Abstract
Objective
To evaluate rates of antiretroviral therapy (ART) initiation within 12 months of a new HIV diagnosis in Durban, South Africa.
Design
Prospective observational cohort.
Methods
Adults (≥18 years) were enrolled before HIV testing at two outpatient clinics into the South African Test, Identify and Link cohort. Both sites offer comprehensive HIV care. HIV test results, CD4 cell counts, dates of ART initiation and dates of death were collected from medical records and 12-month patient/family interviews were conducted. ART eligibility was defined as a CD4 cell count less than 200 cells/μl within 90 days of HIV diagnosis. The primary endpoint was ART initiation within 12 months for ART-eligible subjects.
Results
From November 2006 to October 2008, 1474 newly diagnosed HIV-infected outpatients were enrolled, 1012 (69%) of whom underwent CD4 cell count testing within 90 days. The median CD4 cell count was 159 cells/μl (interquartile range 65–299). Of those who underwent CD4 cell count testing, 538 (53%) were ART-eligible. Only 210 (39%) eligible enrollees were known to have initiated ART within 12 months. Among ART-eligible subjects, there were 108 known deaths; 82% occurred before ART initiation or with unknown ART initiation status. Men [rate ratio (RR) 1.3, 95% confidence interval (CI) 1.1–1.5] and subjects without an HIV-infected family member/friend (RR 1.3, 95% CI 1.1–1.7) were more likely not to start ART.
Conclusion
Less than half of ART-eligible subjects started ART within 12 months. Substantial attrition and mortality follow HIV diagnosis before ART initiation in Durban, South Africa. Major efforts directed towards earlier HIV diagnosis, effective linkage to care and timely ART initiation are urgently needed.
Keywords: HIV-1, HIV testing, linkage to care, loss to care, South Africa
Introduction
Over 5 million people are living with HIV in South Africa; an estimated 300 000 South Africans die of the disease per year [1]. The epidemic has generated a massive response; in collaboration with the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR), The Global Fund for AIDS, Tuberculosis and Malaria, and other partners, South Africa now has the largest antiretroviral therapy (ART) programme in the world [1–6]. Only an estimated 30% of those in need of treatment in South Africa are, however, receiving it [2].
Little is known about the rate at which HIV-infected individuals in resource-limited settings fail to enter care following a new diagnosis. Studies that follow patients from the time of ART initiation or HIV clinic enrollment show high rates of early attrition and mortality from ART programmes [7–12]. Routine monitoring and evaluation data and most cohort studies do not, however, account for patients who fail to enter care or die before HIV clinic enrollment. Understanding both the points of attrition along the care path following a new HIV diagnosis and the predictors of failing to start ART are critical for designing interventions to improve clinical outcomes. Our objective was to determine the rate of ART initiation for eligible outpatients within 12 months of a positive HIV test in Durban, South Africa, and to identify baseline factors associated with failure to be on ART at one year.
Methods
Study setting
Study participants were enrolled in the outpatient setting at two partly government-subsidized hospitals in Kwa-Zulu-Natal, the South African province with the highest HIV prevalence [13]. McCord Hospital is a 142-bed state-aided (public/private partnership) general hospital that serves a mainly urban population from the greater Durban area, as well as more distant parts of KwaZulu-Natal. Study enrollment took place in McCord’s outpatient department, which sees 3000–4000 adults per month [14]. McCord charges subsidized fees for services and treatment; during the study period patients were charged 140–200 ZAR (US$18–26, 2008) for a medical consultation in the outpatient department. St Mary’s Hospital, Mariannhill, located 20 km west of Durban, is a state-aided, 200-bed general district hospital serving a poorer, peri-urban patient base from a defined catchment area in the surrounding communities. A higher state subsidy permits St Mary’s to charge lower service fees than McCord. Subjects were enrolled in the St Anne’s primary health care clinic, located on the grounds of St Mary’s, and were charged 50–70 ZAR (US$6–9, 2008) per visit during the study period.
Both McCord and St Mary’s Hospitals offer rapid HIV testing in the outpatient setting as per South African guidelines [15]. At McCord Hospital, from the start of the study to June 2007, patients newly diagnosed with HIV were referred to the HIV clinic in an adjacent building where they paid a 90 ZAR (US$12, 2008) registration fee, and were offered CD4 cell count testing; starting in June 2007, CD4 cell count testing at the HIV clinic became free of charge to the patient. At St Mary’s Hospital, newly diagnosed HIV-infected individuals were offered CD4 cell count testing free of charge throughout the study period. Both sites have PEPFAR-funded HIV clinics on the hospital grounds with a consistent supply of ART. Patients at both sites are deemed ART-eligible if they have a CD4 cell count less than 200 cells/μl or meet clinical criteria (World Health Organization stage 3 or 4). As is typical of South African ART programmes, ART-eligible patients undergo a three-session HIV literacy training and psychosocial/clinical evaluation in preparation for ART initiation [10,16,17]. Both sites maintain electronic medical records that can be accessed for patient-level information regarding CD4 cell counts, ART initiation and known/documented deaths.
Study participants, eligibility, enrollment and follow-up procedures
English or Zulu speaking adults (≥18 years) who presented for HIV testing at each of the outpatient study sites between November 2006 and October 2008 were eligible for enrollment in the South African Test, Identify and Link (STIAL) cohort [18]. Consenting subjects were included if they were not already known to be HIV infected and were willing to share HIV test results with research personnel. Pregnant women and patients presenting to the outpatient department on stretchers were excluded. Subjects were enrolled before HIV testing, were administered a baseline questionnaire in English or Zulu by a trained research assistant, and were asked to provide the research assistant with telephone contact details. After enrollment, subjects underwent HIV testing; individuals newly diagnosed with HIV were counselled to undergo CD4 cell count testing to ascertain disease severity and to establish ART eligibility. Those deemed ART-eligible were subject to standard procedures of HIV care and ART initiation at both study sites.
Subjects were contacted approximately 6 and 12 months after enrollment to complete follow-up questionnaires and to ascertain whether HIV care or ART was initiated at a non-study site. Follow-up was complete to June 2009. Whenever feasible, an in-person follow-up interview was performed on a day the study subject was scheduled to be at the study site, otherwise follow-up questionnaires were completed over the telephone. A minimum of three phone call attempts at different times of day on different days were undertaken at both follow-up time points before a patient was declared ‘lost to follow-up’. At the 6- and 12-month time points, trained research personnel also performed an electronic medical record review at the study site for each patient to ascertain whether the patient underwent CD4 cell count testing, received the CD4 cell count result and started ART.
The study protocol and data collection instruments were approved by the McCord Hospital Ethics Committee, the St Mary’s Hospital, Mariannhill, Ethics Committee (both in Durban, South Africa) and the Partners Health Care Human Research Committee (Boston, Massachusetts, USA).
Data elements
At the time of study enrollment, we collected demographic data from each subject including age, sex, educational level, marital status, employment status, income sources and household composition, including whether the subject had a known friend or family member infected with HIV. Patients also reported mode of travel, distance and travel time from home to the study site. Clinical information included previous HIV testing history, recent healthcare utilization, tuberculosis history, functional status, emotional health and alcohol and illicit drug use.
At the 6 and 12-month time points, we ascertained all dates and results of CD4 cell counts, documented receipt of these test results and ART initiation based on care received at the study sites (available by electronic medical records), as well as HIV care at other sites self-reported by the patients. In addition, we obtained dates of deaths from clinical records and follow-up telephone contacts.
Statistical analysis
The primary outcome was ART initiation within 12 months of enrollment for eligible participants. Eligible subjects were defined as those who had a CD4 cell count of less than 200 cells/μl within 90 days of their HIV test. ART initiation was defined by documentation of ART dispensing with an ART start date in the electronic medical record at each of the study sites. These strict criteria were chosen to ensure that the primary analysis would be limited only to those study subjects deemed ART-eligible at baseline enrollment. We conducted a sensitivity analysis using more ‘liberal’ criteria for ART initiation by including subjects who: (i) had a CD4 cell count of less than 200 cells/μl at any time during the study period; (ii) were ART-eligible based on clinical criteria; or (iii) who initiated ART as either recorded in the electronic medical record at a study or by self-report at a non-study site at the time of follow-up interviews.
We compared baseline characteristics of ART-eligible study participants who were known to initiate ART within 12 months and those who were not using the χ2 test for categorical data and the Student’s t-test for continuous variables. We used the Wilcoxon rank sums test to compare ordinal or interval variables with non-normal distributions. Predictors that were statistically significant in these bivariate analyses were advanced to a multivariate Poisson regression. Using Poisson regression, we estimated rate ratios (RR) and 95% confidence intervals (CI) for predictors of failure to initiate ART during the study period. Associations were examined at a P <0.05 significance level (two-sided test).
We used the Kaplan–Meier method to estimate time from HIV testing to ART initiation among those with a known ART initiation date. We tested the difference in time to ART initiation by sex using a log rank test. We evaluated the proportion of subjects who died over the course of the study, assessing pre and post-ART initiation mortality among those who were ART-eligible. All analyses were performed using Stata statistical software (Stata Statistical Software Release 10, StataCorp, College Station, Texas, USA).
Results
Cohort characteristics
From November 2006 to October 2008, 3401 patients were screened for enrollment in the study (Fig. 1). Those who reported they were already known to be HIV-infected (n =144), less than 18 years of age (n =143), were on a stretcher (n =5), were unable to consent (n =15), did not speak the study languages (n =1), did not complete the screening process (n =247), or declined to participate (n =69) were excluded. Among the 2777 subjects enrolled, 71 did not complete HIV testing or did not have results available. Of the remaining 2706 subjects, 1226 tested HIV negative, 1474 were HIV-infected (HIV prevalence 55%), and six had indeterminate rapid HIV test results. The median age of the 1474 HIV-infected individuals was 34 years [interquartile range (IQR) 28–41] and 51% were women.
Fig. 1.
Cohort flow diagram showing study enrollment, HIV test results, CD4 cell count test results and antiretroviral therapy initiation
ART, Antiretroviral therapy.
Cohort follow-up
As of June 2009, the median follow-up time was 12 months (IQR 8.0–14.0). Of the 1474 HIV-infected individuals, 1012 (69%) underwent CD4 cell count testing within 90 days of HIV diagnosis; the median CD4 cell count for those who underwent testing was 159 cells/μl (IQR 65–299). Of these, 538 (53%) had a CD4 cell count less than 200 cells/μl and were therefore determined to be ART-eligible; the median CD4 cell count for this group was 81 cells/μl (IQR 36–132). Less than half of ART-eligible subjects were employed full time or lived less than 10 km from the enrollment site (Table 1). Two hundred and ten (39%) ART-eligible patients were known to have initiated ART during the follow-up period. Three-hundred and ninety-one (27%) subjects in the HIV-infected cohort were unreachable by telephone and did not have follow-up information in the medical record; these patients were considered lost to follow-up and to have an unknown ART initiation status.
Table 1.
Baseline characteristics of antiretroviral therapy-eligible patients in two outpatient settings in Durban, South Africa (N = 538).
| n (%) | |
|---|---|
| Demographic characteristics | |
| Men | 272 (51) |
| Women | 266 (49) |
| Age, median in years (IQR) | 35 (29–42) |
| Employment status | |
| Employed full-time | 249 (46) |
| Not employed full-time | 287 (54) |
| Location of employment | |
| Works outside the home | 347 (65) |
| No work outside the home | 187 (35) |
| Known HIV-infected family/friend | |
| Yes | 90 (17) |
| No | 441 (83) |
| Living arrangement | |
| Lives with HIV-infected person | 38 (7) |
| Does not live with HIV-infected person | 499 (93) |
| Lives alone | 73 (14) |
| Does not live alone | 462 (86) |
| Geographical characteristics | |
| Site of care | |
| McCord Hospital | 231 (43) |
| St Mary’s Hospital | 307 (57) |
| Distance from home to clinic | |
| <10 km | 263 (49) |
| ≥10 km | 270 (51) |
| Transportation to the clinic | |
| Public | 467 (90) |
| Private | 49 (10) |
| Baseline CD4 cell count, median/μl (IQR) | 81 (36–132) |
| Ever treated for tuberculosis | |
| No prior tuberculosis treatment | 442 (83) |
| Previous tuberculosis treatment | 93 (17) |
| HIV testing history | |
| Has had previous HIV test | 49 (9) |
| Has not had previous HIV test | 488 (91) |
IQR, Interquartile range. Data are no. (%) of patients in each category, unless otherwise indicated.
Time from HIV diagnosis to initiation of antiretroviral therapy
Figure 2 depicts the time from HIV diagnosis to ART initiation for subjects ART-eligible at baseline (CD4 cell count <200 cells/μl within 90 days) with known ART initiation dates. The median time from HIV diagnosis to ART start was 6.6 months. The proportion of women who had started ART by 6 months (55%, 95% CI 47.8–61.5%) was significantly higher than the proportion of men (40%, 95% CI 32.9–47.7, P <0.001).
Fig. 2.
Kaplan–Meier estimate of time from HIV diagnosis to antiretroviral therapy start for patients antiretroviral therapy eligible at baseline enrollment, stratified by sex
ART, Anti-retroviral therapy.
Factors associated with failure to initiate antiretroviral therapy
We compared the baseline characteristics of ART-eligible subjects who did not initiate ART or had an unknown ART initiation status with subjects who were known to start ART (Table 2). In bivariate analyses, we found that men were more likely to fail to initiate ART (70% of men versus 52% of women, P <0.001). Among those who did not have family or friends who they knew to be HIV-infected, 64% did not start ART within the follow-up period compared with 48% who had a family member or friend known to be HIV-infected (P =0.005). There was no association between failure to initiate ART and median age or baseline CD4 cell count (P =0.30 and P =0.63, respectively).
Table 2.
Baseline characteristics of antiretroviral therapy-eligible patients within 12 months of HIV diagnosis in two outpatient settings in Durban, South Africa (N = 538).
| Not known to start ART N (%) | Known to start ART N (%) | Unadjusted RR (95% CI) | Adjusted RR (95% CI) | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Men | 191 (70) | 81 (30) | 1.36 (1.18–1.57) | 1.32 (1.14–1.54) |
| Women | 137 (52) | 129 (48) | 1.00 | 1.00 |
| Employment status | ||||
| Employed full-time | 159 (64) | 90 (36) | 1.10 (0.96–1.26) | |
| Not employed full-time | 167 (58) | 120 (42) | 1.00 | |
| Location of employment | ||||
| Works outside the home | 223 (64) | 124 (36) | 1.17 (1.00–1.36) | 1.09 (0.94–1.28) |
| No work outside the home | 103 (55) | 84 (45) | 1.00 | 1.00 |
| Known HIV-infected family/friend | ||||
| Yes | 281 (64) | 160 (36) | 1.33 (1.06–1.67) | 1.35 (1.08–1.68) |
| No | 43 (48) | 47 (52) | 1.00 | 1.00 |
| Living arrangement | ||||
| Lives alone | 48 (66) | 25 (34) | 1.10 (0.91–1.32) | |
| Does not live alone | 277 (60) | 185 (40) | 1.00 | |
| Geographical characteristics | ||||
| Site of care | ||||
| St Mary’s Hospital | 199 (65) | 108 (35) | 1.16 (1.01–1.34) | 1.21 (1.05–1.39) |
| McCord Hospital | 129 (56) | 102 (44) | 1.00 | |
| Distance from home to clinic | ||||
| <10 km | 161 (61) | 102 (39) | 1.00 (0.87–1.14) | |
| ≥10 km | 166 (61) | 104 (39) | 1.00 | |
| Transportation to the clinic | ||||
| Public | 292 (63) | 175 (37) | 1.18 (0.90–1.55) | |
| Private | 26 (53) | 23 (47) | 1.00 | |
| Clinical characteristics | ||||
| Baseline CD4 cell count | ||||
| CD4 cell count ≥100 cells/μl | 138 (62) | 84 (38) | 1.03 (0.90–1.19) | 1.07 (0.93–1.22) |
| CD4 cell count <100 cells/μl | 190 (60) | 126 (40) | 1.00 | |
| Ever treated for tuberculosis | ||||
| No previous tuberculosis treatment | 273 (62) | 169 (38) | 1.06 (0.88–1.28) | |
| Previous tuberculosis treatment | 54 (58) | 39 (42) | 1.00 | |
| HIV testing history | ||||
| Has had prior HIV test | 36 (73) | 13 (27) | 1.23 (1.02–1.48) | 1.15 (0.96–1.37) |
| Has not had previous HIV test | 291 (60) | 196 (40) | 1.00 | 1.00 |
ART, Antiretroviral therapy; CI, confidence interval; RR, rate ratio.
Controlling for study site, being male (RR 1.3, 95% CI 1.1–1.5) and not having a family member or friend known to be HIV-infected (RR 1.3, 95% CI 1.1–1.7) remained independently associated with failing to start ART during the follow-up period.
Mortality during the 12 months following HIV diagnosis
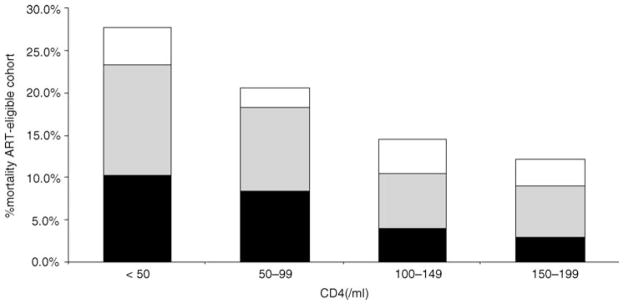
Fifteen per cent (221/1474) of the HIV-infected cohort overall died during the follow-up period. Of the 538 ART-eligible individuals, 108 (20%) died. Those with the lowest CD4 cell counts were most likely to die (test for linear trend P <0.001, Fig. 3), with 28% of those with CD4 cell counts less than 50 cells/μl dying during the study period. In all CD4 cell count strata, the majority of deaths (82%, 89/108) occurred among patients before ART initiation or with unknown ART initiation status (Fig. 3).
Fig. 3.
Mortality among antiretroviral therapy-eligible subjects, based on ART initiation status (NU108 deaths among 536 ART-eligible subjects)
□ On ART;
 unknown ART status; ■ pre-ART. ART, Antiretroviral therapy.
unknown ART status; ■ pre-ART. ART, Antiretroviral therapy.
Results of sensitivity analysis
We performed a sensitivity analysis employing alternative definitions of ART eligibility by extending the definition to those who underwent CD4 cell count testing more than 90 days from HIV testing, who were ART-eligible based on clinical criteria, who started ART at a study site or at a non-study site as reported in patient interviews, and who started ART at any time during the study period. With these alternative definitions, the number of study participants included in the analysis who obtained a CD4 cell count increased to 1092 (74%) subjects and those who were eligible for ART increased to 711 (66%). The proportion who started ART among those who became eligible at any time during the study period increased to 62% (443/711).
When we included all subjects who became ART-eligible at any time during the study period, not only those with CD4 cell counts less than 200 cells/μl within 90 days of HIV diagnosis, the same predictors were significantly associated with failure to initiate ART. Two additional factors became significant in that model: having a job outside of the home and having had a previous HIV test.
Discussion
This study evaluated the rate of ART initiation within 12 months of a new HIV diagnosis in the outpatient setting among ART-eligible patients in Durban, South Africa. Although a substantial proportion of study subjects had CD4 cell counts less than 200 cells/μl (53%, 538/1012), and were therefore eligible for ART according to South African guidelines [15] at the time of study enrollment, only 39% (210/538) of eligible subjects were known to have started ART within 12 months. A substantial number of ART-eligible individuals died during the study period (20%, 108/538), with the vast majority of deaths (82%, 89/108) occurring before ART initiation or among subjects with unknown ART status. This study highlights the substantial losses from care and mortality that occur before entry into ART programmes, a time period for which limited data have previously been available.
Efforts are needed to improve entry into care following a new HIV diagnosis. There are multiple points in the HIV care trajectory from diagnosis to treatment initiation when patients may be lost; as has been seen in a retrospective study from routinely collected data in Mozambique [19], a critical point of attrition is between HIV testing and obtaining a CD4 cell count to evaluate ART eligibility. This may reflect a physical or programmatic disconnect between HIV testing sites and ART treatment programmes.
We document a median 6.6 month interval from the time of HIV diagnosis to ART initiation. Although the delay to treatment initiation may be justified by the need for careful investigation and treatment for opportunistic infections and tuberculosis, as well as HIV literacy training and readiness, it has been identified as a period of substantial mortality [16,20,21]. Providing CD4 cell count testing at the time of HIV diagnosis and allowing for ‘fast-tracking’, or prioritizing, of patients with the most advanced immune deficiency may help. Programmatic efforts to minimize delays are critical, particularly given the severe immune suppression seen in South African programmes at the time of ART programme entry [10,14,17,20]. Both outpatient study sites now offer baseline CD4 cell count testing free of charge following a new HIV diagnosis to facilitate more rapid entry into care [16]. McCord Hospital has also changed its ART literacy training from weekly sessions for 3 weeks to a shorter time frame to facilitate more rapid ART initiation [16].
A major contribution of this study is the active patient follow-up following HIV diagnosis to ascertain vital status. Pre-treatment mortality among ART programme enrollees, particularly those with low baseline CD4 cell counts, has been seen in a model community-based programme in Cape Town [10,21] as well as in Durban [16]. This study shows the substantial burden of mortality occurring before ART programme entry that would otherwise go unaccounted for in PEPFAR statistics and standard ART programme monitoring and evaluation.
Concerns as to whether men are successfully accessing HIV prevention, testing and treatment in Africa have recently been raised [2,22]. Men in this study were significantly less likely to start ART than women (70% men did not start versus 52% of women). These results echo findings from recent reviews of the sex distribution of HIV-infected adults receiving ART in resource-constrained settings [23,24]. The Antiretroviral Treatment in Lower Income Countries (ART–LINC) collaboration pooled patient data from over 28 000 patients from 23 African cohorts and found that although men made up approximately 41% of infected patients, a significantly smaller proportion of ART recipients were men – 32% [22,23]. Although women may have more opportunities to enter care and treatment through antenatal clinics, which may account for some of the observed sex differences [2], the subjects in this study were diagnosed with HIV in a general medical outpatient setting and yet women were still accessing care faster and at higher rates. Innovative efforts are needed to obtain a better understanding of men’s health-seeking behaviours and to improve access to care for men.
Those who failed to start ART during the study period were less likely to have family or friends who they knew to be HIV infected. This finding supports the notion that disclosure within social networks promotes health-seeking behaviour [25,26] and highlights the potential positive impact of disclosure to family and friends on linkage to HIV care for the index patient.
This study has several limitations. A large proportion (27%) of patients could not be reached by telephone during follow-up, and did not have health information available in the electronic medical record to ascertain their status at the 12-month time point. A systematic review and meta-analysis of the mortality among patients lost to follow-up in ART treatment programmes in sub-Saharan Africa found that mortality in this group was 46% [27]. As a result, our mortality estimate of approximately 20% of ART-eligible patients dying during the study period is likely to be an underestimate. In addition, the study sites, which require a fee for patients to be seen in the outpatient department and to receive care in the on-site HIV clinic, may not be representative of public, wholly government-funded sites where patients with HIV may seek care. Ascertainment of ART initiation could be affected by this bias, as patients needed to initiate ART t the study sites to reach the primary outcome. Even when we performed a sensitivity analysis that included patients who self-reported ART initiation at non-study sites during the follow-up interview, the proportion of patients starting ART remained unacceptably low.
This study illustrates that less than half of ART-eligible individuals initiate treatment within the first year after a new HIV diagnosis in Durban, South Africa. Studies that have shown high rates of mortality and losses from ART programmes do not reflect the high burden of mortality in the community before ART programme entry. This study highlights the urgent need to devise interventions to facilitate earlier HIV diagnosis, CD4 cell count availability and rapid ART initiation in resource-limited settings.
Acknowledgments
The authors thank their dedicated research assistants: Lindeni Sangweni, Success Mncwabe, Aletta Maphasa, Yolisa Mgobhozi and Nompumelelo Badumuti and the study participants. They would also like to thank Sarah Bancroft Lorenzana for technical assistance.
Sponsorship: This study was partly supported by the National Institutes of Health: K23 AI068458; R01 AI058736; K24 AI062476; R01 MH073445; P30 AI42851 (Harvard Center for AIDS Research), Harvard University Program on AIDS, Claflin Distinguished Scholar Award of the Massachusetts General Hospital (I.V.B.) and The Doris Duke Charitable Foundation, Clinical Scientist Development Award (R.P.W.).
Footnotes
Presented in part at the 5th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention, 19–22 July 2009, Cape Town, South Africa.
Author contributions: I.V.B., J.G., H.H., D.R., J.N.K., R.P.W., K.A.F. and E.L. conceived and designed the study. I.V.B., S.R. and S.C. collected and assembled data. I.V.B., S.R., S.C., J.G., L.M.U., J.N.K., R.P.W., K.A.F. and E.L. analysed and interpreted the data. S.R. and E.L. performed statistical analysis. I.V.B. drafted the article. S.R., S.C., J.G., L.M.U., H.H., D.R., J.N.K., R.P.W., K.A.F. and E.L. critically revised the article for important intellectual content.
Conflicts of interest: None.
References
- 1.World Health Organization, UNAIDS. [Accessed: 3 August 2009];Epidemiological Country Profile on HIV and AIDS: South Africa. 2008 Available at: http://www.who.int/globalatlas/predefinedReports/EFS2008/short/EFSCountryProfiles2008_ZA.pdf.
- 2.World Health Organization. [Accessed: 3 August 2009];Towards universal access: scaling up priority HIV/AIDS interventions in the health sector: progress report. 2008 Available at: http://www.who.int/hiv/pub/towards_universal_access_report_2008.pdf.
- 3.United States President’s Emergency Plan for AIDS Relief. [Accessed: 3 August 2009];Accountability: Report on PEPFAR partnerships for prevention, treatment and care. Available at: http://www.pepfar.gov/press/fifth_annual_report/113725.htm.
- 4. [Accessed 28 October 2009];The Global Fund ARV fact sheet. Available at: http://www.the-globalfund.org/content/pressreleases/pr_090708_Factsheet.pdf.
- 5.EU at United Nations, Partnership in Action. [Accessed: 28 October 2009];Commission approves substantial programme to strenghten health care and fight HIV/AIDS in South Africa. Available at: http://www.eu-un.europa.eu/articles/en/article_1171_en.htm.
- 6.UNAIDS. [Accessed: 28 October 2009];Country situation: South Africa. 2008 Jul; Available at: http://data.unaids.org/pub/FactSheet/2008/sa08_soa_en.pdf.
- 7.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 8.Brinkhof MW, Dabis F, Myer L, Bangsberg DR, Boulle A, Nash D, et al. Early loss of HIV-infected patients on potent antire-troviral therapy programmes in lower-income countries. Bull WHO. 2008;86:559–567. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19:2141–2148. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 11.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4:e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fairall LR, Bachmann MO, Louwagie GM, van Vuuren C, Chikobvu P, Steyn D, et al. Effectiveness of antiretroviral treatment in a South African program: a cohort study. Arch Intern Med. 2008;168:86–93. doi: 10.1001/archinternmed.2007.10. [DOI] [PubMed] [Google Scholar]
- 13.Republic of South Africa Department of Health. [Accessed: 3 August 2009];National HIV and syphilis antenatal seroprevalence survey in South Africa. 2006 Available at: http://www.doh.gov.za/docs/index.html.
- 14.Bassett IV, Giddy J, Nkera J, Wang B, Losina E, Lu Z, et al. Routine voluntary HIV testing in Durban, South Africa: the experience from an outpatient department. J Acquir Immune Defic Syndr. 2007;46:181–186. doi: 10.1097/QAI.0b013e31814277c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.South African Government Information. [Accessed: 3 August 2009];Operational plan for comprehensive HIV and AIDS care, management and treatment for South Africa. 2003 Available at: http://www.info.gov.za/issues/hiv/careplan.htm.
- 16.Bassett IV, Wang B, Chetty S, Mazibuko M, Bearnot B, Giddy J, et al. Loss to care and death before antiretroviral therapy in Durban, South Africa. J Acquir Immune Defic Syndr. 2009;51:135–139. doi: 10.1097/qai.0b013e3181a44ef2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bekker LG, Myer L, Orrell C, Lawn S, Wood R. Rapid scale-up of a community-based HIV treatment service: programme performance over 3 consecutive years in Guguletu, South Africa. S Afr Med J. 2006;96:315–320. [PubMed] [Google Scholar]
- 18.Losina E, Bassett IV, Giddy J, Chetty S, Regan S, Walensky RP, et al. The “ART” of linkage: early loss to follow up (LTFU) after HIV diagnosis at two PEPFAR sites in Durban, South Africa. XVIIth International AIDS Society Meeting; Mexico City. 3–8 August 2008; [poster TUPE0345] [Google Scholar]
- 19.Micek MA, Gimbel-Sherr K, Baptista AJ, Matediana E, Montoya P, Pfeiffer J, et al. Loss to follow-up of adults in public HIV care systems in central Mozambique: identifying obstacles to treatment. J Acquir Immune Defic Syndr. 2009;52:397–405. doi: 10.1097/QAI.0b013e3181ab73e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coetzee D, Hildebrand K, Boulle A, Maartens G, Louis F, Labatala V, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18:887–895. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 21.Lawn SD, Myer L, Harling G, Orrell C, Bekker LG, Wood R. Determinants of mortality and nondeath losses from an anti-retroviral treatment service in South Africa: implications for program evaluation. Clin Infect Dis. 2006;43:770–776. doi: 10.1086/507095. [DOI] [PubMed] [Google Scholar]
- 22.Mills EJ, Ford N, Mugyenyi P. Expanding HIV care in Africa: making men matter. Lancet. 2009;374:275–276. doi: 10.1016/S0140-6736(09)61348-9. [DOI] [PubMed] [Google Scholar]
- 23.Braitstein P, Boulle A, Nash D, Brinkhof MW, Dabis F, Laurent C, et al. Gender and the use of antiretroviral treatment in resource-constrained settings: findings from a multicenter collaboration. J Womens Health. 2008;17:47–55. doi: 10.1089/jwh.2007.0353. [DOI] [PubMed] [Google Scholar]
- 24.Muula AS, Ngulube TJ, Siziya S, Makupe CM, Umar E, Prozesky HW, et al. Gender distribution of adult patients on highly active antiretroviral therapy (HAART) in Southern Africa: a systematic review. BMC Public Health. 2007;7:63–70. doi: 10.1186/1471-2458-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stirratt MJ, Remien RH, Smith A, Copeland OQ, Dolezal C, Krieger D. The role of HIV serostatus disclosure in antiretro-viral medication adherence. AIDS Behav. 2006;10:483–493. doi: 10.1007/s10461-006-9106-6. [DOI] [PubMed] [Google Scholar]
- 26.Waddell EN, Messeri PA. Social support, disclosure, and use of antiretroviral therapy. AIDS Behav. 2006;10:263–272. doi: 10.1007/s10461-005-9042-x. [DOI] [PubMed] [Google Scholar]
- 27.Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4:e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]