Abstract
Background
Hereditary breast and ovarian cancer risk assessments (CRAs) are underutilized by low-income and racial/ethnic minority women, potentially exacerbating cancer-related disparities observed within these populations. We deployed and evaluated a systems-level intervention designed to identify patients potentially at-risk for hereditary breast/ovarian cancer, refer them for CRAs, and facilitate CRA utilization at an urban community-based breast health care center.
Methods
Cancer family history forms were completed by patients seen at the center during an 18-month period and reviewed by staff for CRA eligibility against published referral criteria. A patient navigator educated eligible patients about the benefits of CRA, navigating interested patients to this service. CRA-specific patient interest and utilization outcomes are reported.
Results
In total, 94.7% of all patients (n = 2,436) completed forms and 65 patients (2.7%) met CRA eligibility criteria. Most eligible patients (72.3%) were interested in CRA. Interested patients had a greater risk for hereditary breast/ovarian cancer (i.e., more affected relatives, greater objective risk scores) than uninterested patients: 57.4% scheduled a CRA appointment and 51.9% of scheduled patients utilized CRAs. Patients scheduling a CRA were contacted in less time and required fewer follow-up contacts by the patient navigator, and were more likely to be African American, than those who declined a CRA or were lost to follow-up (all p’s ≤ .05).
Conclusions
The systems-level intervention successfully identified patients eligible for CRA and linked interested and at-risk patients with CRA resources. More intensive patient navigation addressing the unique barriers encountered within this population may be required to enhance utilization.
Keywords: Cancer risk assessment, Systems intervention, Disparities, Underserved populations
Introduction
Breast cancer is the most commonly diagnosed malignancy among women in the United States, with nearly 27,000 new cases expected in 2011 [1]. While multiple factors contribute to breast cancer risk, including environmental exposures and lifestyle factors, between 5%–10% of all cases are estimated to be hereditary in nature and attributable to germline mutations in two major breast cancer-predisposing genes, BRCA1 and BRCA2 (BRCA1/2) [2]. Women identified as BRCA1/2 mutation carriers have a substantially increased risk for hereditary breast and ovarian cancer (HBOC), with an estimated 40%–85% lifetime risk for breast cancer and a 25%–65% risk of ovarian cancer [3, 4]. Women affected with HBOC are often diagnosed with breast and/or ovarian cancer at ages younger than those observed in the general population.
For women potentially at-risk for HBOC, a cancer risk assessment (CRA) is recommended to provide a personalized risk evaluation and to inform decisions about clinical management [5, 6]. CRAs for HBOC typically include genetic counseling, a discussion about the pros and cons of BRCA1/2 mutation testing and, where indicated, the opportunity to undergo genetic testing for BRCA1/2 [5]. Given the multifactorial nature of HBOC risk and the need to address both health and psychosocial issues in the delivery of care, clinical practice guidelines recommend that CRAs are delivered by a certified genetic counselor or similar clinical consultants with expertise in genetics and HBOC to ensure delivery of high-quality comprehensive care [7–10].
For women who may be at risk for HBOC, CRAs represent an important opportunity for early detection and intervention [11]. CRAs may be especially beneficial to reduce HBOC disparities observed among special populations, including underserved women and racial/ethnic minority women [12]. Female African Americans and Latinas are disproportionately affected by breast cancer-related disparities. Women from these groups are more likely to present with clinical signs of breast cancer at younger ages and with later stages of disease, and experience disparities across the breast cancer continuum [1, 13, 14]. Despite the availability and increasing utilization of CRAs in majority populations, utilization among racial/ethnic minority women remains unacceptably low [15–21].
Many factors contribute to racial/ethnic differences in CRA utilization, including socio-cultural influences, individual level differences, and barriers to CRA delivery within the healthcare system [13, 21, 22]. Recent studies indicate that exposure to and knowledge about genetic testing for general cancer risk [23–25] and for BRCA1/2 mutations specifically [15, 20, 26, 27] are lower among African American women and Latinas than among white women. Research points to additional barriers to CRA utilization among minority women, including access to care (e.g., inability to pay, lack of health insurance, language barriers) and perceptions (e.g., concern regarding discrimination/stigmatization, cancer risk cognitions) [16, 17, 21, 27–29]. Within the healthcare system, barriers to accessing CRAs also include clinicians’ lack of time, knowledge, skills, and screening tools to determine HBOC risk; absence of integrated systems to facilitate identification and referral of patients with risk for HBOC; and lack of clinician follow-up with patients referred for CRAs [30–33]. In short, the system of preventive HBOC cancer care, including CRAs, among special populations is fragile and fractured.
These barriers notwithstanding, racial/ethnic minority women have demonstrated high levels of interest in and favorable attitudes towards CRA [20, 27, 34, 35] . This is especially true among women with elevated risk for HBOC (i.e., among those with family histories suggestive of HBOC), and greater perceived risk for cancer [36–38]. Taken together, these data highlight the critical need for integrated interventions to reduce access barriers to CRA and promote utilization among potentially at-risk, underserved women.
Systems-level interventions designed to increase access to clinical services and provide individually-directed strategies to assist patients in navigating cancer care are complementary approaches that may help to address the complex issues impacting CRAs among underserved women [39] and reduce breast cancer-related disparities affecting this population [12]. Patient navigation is one individually-directed intervention approach that can be integrated into healthcare delivery systems and has been advocated as an important strategy to reduce cancer-related disparities by increasing access to prevention [40]. Patient navigation is an intervention that is particularly well-suited to reduce cancer-related disparities because it is designed to address individual-level barriers to receiving care (e.g., patient knowledge/understanding, practical barriers); enhance access by reducing delays in care delivery; ensure continuity of services (e.g., facilitate referral and care delivery); and track patients over time to ensure receipt of the intended service(s) [41]. A growing body of research demonstrates that patient navigation can be efficacious for improving the delivery of cancer-related care, including increasing utilization of preventive screening (e.g., mammography) and improving diagnostics and timely follow-up of diagnoses [40]. While far fewer studies have implemented patient navigation as a strategy to improve access to CRAs among underserved women, promising evidence is emerging. In particular, the results of two recent studies suggest that coupling systems-level interventions (e.g., provider education, patient identification) with individually-directed patient navigation leads to improved access to CRA for HBOC among Latinas [15] and other special and high-risk populations of women [42].
Despite these encouraging findings, empirical evidence for innovative, integrated care delivery models designed to systematically identify and navigate potentially at-risk patients for CRAs remains sorely lacking [11, 43]. Applications of such systems-level intervention models in community-based health care delivery settings—a primary point of health care for underserved women—remain particularly scarce [15]. Filling these research gaps will be essential in reducing cancer-related disparities and improving access and delivery of CRAs for underserved women at risk for HBOC. In light of these important clinical and public health needs, our study was designed to deploy and evaluate a systems-level intervention to routinely identify women potentially at-risk for HBOC, refer them for CRA services, and navigate them through CRA utilization. As part of this effort, patients’ interest in and utilization of CRA were assessed and CRA-specific outcomes are reported.
Methods
Study Setting
The study was conducted at an urban, community-based breast health center. The center offers comprehensive, culturally-appropriate breast care (e.g., breast exams, mammography) and patient education and support services to predominantly low-income, uninsured or underinsured racial/ethnic minority women in the Washington, DC metropolitan area regardless of ability to pay. We chose this setting because it is a primary point of preventive breast cancer care delivery for underserved women within the local community and addresses a critical need for applications of systems-level interventions to improve access to CRA within community-based healthcare settings. Support services offered by the center include transportation to and from clinical appointments, access to bilingual staff, and patient navigation (e.g., to guide patients through the healthcare system, apply for financial aid, arrange medical and social services, provide emotional support, explain diagnostic procedures, ensure follow-up care/social services are available, locate additional resources as needed). All study procedures were approved by the host institution’s Institutional Review Board. Written informed consent was waived as the intervention was implemented as the standard care for all women, and all data were de-identified.
Systems-Level Intervention
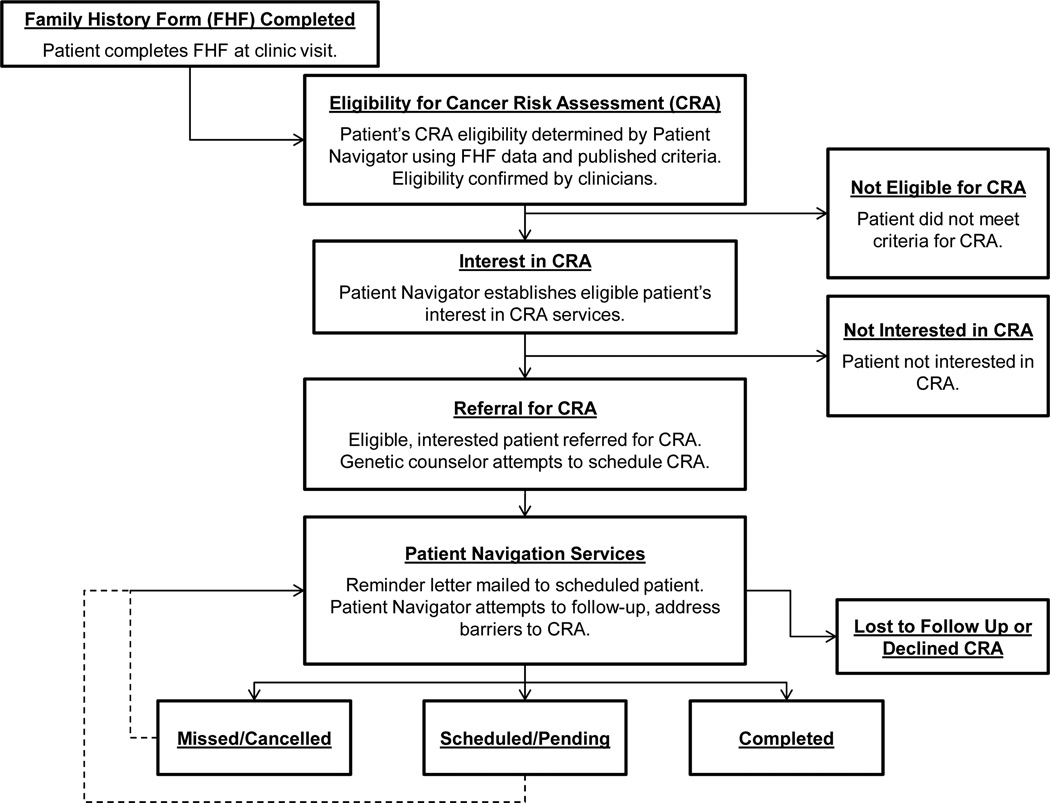
To facilitate access to CRAs among patients seen in this setting, we implemented a systems-level intervention for an 18-month period (January, 2010-June, 2011). The intervention was designed to integrate a cascade of logical decision points into the usual breast cancer preventive care delivery process, as depicted in Figure 1. These systems-directed changes were designed to: (1) routinely gather HBOC risk information from all patients seen during each clinical encounter; (2) determine each patient’s risk for HBOC and eligibility for CRA; (3) refer patients eligible for CRA to this service; and (4) navigate patients to CRA by conducting telephone follow-up calls, tracking patient referral progress, and offering education/counseling and other resources to address barriers to access CRAs [44–46].
Figure 1.
Systems-level intervention to facilitate access to and utilization of cancer risk assessment services
A paper-based family history form (FHF) was added to the center’s patient intake assessment battery to collect data about personal and family history of breast and ovarian cancer. The FHF was adapted based on those used as part of standard care delivery in our longstanding HBOC genetic counseling and testing program, and as informed by research examining the validity of patient-oriented FHFs [42, 47–49]. The FHF captures systematic information about personal and family history of breast/ovarian cancer, and ethnic background relevant to clinical referral criteria for CRA, which are detailed below. The FHF was not intended to quantify HBOC risk (i.e., a risk prediction model), but rather was designed to help identify women who may be at risk based on personal and family history of cancer and to facilitate referral for formal CRA. The FHF was completed by the patient with a patient navigator prior to or during the patient’s clinical appointment for other services. Completed FHF’s were stored in each patient’s medical record, and then reviewed by a patient navigator against published referral criteria (Figure 1).
The patient navigator supported by this project was an African American female with graduate-level training in the health sciences. The navigator underwent an additional eight hours of training led by senior cancer genetic counseling and medical staff to review FHF information against the National Society of Genetic Counselors’ (NSGC) criteria for CRA eligibility, which determines HBOC risk based on patients’ personal and family cancer histories and ethnic backgrounds [7]. Specifically, patients who met one or more of the following NSGC criteria were determined to be eligible for CRA: breast cancer diagnosis at age ≤ 50; ovarian cancer at any age in the patient or one or more first degree relative; two or more primary breast cancers in the patient or first degree relatives; two or more cases of breast cancer in first or second degree relatives, if age ≤ 50; and three or more cases of breast/ovarian cancer among relatives at any age [7]. Medical records of patients identified by the navigator as potentially eligible for CRA were flagged for review by a physician assistant and medical oncologist for confirmation. A genetic counselor was consulted on an as-needed basis for any records requiring additional oversight.
Once CRA eligibility was confirmed, the patient navigator approached the patient to discuss referral to CRA, providing background information and educational materials about the purpose of CRA, the referral process, and available resources. These activities primarily took place on the day of the patient’s appointment at the center. However, if the patient navigator was unable to do so during the clinic visit, follow-up telephone calls were initiated. The patient navigator was also responsible for assessing eligible patients’ initial interest in a CRA (consulting with the physician assistant as appropriate) and responses (yes/no) were recorded (Figure 1).
Patients eligible for and interested in a CRA were then referred to the center’s genetic counselor for follow-up. The genetic counselor subsequently made up to two attempts to reach referred patients by telephone to schedule a CRA. The patient navigator was responsible for mailing all scheduled patients an informational packet about the upcoming CRA appointment. The navigator tracked referral progress and process data, and updated each patient’s disposition in her record. The genetic counselor and patient navigator maintained weekly contact to address individual patient-related issues as they arose, including patient barriers to accessing CRA services. For example, in the event that the genetic counselor was unable to successfully reach a patient by telephone, could not schedule a patient due to patient conflict, or if the patient did not attend a scheduled appointment, the patient navigator initiated multiple drop-out prevention strategies. These included proactive telephone outreach calls and mailed reminders to patients. If no further contact could be reestablished with a patient, the patient was considered lost to follow-up for CRA. Patient navigation procedures (e.g., telephone and mail follow-up) were informed by prior research [41], designed to be compatible with the center’s clinical workflow, and selected to optimize available navigation services for patients given the time and support available for the patient navigator for the project.
Cancer Risk Assessment
Patients were offered the opportunity to attend an in-person genetic counseling session at one of two hospital-based locations, both accessible by public transportation and with logistical assistance provided through patient navigation. For patients unable or unwilling to travel to either location, telephone genetic counseling was offered. A bilingual staff member was available to assist in communicating with Spanish-speaking patients at the center, and a bilingual interpreter was available at CRA appointments. Patients who were un- or underinsured for genetic counseling received this service at no cost to them. Various options for payment for BRCA1/2 genetic testing were explored with the patient navigator and the genetic counselor when appropriate, including patient insurance, research and philanthropic support, and other mechanisms. No patient who was eligible for and interested in BRCA1/2 testing for HBOC was denied this service due to an inability to pay. Options available for patients who were unable to pay for genetic testing included funding from institutional resources and support through a third party financial assistance program. These options were discussed with patients throughout the navigation process and at the time of CRA with the genetic counselor as well.
Data Collection
A breast care center staff member prepared a final de-identified data file spanning the 18-month study period that included: 1) FHF data, 2) HBOC risk data, 3) patient navigation data, and 4) CRA utilization data. Each of these data elements are described below.
Family History Forms
We counted the total number of FHFs completed during the study time period and compared this with the total number of patients seen during this same interval to assure high implementation fidelity and reach. Patients’ eligibility for CRA based on FHF data was recorded. Patient-level data extracted from completed FHFs included demographic characteristics (age, race, ethnicity, insurance status, geocode-estimated household income, parenting status) [50], personal breast/ovarian cancer history, and familial breast/ovarian cancer history.
Risk for Hereditary Breast/Ovarian Cancer
Patients’ objective risk for HBOC was operationalized in three ways using FHF data. For each patient eligible for a CRA, we summed the total number of first- and second-degree relatives reported by the patient to have been diagnosed with breast and/or ovarian cancer. We also calculated each patient’s probability (i.e., % likelihood) of carrying a BRCA1/2 mutation and her lifetime probability of breast cancer using the BRCAPRO model within CancerGene 5.1 (sensitivity = 0.80) [51]. BRCAPRO is a statistical model with accompanying third-party computer software that calculates the probability that an individual carries a BRCA1/2 genetic mutation and lifetime probability of developing breast/ovarian cancer based on personal breast/ovarian cancer history, history of breast/ovarian cancer among first- and second-degree relatives, relatives’ demographic characteristics, and population-based prevalence data [52]. The model was originally developed and validated in populations of European ancestry but has since been demonstrated to be an accurate predictive model among African American and Hispanic women [53–55]. BRCAPRO risk estimates were used only as a post-hoc validation to understand risk for HBOC among patients at each stage of the CRA referral and delivery process; estimates were not used as a criterion to identify and refer patients potentially at-risk for HBOC.
Interest in Cancer Risk Assessment & Utilization Outcomes
Based on FHF information, we first identified the total number of patients eligible for CRA during the study time period. For each patient identified as eligible, we then determined whether or not she was interested in a CRA by recording the outcome of her referral consultation with the patient navigator and/or physician assistant. Finally, we examined CRA utilization by recording whether or not eligible and interested patients were scheduled for (and ultimately completed) CRA appointments.
Patient Navigation Outcomes
Two indices were used to quantify the amount of time and effort devoted to navigation to CRA. For patients who were eligible and expressed interest in CRA, the number of days to contact was defined as the number of days between initial referral to CRA and the time when she scheduled a CRA appointment with a genetic counselor, subsequently declined CRA, or could no longer be reached by the patient navigator for follow-up. We also maintained a count of the number of follow-up contacts made by phone and/or by mail by the patient navigator throughout the navigation process.
Statistical Analysis
Univariate statistics were used to describe patient demographic and clinical characteristics, patient navigation, and CRA interest and utilization outcomes. Among patients determined to be eligible for CRAs, bivariate analyses (e.g., t tests, χ2 tests) compared the demographic and clinical characteristics of those who were and were not interested in CRA. Finally, descriptive statistics were used to examine the CRA and patient navigation outcomes described above.
Results
Family History Forms
Clinic data show that 2,570 patients were seen during the study’s 18-month activity period, and that a total of 2,436 of these patients (94.7%) had their FHFs reviewed for CRA eligibility. While data are not available to further characterize the 5.3% of patients who were missing FHFs, these FHFs were most likely missing due to practical issues affecting implementation within the community-based center (e.g., changes in patient flow, scheduling, and visit timing). Thus, the intervention was deployed with high fidelity and reach.
Cancer Risk Assessment Eligibility
Table 1 displays all captured demographic and clinical characteristics of patients determined to be eligible for a CRA, based upon a review of their FHFs. Of these patients, n = 65 (2.7%) met well-established criteria for referral to CRA and n = 2,371 (97.3%) did not.
Table 1.
Eligible Patient Characteristics, Stratified by Interest in Receiving a Hereditary Breast/Ovarian Cancer Risk Assessment (CRA)
| Characteristic | Total Eligible for CRA | Interested in CRA? | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Statistic | |||||||||||
| (n = 65) | (n = 47) | (n = 18) | |||||||||||
| m | sd | n | % | m | sd | n | % | m | sd | n | % | p-value | |
| Demographic | |||||||||||||
| Age | 48.3 | 8.7 | 48.3 | 9.1 | 48.3 | 7.9 | n.s. | ||||||
| Race | |||||||||||||
| African American | 42 | 64.6 | 31 | 66.0 | 11 | 61.1 | n.s. | ||||||
| Other | 23 | 35.4 | 16 | 34.0 | 7 | 38.9 | |||||||
| Ethnicity | |||||||||||||
| Latina | 16 | 24.6 | 11 | 23.4 | 5 | 27.8 | n.s. | ||||||
| Other | 49 | 75.4 | 36 | 76.6 | 13 | 72.2 | |||||||
| Insurance Status | |||||||||||||
| Uninsured | 32 | 49.2 | 23 | 48.9 | 9 | 50.0 | n.s. | ||||||
| Medicare/Medicaid | 21 | 32.3 | 15 | 31.9 | 6 | 33.3 | |||||||
| Private | 12 | 18.5 | 9 | 19.1 | 3 | 16.7 | |||||||
| Annual Income, $* | 47,694 | 26,654 | 46,809 | 22,304 | 50,006 | 36,341 | n.s. | ||||||
| Raising ≥1 child, Yes** | 43 | 70.5 | 32 | 68.1 | 11 | 78.6 | n.s. | ||||||
| Clinical | |||||||||||||
| Breast or ovarian cancer survivor, Yes | 7 | 10.8 | 4 | 8.5 | 3 | 16.7 | n.s. | ||||||
| Breast/ovarian cancer-affected relatives, # | 1.8 | 1.0 | 1.9 | 1.0 | 1.4 | 1.1 | 0.05 | ||||||
| Probability of carrying BRCA1/2 mutation, %*** | 4.8 | 10.0 | 5.8 | 11.5 | 2.2 | 2.1 | 0.05 | ||||||
| Lifetime probability of breast cancer diagnosis, %*** | 9.7 | 3.3 | 10.2 | 3.5 | 8.2 | 2.1 | 0.03 | ||||||
Note.n.s. = nonsignificant.
Calculated using geocoding based on address at time of referral
Data missing for 4 patients who declined CRA
Calculated using BRCAPRO
Patients meeting eligibility criteria averaged 48 years-old; most were non-Latina African Americans, uninsured or receiving public health insurance (e.g., Medicare, Medicaid), lived in areas falling below the median annual household income for the District of Columbia (i.e., < $59,000), and were raising children at home. Clinically, approximately 11% of patients were breast/ovarian cancer survivors. Patients averaged two first- and/or second-degree relatives diagnosed with breast and/or ovarian cancer, a roughly 5% prior probability of carrying a BRCA1/2 mutation, and an approximately 10% lifetime probability of breast cancer (Table 1).
Interest in Cancer Risk Assessment
Among the subgroup of CRA-eligible patients, a majority were initially interested in obtaining this service (n = 47; 72.3%). There were no demographic differences between patients interested and not interested in CRA. However, compared with women who were uninterested in CRA, those who were interested had significantly more relatives affected by breast and/or ovarian cancer (t [63] = 1.97), a higher probability of having a BRCA1/2 mutation (t [63] = 2.03), and a higher lifetime probability of breast cancer (t [63] = 2.22) (all p’s ≤ .05; see Table 1).
Cancer Risk Assessment Utilization
Among the 47 patients who were initially interested in CRA, n = 27 (57.4%) scheduled appointments with a cancer genetic counselor, n= 11 (23.4%) were lost to follow-up, n = 2 (4.3%) remained interested but uncertain about CRA, and n = 7 (14.9%) declined appointments. Among the n = 27 patients who scheduled appointments with a genetic counselor for a CRA, n = 14 (51.9%) completed a CRA, n = 3 (11.1%) have appointments pending, and n = 10 (37.0%) missed or cancelled their scheduled appointments. Thus, from initial interest to scheduling to completion, patient navigation was successful in retaining almost one-quarter of eligible patients throughout this clinical cascade.
Patient Navigation Outcomes
With respect to the amount of time spent navigating patients to CRA, the average number of days to scheduling was 9.8 (sd = 7.0), with 34.1 days (sd = 16.6) to decline, and 37.5 days (sd = 25.2) to loss to follow-up. With respect to the number of follow-up contacts made by phone and/or mail by the patient navigator throughout the navigation process, patients who scheduled a CRA required an average of 0.7 (sd = 1.1) follow-up contacts, those lost to follow-up required an average of 1.5 (sd = 1.3) contacts, and those who declined averaged 1.3 (sd = 1.3) contacts. Patients who scheduled a CRA appointment could be contacted in less time (t [43] = 4.93, p< .001), received/required fewer follow-up contacts from the patient navigator (t [45] = −2.22, p = .03), and were more likely to be African American (X2 [1] = 3.95, p = .05) than those who declined CRA or were lost to follow-up.
Discussion
Underserved women, including racial/ethnic minority women and low-income populations, are disproportionately impacted by breast cancer and experience poorer clinical outcomes [1, 13]. These disparities may partly stem from barriers to accessing available preventive services, including CRA services among women who may be at-risk for HBOC [16, 17, 21]. In an effort to improve access to CRA services and reduce HBOC disparities, we examined the feasibility of implementing a systems-level intervention designed to routinely collect information about personal and family cancer history, identify patients who may be at risk for HBOC, and facilitate access to CRAs through patient navigation. We did so within a community-based breast health care center providing mammography and other cancer preventive services for low-income, predominantly racial/ethnic minority women. Our findings suggest that the systems-level approach is highly feasible, facilitates identification of potentially at-risk women, and reduces barriers to access needed CRA services.
The systems-level intervention gathered family cancer history information from patients with high implementation fidelity and reach, as nearly 95% of patients visiting the center during the 18 month study period completed a family history form and had it reviewed for CRA eligibility. Based on review of family history form information, 3% of patients met well-established criteria for CRA eligibility. The intervention retained nearly 25% of patients meeting eligibility criteria for CRA and utilizing CRA services. At the time of analyses, more than half of patients meeting eligibility criteria and interested in CRA had visited a genetic counselor or had appointments pending. Our findings suggest patient navigation usefully facilitated access and reduced barriers to CRA among eligible and interested women who otherwise would neither have been screened nor referred for HBOC risk assessments.
The findings reported herein are comparable to recent studies evaluating similar multi-component, systems-level interventions to improve CRA access and increase utilization among underserved women. A similar study compared combined provider-directed education with administration of a cancer FHF among women seeking mammography at a large, urban public hospital in San Francisco [42]. That study sought to increase identification of CRA-eligible patients and facilitate referral within a diverse, underserved patient population (26% Hispanic, 13% African American, and 50% unemployed). The intervention used criteria similar to the NSGC criteria used in the present work to identify women who were at risk for HBOC and eligible for CRA. Overall, 6% of women screened were determined to be at high risk compared with 3% in the current study. However, patients were predominantly comprised of Caucasian women and also differed from our patient population based on socioeconomic characteristics as well (e.g., education) [42]. As a result of the systems-level intervention, 26% of eligible women completed an initial CRA appointment [42], which is comparable to our findings.
In another recent study, satellite genetics clinics were established within two indigent healthcare systems serving primarily Latina women (71%), offering provider education, outreach, and dissemination of HBOC CRA referral guidelines. These system-directed changes improved quality of CRA referral by clinicians (91% appropriate) and led to a 52% CRA utilization rate among referred patients [15]. Finally, another recent study evaluated a systems-level intervention to improve CRA referral and applied patient navigation to increase utilization within a large managed care organization [45]. This study used a randomized design where patients who were referred to CRA were randomly assigned to either patient navigation or usual care. The patient navigation approach significantly decreased time to appointment (83% of patients were seen within 3 months, compared with 32% in usual care) and resulted in a 44% utilization rate (compared with 31% in usual care) [45]. While the differences observed in utilization were not statistically significant, this is likely be due to the fact that the study was not sufficiently powered detect small absolute differences in utilization [45].
Our intervention led to CRA service utilization levels that are comparable to these earlier efforts. However, our work represents a unique contribution to this area of research in several important ways. Ours is among the first studies to specifically evaluate the feasibility of a systems-level intervention designed to routinely collect HBOC risk information, identify, and refer women potentially at-risk for HBOC, with follow-up via an embedded patient navigator to assist patients in accessing care. Furthermore, we implemented our model within a community-based setting serving low-income, predominantly racial/ethnic minority women who most likely would not otherwise have had access to CRAs. Prior efforts applying similar systems-directed intervention strategies have largely focused on increasing referrals through provider-directed education and skills training [15, 42] or were conducted within managed care settings where patient access is a confounder [45].
Evidence regarding effective approaches to facilitate CRA access in community-based settings remains scarce. An important limitation of provider-directed approaches to facilitate CRA referral is lack of follow-up by clinicians among referred patients to ensure CRA services are utilized [33]. Our work advances this line of research by demonstrating the feasibility of implementing a systems-level intervention to facilitate identification and referral of women potentially at risk for HBOC and to integrate individually-directed patient navigation to facilitate access and reduce barriers to care. We implemented this program within a community-based healthcare setting with minimal resources, relying solely on support for the patient navigator who had other administrative duties at the center. This is an important addition to this nascent area of cancer prevention research.
Descriptively, the sample of women eligible and interested in CRA appears to have a relatively low risk of breast cancer and carrying a BRCA1/2 mutation based on objective measures. For example, the prior probability of carrying BRCA1/2 mutation among women interested in CRA was approximately 6% and is lower than the often-used criterion of a 10% prior probability indicated by BRCAPRO [56]. However, the characteristics of our sample should be interpreted in light of population-level data on risk for HBOC, with important limitations in risk prediction models. At the population level, racial/ethnic minority women (including African Americans and Latinas) have a lower probability of developing breast cancer [57] and probability of carrying a BRCA1/2 genetic mutation [58] relative to majority groups. Additionally, while BRCAPRO generally performs well in racial/ethnic minority populations, it may under-predict risk among African American women when based solely on prior probabilities [53, 54]. Finally, it should be recognized that determination of HBOC risk and referral for CRA is determined by multiple factors, including objective risk estimates and personal and family history of cancer [7]. Not all women referred to CRA ultimately obtain BRCA1/2 genetic testing, though they may benefit from an assessment nonetheless as it might identify high risk kindreds [59].
Our results are promising and suggest value in systems-level interventions to facilitate CRA within community-based contexts. Additional research is still needed to inform the development of more intensive systems-directed strategies to increase CRA utilization among underserved and minority women. One important area for future study is to gain a better understanding of the specific barriers that affect CRA utilization among underserved women. These may include factors such as how women’s knowledge of and beliefs about HBOC and CRA and their awareness of available services influence decisions to utilize CRA. One recent study among African American women with possible risk for HBOC found that individual-level sociocultural factors (medical mistrust, self-efficacy) influenced level of engagement with CRAs [29]. These factors were unmeasured attributes within our study. Finally, cultural barriers such as spirituality, perceived susceptibility to cancer, and norms around family communication must be considered as well [13, 60].
The aforementioned factors may be integrated into patient navigation, as they represent potentially modifiable barriers that could be targeted through patient education and counseling. Additional barriers to CRA access should be examined and addressed through systems-based changes, including resources affecting access (e.g., transportation, childcare, availability of non-English language services), cost and/or insurance status, language, and clinician-level factors influencing referral and utilization [13, 17, 33, 61].
Limitations
Our findings should be interpreted in light of the important study limitations noted above. Also, our design was observational and did not include a systematic within-site temporal comparison following the intervention, or a comparison condition at another similar setting. Without such a comparison condition we are unable draw firm conclusions about whether the systems-level intervention directly improved processes and outcomes related to CRA. Our study was also conducted at one clinic site, limiting its external validity. Through the systems-level intervention we offered, HBOC genetic counseling services were delivered at no-cost to all women meeting risk-based criteria. Every effort was made to ensure genetic testing would be available at no cost to women who had risk factors indicative of HBOC.
Conclusions
A systems-level intervention designed to systematically collect information about personal and family cancer history, identify women who may be at risk for HBOC, and facilitate access to cancer risk assessment through patient navigation holds promise to reduce barriers to CRAs among underserved women. Such approaches may be especially important to implement in community-based settings as a strategy to address population-level disparities related to hereditary breast and ovarian cancer. Further research is also needed to more directly examine the impact of such interventions (e.g., using experimental approaches) and to investigate how additional intervention components (e.g., culturally tailored patient education materials) may help to further bolster patient interest in CRA and improve cancer prevention outcomes.
Acknowledgments
Support for this research was provided by the Ethical, Legal, and Social Implications Research Program of the National Human Genome Research Institute at the National Institutes of Health (R01HG002686-S1 to Dr. Tercyak). The project was supported in part by Award Number P30CA051008 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
This is version of the manuscript was provided by the authors for the purpose of publication in PubMed Central. The original publication is available at springerlink.com.
References
- 1.American Cancer Society. Cancer Facts & Figures for African Americans 2009–2010. Atlanta: American Cancer Society; 2009. [Cited 3 Nov 2011]. http://www.cancer.org/acs/groups/content/@nho/documents/document/cffaa20092010pdf.pdf. [Google Scholar]
- 2.Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol. 2009;36:237–249. doi: 10.1053/j.seminoncol.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amir E, Freedman OC, Seruga B, Evans DG. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010;102:680–691. doi: 10.1093/jnci/djq088. [DOI] [PubMed] [Google Scholar]
- 5.Robson ME, Storm CD, Weitzel J, Wollins DS, Offit K American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2010;28:893–901. doi: 10.1200/JCO.2009.27.0660. [DOI] [PubMed] [Google Scholar]
- 6.Peshkin BN, DeMarco TA, Brogan BM, Lerman C, Isaacs C. BRCA1/2 testing: complex themes in result interpretation. J Clin Oncol. 2001;19:2555–2565. doi: 10.1200/JCO.2001.19.9.2555. [DOI] [PubMed] [Google Scholar]
- 7.Berliner JL, Fay AM Practice Issues Subcommittee of the National Society of Genetic Counselors'. Risk assessment and genetic counseling for hereditary breast and ovarian cancer: recommendations of the National Society of Genetic Counselors. J Genet Couns. 2007;16:241–260. doi: 10.1007/s10897-007-9090-7. [DOI] [PubMed] [Google Scholar]
- 8.Ready K, Arun B. Clinical assessment of breast cancer risk based on family history. J Natl Compr Canc Netw. 2010;8:1148–1155. doi: 10.6004/jnccn.2010.0084. [DOI] [PubMed] [Google Scholar]
- 9.Trepanier A, Ahrens M, McKinnon W, et al. Genetic cancer risk assessment and counseling: recommendations of the National Society of Genetic Counselors. J Genet Couns. 2004;13:83–114. doi: 10.1023/B:JOGC.0000018821.48330.77. [DOI] [PubMed] [Google Scholar]
- 10.Weitzel JN. Genetic cancer risk assessment. Putting it all together. Cancer. 1999;86:2483–2492. doi: 10.1002/(sici)1097-0142(19991201)86:11+<2483::aid-cncr5>3.3.co;2-w. [DOI] [PubMed] [Google Scholar]
- 11.Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2010;60:99–119. doi: 10.3322/caac.20063. [DOI] [PubMed] [Google Scholar]
- 12.Dankwa-Mullan I, Rhee KB, Williams K, et al. The science of eliminating health disparities: summary and analysis of the NIH summit recommendations. Am J Public Health. 2010;100(Suppl 1):S12–S18. doi: 10.2105/AJPH.2010.191619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerend MA, Pai M. Social determinants of Black-White disparities in breast cancer mortality: a review. Cancer Epidemiol Biomarkers Prev. 2008;17:2913–2923. doi: 10.1158/1055-9965.EPI-07-0633. [DOI] [PubMed] [Google Scholar]
- 14.Ooi SL, Martinez ME, Li CI. Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Res Treat. 2011;127:729–738. doi: 10.1007/s10549-010-1191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricker C, Lagos V, Feldman N, et al. If we build it … will they come?--establishing a cancer genetics services clinic for an underserved predominantly Latina cohort. J Genet Couns. 2006;15:505–514. doi: 10.1007/s10897-006-9052-5. [DOI] [PubMed] [Google Scholar]
- 16.Forman AD, Hall MJ. Influence of race/ethnicity on genetic counseling and testing for hereditary breast and ovarian cancer. Breast J. 2009;15(Suppl 1):S56–S62. doi: 10.1111/j.1524-4741.2009.00798.x. [DOI] [PubMed] [Google Scholar]
- 17.Simon MS, Petrucelli N. Hereditary breast and ovarian cancer syndrome : the impact of race on uptake of genetic counseling and testing. Methods Mol Biol. 2009;471:487–500. doi: 10.1007/978-1-59745-416-2_25. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA. 2005;293:1729–1736. doi: 10.1001/jama.293.14.1729. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong K, Weber B, Stopfer J, et al. Early use of clinical BRCA1/2 testing: associations with race and breast cancer risk. Am J Med Genet A. 2003;117A:154–160. doi: 10.1002/ajmg.a.10928. [DOI] [PubMed] [Google Scholar]
- 20.Vadaparampil ST, McIntyre J, Quinn GP. Awareness, perceptions, and provider recommendation related to genetic testing for hereditary breast cancer risk among at-risk Hispanic women: similarities and variations by sub-ethnicity. J Genet Couns. 2010;19:618–629. doi: 10.1007/s10897-010-9316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall MJ, Olopade OI. Disparities in genetic testing: thinking outside the BRCA box. J Clin Oncol. 2006;24:2197–2203. doi: 10.1200/JCO.2006.05.5889. [DOI] [PubMed] [Google Scholar]
- 22.Thompson HS, Valdimarsdottir HB, Duteau-Buck C, et al. Psychosocial predictors of BRCA counseling and testing decisions among urban African-American women. Cancer Epidemiol Biomarkers Prev. 2002;11:1579–1585. [PubMed] [Google Scholar]
- 23.Pagan JA, Su D, Li L, Armstrong K, Asch DA. Racial and ethnic disparities in awareness of genetic testing for cancer risk. Am J Prev Med. 2009;37:524–530. doi: 10.1016/j.amepre.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 24.Wideroff L, Vadaparampil ST, Breen N, Croyle RT, Freedman AN. Awareness of genetic testing for increased cancer risk in the year 2000 National Health Interview Survey. Community Genet. 2003;6:147–156. doi: 10.1159/000078162. [DOI] [PubMed] [Google Scholar]
- 25.Peters N, Rose A, Armstrong K. The association between race and attitudes about predictive genetic testing. Cancer Epidemiol Biomarkers Prev. 2004;13:361–365. [PubMed] [Google Scholar]
- 26.Armstrong K, Weber B, Ubel PA, Guerra C, Schwartz JS. Interest in BRCA1/2 testing in a primary care population. Prev Med. 2002;34:590–595. doi: 10.1006/pmed.2002.1022. [DOI] [PubMed] [Google Scholar]
- 27.Halbert CH, Kessler LJ, Mitchell E. Genetic testing for inherited breast cancer risk in African Americans. Cancer Invest. 2005;23:285–295. doi: 10.1081/cnv-58819. [DOI] [PubMed] [Google Scholar]
- 28.Thompson HS, Valdimarsdottir HB, Jandorf L, Redd W. Perceived disadvantages and concerns about abuses of genetic testing for cancer risk: differences across African American, Latina and Caucasian women. Patient Educ Couns. 2003;51:217–227. doi: 10.1016/s0738-3991(02)00219-7. [DOI] [PubMed] [Google Scholar]
- 29.Sheppard VB, Mays D, LaVeist T, Tercyak KP, et al. Medical mistrust influences Black women's engagement in BRCA1/2 genetic counseling and testing. J Natl Med Assoc. 2011 doi: 10.1016/s0027-9684(15)30081-x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 31.Doksum T, Bernhardt BA, Holtzman NA. Does knowledge about the genetics of breast cancer differ between nongeneticist physicians who do or do not discuss or order BRCA testing? Genet Med. 2003;5:99–105. doi: 10.1097/01.GIM.0000055198.63593.32. [DOI] [PubMed] [Google Scholar]
- 32.Scheuner MT, Sieverding P, Shekelle PG. Delivery of genomic medicine for common chronic adult diseases: a systematic review. JAMA. 2008;299:1320–1334. doi: 10.1001/jama.299.11.1320. [DOI] [PubMed] [Google Scholar]
- 33.Graves KD, Christopher J, Harrison TM, Peshkin BN, Isaacs C, Sheppard VB. Providers' perceptions and practices regarding BRCA1/2 genetic counseling and testing in African American women. J Genet Couns. 2011 Aug 6; doi: 10.1007/s10897-011-9396-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durfy SJ, Bowen DJ, McTiernan A, Sporleder J, Burke W. Attitudes and interest in genetic testing for breast and ovarian cancer susceptibility in diverse groups of women in western Washington. Cancer Epidemiol Biomarkers Prev. 1999;8:369–375. [PubMed] [Google Scholar]
- 35.Hughes C, Gomez-Caminero A, Benkendorf J, et al. Ethnic differences in knowledge and attitudes about BRCA1 testing in women at increased risk. Patient Educ Couns. 1997;32:51–62. doi: 10.1016/s0738-3991(97)00064-5. [DOI] [PubMed] [Google Scholar]
- 36.Kinney AY, Croyle RT, Dudley WN, Bailey CA, Pelias MK, Neuhausen SL. Knowledge, attitudes, and interest in breast-ovarian cancer gene testing: a survey of a large African-American kindred with a BRCA1 mutation. Prev Med. 2001;33:543–551. doi: 10.1006/pmed.2001.0920. [DOI] [PubMed] [Google Scholar]
- 37.Ramirez AG, Aparicio-Ting FE, de Majors SS, Miller AR. Interest, awareness, and perceptions of genetic testing among Hispanic family members of breast cancer survivors. Ethn Dis. 2006;16:398–403. [PubMed] [Google Scholar]
- 38.Lipkus IM, Iden D, Terrenoire J, Feaganes JR. Relationships among breast cancer concern, risk perceptions, and interest in genetic testing for breast cancer susceptibility among African-American women with and without a family history of breast cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:533–539. [PubMed] [Google Scholar]
- 39.Legler J, Meissner HI, Coyne C, Breen N, Chollette V, Rimer BK. The effectiveness of interventions to promote mammography among women with historically lower rates of screening. Cancer Epidemiol Biomarkers Prev. 2002;11:59–71. [PubMed] [Google Scholar]
- 40.Paskett ED, Harrop JP, Wells KJ. Patient navigation: an update on the state of the science. CA Cancer J Clin. 2011;61:237–249. doi: 10.3322/caac.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wells KJ, Battaglia TA, Dudley DJ, et al. Patient navigation: state of the art or is it science? Cancer. 2008;113:1999–2010. doi: 10.1002/cncr.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee R, Beattie M, Crawford B, et al. Recruitment, genetic counseling, and BRCA testing for underserved women at a public hospital. Genet Test. 2005;9:306–312. doi: 10.1089/gte.2005.9.306. [DOI] [PubMed] [Google Scholar]
- 43.Hall M, Olopade OI. Confronting genetic testing disparities: knowledge is power. JAMA. 2005;293:1783–1785. doi: 10.1001/jama.293.14.1783. [DOI] [PubMed] [Google Scholar]
- 44.Masi CM, Blackman DJ, Peek ME. Interventions to enhance breast cancer screening, diagnosis, and treatment among racial and ethnic minority women. Med Care Res Rev. 2007;64:195S–242S. doi: 10.1177/1077558707305410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahm AK, Sukhanova A, Ellis J, Mouchawar J. Increasing utilization of cancer genetic counseling services using a patient navigator model. J Genet Couns. 2007;16:171–177. doi: 10.1007/s10897-006-9051-6. [DOI] [PubMed] [Google Scholar]
- 46.Vargas RB, Ryan GW, Jackson CA, Rodriguez R, Freeman HP. Characteristics of the original patient navigation programs to reduce disparities in the diagnosis and treatment of breast cancer. Cancer. 2008;113:426–433. doi: 10.1002/cncr.23547. [DOI] [PubMed] [Google Scholar]
- 47.Gilpin CA, Carson N, Hunter AG. A preliminary validation of a family history assessment form to select women at risk for breast or ovarian cancer for referral to a genetics center. Clin Genet. 2000;58:299–308. doi: 10.1034/j.1399-0004.2000.580408.x. [DOI] [PubMed] [Google Scholar]
- 48.Hoskins KF, Zwaagstra A, Ranz M. Validation of a tool for identifying women at high risk for hereditary breast cancer in population-based screening. Cancer. 2006;107:1769–1776. doi: 10.1002/cncr.22202. [DOI] [PubMed] [Google Scholar]
- 49.Reid GT, Walter FM, Brisbane JM, Emery JD. Family history questionnaires designed for clinical use: a systematic review. Public Health Genomics. 2009;12:73–83. doi: 10.1159/000160667. [DOI] [PubMed] [Google Scholar]
- 50.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: The Public Health Disparities Geocoding Project (US) J Epidemiol Community Health. 2003;57:186–199. doi: 10.1136/jech.57.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998;62:145–158. doi: 10.1086/301670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berry DA, Iversen ES, Jr, Gudbjartsson DF, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes. J Clin Oncol. 2002;20:2701–2712. doi: 10.1200/JCO.2002.05.121. [DOI] [PubMed] [Google Scholar]
- 53.Huo D, Senie RT, Daly M, et al. Prediction of BRCA mutations using the BRCAPRO model in clinic-based African American, Hispanic, and other minority families in the United States. J Clin Oncol. 2009;27:1184–1190. doi: 10.1200/JCO.2008.17.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nanda R, Schumm LP, Cummings S, et al. Genetic testing in an ethnically diverse cohort of high-risk women: a comparative analysis of BRCA1 and BRCA2 mutations in American families of European and African ancestry. JAMA. 2005;294:1925–1933. doi: 10.1001/jama.294.15.1925. [DOI] [PubMed] [Google Scholar]
- 55.Vogel KJ, Atchley DP, Erlichman J, et al. BRCA1 and BRCA2 genetic testing in Hispanic patients: mutation prevalence and evaluation of the BRCAPRO risk assessment model. J Clin Oncol. 2007;25:4635–4641. doi: 10.1200/JCO.2006.10.4703. [DOI] [PubMed] [Google Scholar]
- 56.James PA, Doherty R, Harris M, et al. Optimal selection of individuals for BRCA mutation testing: a comparison of available methods. J Clin Oncol. 2006;24:707–715. doi: 10.1200/JCO.2005.01.9737. [DOI] [PubMed] [Google Scholar]
- 57.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 58.Fackenthal JD, Olopade OI. Breast cancer risk associated with BRCA1 and BRCA2 in diverse populations. Nat Rev Cancer. 2007;7:937–948. doi: 10.1038/nrc2054. [DOI] [PubMed] [Google Scholar]
- 59.Halbert CH, Kessler L, Troxel AB, Stopfer JE, Domchek S. Effect of genetic counseling and testing for BRCA1 and BRCA2 mutations in African American women: a randomized trial. Public Health Genomics. 2010;13:440–448. doi: 10.1159/000293990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kinney AY, Gammon A, Coxworth J, Simonsen SE, Arce-Laretta M. Exploring attitudes, beliefs, and communication preferences of Latino community members regarding BRCA1/2 mutation testing and preventive strategies. Genet Med. 2010;12:105–115. doi: 10.1097/GIM.0b013e3181c9af2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rolnick SJ, Rahm AK, Jackson JM, et al. Barriers in identification and referral to genetic counseling for familial cancer risk: the perspective of genetic service providers. J Genet Couns. 2011;20:314–322. doi: 10.1007/s10897-011-9351-3. [DOI] [PubMed] [Google Scholar]