8 | [70] | |
MDMA: 3,4-methylenedioxy-N-methylamphetamine; OCD: Obsessive–compulsive disorder; SSRI: Selective serotonin-reuptake inhibitor.
Additionally, the patient's living situation and environment play an important role and potentially have a considerable impact on the course of treatment. Most of the patients have a low educational level and are unemployed. Schempf and Strobino reported that 28.8% of the cocaine-using pregnant women and 25.5% of the opiate-using patients in their US sample had not completed high school, and only 11.9 and 7.3%, respectively, were employed [6]. Approximately a third of their pregnant patients relied on public housing and a fifth on subsidized housing, and approximately 60% of the cocaine-using women and 45% of the opiate-using women indicated that they had enough money for necessities ‘less than half of the time’. Additionally, 35 and 22% of the cocaine- and opiate-using women had no relationship with their baby's father, and almost 40 and 30%, respectively, perceived ‘hardly ever’ receiving family support during the course of their pregnancy. Bakstad et al. outlined a similar sociodemographic profile in her Norwegian sample of 41 opioid-dependent women enrolled in maintenance therapy with methadone or buprenorphine. Patients had completed on average 10.6 years of education, over 80% were unemployed and more than a third were single mothers [7]. In a French sample of 159 opioid-dependent pregnant women in buprenorphine therapy, only 3% had a high school diploma or higher education, and 79% were unemployed; 60% were dependent on government help, and 23% of the women had a history of imprisonment [8]. More than a third of the French women had a drug-addicted partner, whereas approximately 12% had a partner who was in prison during the course of the pregnancy; approximately 45% of the women indicated that the current pregnancy had not been planned. These examples demonstrate quite clearly that the typical pregnant patient with a SUD has multiple needs at the same time, and that all need to be addressed and taken into account. Therefore, a comprehensive treatment approach seems most suitable for meeting this requirement.
Multiprofessional, comprehensive treatment
Healthcare professionals from various disciplines need to work together in order to provide the required clinical care for the pregnant patient and her child. Medical and psychosocial interventions that are coordinated and individually tailored to each patient may ideally be delivered by a team of medical doctors, psychologists, social workers and nurses working closely together; moreover, linkages to other institutions/parties that might get involved, such as legal services, obstetrical/gynecological and pediatric care, should be established and made easily available. The Addiction Clinic at the Medical University of Vienna (Austria) has established such a multiprofessional treatment model, and has offered comprehensive care to substance-dependent pregnant women since 1994. Figure 1 represents the clinic with its affiliations that provide medical and psychosocial care throughout the course of pregnancy and the postpartum period for substance-dependent women.
Figure 1. Addiction clinic at the Medical University of Vienna, Austria.
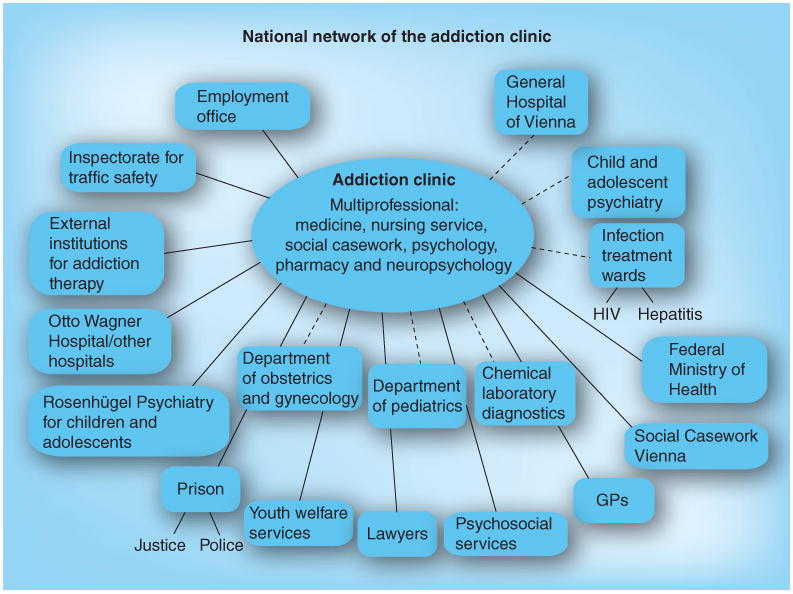
The Otto Wagner Hospital and Rosenhügel Psychiatry are cooperating hospitals in the city of Vienna. Solid lines represent external collaborations, while dashed lines represent in-house collaborations. GP: General practitioner.
To ensure coordinated treatment, the members of the healthcare team have to participate in case conferences regularly; these meetings also have the advantage that difficult decisions might be discussed and taken by the whole team. Nonetheless, supervision for the staff has to be provided so that the caregivers can maintain their own mental health within this particularly stressful and challenging working environment.
Another important aspect is the structure of clinical procedures to guarantee a certain work flow. Although the interventions are individually tailored, standard operating procedures are recommendable to render the therapy more efficient. Treatment protocols should be available for all team members at any time, and the caregivers should get sufficient training before they start working in the team.
Clinical assessment procedures
In order to obtain a comprehensive picture of each patient to plan the required treatment interventions, assessment procedures have to be coordinated carefully to address all potential problem areas for the woman:
Medical history/assessment should include laboratory examinations (blood count, including screening for infectious diseases such as HIV or hepatitis) and gynecological examinations (including screening for sexually transmitted diseases and full obstetrical history);
Psychiatric/psychological assessment of all current and past comorbidities, including previous psychiatric/psychological treatment interventions;
The application of structured clinical interviews and the use of standardized questionnaires such as the Addiction Severity Index [9] are recommendable;
Substance use assessment, including all current and past SUDs (legal and illegal substances and prescription drug abuse) and respective treatment interventions;
Importantly, supervised urine toxicologies and alcohol breath tests are highly recommended in order to obtain an objective measurement of the woman's current substance intake;
Assessment of social status, current living situation and environment (including significant others), vocational situation and legal status.
After the assessment procedures that should take place as early as possible in the course of pregnancy and be completed as quickly as possible, the required interventions for the patient should be discussed and planned by the whole caregiver team. The time aspect is of particular importance in pregnant patients because significant developments already take place in the first trimester of pregnancy and some interventions need to be implemented immediately (e.g., a detoxification treatment of teratogenic substances) [10].
Individually tailored interventions
A range of therapeutic interventions is available and adequate to treat pregnant women with a SUD; the decision on which interventions will be used depends on various factors [4], such as:
The type of illegal or legal substances consumed and their teratogenic potential (main substance and concomitant consumption of further substances);
The time (week) of pregnancy;
The maternal health status, including somatic and psychiatric comorbidities;
The maternal material (income and housing) and social resources (supporting family members, social network and significant others that might have to be taken into account in the frame of treatment);
The particular additional problems (e.g., legal issues and already children in foster care, among others);
The treatment that the patient has already received, including current medications that are effective, as well as the patient's preferences.
Regarding the types of substances used, we summarize in the following sections common treatment interventions in general for mother and child for a range of substances.
Alcohol
Alcohol abuse/dependence during pregnancy is associated with poor neonatal outcomes due to the high teratogenic potential of alcohol, whose detrimental effects might even be worsened in cases of a suboptimal maternal nutritional status [11]. It is thus essential to explore patients thoroughly and to screen/test them regularly for alcohol use throughout the course of pregnancy, even if alcohol is not the primary substance of abuse. Children with prenatal alcohol exposure are at risk for developing a ‘fetal alcohol spectrum disorder’ [105]; this term covers a range of adverse outcomes caused by alcohol consumption during pregnancy, with ‘fetal alcohol syndrome’ (FAS) being the most severe. FAS is a permanent birth defect syndrome characterized by growth deficiency, a unique cluster of facial anomalies and CNS abnormalities [105]. Although it is preventable, it remains the main cause of developmental disabilities in developed countries [12]. Its prevalence is estimated to range between 0.1 and 0.3% of live births [13]. In high-risk populations, prevalences as high as 1.5% have been reported [14]. The physical, cognitive and behavioral deficits observed among individuals with prenatal alcohol exposure may vary considerably.
Due to the high teratogenic potential of alcohol, detection of an alcohol use disorder is crucial; frequently, medical doctors do not educate their pregnant patients regarding the harmful consequences of drinking during pregnancy or do not examine the women thoroughly, and thus the alcohol use disorder remains unrecognized. Tools that have proven effective [15] in detecting at-risk women are the Alcohol Use Disorders Identification Test (AUDIT [16]) and the ‘Cut–Annoyed–Guilty–Eye’ (CAGE [17]) screening test. In the case of an alcohol use disorder, immediate action is indispensable to prevent the highly detrimental effects on the unborn child. An inpatient detoxification treatment (e.g., with supportive medications such as benzodiazepines) seems most appropriate to reach the goal of abstinence as safely and quickly as possible, and has become state-of-the-art [18]. After successful detoxification, close monitoring and intense outpatient care with regular supportive counseling is important to prevent relapses or to intervene as quickly as possible in case of relapses. A trustful relationship with the caregivers is always the most reliable tool in outpatient treatment because it provides the basis to discuss relapses and situations in which the patient is ‘at risk’, among others, despite the common fear of pregnant women regarding losing their newborns if they relapse and cannot maintain alcohol abstinence [19]. The woman should be encouraged and supported in case of relapses, and small goals achieved should be reinforced to enhance the patient's motivation to continue her treatment. The treatment process should be accompanied by psychoeducation of the patient; it is important that the pregnant woman understands why it is crucial to achieve and maintain total abstinence from alcohol and all the necessary interventions that she undergoes, and at the same time that her treatment is voluntary but absolutely necessary in order to deliver a healthy child. Moreover, the patient should have the possibility of postpartal addiction treatment/follow-up visits to maintain her stability.
Nicotine
Although Lamy and Thibaut estimate smoking rates in pregnant women of up to 20–30% worldwide [20], it is unlikely that a pregnant woman attends a specialized substance abuse treatment institution for smoking cessation. We usually encounter pregnant smokers that are dependent on other drugs in addiction centers; however, in any case, the patient should be informed and educated about the health risks of smoking for her and her unborn child, such as spontaneous abortion, low birth weight, premature delivery, sudden infant death syndrome and learning and behavioral problems in the offspring [21]. Moreover, available treatment options for smoking reduction/cessation should be offered to the pregnant woman, so that she might select an intervention. Since it has been shown that daily maternal nicotine intake has a dose–severity association with neonatal abstinence syndrome (NAS), as well as with reduced birth weight in newborns who have been intrauterinally exposed to opioids [22,23], we can conclude that the children would also benefit from a reduction of daily cigarette consumption (e.g., if a heavily smoking women with an intake of more than 20 cigarettes/day decreases her cigarette intake to less than 10 cigarettes/day). Due to its nonproven safety and efficacy, it remains a controversial issue as to whether pregnant patients should receive a nicotine-replacement therapy [24]; however, it is only recommendable if the woman is a daily smoker needing a certain level of nicotine. Rayburn and Bogenschutz suggest monitoring levels of nicotine for the choice of the lowest effective dose and the best route of nicotine delivery [25]. The use of the antidepressant bupropion for smoking cessation during pregnancy has been linked to an elevated risk of congenital heart defects for the newborn [26], while sufficient data on its safety and efficacy for pregnant patients are still lacking [27]. Thus, nonpharmacologic interventions such as counseling or behavioral therapeutic strategies should be preferred; for example, Heil et al. demonstrated that a voucher-based reinforcement intervention (contingency management) was effective in promoting smoking reduction in pregnant smokers, which had highly beneficial effects on the development of the children – ultrasound examinations showed significantly greater growth regarding estimated fetal weight, femur length and abdominal circumference in the group receiving contingency management compared with the group without this intervention [28].
Cannabis/marijuana
Cannabis is the most frequently abused illegal drug during pregnancy, and in many cases its intake takes place within polysubstance abuse/dependence [4]. Its consumption might be associated with mild withdrawal symptoms in the newborn and has been linked to certain cognitive deficits in school-aged children, although no congenital malformations or severe impairments have been shown to be connected with prenatal cannabis exposure [29]. No pharmacological therapeutic options are available for cannabis use disorders during pregnancy. Thus, nonpharmacological interventions have to be implemented to reduce cannabis consumption during pregnancy. Behavioral strategies to reduce smoking could, for example, target nicotine and cannabis consumption at the same time because they focus on the same behavior.
Sedatives/benzodiazepines
Benzodiazepine use during pregnancy could lead to adverse neonatal outcomes, but research results are often controversial, particularly with regard to congenital malformations; however, no benzodiazepines have ever been tested directly with pregnant patients – thus, we do not know if the benefits outweigh the risks for taking these drugs [30]. Iqbal et al. further conclude in their review on benzodiazepine consumption during pregnancy and the postpartal period that benzodiazepines are not absolutely contraindicated during pregnancy, but their intake in the first trimester of pregnancy should be avoided since this is the critical period for organogenesis [30]. We suggest a gradual reduction of daily benzodiazepine intake until total abstinence is achieved, which should represent the goal during pregnancy for safety reasons.
Stimulants: amphetamines/cocaine
Chronic stimulant use during pregnancy has been linked to poor pregnancy outcomes such as intrauterine growth retardation, prematurity, congenital malformations and even mild withdrawal symptoms and CNS stress symptoms [31–34]. Bandstra et al. also reported subtle deficits in neurobehavioral, cognitive and language function in preschool children after in utero cocaine exposure [35]. Abstinence from these substances represents the ideal goal in antiaddictive therapy; since somatic dependence on stimulants is marginal, intense psychological care is recommendable to encourage and stabilize the patient during time of withdrawal. Since no pharmacological intervention has proven safety and efficacy during pregnancy, only nonpharmacological interventions should be recommended. Jones et al. recently showed that reinforcement-based treatment improved treatment retention of the mothers and reduced postnatal hospital stays of the neonates, although it did not reduce maternal illicit drug consumption during pregnancy [36]. Thus, reinforcement-based interventions represent a promising adjunctive treatment approach for promoting abstinence in cocaine-dependent pregnant patients.
Opioids
Opioid abuse/dependence during pregnancy is linked to a number of pregnancy complications and adverse neonatal outcomes such as intrauterine growth retardation, premature birth, low birth weight and increased risk for sudden infant death syndrome [29]. The development of withdrawal symptoms is characteristic of prenatal opioid exposure and comprises the typical symptom complex known as NAS, affecting 55–94% of newborns [37]. Its clinical presentation varies individually and might include neurologic excitability, gastrointestinal dysfunction or autonomic signs, as well as varying in its severity, which might render pharmacological treatment – ideally with opioids – necessary [37]. However, nonpharmacologic interventions such as rooming-in with the mothers or supportive care measures have been shown to alleviate neonatal withdrawal [38,39]. Specially trained staff for postnatal care are required for the challenging management of NAS.
For maternal opioid dependence during pregnancy, pharmacologic maintenance therapy represents the current state-of-the-art treatment. Most experiences and research data are available for methadone, whose safety and efficacy is recognized by the scientific community; additionally, buprenorphine has been shown to be a valuable alternative to methadone because it is associated with less severe neonatal withdrawal, although it is linked to a lower maternal treatment retention rate [40,41]. Thus, buprenorphine can only be recommended for pregnant opioid-dependent women who are responding well to this partial μ-agonist, whereas for many severely addicted, polyaddicted patients, methadone might still be the primary choice of medication. Close monitoring of the patients, as well as accompanying psychosocial care, should be provided throughout the course of pregnancy and the postpartum period for all patients.
Pharmacological treatment interventions
Due to the potential for teratogenesis and the risk for pregnancy complications and further adverse consequences for the fetus or the newborn, a great deal of caution, as well as weighing the risks against the benefits, is indicated when deciding on a pharmacological intervention. This is of particular importance regarding psychiatric comorbidities, where psychological interventions might be highly efficient as well; however, there are cases where counseling/psychotherapeutic approaches are not sufficient, and subsequently, a pharmacological intervention is indicated and important [42]. There might even be cases where pharmacotherapy is the only possible intervention that is efficient and necessary to protect the unborn child (e.g., antiretroviral therapy/highly active antiretroviral therapy for HIV-infected patients).
Nonpharmacological treatment interventions
The amount and type of nonpharmacological interventions might vary considerably; however, there are some specific issues for pregnant women with SUDs that should at least be addressed in the frame of counseling or a psychoeducative approach:
Nutrition during pregnancy and the lactation period;
Intake of vitamin/mineral supplements, over-the-counter medications and prescribed drugs (including possible interaction effects of their respective drug of abuse) during pregnancy and the lactation period;
Concomitant consumption of nonprescribed drugs and possible effects on the fetus/newborn;
Breastfeeding (including contraindications);
Future family planning and contraception;
Individual questions/topics.
Obstetrical/gynecological care & postpartal care
Regular prepartal visits and gynecological care are essential for women having a high-risk pregnancy.
For all mothers with SUDs, postpartal care and support in the early stages of motherhood are recommendable. Some women might not be allowed to keep custody of their children because they are too unstable and the newborns are, for example, kept in foster care; for these women, it is especially important to retain them in treatment because they might have an even higher risk of relapse after the loss of their child.
Economic considerations
Careful choice of maternal and neonatal treatment interventions are of particular importance in this patient group because adequate interventions might help save healthcare costs, which might become extremely high in cases of complications or poor neonatal outcome. Since there is a severe lack of detailed health cost analyses in this domain, we outline some concrete results to give an impression of the dimension of costs and to encourage further evaluations.
Svikis et al. already showed in the late 1990s in the USA that multiprofessional intensive treatment for pregnant women with SUDs led to better clinical outcomes at delivery compared with those of women with SUDs who did not receive drug abuse treatment [43]. The treatment group had less maternal substance use as well as superior neonatal outcome parameters such as higher gestational age, increased birth weight and better Apgar scores. Moreover, newborns of women in treatment were less likely to require neonatal intensive care unit services, and those that did had shorter stays. Total costs for treatment were examined, and multiprofessional treatment resulted in mean net savings for patients in the treatment group of US$4644 per mother/infant pair.
Coyle et al. calculated hospital costs for opioid-exposed newborns comparing a group of infants whose NAS was treated with a diluted tincture of opium (mean hospital stay after birth: 79 days; mean costs: $69,200) with a group treated with diluted tincture of opium and phenobarbital (mean hospital stay: 38 days; mean costs: $33,344), and found that mean costs differed by $35,856 between the two groups [44]. Thus, the adequate choice of treatment may reduce costs substantially; however, the current NAS treatment standard for in utero opioid-exposed neonates are opiates, with morphine preparations most commonly used [45].
Daley et al. compared outcome parameters of newborns from women in five different settings of substance abuse treatment (residential, outpatient, residential/outpatient, pharmacological and detoxification) and detected a linear relationship between birth weight and amount of maternal treatment received; women who had received more specialized treatment (e.g., residential treatment) had heavier infants than those receiving less specialized treatment (e.g., detoxification) [46]. Treatment costs for mothers and infants were compared and it was shown that the outpatient treatment approach was most cost–effective, yielding an increase in birth weight of 139 g (compared with the detoxification group) for an investment of only $1788 in additional healthcare and treatment costs. Moreover, the authors showed that improved nutritional status and less drug use were linked to higher birth weight factors, which are more easily manageable in more intense treatment. Generally, low birth weight is found in premature infants, with prematurity rates in the general population ranging between 7.0% of live births in the UK (2006) [106] and 12.7% in the USA (2007) [47], and entailing enormous costs. Mangham et al. clearly demonstrated an inverse relationship between gestational age at birth and healthcare costs arising not only during peri- and post-natal care, but throughout childhood and adolescence due to preterm-associated morbidity [48]. The authors compared costs for preterm children until the age of 18 years in England. The evaluation clearly showed that the earlier the infants were born, the higher the healthcare expenditures were (i.e., €90,734 for preterm infants [born prior to 37 weeks of gestation], €245,692 for very preterm infants [born prior to 33 weeks of gestation] and €466,250 for extremely preterm infants [born prior to 28 weeks of gestation]) when taking into account costs of delivery and neonatal care and inpatient and outpatient medical care, as well as community health and social care until 18 years of age.
In a multicenter, double-blind, double-dummy clinical trial comparing the safety and efficacy of methadone versus buprenorphine therapy in opioid-dependent pregnant women, by applying an intensive outpatient care approach with daily maternal visits, Jones et al. found preterm birth rates of 19% (methadone) versus 7% (buprenorphine), with corresponding mean hospital stays of 17.5 versus 10.0 days [40]. These rates are in line with previous research findings showing higher prematurity for children of women with SUDs, although the result for the buprenorphine group is still under the average preterm rate of the USA or Austria, where Kiss et al. have recently shown that antenatal screen-and-treat programs might reduce the preterm rate (from 12.1 to 8.2%), which could subsequently reduce costs [49].
A current estimation of postnatal hospital costs for neonates born at the General Hospital of Vienna with an International Classification of Diseases (ICD)-10 diagnosis P96.1 ‘neonatal withdrawal symptoms from maternal use of drugs of addiction’ showed that their mean costs were €7700 if they had a hospitalization duration of between 7 and 22 days (without further complications); if the duration of hospital stay was shorter than 7 days and the newborn was roomed-in with the mother, only maternal costs and no extra costs for the infants would be charged, whereas hospitalization durations exceeding 22 days could entail considerable cost increases (e.g., ˜€11,500 for 27 days) [Fischer G, Metz V, Unpublished Data]. However, these considerations only concern postnatal care. Unfortunately, no current cost estimations for short- and long-term costs for intrauterinally exposed children are available, but Brown et al. already estimated the necessary lifetime treatment costs of in utero substance-exposed infants (alcohol, tobacco and other drugs) in the USA of approximately $750,000–1,400,000 [50], giving an idea of the scope of the economic burden. Therefore, it is even more important to provide adequate maternal and neonatal treatment for this special patient population.
Conclusion & future perspective
Specialized care is indispensable for pregnant women with SUDs and their offspring. A multidisciplinary outpatient treatment setting with the flexibility of tailoring interventions individually has been shown to be effective for coping with the multiple medical and psychosocial needs of these women throughout pregnancy and in the postpartum period. Stabilization of the patient and the goal of the mother being able to care for her child should be a focus of treatment, as well as preparation for motherhood. Careful assessment and screening procedures are essential to plan the respective interventions, while nonpharmacological care should be preferred wherever possible. In case of pharmacological interventions, the risks have to be weighed against the benefits, taking into account possible short- and long-term consequences for the children. Particular attention should be dedicated to special education, training and supervision for caregivers working with this vulnerable patient population. We consider diversification and individual adaptation of treatment interventions for pregnant patients with SUDs as most recommendable for clinical practice in the future. Moreover, clinical studies of pharmacological and nonpharmacological treatment interventions are encouraged in order to establish a universal standard of care for pregnant women with SUDs and their children. Finally, health professionals should keep in mind that adequate treatment might reduce pregnancy complications and poor neonatal outcomes, yielding a reduction of healthcare costs and thus a decrease of the socioeconomic burden for the patients and society.
Practice points.
▪ Specialized management
Specialized care of healthcare professionals from multiple disciplines is most appropriate to manage pregnant patients with substance use disorders.
Special training and education for the ethical and clinical management of pregnant women with substance use disorders and their offspring is recommendable for healthcare providers.
▪ Caregiver attitude
A nonjudgmental, respectful attitude from caregivers is necessary to create a trustful environment for the challenging treatment process.
▪ Comprehensive, individually tailored interventions
Comprehensive medical and psychosocial assessment and subsequent individually tailored interventions are indispensable for effective treatment.
In case of pharmacological interventions, the benefits have to be outweighed carefully against the risks, taking into account the potentially detrimental impact on the unborn child.
▪ Postpartal care
Postpartal support for the mother is as important for her when she is discharged with her newborn as it is when she cannot keep her child, which might entail relapse.
▪ Economic considerations
Adequate interventions lead to better maternal and neonatal outcomes and help save healthcare costs.
Acknowledgments
The work was supported by the National Institute on Drug Abuse grant R01DA018417.
Footnotes
Financial & competing interests disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Gyarmathy VA, Giraudon I, Hedrich D, Montanari L, Guarita B, Wiessing L. Drug use and pregnancy – challenges for public health. Eur Surveill. 2009;14(9):33–36. [PubMed] [Google Scholar]
- 2.Anstice S, Strike CJ, Brands B. Supervised methadone consumption: client issues and stigma. Subst Use Misuse. 2009;44(6):794–808. doi: 10.1080/10826080802483936. [DOI] [PubMed] [Google Scholar]
- 3.Tuten M, Jones HE. A partner's drug-using status impacts women's drug treatment outcome. Drug Alcohol Depend. 2003;70(3):327–230. doi: 10.1016/s0376-8716(03)00030-9. [DOI] [PubMed] [Google Scholar]
- ▪▪4.Winklbaur B, Kopf N, Ebner N, Jung E, Thau K, Fischer G. Treating pregnant women dependent on opioids is not the same as treating pregnancy and opioid dependence: a knowledge synthesis for better treatment for women and neonates. Addiction. 2008;103(9):1429–1440. doi: 10.1111/j.1360-0443.2008.02283.x. Comprehensive review on evidence-based treatment options for opioid-dependent pregnant women and newborns with neonatal abstinence syndrome. [DOI] [PubMed] [Google Scholar]
- 5.Morton J, Konrad SC. Introducing a caring/relational framework for building relationships with addicted mothers. J Obstet Gynecol Neonatal Nurs. 2009;38(2):206–213. doi: 10.1111/j.1552-6909.2009.01006.x. [DOI] [PubMed] [Google Scholar]
- 6.Schempf AH, Strobino DM. Illicit drug use and adverse birth outcomes: is it drugs or context? J Urban Health. 2008;85(6):858–873. doi: 10.1007/s11524-008-9315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakstad B, Sarfi M, Welle-Strand GK, Ravndal E. Opioid maintenance treatment during pregnancy: occurrence and severity of neonatal abstinence syndrome. A national prospective study. Eur Addict Res. 2009;15(3):128–134. doi: 10.1159/000210042. [DOI] [PubMed] [Google Scholar]
- 8.Simmat-Durand L, Lejeune C, Gourarier L Groupe d'Etudes Grossesse et Addictions (GEGA) Pregnancy under high-dose buprenorphine. Eur J Obstet Gynecol Reprod Biol. 2009;142(2):119–123. doi: 10.1016/j.ejogrb.2008.10.012. [DOI] [PubMed] [Google Scholar]
- ▪9.McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. Paper on an important assessment tool in addiction medicine. [DOI] [PubMed] [Google Scholar]
- 10.Ornoy A, Ergaz Z. Alcohol abuse in pregnant women: effects on the fetus and newborn, mode of action and maternal treatment. Int J Environ Res Public Health. 2010;7:364–379. doi: 10.3390/ijerph7020364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keen CL, Uriu-Adams JY, Skalny A, et al. The plausibility of maternal nutritional status being a contributing factor to the risk for fetal alcohol spectrum disorders: the potential influence of zinc status as an example. Biofactors. 2010;36(2):125–135. doi: 10.1002/biof.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abel EL, Sokol RJ. Incidence of fetal alcohol syndrome and economic impact of FAS-related anomalies. Drug Alcohol Depend. 1987;19(1):51–70. doi: 10.1016/0376-8716(87)90087-1. [DOI] [PubMed] [Google Scholar]
- 13.Stratton K, Howe C, Battaglia F, editors. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment. National Academy Press; Washington, DC, USA: 1996. [Google Scholar]
- 14.Astley SJ, Stachowiak J, Clarren SK, Clausen C. Application of the fetal alcohol syndrome facial photographic screening tool in a foster care population. J Pediatr. 2002;141(5):712–717. doi: 10.1067/mpd.2002.129030. [DOI] [PubMed] [Google Scholar]
- ▪15.Fiellin DA, Reid MC, O'Connor PG. Screening for alcohol problems in primary care: a systematic review. Arch Intern Med. 2000;160(13):1977–1989. doi: 10.1001/archinte.160.13.1977. Interesting review on screening for alcohol use disorders, including a description of the most common and important screening tools. [DOI] [PubMed] [Google Scholar]
- 16.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption – II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 17.Ewing JA. Detecting alcoholism: the CAGE questionnaire. JAMA. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- ▪▪18.Wong S, Ordean A, Kahan M Maternal Fetal Medicine Committee. Family Physicians Advisory Committee; Medico–Legal Committee; Society of Obstetricians and Gynaecologists of Canada. Substance use in pregnancy. J Obstet Gynaecol Can. 2011;33(4):367–384. doi: 10.1016/S1701-2163(16)34855-1. Up-to-date clinical practice guideline paper on interventions for pregnant substance users. [DOI] [PubMed] [Google Scholar]
- 19.Elstein SG. Working with substance abuse practitioners on behalf of children and families. ABA Child Law Pract. 2001;19(9):185–190. [Google Scholar]
- 20.Lamy S, Thibaut F. Psychoactive substance use during pregnancy: a review. Encephale. 2010;36(1):33–38. doi: 10.1016/j.encep.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Oncken CA, Kranzler HR. Pharmacotherapies to enhance smoking cessation during pregnancy. Drug Alcohol Rev. 2003;22(2):191–202. doi: 10.1080/09595230100100633. [DOI] [PubMed] [Google Scholar]
- 22.Choo RE, Huestis MA, Schroeder JR, Shin AS, Jones HE. Neonatal abstinence syndrome in methadone-exposed infants is altered by level of prenatal tobacco exposure. Drug Alcohol Depend. 2004;75:253–260. doi: 10.1016/j.drugalcdep.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Winklbaur B, Baewert A, Jagsch R, et al. Association between prenatal tobacco exposure and outcome of neonates born to opioid-maintained mothers. Implications for treatment. Eur Addict Res. 2009;15(3):150–156. doi: 10.1159/000216466. [DOI] [PubMed] [Google Scholar]
- 24.Coleman T, Chamberlain C, Cooper S, Leonardi-Bee J. Efficacy and safety of nicotine replacement therapy for smoking cessation in pregnancy: systematic review and meta-analysis. Addiction. 2010;106:52–61. doi: 10.1111/j.1360-0443.2010.03179.x. [DOI] [PubMed] [Google Scholar]
- ▪▪25.Rayburn WF, Bogenschutz MP. Pharmacotherapy for pregnant women with addictions. Am J Obstet Gynecol. 2004;191(6):1885–1897. doi: 10.1016/j.ajog.2004.06.082. Systematic review that summarizes findings with regard to pharmacotherapy for pregnant women with specific substance use disorders. [DOI] [PubMed] [Google Scholar]
- 26.Alwan S, Reefhuis J, Botto LD, Rasmussen SA, Correa A, Friedman JM. National Birth Defects Prevention Study: maternal use of bupropion and risk for congenital heart defects. Am J Obstet Gynecol. 2010;203(1):52.E1–52.E6. doi: 10.1016/j.ajog.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Clark SM, Nakad R. Pharmacotherapeutic management of nicotine dependence in pregnancy. Obstet Gynecol Clin North Am. 2011;38(2):297–311. doi: 10.1016/j.ogc.2011.02.017. X. [DOI] [PubMed] [Google Scholar]
- ▪28.Heil SH, Higgins ST, Bernstein IM, et al. Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction. 2008;103(6):1009–1018. doi: 10.1111/j.1360-0443.2008.02237.x. Interesting article on contingency management for smoking cessation in pregnant women. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ▪▪29.Huestis MA, Choo RE. Drug abuse's smallest victims: in utero drug exposure. Forensic Sci Int. 2002;128(1–2):20–30. doi: 10.1016/s0379-0738(02)00160-3. Review that gives a synopsis of the consequences of in utero drug exposure to various substances, including detection and treatment strategies. [DOI] [PubMed] [Google Scholar]
- 30.Iqbal MM, Sobhan T, Ryals T. Effects of commonly used benzodiazepines on the fetus, the neonate, and the nursing infant. Psychiatr Serv. 2002;53(1):39–49. doi: 10.1176/appi.ps.53.1.39. [DOI] [PubMed] [Google Scholar]
- 31.Smith LM, LaGasse LL, Derauf C, et al. The Infant Development, Environment, and Lifestyle Study: effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics. 2006;118(3):1149–1156. doi: 10.1542/peds.2005-2564. [DOI] [PubMed] [Google Scholar]
- 32.Gouin K, Murphy K, Shah PS. Am J Obstet Gynecol. 2011;204(4):340.E1–340.E2. doi: 10.1016/j.ajog.2010.11.013. Knowledge synthesis group on determinants of low birth weight and preterm births: effects of cocaine use during pregnancy on low birth weight and preterm birth: systematic review and metaanalyses. [DOI] [PubMed] [Google Scholar]
- 33.National Toxicology Program. NTP-CERHR monograph on the potential human reproductive and developmental effects of amphetamines. NTP CERHR MON 16. 2005:VII–III1. [PubMed] [Google Scholar]
- 34.Smith LM, LaGasse LL, Derauf C, et al. Motor and cognitive outcomes through three years of age in children exposed to prenatal methamphetamine. Neurotoxicol Teratol. 2011;33(1):176–184. doi: 10.1016/j.ntt.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bandstra ES, Morrow CE, Mansoor E, Accornero VH. Prenatal drug exposure: infant and toddler outcomes. J Addict Dis. 2010;29(2):245–258. doi: 10.1080/10550881003684871. [DOI] [PubMed] [Google Scholar]
- 36.Jones HE, O'Grady KE, Tuten M. Reinforcement-based treatment improves the maternal treatment and neonatal outcomes of pregnant patients enrolled in comprehensive care treatment. Am J Addict. 2011;20(3):196–204. doi: 10.1111/j.1521-0391.2011.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neonatal drug withdrawal American Academy of Pediatrics Committee on Drugs. Pediatrics. 1998;101(6):1079–1088. erratum in: Pediatrics 102(3 Pt 1), 660 (1998) [PubMed] [Google Scholar]
- 38.Saiki T, Lee S, Hannam S, Greenough A. Neonatal abstinence syndrome – postnatal ward versus neonatal unit management. Eur J Pediatr. 2010;169(1):95–98. doi: 10.1007/s00431-009-0994-0. [DOI] [PubMed] [Google Scholar]
- ▪▪39.Kuschel C. Managing drug withdrawal in the newborn infant. Semin Fetal Neonatal Med. 2007;12(2):127–133. doi: 10.1016/j.siny.2007.01.004. Review that gives an overview of pharmacological and nonpharmacological treatment interventions for in utero substance-exposed newborns. [DOI] [PubMed] [Google Scholar]
- ▪40.Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363(24):2320–2331. doi: 10.1056/NEJMoa1005359. Recent paper on a clinical trial comparing methadone versus buprenorphine in opioid-dependent pregnant women and the subsequent neonatal outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Unger A, Jung E, Winklbaur B, Fischer G. Gender issues in the pharmacotherapy of opioid-addicted women: buprenorphine. J Addict Dis. 2010;29(2):217–230. doi: 10.1080/10550881003684814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ▪42.Committee on Drugs. Pediatrics. 4. Vol. 105. American academy of pediatrics; 2000. Use of psychoactive medication during pregnancy and possible effects on the fetus and newborn; pp. 880–887. Paper that describes the risks of the most common psychiatric medications when administered during pregnancy. [DOI] [PubMed] [Google Scholar]
- 43.Svikis DS, Golden AS, Huggins GR, et al. Cost–effectiveness of treatment for drug-abusing pregnant women. Drug Alcohol Depend. 1997;45(1–2):105–113. doi: 10.1016/s0376-8716(97)01352-5. [DOI] [PubMed] [Google Scholar]
- 44.Coyle MG, Ferguson A, Lagasse L, Oh W, Lester B. Diluted tincture of opium (DTO) and phenobarbital versus DTO alone for neonatal opiate withdrawal in term infants. J Pediatr. 2002;140(5):561–564. doi: 10.1067/mpd.2002.123099. [DOI] [PubMed] [Google Scholar]
- 45.Osborn DA, Jeffery HE, Cole M. Opiate treatment for opiate withdrawal in newborn infants. Cochrane Database Syst Rev. 2005;3:CD002059. doi: 10.1002/14651858.CD002059.pub2. [DOI] [PubMed] [Google Scholar]
- 46.Daley M, Argeriou M, McCarty D, Callahan JJ, Jr, Shepard DS, Williams CN. The impact of substance abuse treatment modality on birth weight and health care expenditures. J Psychoact Drugs. 2001;33(1):57–66. doi: 10.1080/02791072.2001.10400469. [DOI] [PubMed] [Google Scholar]
- 47.Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2007. Natl Vital Stat Rep. 2010;58(24):1–85. [PubMed] [Google Scholar]
- 48.Mangham LJ, Petrou S, Doyle LW, Draper ES, Marlow N. The cost of preterm birth throughout childhood in England and Wales. Pediatrics. 2009;123:E312–E327. doi: 10.1542/peds.2008-1827. [DOI] [PubMed] [Google Scholar]
- 49.Kiss H, Petricevic L, Martina S, Husslein P. Reducing the rate of preterm birth through a simple antenatal screen-and-treat programme: a retrospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2010;153(1):38–42. doi: 10.1016/j.ejogrb.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 50.Brown HL, Hiett AK, Gath S. Prevalence of Alcohol and Other Drug Use in Pregnant Women: Indiana Demand and Needs Assessment Studies: Alcohol and Other Drugs. Indiana University; School of Medicine, Department of Obstetrics and Gynecology, IN, USA: 1997. [Google Scholar]
- 51.Wachman EM, Byun J, Philipp BL. Breastfeeding rates among mothers of infants with neonatal abstinence syndrome. Breastfeed Med. 2010;5(4):159–164. doi: 10.1089/bfm.2009.0079. [DOI] [PubMed] [Google Scholar]
- 52.Dryden C, Young D, Hepburn M, Mactier H. Maternal methadone use in pregnancy: factors associated with the development of neonatal abstinence syndrome and implications for healthcare resources. BJOG. 2009;116(5):665–671. doi: 10.1111/j.1471-0528.2008.02073.x. [DOI] [PubMed] [Google Scholar]
- 53.Vucinovic M, Roje D, Vucinovic Z, Capkun V, Bucat M, Banovic I. Maternal and neonatal effects of substance abuse during pregnancy: our ten-year experience. Yonsei Med J. 2008;49(5):705–713. doi: 10.3349/ymj.2008.49.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kashiwagi M, Arlettaz R, Lauper U, Zimmermann R, Hebisch G. Methadone maintenance program in a Swiss perinatal center: (I): management and outcome of 89 pregnancies. Acta Obstet Gynecol Scand. 2005;84(2):140–144. doi: 10.1111/j.0001-6349.2005.00497.x. [DOI] [PubMed] [Google Scholar]
- 55.Ziegler M, Poustka F, von Loewenich V, Englert E. Postpartum risk factors in the development of children born to opiate-addicted mothers; comparison between mothers with and without methadone substitution. Nervenarzt. 2000;71(9):730–736. doi: 10.1007/s001150050657. [DOI] [PubMed] [Google Scholar]
- 56.Rohrmeister K, Bernert G, Langer M, Fischer G, Weninger M, Pollak A. Opiate addiction in gravidity – consequences for the newborn. Results of an interdisciplinary treatment concept. Z Geburtshilfe Neonatol. 2001;205(6):224–230. doi: 10.1055/s-2001-19054. [DOI] [PubMed] [Google Scholar]
- 57.Almario CV, Seligman NS, Dysart KC, Berghella V, Baxter JK. Risk factors for preterm birth among opiate-addicted gravid women in a methadone treatment program. Am J Obstet Gynecol. 2009;201(3):326.E1–326.E6. doi: 10.1016/j.ajog.2009.05.052. [DOI] [PubMed] [Google Scholar]
- 58.Kelly JJ, Dais PG, Henschke PN. The drug epidemic: effects on newborn infants and health resource consumption at a tertiary perinatal centre. J Paediatr Child Health. 2000;36:262–264. doi: 10.1046/j.1440-1754.2000.00492.x. [DOI] [PubMed] [Google Scholar]
- 59.Cavanaugh CE, Hedden SL, Latimer WW. Sexually transmitted infections among pregnant heroin- or cocaine-addicted women in treatment: the significance of psychiatric co-morbidity and sex trade. Int J STD AIDS. 2010;21(2):141–142. doi: 10.1258/ijsa.2009.009172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fiocchi FF, Kingree JB. Treatment retention and birth outcomes of crack users enrolled in a substance abuse treatment program for pregnant women. J Subst Abuse Treat. 2001;20(2):137–142. doi: 10.1016/s0740-5472(00)00159-8. [DOI] [PubMed] [Google Scholar]
- 61.Spitzer RL, Williams JB, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R (SCID) American Psychiatric Press; Washington, DC, USA: 1989. [Google Scholar]
- 62.Kissin WB, Svikis DS, Morgan GD, Haug NA. Characterizing pregnant drug-dependent women in treatment and their children. J Subst Abuse Treat. 2001;21:27–34. doi: 10.1016/s0740-5472(01)00176-3. [DOI] [PubMed] [Google Scholar]
- 63.Goel N, Beasley D, Rajkumar V, Banerjee S. Perinatal outcome of illicit substance use in pregnancy – comparative and contemporary socio-clinical profile in the UK. Eur J Pediatr. 2011;170(2):199–205. doi: 10.1007/s00431-010-1284-6. [DOI] [PubMed] [Google Scholar]
- 64.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiat. 1998;59(Suppl. 20):S22–S33. [PubMed] [Google Scholar]
- 65.Benningfield MM, Arria AM, Kaltenbach K, et al. Co-occurring psychiatric symptoms are associated with increased psychological, social, and medical impairment in opioid dependent pregnant women. Am J Addict. 2010;19(5):416–421. doi: 10.1111/j.1521-0391.2010.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mayet S, Groshkova T, Morgan L, MacCormack T, Strang J. Drugs and pregnancy – outcomes of women engaged with a specialist perinatal outreach addictions service. Drug Alcohol Rev. 2008;27(5):497–503. doi: 10.1080/09595230802245261. [DOI] [PubMed] [Google Scholar]
- 67.Liu AJ, Jones MP, Murray H, Cook CM, Nanan R. Perinatal risk factors for the neonatal abstinence syndrome in infants born to women on methadone maintenance therapy. Aust NZ J Obstet Gynaecol. 2010;50(3):253–258. doi: 10.1111/j.1479-828X.2010.01168.x. [DOI] [PubMed] [Google Scholar]
- 68.Little BB, Snell LM, Van Beveren TT, Crowell RB, Trayler S, Johnston WL. Treatment of substance abuse during pregnancy and infant outcome. Am J Perinatol. 2003;20(5):255–262. doi: 10.1055/s-2003-42336. [DOI] [PubMed] [Google Scholar]
- 69.McCarthy JJ, Leamon MH, Parr MS, Anania B. High-dose methadone maintenance in pregnancy: maternal and neonatal outcomes. Am J Obstet Gynecol. 2005;193(3 Pt 1):606–610. doi: 10.1016/j.ajog.2005.03.072. [DOI] [PubMed] [Google Scholar]
- 70.Ho E, Karimi-Tabesh L, Koren G. Characteristics of pregnant women who use ecstasy (3, 4-methylenedioxymethamphetamine) Neurotoxicol Teratol. 2001;23(6):561–567. doi: 10.1016/s0892-0362(01)00178-7. [DOI] [PubMed] [Google Scholar]
Websites
- 101.UNODC/WHO. Principles of drug dependence treatment. Discussion paper. [Accessed 13 September 2011];2008 www.unodc.org/documents/drug-treatment/UNODC-WHO-Principles-of-Drug-Dependence-Treatment-March08.pdf.
- 102.SAMHSA. The NSDUH Report. Substance use during pregnancy – 2002 and 2003 update. [Accessed 7 September 2011];2005 http://oas.samhsa.gov/2k5/pregnancy/pregnancy.htm.
- 103.UK Home Office. Hidden harm – full report. [Accessed 7 September 2011];2003 www.homeoffice.gov.uk/publications/agencies-public-bodies/acmd1/hidden-harm-full.
- 104.EMCDDA. Office for Official Publications of the European Communities; Luxembourg: 2009. [Accessed 8 September 2011]. EMCDDA thematic paper – women's voices: experiences and perceptions of women who face drug-related problems in Europe. www.emcdda.europa.eu/attachements.cfm/att_71266_EN_EMCDDA-TP_women's%20voices.pdf. [Google Scholar]
- 105.Astley S. Diagnostic Guide for Fetal Alcohol Spectrum Disorders: The 4-Digit Diagnostic Code. 3rd. University of Washington; WA, USA: [Accessed 8 September]. http://depts.washington.edu/fasdpn/pdfs/guide2004.pdf. [Google Scholar]
- 106.Richardson A, Mmata C. NHS Maternity Statistics; England: 2007. [Accessed 13 September 2011]. pp. 2005–06. The Information Centre. www.ic.nhs.uk/webfiles/publications/maternity0506/NHSMaternityStatsEngland200506_fullpublication%20V3.pdf. [Google Scholar]


