Abstract
A facile synthetic strategy for introducing catecholic moieties into polymeric materials based on a readily available precursor – eugenol – and efficient chemistries – tris(pentafluorophenyl)borane catalyzed silation and thiol-ene coupling is reported. Silyl-protection is shown to be critical for the oxidative stability of catecholic moieties during synthesis and processing which allows functionalized polysiloxane derivatives to be fabricated into 3-D microstructures as well as 2-D patterned surfaces. Deprotection gives stable catechol surfaces with adhesion to a variety of oxide surfaces being precisely tuned by the level of catechol incorporation. The advantage of silyl-protection for catechol functionalized polysiloxanes is demonstrated and represents a promising and versatile new platform for underwater surface treatments.
Keywords: Catechol, Deprotection, Polysiloxane, Wet adhesion, Lithography
INTRODUCTION
The harsh, aqueous environment endured by different marine species has inspired the development of a range of synthetic materials based on the functional groups/nanostructures1,2 and assembly processes3,4 present in the natural system. This is especially true for adhesive proteins derived from marine fouling organisms (e.g. mussels, hydroids, and tubeworms) which have attracted considerable interest because of their rapid and strong binding to nearly all surfaces.5–7 A key chemical functionality present in the adhesive proteins of mussels and sandcastle worms is the amino acid, 3,4-dihydroxy-L-phenylalanine (DOPA),8,9 the catecholic moiety of which has an attractive and diverse range of chemistries including: a) strong coordination complexes with diverse metal ions, b) ability to undergo covalent crosslinking, c) rich oxidation/reduction chemistry and d) a variety of energetic surface interactions.10–14 Mussels appear to have spatial control over all four of the above features within their adhesives11,15–17 but, with the exception of adhesion to living tissues, most efforts using synthetic catechol-functionalized polymers have been stymied by the oxidative instability of unprotected catechols in the neutral to basic pH range. Our goal was to develop a strategy that overcomes this instability, thereby enabling the preparation of a diverse range of 2-D and 3-D features for demanding underwater applications.
Synthetically, the strategy relies upon the preparation of catechol functionalized polyorganosiloxanes (alternatively referred to as silicones or polysiloxanes) from readily available building blocks that are designed to serve as broadly applicable and crosslinkable artificial adhesives. Silicones, as a general class of materials, are ubiquitous in technology, with applications ranging widely from electrical materials to biomaterials as a result of their unique properties such as low glass transition temperature, low surface energy, transparency, good thermal and oxidative stability, low modulus, high flexibility and excellent moldability.18–23 Although polysiloxanes are capable of molding and patterning as crosslinked microstructures, the inherently low mechanical and anti-adhesive properties, as well as side reactions that occur during traditional thermal curing processes, pose complications for employing them as a catechol-based wet adhesive material. Lee and co-workers reported polysiloxane structural pillars having wet/dry adhesiveness resulting from coating of an adhesive polymer onto pre-fabricated polysiloxane pillars inspired by gecko and mussels24 biological nanostructures. While this method forms polysiloxane arrays that are useful for a reversible wet/dry adhesive, it is costly and complicated requiring formation of nanostructured polysiloxanes through electron-beam lithography, followed by coating with under-water adhesive polymers. Of particular note is the care that must be taken to prevent catechol oxidation, which significantly undermines polymer adhesion.25,26
Our strategy overcomes these issues by employing a common building block, eugenol (the active ingredient of clove oil and readily available in large quantities), and two efficient and orthogonal reactions to prepare adhesive catechol functionalized polysiloxanes: (1) tris(pentafluorophenyl)borane (TPFPB)-catalyzed silation and (2) thiol-ene coupling. In turn, this new class of marine-inspired adhesive polysiloxanes has a number of advantages including ease of synthesis, versatility, and inherent stability with the resulting air- and moisture-stable materials being activated through a simple deprotection process. The first enabler in our strategy, the TPFPB-catalyzed silylation, has been used for hydrosilylation of various nucleophilic groups,27–35 including ethers, and is highly efficient and insensitive to moisture.36–38 Moreover, the orthogonal reactivity of TPFPB allows the facile one-step transformation of eugenol into a reactive, bis-silyl-protected DOPA mimic. The silyl protecting groups are key to maintaining the stability of the catechol unit throughout the thiol-ene polymer functionalization, processing and subsequent crosslinking reactions. Moreover, these silyl protecting groups serve to provide long shelf-life to the assembled materials but can be selectively deprotected under mild acidic or basic conditions such as during conventional contact printing strategies, essentially activating the adhesive properties just prior to use.
The second enabler is the thiol-ene reaction that allows the attachment of the protected eugenol moieties to the polymer backbone. Significantly, this reaction proceeds in high yields with tolerance for various functional groups even under solvent-free conditions and has been widely used for the modification of polymers,39–44 thin-film fabrication45–47 and dendrimer synthesis.39,48,49 Due to its high efficiency and simplicity the thiol-ene reaction is an ideal partner for the quantitative functionalization of the siloxane backbone and subsequent preparation of thin films and functionalized polymers.50–52
Herein, we demonstrate the preparation of novel, bio-inspired polysiloxanes with controlled catechol functional group incorporation through facile and efficient chemistry. These building blocks are then used to fabricate three-dimensional microstructures with tunable adhesion and high stability due to reduction of oxidative side reactions for the key catechol units.
RESULTS AND DISCUSSION
The investigation of marine organisms has provided insights into the design criteria needed for the preparation of synthetic underwater adhesive polymers. First of all, the synthetic materials should have unoxidized catecholic moieties for adhesion and the concentration of catecholic moieties should be adjustable to mimic the various adhesive proteins of marine organisms and conversely, various potential applications. Second, facile fabrication strategies for both surface modification and for the construction of 3-D microstructures are highly desirable. In addition, minimal loss of material through solubilization in the surrounding aqueous solution is required for the molded structures to endure, necessitating a degree of hydrophobicity. Lastly, simple protection and deprotection methods are desired to prevent premature oxidation of the catecholic moieties during synthesis and storage of the synthetic adhesive materials. Traditionally, catecholic units are prone to oxidization during synthesis and processing to o-quinone groups which then undergo secondary reaction with nucleophiles, such as amines, thiols and other catechols causing extensive crosslinking and aggregation, resulting in reduced adhesion and poor performance. To address these challenges, silyl-protected catechol (SPC) functionalized polysiloxanes, employing eugenol as a naturally-derived and economically-viable precursor, were prepared and their fabrication into thin films and microstructures studied.
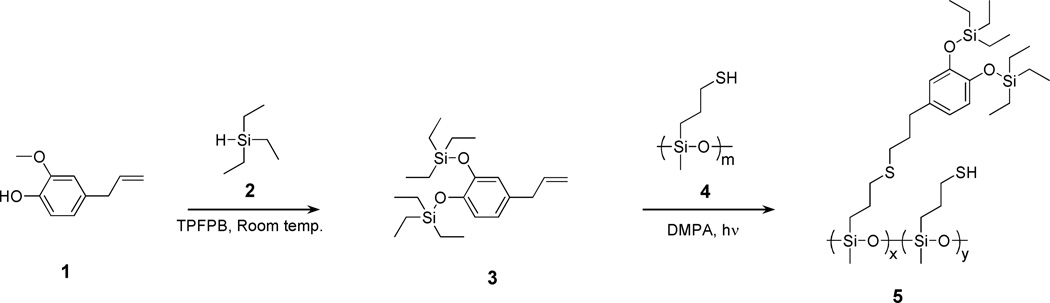
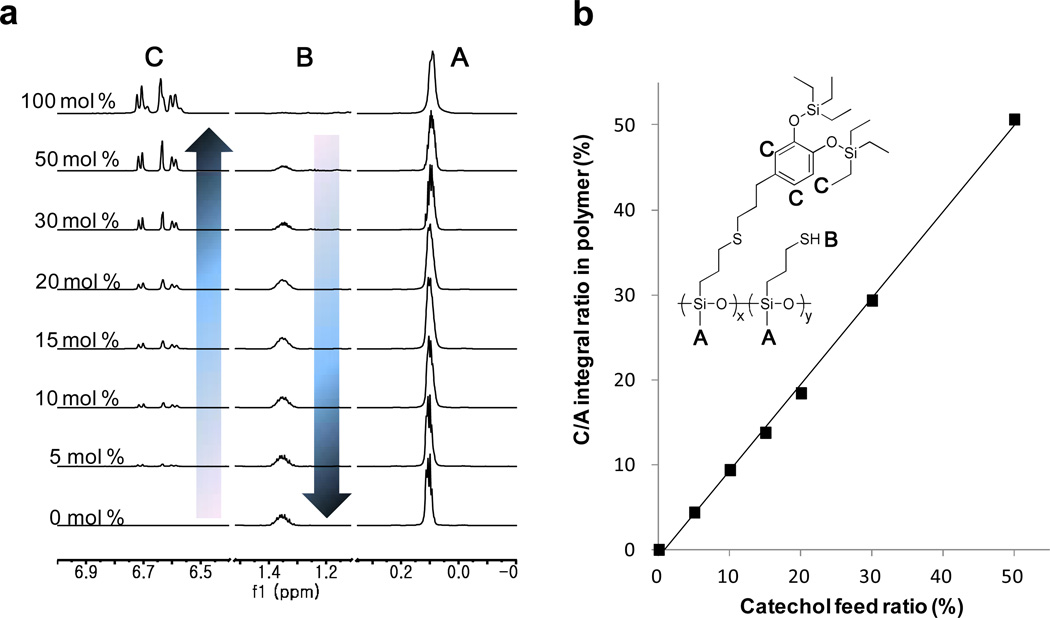
The synthetic strategy for the key silyl-protected catecholic intermediate, 3, and the corresponding SPC-functionalized polysiloxane, 5, is illustrated in Scheme 1. Hydrosilylation of both the phenolic and methyl ether units of eugenol, 1, was achieved through a one-pot transformation catalyzed by tris(pentafluorophenyl)borane (TPFPB) in the presence of triethylsilane, 2. The reaction of 1 and 2 proved to be facile at room temperature under ambient conditions with complete consumption of starting materials occurring within 10 minutes. The crude silyl-protected derivative, 3, was then filtered through neutral alumina to remove the TPFPB, to give 3 as a pure product in quantitative yields without any need for further purification. Auspiciously, the resulting silyl-protected catecholic moiety, 3, retains the terminal alkene group that can be used as a reactive handle for attachment to a wide variety of polymer backbones through thiol-ene chemistry. Employing commercially available poly[(mercaptopropyl) methylsiloxane] (PMMS) (Figure 1a) as the thiol-functional siloxane precursor, polymers functionalized with protected catechol units could be routinely prepared with varying levels of incorporations (5–50 mol%). The facile nature of this chemistry allows a neat mixture of the polymer, 4, varying mole percentages of the silyl-protected catechol, 3, and 2,2-dimethoxy-2-phenylacetophenone (DMPA) to be photoirradiated at room temperature for 30 minutes with quantitative incorporation of the protected catechol, allowing the percent incorporation of catechol to be simply controlled through the feed ratio of ene to thiol (Figure 1b). It should be noted that all SPC-functionalized were stable and could be kept under ambient conditions for weeks or stored at 0°C for months without any observable degradation.
Scheme 1.
Synthesis of silyl-protected catecholic derivative, 3, and reactive silyl-protected catechol functionalized polysiloxane building block, 5.
Figure 1.
Characterization of the level of catechol incorporation for the SPC-functionalized polysiloxanes (a) 1H-NMR spectra of SPC-functionalized polysiloxane with different ratios of silyl-protected catecholic units attached. All spectra are normalized to the intensity of the Si-methyl resonance at ca. 0.1 ppm (b) Integral ratio of catechol/Si-methyl (C/A) ratio groups from 1H-NMR versus catechol to thiol repeat unit feed ratio.
Figure 1a displays the 1H-NMR spectra for a series of SPC-functionalized polysiloxanes and clearly demonstrates unique resonances for each repeat unit which allows the calculation of percent incorporation of the silyl-protected catecholic units as a function of the feed ratio. While the PMMS backbone could be fully functionalized with catecholic units (100 mol %), the highest loading used for this study was 50 mol %. In comparison, naturally-occurring mussel foot protein-5 (mfp-5) of the Mytilus genus contains the highest DOPA loading in nature with ~30 mol% loading of DOPA units along the backbone.53 From a total catechol concentration viewpoint, the polymers in this study are therefore good analogs of those employed by a wide range of marine organisms. Indeed, there are no other reports of polymers functionalized with catecholic moieties to 50 mol % levels, presumably due to oxidative instability and uncontrolled crosslinking. Notably, the silyl protecting groups are stable throughout the synthesis and handling of these materials (as indicated by the resonances at 0.98 and 0.74 ppm in the 1H-NMR spectra) and their presence ensures that the catechol groups will not participate in any undesirable, premature adhesion and are stable to unwanted side reactions involving oxidative pathways.
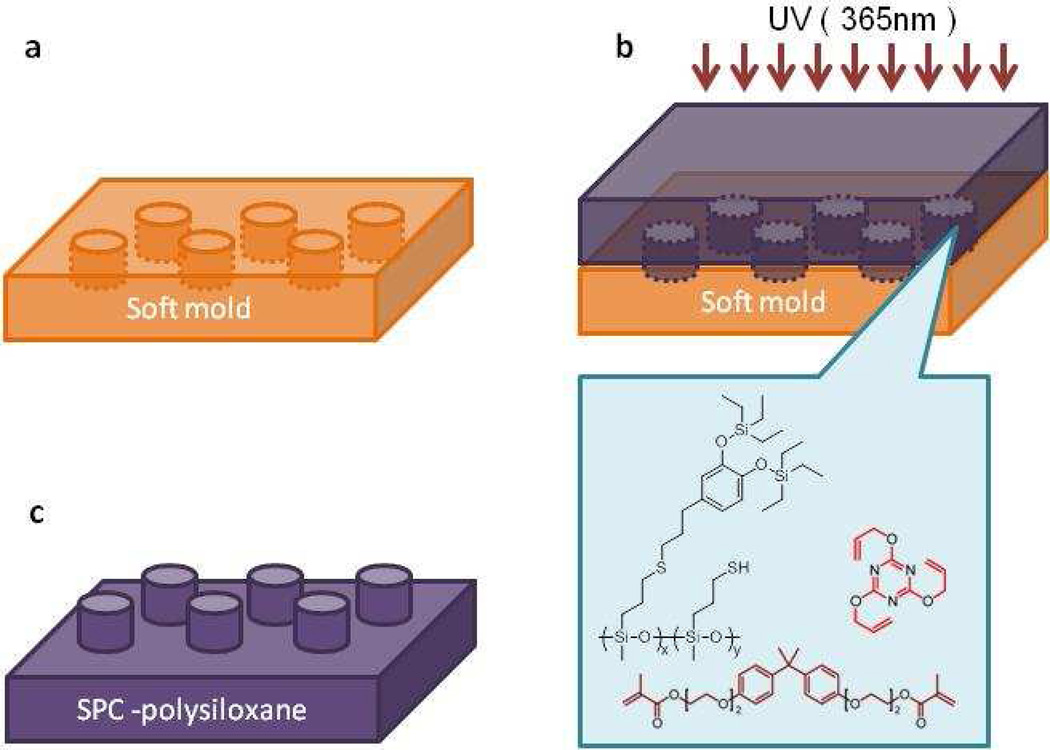
The availability of SPC-functionalized polysiloxanes bearing both protected catechol as well as unreacted thiol groups represents a stable and highly functional platform for the fabrication of crosslinked microstructures. Using imprint lithography we were particularly interested in the generation of micro-pillar arrays and the measurement of adhesive forces with a modified atomic force microscopy (AFM) strategy. A liquid pre-polymer mixture of the SPC-functionalized PMMS, triallyl cyanurate (TAC), dimethacrylate of ethoxylated bisphenol A (BPADMA), and DMPA was therefore applied to a patterned soft imprint mold and cured through UV irradiation (λ = 365 nm, 4.6 mW cm−2) for four minutes under ambient conditions (Figure 2b). This soft mold was prepared from a hard Si master through soft imprint lithography methods using thiol-ene chemistry50 (see Supplementary Figure S2). The photo-cured SPC-functionalized polysiloxane-based replica films were then easily peeled from the soft mold and to ensure that a uniform contact area was being measured in each case, pillars were designed with dimensions smaller than the contact area of the tipless AFM cantilever (100 µm length and 13.5 µm width) and with sufficient spacing between pillars to insure that the contact adhesion of a single pillar was being measured. Figure 3a shows a representative scanning electron microscopy (SEM) image of the fabricated microstructures based on SPC-functionalized polysiloxane-based materials with pillar diameters of 5 µm and height of 10 µm (aspect ratio 2:1).
Figure 2.
Process for fabrication of microstructures using SPC-functionalized polysiloxane and imprint lithography. (a) fabrication of soft imprint mold, (b) casting of a mixture of SPC-functionalized polysiloxane and alkene crosslinkers onto the patterned soft imprint mold followed by photocuring and (c) removal of mold to give SPC-functionalized polysiloxane-based microstructures.
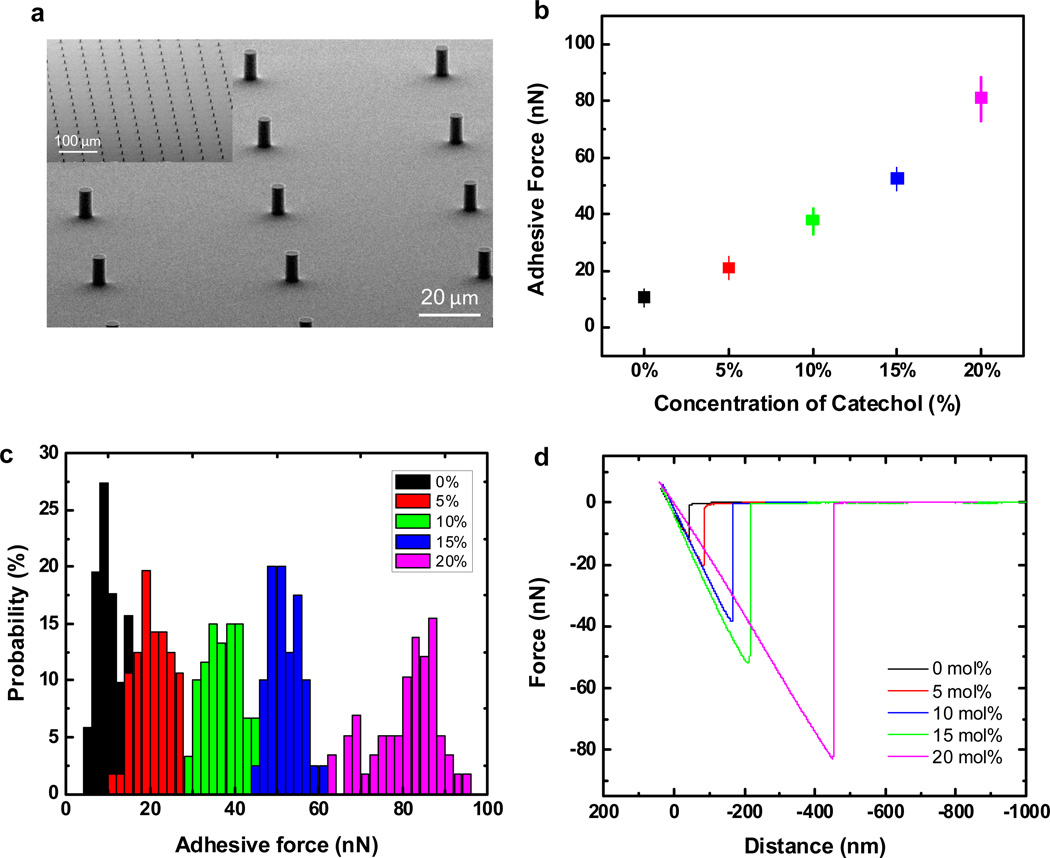
Figure 3.
Measurement of adhesive force for fabricated microstructures based on catechol-functionalized polysiloxanes after deprotection. (a) SEM images of fabricated microstructures; 5 µm diameter, 10 µm height, AR = 2. (b) Adhesive force versus concentration of catechol units in the starting polysiloxane. (c) Histogram of adhesive force versus concentration of catechol units in the starting polysiloxane. (d) Representative force-distance curves of fabricated microstructures incorporating different concentration of catechol from 0 mol% to 20 mol%.
To demonstrate the ability to fabricate microstructures with photo-cured SPC-functionalized polysiloxane-based materials while retaining and tuning their adhesive properties, surface forces were measured in aqueous solutions using an AFM system coupled with a tipless cantilever (Si3N4). The cantilever was coated with a 10 nm thick layer of titanium oxide by e-beam evaporation of Ti followed by O2 plasma treatment to form titanium oxide (TiO2). The oxide surface then provides specific and reversible binding with the catecholic moieties on the surface of photo-cured SPC-functionalized microstructures/films.54 After coating with titanium oxide, the spring constant of each cantilever was calibrated based on their thermal vibration factors before measuring adhesive force. In a typical adhesion experiment, the tipless cantilever first approached the wet surface of the film/microstructure and then, during separation, the retracting force versus extension was measured. The force measurements were performed in aqueous solution buffered at pH 3.0 in order to deprotect the silylated catechol units at the surface of the photo-cured polysiloxane-based films.
Following initial experiments with microstructures of different contact areas (i.e. 5, 10, 20 and 50 µm diameter pillars), it was determined that 5 µm pillars were best suited to the measurable range of adhesion values based on the AFM strategy described above (see Supplementary Figure S6). In addition, to demonstrate the correlation between the adhesive force and the concentration of catecholic moieties, five different ratios (0, 5, 10, 15 and 20 mol%) of silyl-protected catecholic moieties were examined. Significantly, the mean values and standard deviations of single pillars adhesive forces were found to vary from 9.8 to 83 nN as the molar ratio of catechol was varied from 0 to 20 mol% (Figure 3b and 3c). The linear relationship between adhesive force and concentration of catechol units clearly demonstrates the power of this strategy to synthesize and functionalize the surface of crosslinked films with a defined number of catechol moieties in order to modulate physical properties, analogous to marine organisms and the various proteins they produce.17 The representative force-distance curve is shown in Figure 3d with the adhesive force determined from an average of 60 curves.
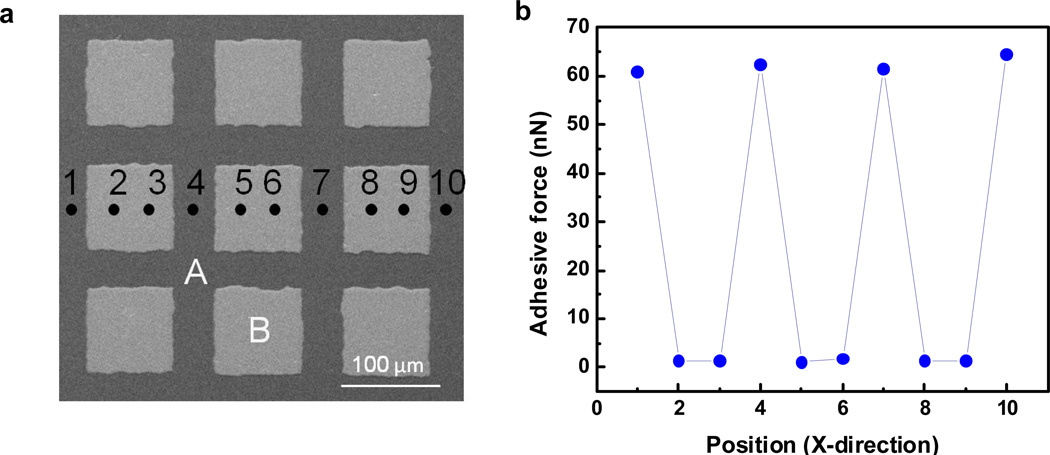
To further demonstrate the utility of protected catechols as a building block for adhesive materials, continuous polysiloxane-based films were patterned by coating with metal (Au on Ti) films deposited by e-beam evaporation using transmission electron microscopy (TEM) grids as a shadow mask. Figure 4a shows a SEM image of the patterned polysiloxane/metal films patterned with dark areas (A) being the thiol-ene derived films with catecholic moieties and bright area (B) being the areas covered with evaporated metal. As the tipless AFM cantilever is moved across the surface a dramatic change in adhesive force is observed as the surface change in adhesive force is observed as the surface changes from catechol (ca. 60nN) to metal (< 5nN) which correlates exactly with the patterned areas and is a further indication of the specific and reversible interaction of the oxide cantilever surface with the catechol groups (Figure 4b). While these results were from thin films containing 15 % catechol groups, it should be noted that similar differential adhesion were obtained for other patterned films with different catechol loadings. These results also demonstrate the versatility and robustness of silane-protected catecholic moieties as the adhesive properties of the final films are not changed during micro-patterning which employs harsh metal deposition techniques. Attempts to perform the same experiments with unprotected catechol groups or after deprotection of the silylated catechols units were not successful, presumably due to the catechol units undergoing unwanted oxidation/side reactions during patterning, processing or deposition.
Figure 4.
(a) SEM of patterned catechol-functionalized polysiloxane film (A: catechol-functionalized polysiloxane area, B: Ti/Au metal coated area). (b) Measured adhesive force versus position across wafer.
The tunable, building block nature of the materials described above, coupled with the oxidative stability of the silyl-protected catechol groups, now permits a wide range of formats and functions to be developed for these systems which in turn allow control over macroscopic properties such as adhesion. To illustrate these features, the application of these materials as bio-inspired underwater adhesives and as active surfaces for capturing/retaining oxide particles was investigated.
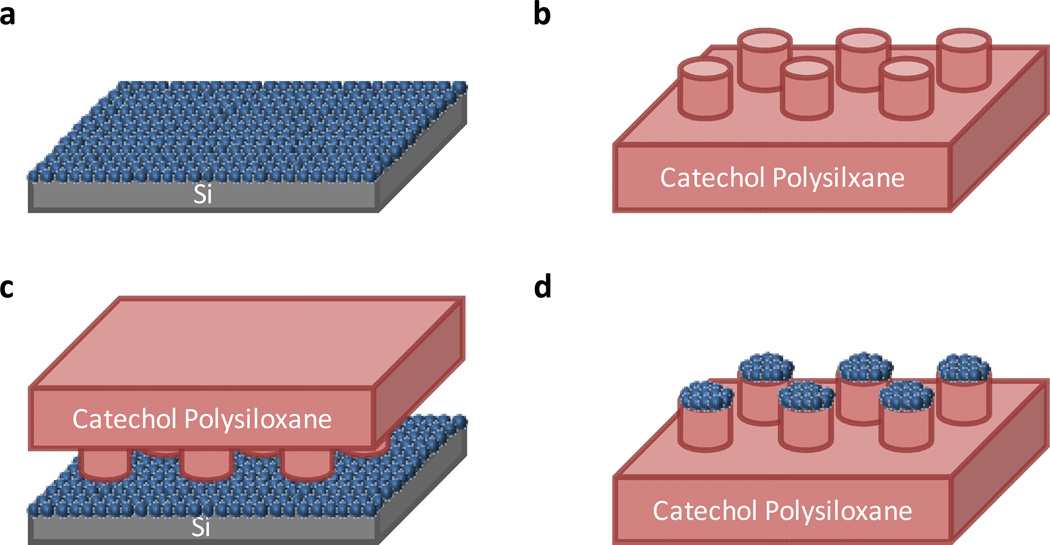
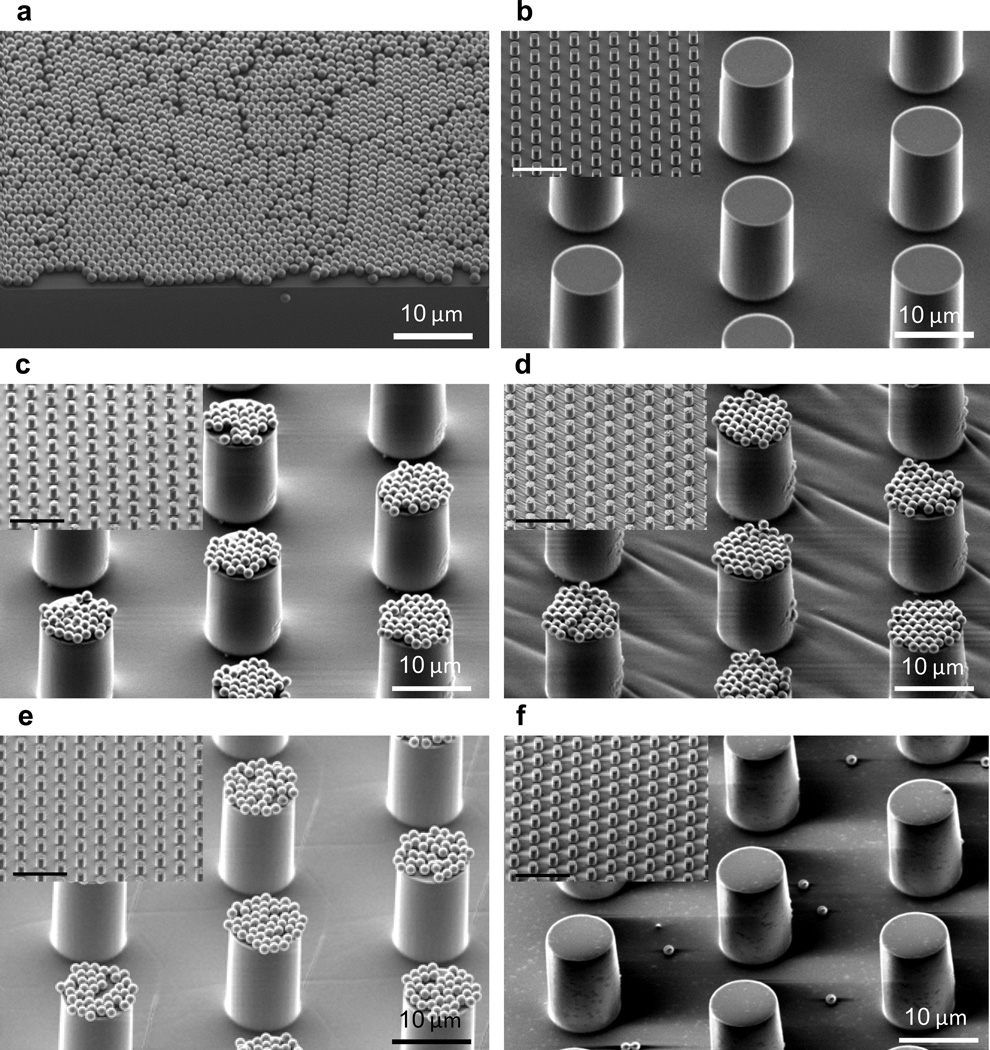
A simple and quantitative strategy was therefore developed through a combination of soft imprint lithography55 and transfer printing56,57 to investigate the 3-D assembly and adhesion of microparticle-based oxide super-structures. The design of these experiments allows many aspects of the power of this protected catechol strategy to be illustrated. For example, the stability of the protected catechol units during process and 3-D fabrication and the ability to control the assembly of complex microstructures through pre-defined adhesion properties.58 It should also be noted that this 3-D assembly strategy represents a variation of the recent subtractive patterning via chemical lift-off lithography developed by Weiss and Andrews. In this strategy, strong reversible interactions (catecholoxide) are exploited for lift-off compared to covalent (Si-O) bonding.59 As shown schematically in Figure 5, arrays of microstructured pillars were brought into contact with oxide particles supported on a solid substrate and due to the lower modulus of the polymeric pillars, these particles were transferred to the tops of the pillars after lift-off. Sonication and extensive washing then served as a quantitative analysis of the adhesion between the particle and the pillar. Using SPC-functionalized polysiloxanes with a 20 % loading of catechols, arrays of pillars (10 µm diameter and 20 µm in height; figure 6b) were prepared, the silyl protecting groups removed by acidic hydrolysis, and a layer of silica particles were transferred to the top surface (Figure 6c). As a control, the same assembly was fabricated using pillars that did not have the silyl protecting groups removed (Figure 6d). Significantly, after sonication and washing of the surfaces, the silica particles remained strongly attached to the tops of the pillars only in the case of the deprotected materials where there is a direct interaction between the oxide surface of the particles and the catechol units of the pillars (Figure 6e). In direct contrast, the silica particles were easily and completely dislodged from the tops of the pillars for the silyl-protected systems (Figure 6f).
Figure 5.
General strategy for qualitatively testing the adhesion of silica particles to functionalized siloxane-based micropillars. (a) preparation of silica particles coated Si substrate; (b) fabrication of SPC-functionalized polysiloxane-based microstructures and activation of catechol; (c) stamping activated catechol-functionalized polysiloxane-based microstructures on coated silica particles; and (d) removal of catechol-functionalized polysiloxane-based film from Si substrate.
Figure 6.
Representative SEM images demonstrating the adhesive capabilities of the fabricated catechol microstructures with silica particles. (a) Silica particles, 1 µm diameter, employed in experiments; (b) Fabricated catechol microstructures, 10 µm diameter and 20 µm height; (c) Silica particles directly transferred to tops of catechol-functionalized pillars; (d) Silica particles directly transferred to tops of silyl-protected catechol-functionalized pillars; (e) Strong adhesion demonstrated by the stability of assembled silica particles on catechol-functionalized pillars after sonication and extensive washing; (f) Loss of adhesion for silyl-protected catechol-functionalized pillars after sonication and extensive washing. Scale bars in small inserts of (b) to (f) are 50 µm.
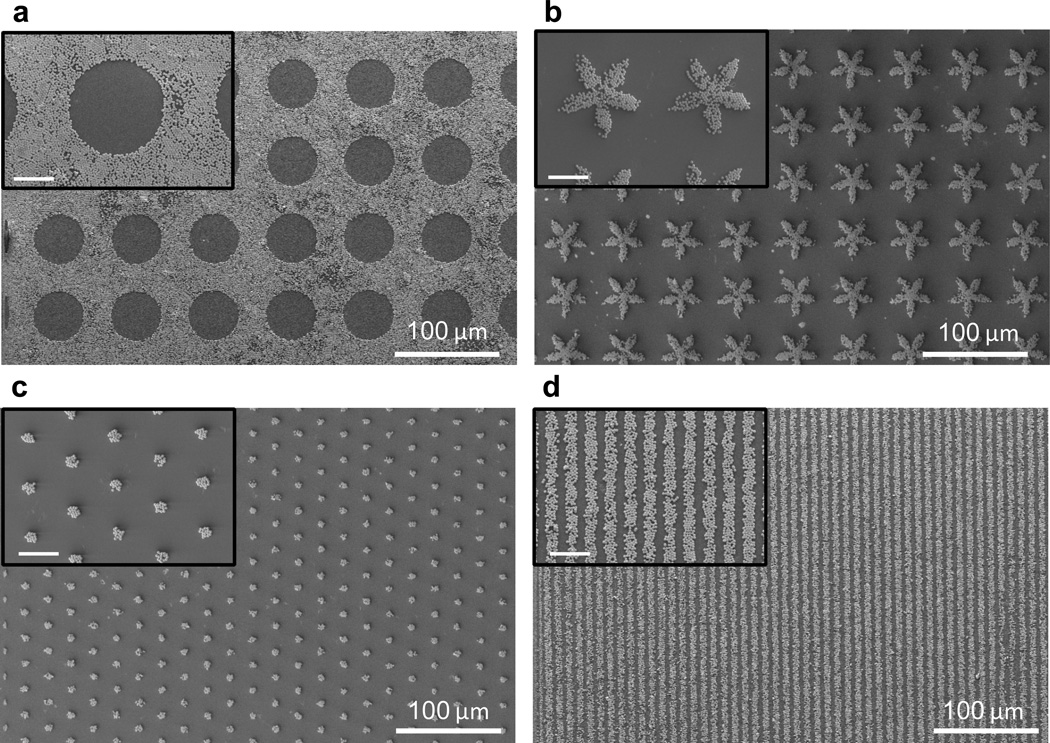
A further demonstration of the bio-mimetic nature of this system was obtained from the fabrication of metal oxide particle assemblies on the surfaces of flexible films. In this example, continuous films were prepared from the SPC-functionalized polysiloxane-based thiol-ene mixture followed by photocrosslinking, and the silica particles were transferred by contact printing between an ‘inked’ conventional polydimethylsiloxane (PDMS) elastomeric stamp and the adhesive surface. Using the approach, various patterns of silica microparticles were easily assembled on the adhesive catechol surfaces (Figure 7) and resulted in strong anchoring that resisted washing and sonication.
Figure 7.
Representative SEM images of assembles formed after transfer printing using micron-sized silica particles: (a) 50 µm diameter circles; (b) 50 µm flower shapes in a square lattice; (c) 7 µm diameter circles in a hexagonal lattice; and (d) 5 µm line and space patterns. Scale bars in small inserts of (a) to (d) are 20 µm.
CONCLUSION
The importance of silyl-protection in the synthesis, processing, and utilization of catechol-based materials has been demonstrated through the development of a facile and efficient strategy for the preparation of polysiloxane derivatives. These silyl-protected catechol polysiloxanes could be fabricated into a variety of 2-D and 3-D patterned surfaces using thiol-ene chemistry with straightforward pH-activated, catechol-mediated adhesion. Significantly, the adhesion is tunable by simply increasing or decreasing the concentration of protected catechol units along the polysiloxane backbone with a key feature of these SPC-functionalized materials being their oxidative stability, which is not affected by processing or fabrication procedures and provides long shelf-life under ambient conditions. The user-friendly nature of their synthesis and subsequent activation chemistry makes these SPC-functionalized polysiloxanes a promising and versatile new platform for underwater surface treatments including paints, coatings, adhesive tapes and glues.
Supplementary Material
Acknowledgments
Funding Sources
This material is based upon work supported by the National Science Foundation (MRSEC Program – DMR-1121053, JH, TK, JMS, JHW, CJH). This work was also partially supported by the Institute for Collaborative Biotechnologies through grant numbers W911NF-07-1-0279 and W911NF-09-D-0001 from the US Army Research Office (SGJ, KLK, CJH), the National Institutes of Health (R01 DE018468 - JHW) and by fellowship support from Samsung (TK).
ABBREVIATIONS
- SPC
Silyl-Protected Catechol.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Complete experimental procedures along with additional supporting data, this material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Capadona JR, Shanmuganathan K, Tyler DJ, Rowan SJ, Weder C. Science. 2008;319:1370–1374. doi: 10.1126/science.1153307. [DOI] [PubMed] [Google Scholar]
- 2.Cho Y, Sundaram HS, Finlay JA, Dimitriou MD, Callow ME, Callow JA, Kramer EJ, Ober CK. Biomacromolecules. 2012;13:1864–1874. doi: 10.1021/bm300363g. [DOI] [PubMed] [Google Scholar]
- 3.Aida T, Meijer EW, Stupp SI. Science. 2012;335:813–817. doi: 10.1126/science.1205962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miesch C, Kosif I, Lee E, Kim J-K, Russell TP, Hayward RC, Emrick T. Angew. Chem.-Int. Edit. 2012;51:145–149. doi: 10.1002/anie.201106665. [DOI] [PubMed] [Google Scholar]
- 5.Waite JH. Integrative and Comparative Biology. 2002;42:1172–1180. doi: 10.1093/icb/42.6.1172. [DOI] [PubMed] [Google Scholar]
- 6.Wiegemann M. Aquat. Sci. 2005;67:166–176. [Google Scholar]
- 7.Silverman HG, Roberto FF. Mar. Biotechnol. 2007;9:661–681. doi: 10.1007/s10126-007-9053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waite JH, Tanzer ML. Science. 1981;212:1038–1040. doi: 10.1126/science.212.4498.1038. [DOI] [PubMed] [Google Scholar]
- 9.Waite JH, Tanzer ML. Biochem. Biophys. Res. Commun. 1980;96:1554–1561. doi: 10.1016/0006-291x(80)91351-0. [DOI] [PubMed] [Google Scholar]
- 10.Waite JH. Int. J. Biol. Macromol. 1990;12:139–144. doi: 10.1016/0141-8130(90)90065-i. [DOI] [PubMed] [Google Scholar]
- 11.Zeng H, Hwang DS, Israelachvili JN, Waite JH. Proc. Natl. Acad. Sci. U. S. A. 2010;107:12850–12853. doi: 10.1073/pnas.1007416107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holten-Andersen N, Harrington MJ, Birkedal H, Lee BP, Messersmith PB, Lee KYC, Waite JH. Proc. Natl. Acad. Sci. U. S. A. 2011;108:2651–2655. doi: 10.1073/pnas.1015862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haeshin L, Dellatore SM, Miller WM, Messersmith PB. Science. 2007;318:426–430. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling D, Park W, Park YI, Lee N, Li F, Song C, Yang S-G, Choi SH, Na K, Hyeon T. Angew. Chem.- Int. Edit. 2011;50:11360–11365. doi: 10.1002/anie.201101521. [DOI] [PubMed] [Google Scholar]
- 15.Sun CJ, Waite JH. Journal of Biological Chemistry. 2005;280:39332–39336. doi: 10.1074/jbc.M508674200. [DOI] [PubMed] [Google Scholar]
- 16.Harrington MJ, Masic A, Holten-Andersen N, Waite JH, Fratzl P. Science. 2010;328:216–220. doi: 10.1126/science.1181044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waite JH, Andersen NH, Jewhurst S, Sun CJ. J. Adhes. 2005;81:297–317. [Google Scholar]
- 18.Polmanteer KE. Rubber Chem. Technol. 1988;61:470–502. [Google Scholar]
- 19.Quinn KJ, Courtney JM. British Polymer Journal. 1988;20:25–32. [Google Scholar]
- 20.Voronokov MG, Milenshkevich VP, Yuzhelevskii YA. The Siloxane Bond. New York: Consultants Bureau; 1978. [Google Scholar]
- 21.Noll W. Chemistry and Technology of Silicones. New York: Academic Press; 1968. [Google Scholar]
- 22.Abbasi F, Mirzadeh H, Katbab AA. Polym. Int. 2001;50:1279–1287. [Google Scholar]
- 23.Yilgor I, McGrath JE. Advances in Organosiloxane Copolymers. Heidelberg: Springer-Verlag; 1988. [Google Scholar]
- 24.Lee H, Lee BP, Messersmith PB. Nature. 2007;448:338–341. doi: 10.1038/nature05968. [DOI] [PubMed] [Google Scholar]
- 25.Yu J, Wei W, Danner E, Israelachvili JN, Waite JH. Advanced Materials. 2011;23:2362–2366. doi: 10.1002/adma.201003580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu J, Wei W, Danner E, Ashley RK, Israelachvili JN, Waite JH. Nature Chemical Biology. 2011;7:588–590. doi: 10.1038/nchembio.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blackwell JM, Foster KL, Beck VH, Piers WE. J. Org. Chem. 1999;64:4887–4892. doi: 10.1021/jo9903003. [DOI] [PubMed] [Google Scholar]
- 28.Chojnowski J, Rubinsztajn S, Cella JA, Fortuniak W, Cypryk M, Kurjata J, Kazmierski K. Organometallics. 2005;24:6077–6084. [Google Scholar]
- 29.Chojnowski J, Fortuniak W, Kurjata J, Rubinsztajn S, Cella JA. Macromolecules. 2006;39:3802–3807. [Google Scholar]
- 30.Thompson DB, Brook MA. Journal of the American Chemical Society. 2008;130:32–33. doi: 10.1021/ja0778491. [DOI] [PubMed] [Google Scholar]
- 31.Rubin M, Schwier T, Gevorgyan V. J. Org. Chem. 2002;67:1936–1940. doi: 10.1021/jo016279z. [DOI] [PubMed] [Google Scholar]
- 32.Blackwell JM, Sonmor ER, Scoccitti T, Piers WE. Org. Lett. 2000;2:3921–3923. doi: 10.1021/ol006695q. [DOI] [PubMed] [Google Scholar]
- 33.Ishihara K, Yamamoto H. Eur. J. Org. Chem. 1999:527–538. [Google Scholar]
- 34.Blackwell JM, Morrison DJ, Piers WE. Tetrahedron. 2002;58:8247–8254. [Google Scholar]
- 35.Gevorgyan V, Rubin M, Benson S, Liu JX, Yamamoto Y. J. Org. Chem. 2000;65:6179–6186. doi: 10.1021/jo000726d. [DOI] [PubMed] [Google Scholar]
- 36.Erker G. Dalton Trans. 2005:1883–1890. doi: 10.1039/b503688g. [DOI] [PubMed] [Google Scholar]
- 37.Roesler R, Har BJN, Piers WE. Organometallics. 2002;21:4300–4302. [Google Scholar]
- 38.Piers WE, Chivers T. Chem. Soc. Rev. 1997;26:345–354. [Google Scholar]
- 39.Killops KL, Campos LM, Hawker CJ. Journal of the American Chemical Society. 2008;130:5062–5064. doi: 10.1021/ja8006325. [DOI] [PubMed] [Google Scholar]
- 40.Campos LM, Killops KL, Sakai R, Paulusse JMJ, Damiron D, Drockenmuller E, Messmore BW, Hawker CJ. Macromolecules. 2008;41:7063–7070. [Google Scholar]
- 41.Gress A, Volkel A, Schlaad H. Macromolecules. 2007;40:7928–7933. [Google Scholar]
- 42.Justynska J, Schlaad H. Macromol. Rapid Commun. 2004;25:1478–1481. [Google Scholar]
- 43.David RLA, Kornfield JA. Macromolecules. 2008;41:1151–1161. [Google Scholar]
- 44.ten Brummelhuis N, Diehl C, Schlaad H. Macromolecules. 2008;41:9946–9947. [Google Scholar]
- 45.Hoyle CE, Lee TY, Roper T. J. Polym. Sci. Pol. Chem. 2004;42:5301–5338. [Google Scholar]
- 46.Cole MA, Bowman CN. J. Polym. Sci. Pol. Chem. 2012;50:4325–4333. doi: 10.1002/pola.26245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lo Conte M, Robb MJ, Hed Y, Marra A, Malkoch M, Hawker CJ, Dondoni A. J. Polym. Sci. Pol. Chem. 2011;49:4468–4475. doi: 10.1002/pola.24888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang T, Amir RJ, Khan A, Ohshimizu K, Hunt JN, Sivanandan K, Montanez MI, Malkoch M, Ueda M, Hawker CJ. Chem. Commun. 2010;46:1556–1558. doi: 10.1039/b921598k. [DOI] [PubMed] [Google Scholar]
- 49.Amir RJ, Albertazzi L, Willis J, Khan A, Kang T, Hawker CJ. Angew. Chem.-Int. Edit. 2011;50:3425–3429. doi: 10.1002/anie.201007427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campos LM, Meinel I, Guino RG, Schierhorn M, Gupta N, Stucky GD, Hawker CJ. Advanced Materials. 2008;20:3728–3733. [Google Scholar]
- 51.Xu T, Stevens J, Villa JA, Goldbach JT, Guarim KW, Black CT, Hawker CJ, Russell TR. Advanced Functional Materials. 2003;13:698–702. [Google Scholar]
- 52.Barner-Kowollik C, Du Prez FE, Espeel P, Hawker CJ, Junkers T, Schlaad H, Van Camp W. Angew. Chem.-Int. Edit. 2011;50:60–62. doi: 10.1002/anie.201003707. [DOI] [PubMed] [Google Scholar]
- 53.Waite JH, Qin XX. Biochemistry. 2001;40:2887–2893. doi: 10.1021/bi002718x. [DOI] [PubMed] [Google Scholar]
- 54.Lee H, Scherer NF, Messersmith PB. Proc. Natl. Acad. Sci. U. S. A. 2006;103:12999–13003. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Odom TW, Love JC, Wolfe DB, Paul KE, Whitesides GM. Langmuir. 2002;18:5314–5320. [Google Scholar]
- 56.Meitl MA, Zhu ZT, Kumar V, Lee KJ, Feng X, Huang YY, Adesida I, Nuzzo RG, Rogers JA. Nature Materials. 2006;5:33–38. [Google Scholar]
- 57.Park J, Hammond PT. Advanced Materials. 2004;16:520–525. [Google Scholar]
- 58.Kim T-H, Cho K-S, Lee EK, Lee SJ, Chae J, Kim JW, Kim DH, Kwon J-Y, Amaratunga G, Lee SY, Choi BL, Kuk Y, Kim JM, Kim K. Nature Photonics. 2011;5:176–182. [Google Scholar]
- 59.Liao WS, Cheunkar S, Cao HH, Bednar HR, Weiss PS, Andrews AM. Science. 2012;337:1517–1521. doi: 10.1126/science.1221774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.