Abstract
We evaluated the prevalence and correlates of depressive symptoms prior to HIV diagnosis and determined the effect of these symptoms on seeking HIV care at an urban and rural clinic in Durban, South Africa. Adults were administered a questionnaire which included the 5-item Mental Health Index (MHI-5) before HIV testing. We determined the depressive symptoms among HIV-infected subjects. Of 1,545 newly-diagnosed HIV-infected subjects, 55% had depressive symptoms by MHI-5 score. Enrolling at the urban clinic and decreasing functional activity score were associated with depressive symptoms. Subjects with depressive symptoms who were referred for HIV testing by a healthcare provider were less likely to obtain a CD4 count than those without depressive symptoms who self-referred for testing. Depressive symptoms were common among newly-diagnosed HIV-infected participants and impacted CD4 uptake. Depression screening at the time of HIV diagnosis is critical for improving linkage to mental health and HIV services in South Africa.
Keywords: HIV, Linkage to care, Depressive symptoms, Depression, South Africa, Africa
Introduction
Depression is common among HIV-infected patients in resource-limited settings [1]. Cross-sectional South African studies have reported an 11–60% prevalence of depression when screening patients after their HIV diagnosis [2–8]. Depression has been shown to negatively affect antiretroviral treatment (ART) initiation and adherence for patients enrolled in longitudinal HIV care [9–14]. However, there are limited studies on the prevalence and correlates of depressive symptoms immediately preceding a new HIV diagnosis [8], and the impact of depressive symptoms on linking to HIV care.
We examined the prevalence and correlates of depressive symptoms in adults who were surveyed before HIV testing and subsequently diagnosed with HIV in two out-patient clinics in Durban, South Africa. We also analyzed the effects of depressive symptoms on HIV related health-seeking behavior in this newly-diagnosed population. Guided by previously published work, we hypothesized that newly-diagnosed HIV-infected participants who were younger [6] and female [3] would have depressive symptoms and participants with depressive symptoms would be less likely to obtain a CD4 count.
Methods
Clinic Setting
This secondary analysis of a prospective adult cohort was conducted in the outpatient clinic of an urban and a rural hospital in KwaZulu-Natal, the province with the highest HIV prevalence in South Africa [15]. McCord Hospital is an urban, state-aided general hospital in Durban where patients pay a subsidized fee for services. The outpatient clinic serves 3,000–4,000 adult patients per month and approximately 70% are black African, Zulu speakers, 20% are Indian, and 10% are White [16, 17]. The McCord Hospital HIV clinic has served >14,000 patients over the last 10 years and charges an inclusive fee (ZAR 180 = US $25 per visit, 2008) for comprehensive HIV services including ART [17]. The HIV clinic is subsidized by the United States President’s Emergency Plan for AIDS Relief (PEPFAR) and the KwaZulu-Natal Department of Health [16].
St. Mary’s Hospital is a Catholic, state-aided rural hospital that is located 20 km from Durban in Mariannhill. The hospital serves a peri-urban and rural population and charges a subsidized fee for outpatient clinic services. The St. Mary’s outpatient clinic serves 4,000 adult patients per month. The HIV clinic has served >10,000 patients over the last 10 years and offers comprehensive HIV care for a subsidized fee (ZAR 50–70 = US $6–9 per visit, 2008) through PEPFAR and KwaZulu-Natal Department of Health subsidies [16].
McCord Hospital offers rapid fingerstick HIV testing to patients presenting to the outpatient clinic. At St. Mary’s Hospital, HIV ELISA testing was initially offered until August 2007 when rapid fingerstick HIV testing was adopted [16, 17]. At both outpatient clinic sites, patients can self-refer for HIV testing or are tested following physician referral. Newly-diagnosed HIV-infected patients were immediately referred for CD4 testing at both clinic sites. Patients at McCord Hospital were charged a ZAR 90 fee ($12 US, 2008) for HIV clinic registration and CD4 testing; CD4 testing became free of charge in November 2008 in the McCord outpatient clinic. At St. Mary’s Hospital, CD4 testing was offered free of charge. Patients were then offered an HIV clinical assessment at both sites [16]. Patients with a CD4 count <200 cells/μL or who were WHO clinical stage 3 or 4 were eligible for ART at both HIV clinics during the study period [18]. ART-eligible patients underwent standard procedures of HIV care and three literacy sessions prior to ART initiation at both sites [16].
The STIAL Cohort
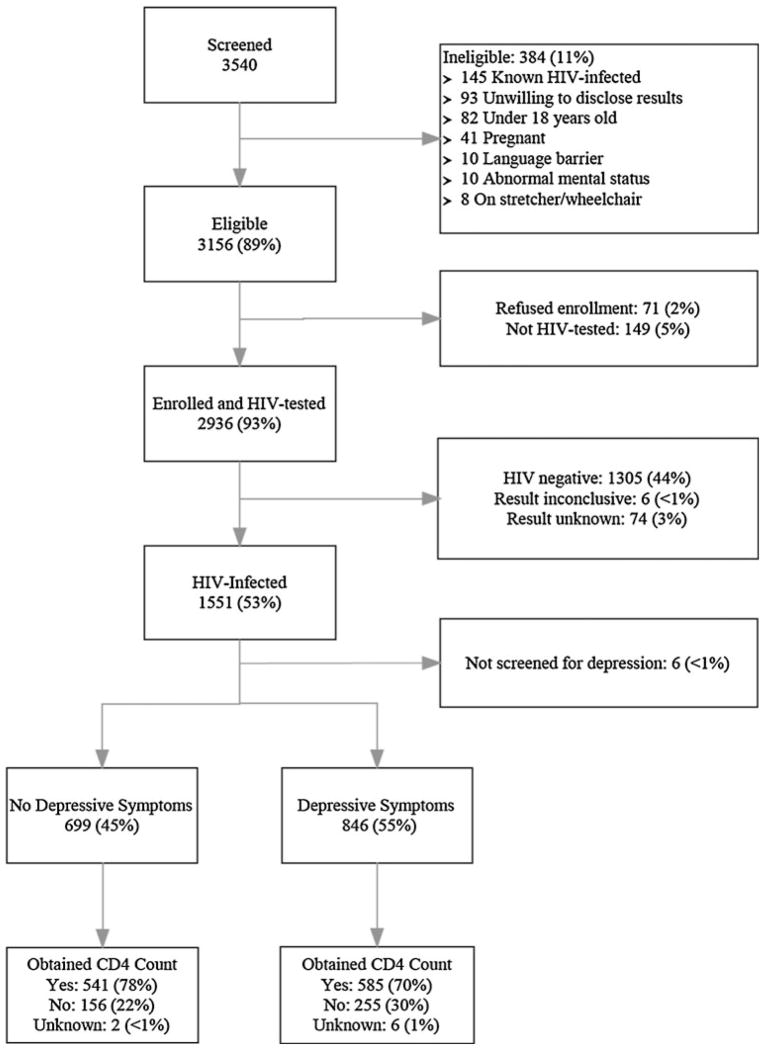
Adults (≥18 years) who presented for HIV testing at the McCord or St. Mary’s outpatient clinic between August 6, 2006 and December 31, 2008 were eligible for enrollment in the South African Test, Identify, and Link (STIAL) cohort (Fig. 1) [16, 19]. Enrolled participants were administered a verbal questionnaire, in English or Zulu, and subsequently underwent HIV testing. Subjects did not know their HIV status when administered the questionnaire. The current analysis only includes participants who were found to be HIV-infected and who collected their HIV results. HIV-infected participants were referred for CD4 testing at the time of diagnosis to assess immunologic status and ART-eligibility.
Fig. 1.
Study enrollment flow chart. Depressive symptoms are defined as an Mental Health Index-5 (MHI-5) score ≤60
The study questionnaire was administered by five bilingual (English/Zulu) research assistants who underwent a month-long orientation and had interval follow-up training during the study period. In addition, training was provided to standardize the verbal administration of the questionnaire across participants of different ages, gender, and educational level. The study questionnaire included the following data elements:
Demographic and Geographic: Questions included age, sex, education (no school, primary, some high school, high school, tertiary), employment status, distance from their home to the clinic, living arrangement (lives alone, with partner, with relatives/friends, employer), marital status, and whether they had an HIV-infected family member or friend.
Clinical: Clinical questions included HIV testing referral pattern (referral by a healthcare provider vs. self-referred), history of tuberculosis (TB) treatment, and health rating (very good, good, fair, poor/bad). Functional activity was assessed using the 10-item Physical Functioning Scale (PFS) of the Medical Outcomes Study 36-Item Short Form Health Survey (SF-36) [20]. The 10 PFS questions included ability to walk, carry groceries, and climb stairs, and were scored from 0 to 100 [20].
Mental Health: Subjects were asked 5 mental health screening questions from the Mental Health Index-5 (MHI-5) [21, 22]. The MHI-5 tool is the Mental Health domain of the SF-36. The SF-36 is a measure of health status that has been validated in HIV-infected populations [23, 24] in South Africa [25, 26]. The MHI-5 has been validated and used to screen for depression in international settings [22] and in the outpatient setting [21], though has not been validated in South Africa. The MHI-5 screening tool was chosen based on its suitability to the outpatient study setting and lack of other brief, validated, depression screening tools in South African HIV-infected populations at the time the study was designed in 2005. The participants were asked during the past month, how much of the time: (1) were you a happy person, (2) have you felt calm and peaceful, (3) have you been a nervous person, (4) have you felt very sad, and (5) did you feel so sad that nothing could cheer you up? Subjects’ responses were captured on a frequency scale ranging from 1 (“all of the time”) to 6 (“none of the time”). The MHI-5 responses were rescaled to a score of 0–100. Since the MHI-5 has no formal thresholds, we applied categories reported in a study that evaluated the performance of the MHI-5 in the general Japanese population [22]. In the Japanese study, participants with a score of ≤52 were categorized as severely depressed, 53–60 moderately depressed, 61–68 mildly depressed, and ≥69 not depressed [22]. For this analysis, we categorized participants with a score ≤60 as having depressive symptoms.
Participant Follow-up: We collected the dates and results of CD4 testing via chart abstraction. We also collected the proportion of ART-eligible subjects who started ART.
Written informed consent was obtained from all study participants. Patients unable to give informed consent, were known HIV-infected, <18 years old, pregnant, not willing to share their HIV test results with the study staff, or were critically ill were excluded from study enrollment. An additional eight subjects who were categorized as having a CD4 count were excluded from the study because they did not have a CD4 value or date of test available. Ethics approval was obtained from the McCord Hospital Ethics Committee, the St. Mary’s Hospital Mariannhill Ethics Committee, and the Partners HealthCare Human Research Committee in Boston, Massachusetts, USA (protocol #2006-P-001379).
Statistical Methods
The primary outcome was prevalence of depressive symptoms, defined by an MHI-5 scaled composite score of ≤60, among newly-diagnosed HIV-infected subjects. A secondary outcome was obtaining a CD4 count after HIV-diagnosis. An additional secondary outcome was initiating ART, if eligible, after HIV-diagnosis.
Bivariate analyses were performed to determine predictors of the study outcomes. We used the χ2 test for categorical data, t-tests for continuous variables, and the Wilcoxon rank-sum test for continuous variables with non-normal distributions. For each outcome, we constructed a multivariate model using variables that exhibited statistically significant associations with the outcome in the bivariate analyses, that were associated with linkage to care in previous analyses of this cohort [16, 19], or other published studies. Multiple imputation by chained equations [27, 28] was used to retain subjects with incomplete covariate data. The multivariate model for obtaining a CD4 count included a term to account for the observed interaction between depressive symptoms and HIV testing referral pattern (referral by a healthcare provider vs. self-referred). We assessed the multivariate models using a generalized linear model with a Poisson distribution and log link function. We present relative risks (RR), adjusted relative risks (ARR) and 95% confidence intervals (CI). All analyses were carried out using Stata statistical software (Stata Statistical Software Release 10, StataCorp, College Station, TX, USA).
Results
Cohort Characteristics
During the study period, a total of 1,545 subjects completed the study questionnaire, underwent HIV testing, and were subsequently found to be HIV-infected at the two outpatient clinic sites. Among these participants, 846 (55%) had depressive symptoms based on MHI-5 score (Table 1). The median age of the newly-diagnosed HIV-infected cohort was 34 years (interquartile range (IQR) 28–41 years), and 777 (50%) were female (Table 1). The median age was higher (35 vs. 33 years, p < 0.001) and functional activity scores were lower (90 vs. 95, p < 0.001) among those with depressive symptoms. Depressive symptoms were more common among participants at the urban compared to the rural clinic (83% vs. 34%, p < 0.001), and those who were referred for HIV testing by a healthcare provider compared to those who self-referred (76% vs. 35%, p < 0.001).
Table 1.
Baseline characteristics of a cohort of newly-diagnosed HIV-infected adults in Durban, South Africa surveyed before HIV testing and diagnosis by depressive symptoms
| No depressive symptomsa (%) | Depressive symptoms (%) | Test statistic χ2 unless indicated | p value | |
|---|---|---|---|---|
| Total | 699 (45) | 846 (55) | ||
| Demographic factors | ||||
| Age (years, median, IQRb) | 33 (27–40) | 35 (29–42) | −4.42c | < 0.001 |
| Sex | ||||
| Female | 335 (43) | 442 (57) | 2.86 | 0.09 |
| Male | 364 (47) | 404 (53) | ||
| Education | ||||
| None or primary | 148 (43) | 199 (57) | 14.46 | 0.001 |
| Some high school | 354 (51) | 344 (49) | ||
| High school or more | 189 (40) | 283 (60) | ||
| Employment | ||||
| No full-time job | 368 (46) | 437 (54) | 0.15 | 0.70 |
| Full-time job | 327 (45) | 404 (55) | ||
| Clinic site | ||||
| Urban | 110 (17) | 543 (83) | 368.17 | < 0.001 |
| Rural | 589 (66) | 303 (34) | ||
| Distance from clinic | ||||
| < 10 km | 354 (47) | 393 (53) | 2.62 | 0.10 |
| ≥10 km | 341 (43) | 447 (57) | ||
| Household factors | ||||
| Living arrangement | ||||
| Lives alone | 113 (55) | 93 (45) | 19.80 | < 0.001 |
| Lives with partner | 135 (53) | 121 (47) | ||
| Lives with relatives/friends | 426 (41) | 604 (59) | ||
| Lives with employer | 22 (47) | 25 (53) | ||
| Marital status | ||||
| Currently married | 85 (36) | 150 (64) | 10.48 | 0.001 |
| Not married | 610 (48) | 671 (52) | ||
| HIV-infected family member or friend | ||||
| Yes | 170 (60) | 115 (40) | 29.37 | < 0.001 |
| No | 523 (42) | 724 (58) | ||
| Clinical characteristics | ||||
| HIV testing referral pattern | ||||
| Referred by healthcare provider | 182 (24) | 567 (76) | 257.74 | < 0.001 |
| Self-referral | 515 (65) | 277 (35) | ||
| History of TB treatment | ||||
| Yes | 105 (45) | 126 (55) | 0.02 | 0.89 |
| No | 588 (45) | 720 (55) | ||
| Self health rating | ||||
| Very good | 123 (81) | 28 (19) | 95.69 | < 0.001 |
| Good | 342 (44) | 444 (56) | ||
| Fair | 192 (40) | 285 (60) | ||
| Poor/bad | 34 (30) | 78 (70) | ||
| Functional activity score (IQR) | 95 (75–100) | 90 (55–100) | 4.85c | < 0.001 |
Depressive symptoms are defined as an Mental Health Index-5 (MHI-5) score ≤60
IQR: 25% and 75% interquartile range
Test statistic: t-test for continuous variables
Predictors of Depressive Symptoms
We tested a multivariate model of depressive symptoms that included age, sex, educational level (high school or more vs. no, primary, or some high school), clinic site, living arrangement (lives with partner vs. other) and functional activity score. Increased risk of depressive symptoms was associated with enrolling at the urban clinic (ARR 2.45, 95% CI 2.21–2.70). Every 10 point decrease in functional activity score was associated with a 5% increase in depressive symptoms (ARR 1.05, 95% CI 1.04–1.07) (Table 2).
Table 2.
Correlates of depressive symptomsa in newly-diagnosed HIV-infected adults in Durban, South Africa surveyed before HIV testing and diagnosis
| Unadjusted RR | Test statistic | 95% CI | p value | Adjusted RRb | Test statistic | 95% CI | p value | |
|---|---|---|---|---|---|---|---|---|
| Age (10 years increments) | 1.10 | 4.10 | 1.05–1.15 | < 0.001 | 1.03 | 1.36 | 0.99–1.08 | 0.17 |
| Sex | ||||||||
| Female | 1.08 | 1.69 | 0.99–1.18 | 0.09 | 1.05 | 1.20 | 0.97–1.14 | 0.23 |
| Education | ||||||||
| High school or more | 1.15 | 2.98 | 1.05–1.27 | 0.003 | 1.01 | 0.33 | 0.93–1.11 | 0.75 |
| Clinic site | ||||||||
| Urban | 2.45 | 17.94 | 2.22–2.70 | < 0.001 | 2.45 | 17.69 | 2.21–2.70 | < 0.001 |
| Living arrangement | ||||||||
| Lives with partner | 0.84 | −2.48 | 0.73–0.96 | 0.013 | 0.97 | −0.52 | 0.85–1.10 | 0.60 |
| Functional activity score | ||||||||
| Lower score (per 10 unit decrease in functional score) | 1.04 | 5.24 | 1.03–1.06 | < 0.001 | 1.05 | 7.08 | 1.04–1.07 | < 0.001 |
Test statistic: χ2 test for categorical and t-test for continuous variables. Generalized linear models with a Poisson distribution and log link function for the adjusted relative risks
RR relative risk, 95% CI confidence interval
Depressive symptoms are defined as an Mental Health Index-5 (MHI-5) score ≤ 60
Adjusted relative risk: adjusted for age, sex, educational level, clinic site, living arrangement, and functional activity score
Effect of Depressive Symptoms on Obtaining a CD4 Count and Initiating ART
A total of 1,126 (73%) subjects obtained a CD4 count and of those who had results available, 607 (59%) had a CD4 count <200 cells/μL. Newly-diagnosed HIV-infected subjects who presented to the urban clinic were less likely to have a CD4 count compared to those at the rural clinic (64% vs. 80%, p < 0.001). Participants with depressive symptoms were less likely than those without depressive symptoms to obtain a CD4 count (70% vs. 78%, p < 0.001) (Fig. 1). The median CD4 count was lower in those with depressive symptoms (137 cells/μL; IQR 57–273) compared to those with no symptoms (172 cells/μL; IQR 78–315) (p < 0.005). Participants with depressive symptoms were more likely to have a CD4 count <200 cells/μL (63% vs. 56%, p = 0.03). The median time to obtain a CD4 count was also longer for those participants with depressive symptoms compared to those with no symptoms (1 day vs. 0 days, p < 0.001).
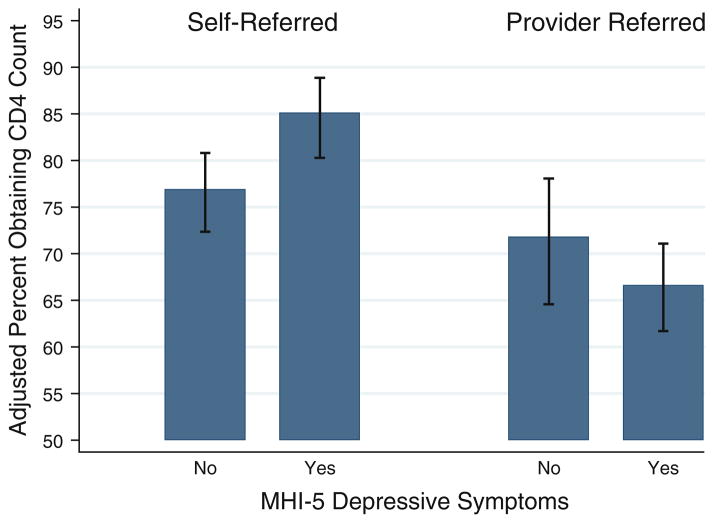
Participants referred for HIV testing by a healthcare provider compared to those who self-referred (65% vs. 81%, p < 0.001) were less likely to obtain a CD4 count after HIV diagnosis. A statistically significant interaction was found between depressive symptoms and HIV testing referral pattern (Fig. 2). The effect of depressive symptoms on obtaining a CD4 count differed by whether the participant was referred for HIV testing by a healthcare provider or self-referred.
Fig. 2.
Adjusted proportion of newly-diagnosed HIV-infected patients in Durban, South Africa who underwent CD4 count by depressive symptoms and HIV testing referral pattern. Mental Health Index-5 (MHI-5) differentiates participants with depressive symptoms and no depressive symptoms. Depressive symptoms were defined as an MHI-5 score ≤60. ‘Self-referred’ subjects were those who self-referred for HIV testing. ‘Provider referred’ subjects were those who were referred for HIV testing by a healthcare provider. Generalized linear models with a Poisson distribution and log link function were used for the adjusted proportions. Proportions were adjusted for age, sex, clinic site, distance from clinic, having an HIV-infected family member or friend, history of TB treatment, functional activity score, and the interaction term HIV testing referral pattern and depressive symptoms
We tested a multivariate model of obtaining a CD4 count that included age, sex, clinic site, distance to clinic, having an HIV-infected family member or friend, history of TB treatment, functional activity score, HIV testing referral pattern (referred by a healthcare provider vs. self-referred), depressive symptoms, and the interaction term HIV testing referral pattern and depressive symptoms (Table 3). Subjects with depressive symptoms who were referred by a healthcare provider for HIV testing were less likely to obtain a CD4 count than those with no depressive symptoms who self-referred for testing (ARR 0.82, 95% CI 0.72–0.94). Every 10 year increase in age was associated with a 4% increase in obtaining a CD4 count (ARR 1.04, 95% CI 1.01–1.08) (Table 3). Participants at the urban clinic were less likely than those at the rural clinic to obtain a CD4 count (ARR 0.86, 95% CI 0.79–0.93).
Table 3.
Correlates of obtaining a CD4 count in a cohort of newly-diagnosed HIV-infected adults in Durban, South Africa surveyed before HIV testing and diagnosis
| Unadjusted RR | Test statistic | 95% CI | p value | Adjusted RRa | Test statistic | 95% CI | p value | |
|---|---|---|---|---|---|---|---|---|
| Age (10 years increments) | 1.02 | 1.36 | 0.99–1.05 | 0.17 | 1.04 | 2.77 | 1.01–1.08 | 0.006 |
| Sex | ||||||||
| Female | 1.03 | 1.09 | 0.97–1.10 | 0.28 | 1.05 | 1.67 | 0.99–1.12 | 0.09 |
| Clinic site | ||||||||
| Urban | 0.80 | −6.63 | 0.75–0.85 | < 0.001 | 0.86 | −3.70 | 0.79–0.93 | < 0.001 |
| Distance from clinic | ||||||||
| ≥10 km | 1.05 | 1.51 | 0.99–1.11 | 0.13 | 1.02 | 0.77 | 0.96–1.09 | 0.44 |
| HIV-infected family member/friend | ||||||||
| Yes | 1.08 | 2.01 | 1.00–1.15 | 0.04 | 0.97 | −0.96 | 0.90–1.04 | 0.34 |
| History of TB treatment | ||||||||
| Yes | 1.00 | 0.03 | 0.92–1.09 | 0.97 | 0.97 | −0.66 | 0.89–1.06 | 0.51 |
| Functional activity score | ||||||||
| Lower score (per 10 unit decrease in functional score) | 1.00 | −0.38 | 0.99–1.01 | 0.70 | 1.00 | −0.67 | 0.98–1.01 | 0.50 |
| HIV testing referral pattern | ||||||||
| Referred by healthcare provider | 0.80 | −7.15 | 0.75–0.85 | < 0.001 | 0.94 | −1.25 | 0.85–1.04 | 0.21 |
| Depressive symptomsb | ||||||||
| Yes | 0.90 | −3.55 | 0.85–0.95 | < 0.001 | 1.10 | 2.86 | 1.03–1.18 | 0.004 |
| Interaction term | ||||||||
| Depressive symptoms/referred by healthcare provider | – | – | – | – | 0.82 | −2.89 | 0.72–0.94 | 0.004 |
Test statistic: χ2 test for categorical and t-test for continuous variables. Generalized linear models with a Poisson distribution and log link function for the adjusted relative risks
RR relative risk, 95% CI confidence interval
ARR: adjusted for age, sex, clinic site, distance from clinic, having a HIV-infected family member/friend, history of TB treatment, functional activity score, HIV testing referral pattern, depressive symptoms, and the interaction term depressive symptoms and HIV testing referral pattern
Depressive symptoms are defined as an Mental Health Index-5 (MHI-5) score ≤60
There were 628 ART-eligible patients based on a CD4 count of <200 cells/μL or WHO clinical stage 3 or 4. Of the ART-eligible participants, 249 (40%) started ART, 143 (23%) did not start ART, and 236 (38%) had an unknown ART status. In bivariate analyses excluding those with unknown ART status, the number of subjects with depressive symptoms initiating ART was 142 (57%) compared to 107 (75%) with no depressive symptoms (p < 0.001). However, there was no significant difference in ART initiation by depressive symptoms in multivariate analysis controlling for age, sex, clinic site, distance to clinic, functional activity score, and HIV testing referral pattern.
Discussion
This study evaluated the prevalence and correlates of depressive symptoms in adults surveyed before HIV diagnosis and determined their impact on obtaining a CD4 count in two outpatient clinics in Durban, South Africa. Fifty-five percent of subjects surveyed before HIV diagnosis had depressive symptoms by MHI-5 score. The factors associated with depressive symptoms were enrolling at the urban clinic and a lower functional activity score. We also found that subjects with depressive symptoms who were referred for HIV testing by a healthcare provider were less likely to obtain a CD4 count than those with no depressive symptoms who self-referred for testing. This study highlights that depressive symptoms are common, and that these symptoms have a negative impact on the initial health-seeking behavior of newly-diagnosed HIV-infected subjects.
Although the high prevalence of depressive symptoms in this newly-diagnosed HIV-infected cohort is consistent with other South African studies, the majority of previous studies have screened HIV-infected patients for depression after they are enrolled in continuity HIV care, rather than prior to diagnosis [2–8]. Among a cohort of South African HIV-infected patients screened more than 6 months after their diagnosis, 35% had major depression [2], and in a semirural cohort of patients diagnosed with HIV within the prior year, nearly 40% had depression scores in or above the moderately depressed range [7]. The prevalence of depression was 60% in HIV-infected inpatients in Durban, South Africa [5]. The wide variability of depression estimates may be due to the use of different psychiatric rating scales, study location, and cohort composition, such as age, sex, and clinical HIV stage [1]. The timing of the depression assessment relative to the patient’s HIV diagnosis could also affect prevalence estimates [1].
Few studies in South Africa have assessed depressive symptoms before HIV testing. A survey of the literature yielded one study in a resource-limited setting of pregnant women who were screened for depression directly prior to HIV testing [8]. HIV-negative women presenting to a rural clinic in Kwa-Zulu Natal were screened for depression using the Edinburgh Postnatal Depression Scale (EPDS) and then offered HIV testing as part of a prevention of mother to child transmission (PMTCT) program [8]. An EPDS score consistent with depression was found in 41% of the women. Forty-one percent of women were subsequently found to be HIV-infected, though no significant relationship was found between HIV status and depression. In our study we screened participants for depressive symptoms before HIV testing and diagnosis and found that symptoms are common not only in women, but also in men. This high prevalence of depressive symptoms highlights that efforts are needed to integrate depression and mental health screening at the time of HIV testing and diagnosis.
We found that enrolling at the urban clinic was a significant predictor of depressive symptoms. Although some studies have found higher prevalence of depression in urban compared to rural settings, others have found no significant difference [29, 30]. The higher prevalence of depression in urban locations is thought to be mediated by the stress of urbanization, including social isolation, crowding, unemployment, crime and violence, poverty, and pollution [29, 30]. We also found that a lower functional activity score correlates with depressive symptoms which is consistent with previous studies in HIV-infected adults in South Africa [3, 4]. Unlike other South African studies, female gender [3], younger age [6], speaking Afrikaans [6], unplanned current pregnancy [8], and absence of a regular income [8], were not associated with increased risk of depressive symptoms in newly-diagnosed HIV-infected patients.
This study highlights the effect of depressive symptoms on obtaining a CD4 count, the first step in initiating care for newly-diagnosed HIV-infected patients. The subjects who had depressive symptoms and were referred for HIV testing by a health care provider were less likely to have a CD4 count than those with no depressive symptoms who self-referred for testing. A previous analysis of this cohort showed that newly-diagnosed HIV-infected patients who were referred for testing by a healthcare provider had a higher rate of early loss to follow-up [19]. The patients with depressive symptoms who were referred for HIV testing may be less likely to seek HIV care in the short-term because they are less prepared to learn their diagnosis [19]. Self-referred patients may also be a more motivated group of patients who will access services irrespective of their mental health state [19]. Our findings suggest that depressive symptoms may be an additional factor associated with poor uptake of initial HIV services for newly-diagnosed HIV-infected patients.
Previous studies have assessed the effect of depression on ART initiation [1, 9–11] and adherence [12–14] in HIV-infected patients who are enrolled in continuity HIV services. Although we report no difference in ART initiation by depressive symptoms in multivariate analysis, the statistical power to address this linkage question was lacking because the number of subjects with unknown ART initiation status was large. Further studies should be undertaken to evaluate the effect of depressive symptoms at the time of HIV diagnosis on ART uptake.
The study has several limitations. At the time the study was designed there were no brief validated depression tools in HIV-infected patients in resource-limited settings. The MHI-5 depression instrument has not been validated in South Africa. The MHI-5 has been used among a stratified sampling of the Japanese population ≥16 years [22]. The MHI-5 has also been used in a sample of 20–64 year olds enrolled in a Health Maintenance Organization (HMO) in the United States [21]. Although both these populations are not relevant to our study population, the MHI-5 was chosen because its brevity made it appropriate for the fast-paced clinic setting. The MHI-5 is also the Mental Health domain of the SF-36, which has been validated in HIV-infected populations [23, 24] in South Africa [25, 26]. Additionally, our study population may not be representative of government clinics where care is free because participants paid a subsidized fee for outpatient services. In addition, some potential confounders such as cognitive impairment were not addressed in our survey and were therefore not measurable in our analysis [31, 32]. We cannot comment on the prevalence of depressive symptoms among all participants who had an HIV test or whether depressive symptoms are associated with a new HIV diagnosis because we did not include participants who were HIV-negative in this analysis. Also, since newly-diagnosed HIV-infected subjects at the urban clinic were charged a CD4 count fee during the majority of the study period and those at the rural clinic were not, the effect of clinic site on obtaining a CD4 count may be explained by the fee.
Conclusion
The very high prevalence of depressive symptoms at the time of HIV diagnosis merits further research and could have important policy implications, particularly because an initiative to expand HIV counseling and testing to all South Africans is currently underway [33]. As a result of this HIV testing campaign, a substantial number of people will learn their HIV status, and many could benefit from depression screening prior to diagnosis in order to facilitate referral to mental health services [34, 35]. Identifying HIV-infected patients with depressive symptoms is also important because it affects uptake of CD4 testing, clinic attendance, and ART initiation and adherence [1, 9–14, 34]. Given the high prevalence of depressive symptoms among newly-diagnosed HIV-infected patients, policy makers should consider integrating mental health services and HIV testing and treatment programs.
Acknowledgments
The authors thank the study participants and the dedicated study research assistants at McCord and St. Mary’s Hospitals. This study was supported in part by the Harvard Institute of Global Health, National Institute of Allergy and Infectious Diseases (NIAID) T32 AI 007433; National Institute of Child Health and Human Development (NICHD) T32 HD 055148-02 (L.R.); NIAID K23 AI068458; National Institute of Mental Health (NIMH) R01MH090326; NIAID R01 AI058736; NIAID K24 AI062476; NIMH R01 MH073445; NIAID P30 AI42851 (Harvard Center for AIDS Research), Harvard University Program on AIDS, Claflin Distinguished Scholar Award of the Massachusetts General Hospital (I.V.B.), and the Doris Duke Charitable Foundation, Clinical Scientist Development Award (R.P.W.).
Footnotes
Presented, in part, at the International AIDS Society Meeting July 17–20, 2011, Rome, Italy.
Contributor Information
Lynn Ramirez-Avila, Email: lynn.ramirez@childrens.harvard.edu, Division of Infectious Diseases, Children’s Hospital Boston, 333 Longwood Avenue, Boston, MA 02139, USA. Division of General Medicine, Massachusetts General Hospital, Boston, MA, USA. Medical Practice Evaluation Center, Massachusetts General Hospital, Boston, MA, USA.
Susan Regan, Division of General Medicine, Massachusetts General Hospital, Boston, MA, USA. Medical Practice Evaluation Center, Massachusetts General Hospital, Boston, MA, USA.
Janet Giddy, McCord Hospital, Durban, South Africa.
Senica Chetty, McCord Hospital, Durban, South Africa.
Douglas Ross, St. Mary’s Hospital, Mariannhill, Durban, South Africa.
Jeffrey N. Katz, Department of Orthopedic Surgery, Brigham and Women’s Hospital, Boston, MA, USA. Division of Rheumatology, Brigham and Women’s Hospital, Boston, MA, USA. Department of Epidemiology, Harvard School of Public Health, Boston, MA, USA
Kenneth A. Freedberg, Division of General Medicine, Massachusetts General Hospital, Boston, MA, USA. Medical Practice Evaluation Center, Massachusetts General Hospital, Boston, MA, USA. Center for AIDS Research, Harvard Medical School, Boston, MA, USA. Department of Epidemiology, Boston University School of Public Health, Boston, MA, USA
Rochelle P. Walensky, Division of General Medicine, Massachusetts General Hospital, Boston, MA, USA. Medical Practice Evaluation Center, Massachusetts General Hospital, Boston, MA, USA. Center for AIDS Research, Harvard Medical School, Boston, MA, USA. Division of Infectious Disease, Massachusetts General Hospital, Boston, MA, USA. Division of Infectious Disease, Brigham and Women’s Hospital, Boston, MA, USA
Elena Losina, Division of General Medicine, Massachusetts General Hospital, Boston, MA, USA. Medical Practice Evaluation Center, Massachusetts General Hospital, Boston, MA, USA. Department of Orthopedic Surgery, Brigham and Women’s Hospital, Boston, MA, USA. Division of Rheumatology, Brigham and Women’s Hospital, Boston, MA, USA. Center for AIDS Research, Harvard Medical School, Boston, MA, USA. Department of Biostatistics, Boston University, Boston, MA, USA.
Ingrid V. Bassett, Division of General Medicine, Massachusetts General Hospital, Boston, MA, USA. Medical Practice Evaluation Center, Massachusetts General Hospital, Boston, MA, USA. Center for AIDS Research, Harvard Medical School, Boston, MA, USA. Division of Infectious Disease, Massachusetts General Hospital, Boston, MA, USA
References
- 1.Collins PY, Holman AR, Freeman M, Patel V. What is the relevance of mental health to HIV/AIDS care and treatment programs in developing countries? A systematic review. AIDS. 2006;20:1571–82. doi: 10.1097/01.aids.0000238402.70379.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olley BO, Gxama F, Seedat S, et al. Psychopathology and coping in recently diagnosed HIV/AIDS patients—the role of gender. SAMJ. 2003;93:928–31. [PubMed] [Google Scholar]
- 3.Olley BO, Seedat S, Nei DG, Stein DJ. Predictors of major depression in recently diagnosed patients with HIV/AIDS in South Africa. AIDS Patient Care STD. 2004;18:481–7. doi: 10.1089/1087291041703700. [DOI] [PubMed] [Google Scholar]
- 4.Olley BO, Seedat S, Stein DJ. Persistence of psychiatric disorders in a cohort of HIV/AIDS patients in South Africa: a 6-month follow-up study. J Psychosoc Res. 2006;61:479–84. doi: 10.1016/j.jpsychores.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Singh D, Sunpath H, John S, Eastham L, Gouden R. The utility of a rapid screening tool for depression and HIV dementia amongst patients with low CD4 counts—a preliminary report. Afr J Pyschiatry. 2008;11:282–6. [PubMed] [Google Scholar]
- 6.Myer L, Smit J, Le Roux L, Parker S, Stein DJ, Seedat S. Common mental disorders among HIV-infected individuals in South Africa: prevalence, predictors, and validation of brief psychiatric rating scales. AIDS Patient Care STD. 2008;22:147–58. doi: 10.1089/apc.2007.0102. [DOI] [PubMed] [Google Scholar]
- 7.Kagee A, Martin L. Symptoms of depression and anxiety among a sample of South African patients living with HIV. AIDS Care. 2010;22:159–65. doi: 10.1080/09540120903111445. [DOI] [PubMed] [Google Scholar]
- 8.Rochat TJ, Richter LM, Doll HA, Buthelezi NP, Tompkins A, Stein A. Depression among pregnant rural South African women undergoing HIV testing. JAMA. 2006;295:1376–8. doi: 10.1001/jama.295.12.1376. [DOI] [PubMed] [Google Scholar]
- 9.Himelhoch S, Moore RD, Treisman G, Gebo KA. Does the presence of a current psychiatric disorder in AIDS patients affect the initiation of antiretroviral treatment and duration of therapy? JAIDS. 2004;37:1457–63. doi: 10.1097/01.qai.0000136739.01219.6d. [DOI] [PubMed] [Google Scholar]
- 10.Fairfield KM, Libman H, Davis RB, Eisenberg DM, Phillips RS. Delays in protease inhibitor use in clinical practice. J Gen Intern Med. 1999;14:395–401. doi: 10.1046/j.1525-1497.1999.08198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tegger MK, Crane HM, Tapia KA, Uldall KK, Holte SE, Kitahata MM. The effect of mental illness, substance use, and treatment for depression on the initiation of highly active anti-retroviral therapy among HIV-infected individuals. AIDS Patient Care STD. 2008;22:233–43. doi: 10.1089/apc.2007.0092. [DOI] [PubMed] [Google Scholar]
- 12.Wagner GJ, Goggin K, Remien RH, et al. A closer look at depression and its relationship to HIV antiretroviral adherence. Ann Behav Med. 2011;42(3):352–60. doi: 10.1007/s12160-011-9295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vranceanu AM, Safren S, Lu M, et al. The relationship of post-traumatic stress disorder and depression to antiretroviral medication adherence in persons with HIV. AIDS Patient Care STD. 2008;22:313–21. doi: 10.1089/apc.2007.0069. [DOI] [PubMed] [Google Scholar]
- 14.Peltzer K, Friend-du Preez N, Ramlagan S, Anderson J. Anti-retroviral treatment adherence among HIV patients in KwaZulu-Natal, South Africa. BMC Pub Health. 2010;10:1–10. doi: 10.1186/1471-2458-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Republic of South Africa Department of Health. [Accessed 15 Sep 2011];National antenatal sentinel HIV and syphilis prevalence survey in South Africa. 2009 Available at: http://www.health-e.org.za/documents/85d3dad6136e8ca9d02cceb7f4a36145.pdf.
- 16.Bassett IV, Regan S, Chetty S, et al. Who starts antiretroviral therapy in Durban, South Africa? Not everyone who should. AIDS. 2010;24:S37–44. doi: 10.1097/01.aids.0000366081.91192.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bassett IV, Giddy J, Nkera J, et al. Routine voluntary HIV testing in Durban, South Africa: the experience from an outpatient department. JAIDS. 2007;46:181–6. doi: 10.1097/QAI.0b013e31814277c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. [Accessed 1 Apr 2011];HIV/AIDS program: antiretroviral therapy for HIV infection in adults and adolescents. Available at: http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf.
- 19.Losina E, Bassett IV, Giddy J, et al. The “ART” of Linkage: pre-treatment loss to care after HIV diagnosis at two PEPFAR sites in Durban, South Africa. PLoS One. 2010;5:1–8. doi: 10.1371/journal.pone.0009538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 health survey: manual and Interpretation Guide. Boston: The Health Institute, New England Medical Center; 1989. [Google Scholar]
- 21.Berwick DM, Murphy JM, Goldman PA, Ware JE, Barsky AJ, Weinstein MC. Performance of a five-item mental health screening test. Med Care. 1991;29:169–76. doi: 10.1097/00005650-199102000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Yamazaki S, Fukuhara S, Green J. Usefulness of five-item and three-item Mental Health Inventories to screen for depression in the general population of Japan. Health Qual Life Outcomes. 2005;3:48–54. doi: 10.1186/1477-7525-3-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu AW, Hays RD, Kelly S, Malitz F, Bozzette SA. Applications of the Medical Outcomes Study health-related quality of life measures in HIV-AIDS. Qual Life Res. 1997;6:531–54. doi: 10.1023/a:1018460132567. [DOI] [PubMed] [Google Scholar]
- 24.Magafu MG, Moji K, Igumbor EU, et al. Usefulness of highly active antiretroviral therapy on health-related quality of life of adult recipients in Tanzania. AIDS Patient Care STDS. 2009;23:563–70. doi: 10.1089/apc.2008.0278. [DOI] [PubMed] [Google Scholar]
- 25.Ncama BP, McInerney PA, Bhengu BR, et al. Social support and medication adherence in HIV disease in KwaZulu-Natal, South Africa. Int J Nurs Stud. 2008;45:1756–63. doi: 10.1016/j.ijnurstu.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 26.O’Keefe EA, Wood R. The impact of human immunodeficiency virus (HIV) infection on quality of life in a multiracial South African population. Qual Life Res. 1996;5:275–80. doi: 10.1007/BF00434749. [DOI] [PubMed] [Google Scholar]
- 27.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18:681–94. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 28.Royston P. Multiple imputation of missing values. Stat J. 2004;4:227–41. [Google Scholar]
- 29.Wang JL. Rural-urban differences in the prevalence of major depression and associated impairment. Soc Psychiatry Psychiatr Epidemiol. 2004;39:19–25. doi: 10.1007/s00127-004-0698-8. [DOI] [PubMed] [Google Scholar]
- 30.Peen J, Schoevers RA, Beekman AT, Dekker J. The current status of urban-rural differences in psychiatric disorders. Acta Psychiatr Scand. 2010;121:84–93. doi: 10.1111/j.1600-0447.2009.01438.x. [DOI] [PubMed] [Google Scholar]
- 31.Nakasujja N, Skolasky RL, Musisi S, et al. Depression symptoms and cognitive function among individuals with advanced HIV infection initiating HARRT in Uganda. BMC Psychiatry. 2010;10:1–7. doi: 10.1186/1471-244X-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawler K, Mosepele M, Ratcliffe S, et al. Neurocognitive impairment among HIV-positive individuals in Botswana: a pilot study. J IAS. 2010;13:1–9. doi: 10.1186/1758-2652-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.South African National AIDS Council. [Accessed 16 Mar 2010];The national HIV counseling and testing campaign strategy. 2010 Available at: http://www.avert.org/media/pdfs/HCT-Campaign-Strategy-2_3_10-final.pdf.
- 34.Freeman MC, Patel V, Collins PY, Bertolote JM. Integrating mental health in global initiatives for HIV/AIDS. Br J Psychiatry. 2005;187:1–3. doi: 10.1192/bjp.187.1.1. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. [Accessed 1 Dec 2010];HIV/AIDS programme: essential prevention and care interventions for adults and adolescents living with HIV in resource-limited settings. 2008 Available at: http://www.who.int/hiv/pub/plhiv/plhiv_treatment_care.pdf.