RhoD has a role in actin dynamics that is distinct from RhoA, Rac1, and Cdc42. Data presented here indicate that RhoD binds the actin nucleation–promoting factor WASp homologue associated with actin Golgi membranes and microtubules (WHAMM) and the related filamin A–binding protein FILIP1. WHAMM acts downstream of RhoD, and both proteins coordinate vital cellular processes, such as actin dynamics, cell attachment, and cell migration.
Abstract
The Rho GTPases have mainly been studied in association with their roles in the regulation of actin filament organization. These studies have shown that the Rho GTPases are essential for basic cellular processes, such as cell migration, contraction, and division. In this paper, we report that RhoD has a role in the organization of actin dynamics that is distinct from the roles of the better-studied Rho members Cdc42, RhoA, and Rac1. We found that RhoD binds the actin nucleation–promoting factor WASp homologue associated with actin Golgi membranes and microtubules (WHAMM), as well as the related filamin A–binding protein FILIP1. Of these two RhoD-binding proteins, WHAMM was found to bind to the Arp2/3 complex, while FILIP1 bound filamin A. WHAMM was found to act downstream of RhoD in regulating cytoskeletal dynamics. In addition, cells treated with small interfering RNAs for RhoD and WHAMM showed increased cell attachment and decreased cell migration. These major effects on cytoskeletal dynamics indicate that RhoD and its effectors control vital cytoskeleton-driven cellular processes. In agreement with this notion, our data suggest that RhoD coordinates Arp2/3-dependent and FLNa-dependent mechanisms to control the actin filament system, cell adhesion, and cell migration.
INTRODUCTION
The Rho GTPases are key operators in signal transduction pathways that control cell behavior in response to signals from the extracellular environment. The Rho GTPases comprise a distinct family within the superfamily of Ras-related small GTPases. The classical Rho GTPases act as molecular switches through their cycling between GDP-bound (inactive) and GTP-bound (active) conformations to control different signal transduction pathways (Jaffe and Hall, 2005). In their active, GTP-bound conformations, the Rho GTPases can interact with effector proteins that evoke a variety of intracellular responses. The cycling between the inactive, GDP-bound conformation and the active, GTP-bound conformation is tightly regulated by three groups of proteins: the guanine nucleotide exchange factors (GEFs), which catalyze the exchange of GDP for GTP to activate the Rho proteins; the GTPase-activating proteins (GAPs), which stimulate the intrinsic GTPase activity to inactivate the Rho proteins; and the guanine nucleotide disassociation inhibitors (GDIs), which sequester the Rho GTPases in their inactive conformation. Although extracellular signals can regulate this switch by modifying any of these regulatory proteins, in general, they appear to act mostly through GEFs (Jaffe and Hall, 2005).
The mammalian Rho GTPases comprise 20 members, several of which share a common role in the regulation of actin filament organization (Aspenström et al. 2004). Actin fibers can be linked to each other in either a parallel or a perpendicular manner, which determines the organization of the resulting actin network. While parallel actin filaments can be found in bundles, stress fibers, or filopodia, perpendicular actin filaments form mesh networks of filamentous actin, as found in membrane ruffles of lamellipodia (Rottner and Stradal, 2011). These distinct actin filament assemblies have unique and specialized properties. Indeed, pivotal cellular functions, such as cell contraction, migration, and division, require an adequate balance among these different modes of actin filament assembly. The Rho GTPases can regulate this balance; for instance, RhoA can regulate the formation of stress fibers, Rac1 can regulate the formation of lamellipodia, and Cdc42 can regulate the production of filopodia (Jaffe and Hall, 2005).
The majority of studies still focus on the three archetypical Rho members, RhoA, Rac1, and Cdc42. There are several reasons for the disproportion in our knowledge of these three Rho GTPases compared with the remaining members of the Rho GTPase subfamily. One obvious reason is that RhoA, Rac1, and Cdc42 were isolated and characterized before the other Rho GTPases were identified, and they are expressed in virtually all cell types. Another indication of their importance is that inactivation or disruption of the RhoA, Rac1, and Cdc42 genes in mice results in early embryonic lethality (Heasman and Ridley, 2008). Although several of the less-studied Rho GTPases have a more tissue-specific expression, they have fundamental roles in many cell types (Aspenström et al. 2004, 2007). RhoD is an example of a less-studied member of the Rho GTPase family, and it was identified by PCR cloning almost 20 yr ago (Chavrier et al., 1992; Murphy et al., 1996). Together with Rif, RhoD constitutes the RhoD subgroup of the classical Rho GTPases (Ellis and Mellor, 2000; Aspenström et al., 2007; Boureux et al., 2007). From an evolutionary point of view, Rif is present in eukaryotes from Drosophila onward. A gene duplication event resulting in RhoD appears to have occurred in mammals, which express both RhoD and Rif (Boureux et al., 2007). RhoD is expressed in most tissues, and also in several commonly used cell lines. In contrast, Rif has a more tissue-specific distribution (Ellis and Mellor, 2000; Gad and Aspenström, 2010).
RhoD has been shown to integrate early endosome motility and actin reorganization (Murphy et al., 1996; Aspenström et al., 2004). However, the molecular mechanisms underlying the effects elicited by RhoD on endocytosis and cytoskeletal reorganization have remained elusive. In this study, we present two binding partners of RhoD that are distantly related, filamin A (FLNa)–interacting protein (FILIP1) and the actin nucleation–promoting factor WASp homologue associated with actin Golgi membranes and microtubules (WHAMM; Nagano et al., 2002; Campellone et al., 2008). It was previously known that FILIP1 interacts with FLNa (also known as actin-binding protein/ABP 280), an actin-binding protein that is required for the regulation of actin dynamics (Nagano et al., 2002; Zhou et al., 2010). We show that FILIP1 and WHAMM belong to a group of proteins that possess similarities in their domain organization. Although both FILIP1 and WHAMM were found to possess a RhoD-binding capacity, they showed unique functions in the control of the actin filament system. In agreement with the distinct functions of these RhoD effectors, FILIP1 links to actin filaments via FLNa, whereas WHAMM acts through its C-terminal Arp2/3-binding motifs (Nagano et al., 2002; Campellone et al., 2008). In accordance with an instrumental role of RhoD in cytoskeletal dynamics, RhoD and its effectors were found to control vital cytoskeleton-driven cellular processes, most notably cell attachment and cell migration.
RESULTS
RhoD regulates the formation of filopodia and the organization of actin bundles
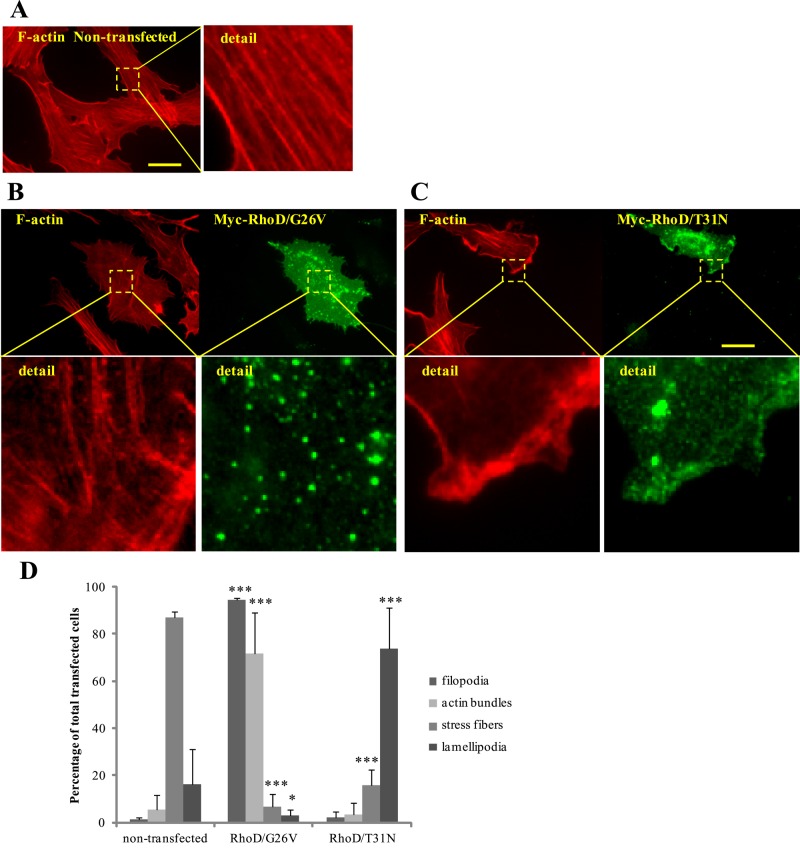
Our previous studies demonstrated that ectopic expression of RhoD in PAE/PDGFRβ cells induced the assembly of short bundles of actin filaments, concomitant with the formation of filopodia (Aspenström et al., 2004). To analyze these phenotypes in detail, we transfected the PAE/PDGFRβ cells with a Myc-tagged constitutively active mutant of RhoD (RhoD/G26V) or a dominant-negative mutant of RhoD (RhoD/T31N). We analyzed the resulting effects on cell morphology and actin organization using fluorescence microscopy. In agreement with the previous observations, RhoD/G26V expression effectively induced filopodia formation (Figure 1, B and D). Additionally, RhoD/G26V expression induced the assembly of actin filament bundles. These bundles were not organized into conventional stress fibers, which predominantly form parallel fibers that transverse the cells in a longitudinal manner. In contrast, the RhoD/G26V-induced bundles appeared less dense, shorter, and organized in a multidirectional manner (Figure 1, B and D). Comparatively, the control mock-transfected cells contained conventional stress fibers (Figure 1A). Strikingly, the RhoD/T31N expression induced a marked increase in edge ruffles, or lamellipodia (Figure 1, C and D). Similar results were observed in immortalized human foreskin fibroblast cells (BJ/SV40T; unpublished data). This suggests that RhoD regulates actin filament bundling and lamellipodia formation.
FIGURE 1:
RhoD induces the formation of filopodia and actin bundles. Nontransfected PAE/PDGFRβ cells (A), Myc-tagged constitutively active RhoD/G26V (B), or dominant-negative RhoD/T31N (C) in transiently transfected PAE/PDGFRβ cells were visualized using rabbit anti-Myc antibodies followed by Alexa Fluor 488–conjugated anti-rabbit antibodies. Filamentous actin was visualized using TRITC-conjugated phalloidin. Scale bar: 20 μm. Magnified images show detail of the actin bundles that are shorter and thinner than conventional stress fibers in RhoD/G26V-expressing cells (B) and RhoD/T31N-induced ruffles (C). (D) Quantification of the specific effects on the organization of the actin filament system. At least 100 transfected cells from three independent experiments were scored for formation of filopodia, actin bundles, stress fibers, and lamellipodia. The error bars represent SD. *, p < 0.05; ***, p < 0.001.
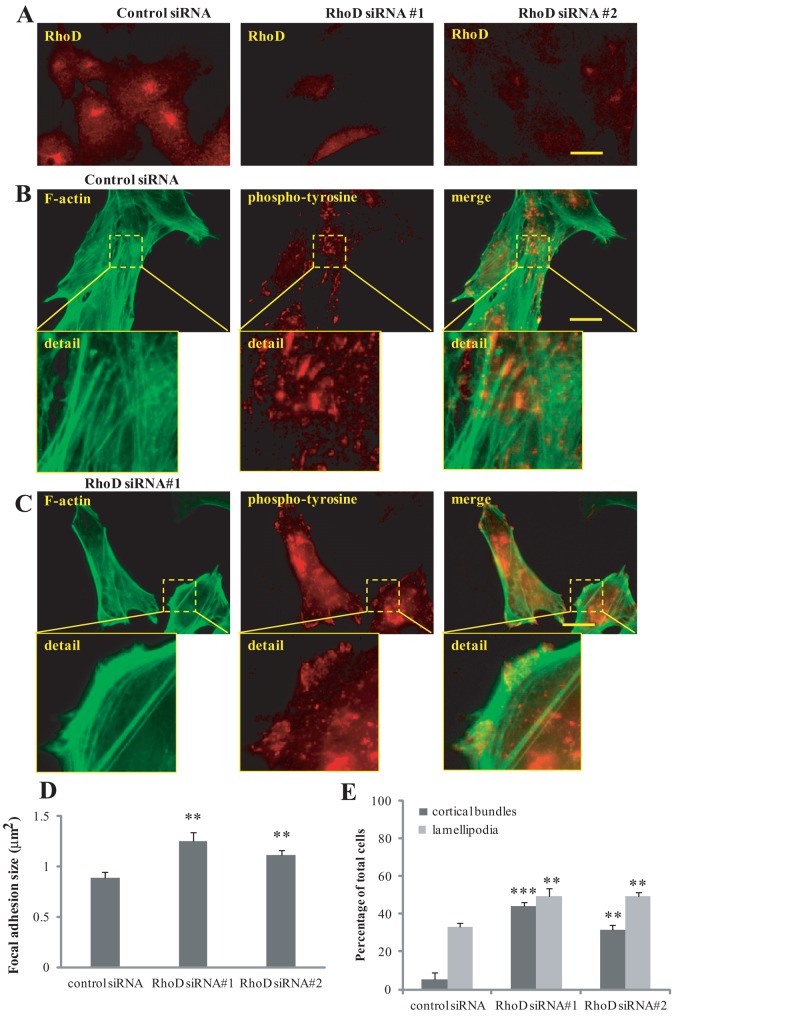
To investigate the RhoD-dependent cellular effects further, we silenced the expression of RhoD with small interfering RNA (siRNA) and analyzed the resulting effects on the actin filament system. For these experiments, we used the human BJ/SV40T fibroblasts. Endogenous RhoD was found to localize to the perinuclear area, and the two independent RhoD-specific siRNAs quenched the RhoD-specific signals, demonstrating the efficiency of the RhoD-targeted siRNA (Figure 2A). The cells treated with the control, nontargeting, siRNA showed normal organization of actin filaments, stress fibers, and focal adhesions (Figure 2B). In contrast, cells treated with RhoD-specific siRNAs showed an accumulation of thick cortical bundles of actin filaments (in 42% of the cells; Figure 2, C and E), together with a significant increase in the size of the focal adhesions (Figure 2D). Interestingly, similar to the cells expressing RhoD/T31N, the cells with reduced RhoD expression had increased edge ruffling (Figure 2, C and E).
FIGURE 2:
Knockdown of RhoD results in the formation of thick cortical actin bundles and increased focal adhesion size. (A) Human BJ/SV40T fibroblasts were treated with control or two different RhoD-specific siRNAs and stained for endogenous RhoD with rabbit anti-RhoD antibodies followed by TRITC-conjugated anti-rabbit antibodies. (B and C) Human fibroblasts were treated with control or RhoD-specific siRNAs. Focal adhesions were visualized using mouse monoclonal anti-phosphotyrosine antibodies followed by TRITC-conjugated anti-mouse antibodies. Filamentous actin was visualized using Alexa Fluor 488–conjugated phalloidin. Scale bar: 20 μm. (D) Quantification of the mean focal adhesion size, using the ImageJ software. Ten randomly selected fields of view from each condition were photographed and used for the image analysis. The experiment was repeated three times. The error bars represent SE of the mean. (E) Quantification of the specific effects on the organization of the actin filament system. At least 100 transfected cells from three independent experiments were scored for cortical actin bundles and lamellipodia. The error bars represent SD. Images of representative cells are shown in (B) and (C). **, p < 0.01; ***, p < 0.001.
FILIP1 is a RhoD/Rif effector
We sought to find out more about the molecular mechanisms that underlie these RhoD-dependent effects on the actin filament system. We performed a yeast two-hybrid system screen, with the constitutively active RhoD/G26V mutant fused to the DNA-binding domain of GAL4 as the bait, to screen a human mammary gland cDNA library fused to the GAL4 activation domain. One set of clones encoded the transmembrane receptor PlexinA1, which is in agreement with the finding that both PlexinA1 and PlexinB1 function as RhoD binding partners (Zanata et al., 2002; Tong et al., 2007). Another set of positive clones encoded the FLNa-interacting protein FILIP1 (Nagano et al., 2002). Searches in domain databases revealed that this protein has a cortactin-binding protein 2 (CortBP2) domain at the N-terminus and a central structural maintenance of chromosome (SMC) domain, which is a leucine-zipper type of coiled-coil domain (Supplemental Figure S1A). The functional roles of these domains are currently unknown. Further, the database analysis indicated that the region between amino acid residues 200 and 800 of FILIP1 is likely to fold into a coiled-coil structure. The FLNa-binding motif resides in a domain that encompasses the 450 C-terminal amino acids of FILIP1 (Nagano et al., 2002). The shortest interacting clone that was isolated in the yeast two-hybrid system screen encompassed amino acid residues 486–1213 of FILIP1 (Figure S1A). We tested whether a full-length FLAG-epitope–tagged cDNA encoding FILIP1 could interact with RhoD in coimmunoprecipitation experiments. To this end, HEK293T cells were transiently transfected with FLAG-FILIP1 and Myc-tagged RhoD variants. The presence of FILIP1 in the Myc-RhoD precipitates was examined using Western blotting. The wild-type and constitutively active RhoD/G26V interacted with FLAG-FILIP1 (Figure S1B). In contrast, the dominant-negative RhoD/T31N mutant did not bind to FILIP1. This suggests that RhoD binds FILIP1 in a GTP-dependent manner.
We next sought to identify the RhoD-binding site on FILIP1 by coexpressing a series of deletion mutants of FILIP1 in HEK293T cells. We noted a strong interaction with RhoD to a fragment that encompasses the central amino acids 431–778 domain of FILIP1, as well as the longer amino acids 431–1213 fragment of FILIP1 (Figure S1C). A weak interaction with the N-terminal amino acids 1–484 fragment of FILIP1 was also observed. This suggests that the FILIP1 interaction domain for RhoD is likely to reside in the FILIP1 SMC domain. The fragment encompassing amino acids 1–778 of FILIP1 could also be expected to have the ability to bind RhoD. However, the unique subcellular localization of this FILIP1 mutant in giant vacuoles makes it less likely to function as a binding partner (Figure S1D). A glutathione S-transferase (GST)-fusion protein encompassing amino acids 431–778 of FILIP1 also bound Myc-tagged RhoD in a pull-down assay (Figure S1E).
FILIP1 is related to the actin nucleation–promoting factor WHAMM
Using homology searches, we aimed to identify additional FILIP1-like proteins with tentative RhoD-binding capacity. We found three FILIP1-like proteins; the FILIP1-like (or down-regulated in ovary cancer-1), the CortBP2 N-terminal like (CTTNBP2 N-terminal like), and the leucine zipper protein1 (LUZP1; Figure S2A; Cheung et al., 2001; Hsu et al., 2008; Kwon et al., 2008). Interestingly, we noticed a similarity between FILIP1 and the Arp2/3-binding proteins WHAMM and the junction-mediating and regulatory protein (JMY; Figure S2A; Campellone et al., 2008; Zuchero et al., 2009). WHAMM and FILIP1 are only distantly related, but their SMC domains appear to be relatively similar. The identity between their SMC domains is around 16%; however, the similarity is around 45% (Figure S2, B and C).
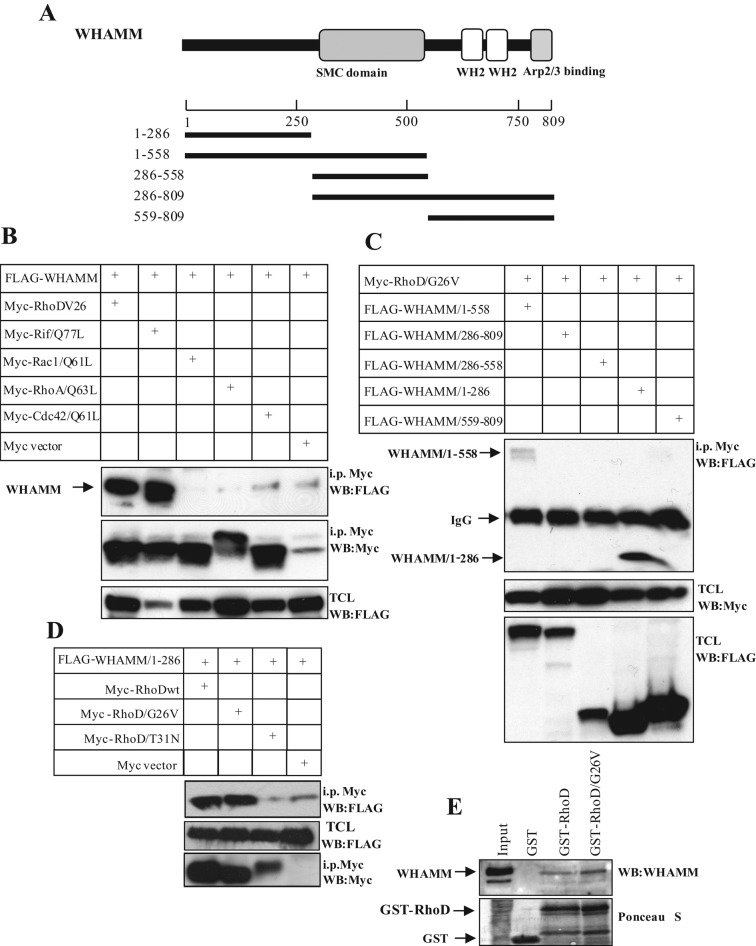
We next tested whether WHAMM can serve as a binding partner for Rho GTPases. To this end, a selection of Rho GTPases was transiently transfected together with the full-length FLAG-tagged WHAMM in HEK293T cells. Interestingly, WHAMM was found to interact with the constitutively active RhoD/G26V and Rif/Q77L, showing only modest interactions to other members of the Rho GTPases (Figure 3B). To identify the RhoD-binding domain of WHAMM, we transfected deletion mutants of WHAMM (Figure 3A) together with the constitutively active RhoD/G26V or Rif/Q77L. We detected strong binding of RhoD/G26V and Rif/Q77L to WHAMM/1-286 and to WHAMM/1-558, but not to WHAMM/286-809 or to WHAMM/559-809 (Figures 3C and S2D). This indicates that the domain for the binding of Rif to WHAMM resides in the N-terminal part of WHAMM (between amino-acid residues 1 and 286). We next tested the GTP-dependency of the RhoD:WHAMM interaction. To this end, FLAG-WHAMM/1-286 was cotransfected with Myc-tagged RhoD variants in HEK293T cells. The wild-type and constitutively active RhoD/G26V interacted with FLAG-WHAMM/1-286 (Figure 3D). In contrast, the dominant-negative RhoD/T31N mutant did not bind, suggesting that RhoD binds WHAMM in a GTP-dependent manner. This observation was subsequently confirmed by analyzing the interaction between endogenous proteins we used GST-RhoD and GST-RhoD/G26V to pull down endogenous WHAMM from lysed HEK293T cells. Also in this case, the constitutively active mutant was slightly better than the wild-type RhoD to pull down WHAMM (Figure 3E). We used the pull-down strategy because, for unknown reasons and despite several attempts, we have not been able to generate any RhoD- or Rif-specific antiserum that recognizes the endogenous proteins useful for immunoprecipitation or Western blotting.
FIGURE 3:
WHAMM is a RhoD-binding protein. (A) The domain organization of WHAMM. Dark gray box, cortactin-binding protein 2 (CTTNBP2) domain; gray box, an SMC domain; white boxes, WH2 domains; light gray box, Arp2/3-binding site. (B) Interactions between selected Rho GTPases and WHAMM were revealed by immunoprecipitation in transiently transfected HEK293T cells. The presence of FLAG-WHAMM in the Myc precipitates was revealed by Western blotting, using anti-FLAG antibodies. (C) Mapping the RhoD-binding domain on WHAMM. The presence of FLAG-WHAMM deletion mutants in the constitutively active Myc-RhoD/G26V precipitates from transiently transfected HEK293T cells was revealed by Western blotting, using anti-FLAG antibodies. (D) The interaction between RhoD mutants and WHAMM/1-286 was investigated by immunoprecipitation in transiently transfected HEK293T cells. The presence of FLAG-WHAMM/1-286 in the immunoprecipitates of RhoDwt, RhoD/G26V, and RhoD/T31N was revealed by Western blotting. (E) Pull down of endogenous WHAMM from lysed HEK293T cells transfected with GST-RhoD and GST-RhoD/G26V.
FILIP1, but not WHAMM, is an FLNa-binding protein
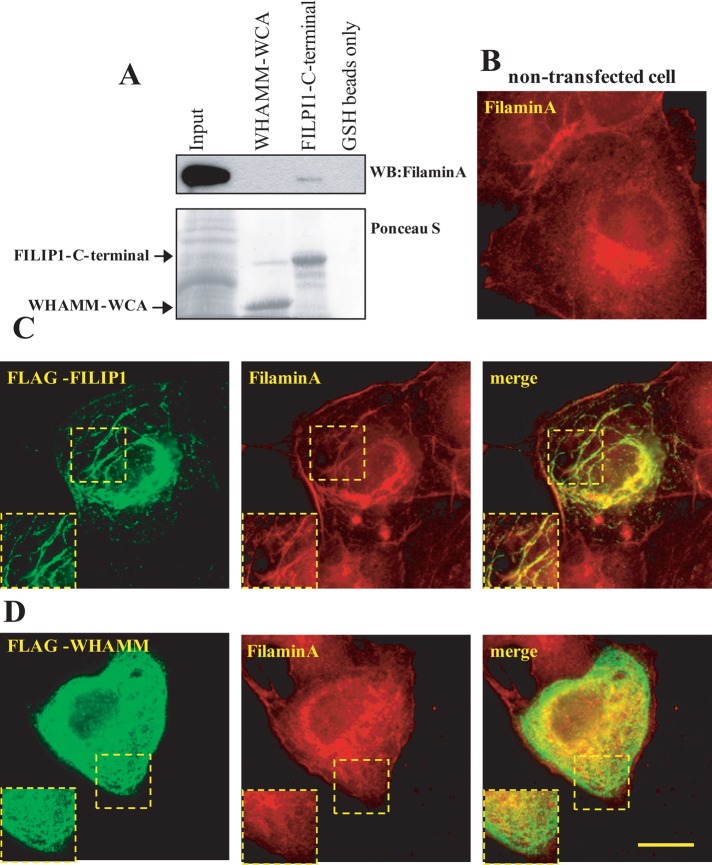
Because FILIP1 was originally identified as a FLNa-interacting protein, we performed GST pull-down assays to determine whether the C-terminal part of FILIP1 or WHAMM interact with FLNa. FILIP1, but not WHAMM, interacted with FLNa, confirming previous observations (Nagano et al. 2002; Figure 4A). These authors reported that ectopic expression of FILIP1 resulted in calpain cleavage and degradation of FLNa. However, we were not able to find any alterations in the FLNa levels in FILIP1-expressing HEK293T cells (Figure S3). Ectopic expression of FILIP1 induced a rather characteristic localization of FILIP1 in thread-like filaments in the cytoplasm (Figure 4C). FILIP1 localized along FLNa-positive fibers in transfected COS1 cells. Ectopic WHAMM was also found in similar filaments, which had an appearance like birds’ nests (Figure 4D). However, in contrast with FILIP1, WHAMM did not colocalize with FLNa, further indicating that WHAMM lacks an FLNa-binding site. These results underline the functional differences between FILIP1 and WHAMM.
FIGURE 4:
FILIP1, but not WHAMM, is an FLNa-binding protein. (A) Endogenous FLNa from lysed HEK293T cells was precipitated using FILIP1/775-1213 and GST-WHAMM/589-809 in GST pull-down assays. The presence of FLNa in the precipitated material was revealed by Western blotting, using anti-FLNa antibodies. (B–D) Colocalization between FILIP1 or WHAMM and FLNa was revealed by transiently transfecting FLAG-tagged FILIP1 or WHAMM in COS1 cells. FLAG-tagged FILIP1 or WHAMM was visualized with mouse monoclonal anti-FLAG antibodies followed by Alexa Fluor 488–conjugated anti-mouse antibodies. FLNa was visualized using a rabbit anti-FLNa antibody, followed by TRITC-conjugated anti-rabbit antibodies. Scale bar: 20 μm.
WHAMM is required for RhoD-dependent actin reorganization
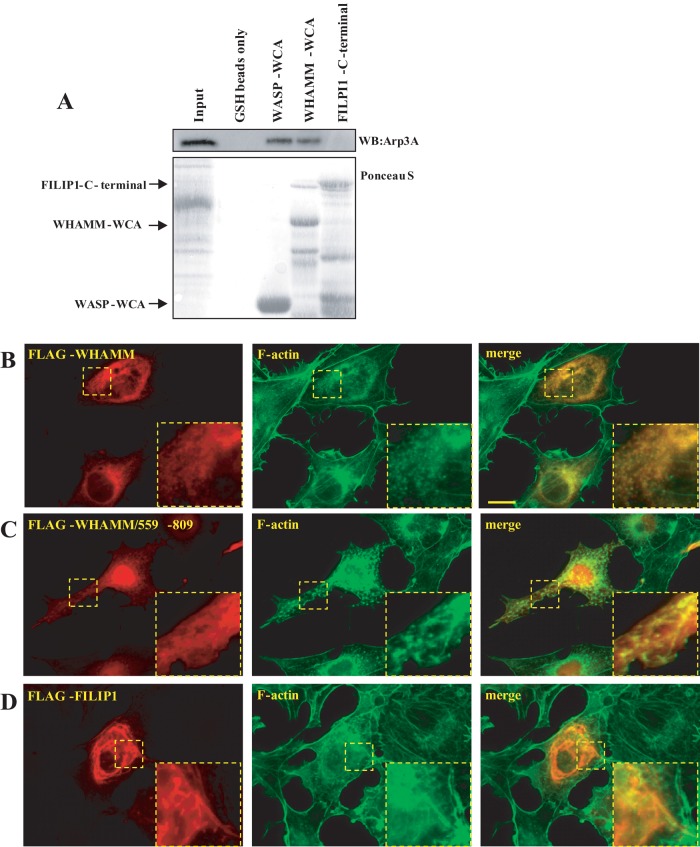
WHAMM has been shown to have a C-terminal WH2 domain and an Arp2/3 complex–binding C-terminal domain. We did not find FILIP1 to possess any detectable Arp2/3-binding capacity in comparison with WHAMM, which bound to components of the Arp2/3 complex as expected (Figure 5A). WHAMM expression was associated with a strong reorganization of actin into multiple foci (Figure 5B). The C-terminal fragment of WHAMM encompassing the Arp2/3-binding domain was particularly efficient in inducing actin foci (Figure 5C), which most likely reflects the nucleation ability of the WHAMM Arp2/3-binding domain. Although FILIP1 localized to a certain extent to filamentous actin, ectopic expression of FILIP1 did not result in the robust actin reorganization seen in WHAMM-expressing cells (Figure 5D).
FIGURE 5:
WHAMM, but not FILIP1, binds the Arp2/3 complex. (A) The Arp2/3-binding potential of FILIP1 and WHAMM was tested using GST pull-down assays. Lysates of HEK293T cells were precipitated using GST fusion proteins of FILIP1/775-1213 and GST-WHAMM/589-809. GST-WASP/442-501 was used as a control Arp2/3-binding protein. The presence of the Arp2/3 complex in the immunoprecipitates was revealed using antibodies against Arp3A. (B–D) WHAMM has a profound influence on the organization of the actin filament system. WHAMM (B), WHAMM/559-809 (C), or FILIP1 (D) were transfected into COS1 cells. FLAG-tagged constructs were visualized using rabbit anti-FLAG antibodies followed by TRITC-conjugated anti-rabbit antibodies. Filamentous actin was visualized using Alexa Fluor 488–conjugated phalloidin. Scale bar: 20 μm.
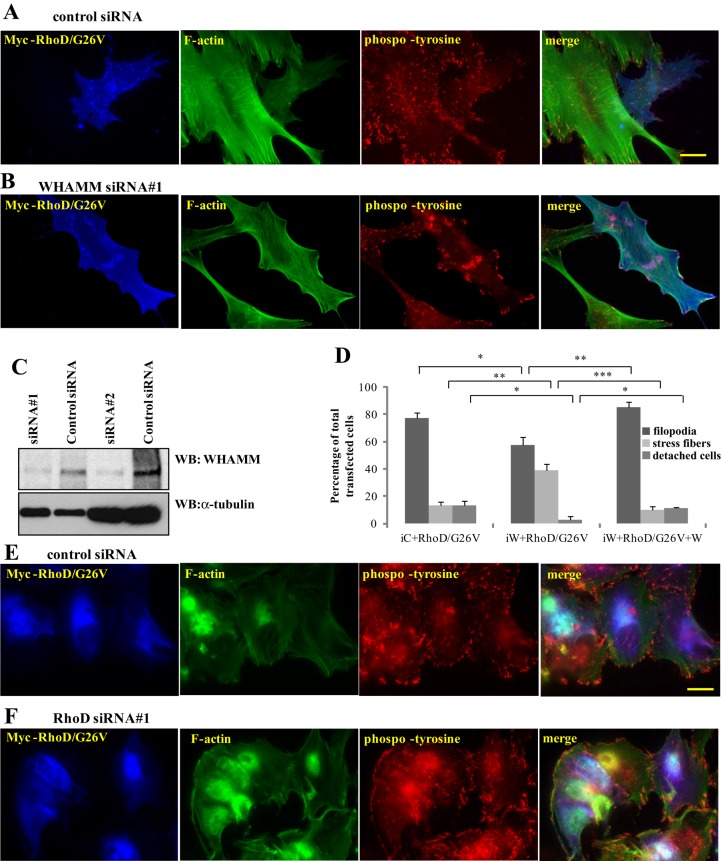
To investigate whether RhoD-dependent reorganization of actin requires WHAMM, we silenced the expression of WHAMM in BJ/SV40T cells using siRNA (Figure 6C). Subsequently, we expressed the constitutively active RhoD/G26V in the presence or absence of WHAMM. We noted a small, yet significant, decrease in filopodia formation in the WHAMM knockdown cells (Figure 6, B and D). The number of RhoD/G26V-induced rounded-up and detached cells also decreased in the WHAMM knockdown cells (Figure 6D). More significantly, RhoD/G26V failed to induce stress fiber dissolution associated with the formation of short actin bundles in the presence of WHAMM siRNA (Figure 6, B and D). These phenotypes could be rescued by ectopically expressing WHAMM together with RhoD/G26V (Figure 6D). In contrast, when WHAMM was ectopically expressed in RhoD-silenced cells, there was no clear difference on the WHAMM-induced actin reorganization, and WHAMM was seen to induce actin foci in both control and RhoD siRNA-treated cells (compare Figure 6, E and F, with Figure 5B). Taken together, these observations suggest that WHAMM acts downstream of RhoD in actin regulation.
FIGURE 6:
WHAMM is required for RhoD-dependent actin reorganization. (A and B) Constitutively active RhoD/G26V has a more profound influence on the organization of the actin filament system in BJ/SV40T cells treated with a control siRNA than in cells treated with a WHAMM-specific siRNA. Myc-RhoD/G26V was visualized using rabbit anti-Myc antibodies followed by AMCA-conjugated anti-rabbit antibodies. Filamentous actin was visualized using Alexa Fluor 488–conjugated phalloidin. Focal adhesions were visualized using mouse anti-phosphotyrosine antibodies followed by TRITC anti-mouse antibodies. Scale bar: 20 μm. (C) The efficiency of a WHAMM-specific siRNA was revealed by transfecting a control siRNA or the WHAMM-specific siRNAs into human fibroblasts. WHAMM was detected using a rabbit anti-WHAMM antibody. The two siRNAs were analyzed in separate experiments, which explains why the cell number differs as demonstrated by the loading control for the two sets of experiments. (D) Quantification of the constitutively active RhoD/G26V-induced effects on the organization of the actin filament system (filopodia, stress fibers, and detached cells). iC, control siRNA; iW, WHAMM siRNA#1; W, FLAG-tagged WHAMM used to rescue the phenotype induced by knockdown of WHAMM. At least 100 transfected cells from three independent experiments were scored for cortical actin bundles and lamellipodia. The error bars represent SD. *, p < 0.05; **, p < 0.01; ***, p < 0.001. (E and F) FLAG-WHAMM was visualized using rabbit anti-FLAG antibodies followed by AMCA-conjugated anti-rabbit antibodies. Filamentous actin was visualized using Alexa Fluor 488–conjugated phalloidin. Focal adhesions were visualized using mouse anti-phosphotyrosine antibodies followed by TRITC-labeled anti-mouse antibodies. Scale bar: 20 μm.
RhoD and WHAMM are required for cell migration and cell adhesion
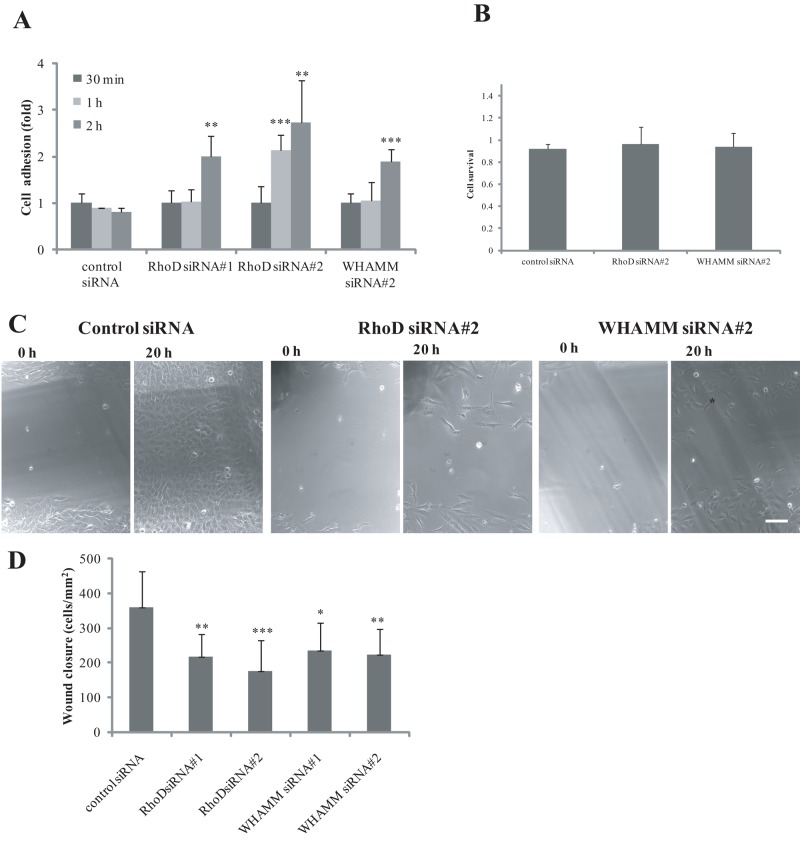
The increased focal adhesion size in cells knocked down for RhoD expression, and the involvement of RhoD and its effectors in actin dynamics, suggested that RhoD and WHAMM have roles in cell adhesion and cell migration. To test this hypothesis, we analyzed the involvement of RhoD and WHAMM in cell adhesion. To this end, we trypsinized cells that had been treated with RhoD- or WHAMM-specific siRNAs for 48 h and seeded them on coverslips. The cells were allowed to adhere for intervals between 30 min and 2 h and were then washed, fixed, and counted under the microscope. After 2 h, the cells knocked down for RhoD and WHAMM were significantly better at adhering to the surface than the control cells. The cells treated with RhoD siRNA#2 also showed a significantly better adhesion after 1 h (Figure 7A).
FIGURE 7:
RhoD and WHAMM are required for cell adhesion and cell migration. (A) Efficiency of cell adhesion in BJ/SV40T cells treated with siRNAs for RhoD and WHAMM. Cells were treated with siRNAs for 48 h. The cells were then trypsinized and seeded on coverslips precoated with serum. The adhering cells were fixed after 30 min, 1 h, and 2 h. The cells were analyzed on the microscope, and the cells attaching to the coverslips under the different conditions were counted under the microscope. The data represent quantifications from 5 to 10 random sites at the coverslips and were normalized to the amount of cells attaching at the initial time point. **, p < 0.01; ***, p < 0.001. (B) Survival of cells treated with siRNAs for RhoD and WHAMM was analyzed after 48 and 72 h of siRNA treatment by the calcein AM cell viability assay. The graph shows, for each siRNA, the relative change in cell viability from 48 to 72 h. (C) The efficiency of cell migration in BJ/SV40T cells treated with siRNAs for RhoD and WHAMM was tested in a wound closure assay. Cells in six-well plates were treated with siRNAs for 48 h, during which time the cells reached confluence. Wounds were made by scratching the cell monolayers with a plastic tip. The wounded areas were photographed directly after the wounding and after 20 h. Scale bar: 100 μm. (D) Quantification of cells moving into the wounded area. The measurements represent number of cells per square millimeter. The data represent five independent experiments with two to three wounded areas for each condition.
We performed a wound closure assay in BJ/SV40T cells knocked down for RhoD or WHAMM expression with siRNA. This analysis demonstrated that cells knocked down for RhoD and cells knocked down for WHAMM exhibited approximately 50% and 60% wound closure, respectively, compared with control cells (Figure 7, C and D). To control for the possibility that the reduced wound closure was simply caused by decreased cell survival, we performed a cell viability assay 48 and 72 h after transfection. A slight decrease in the number of surviving cells was observed for all siRNA conditions (Figure 7B). Nevertheless, this decrease was subtle and could not account for the strong effects seen on wound closure. Thus these results suggest that a RhoD–WHAMM signaling pathway is able to control directed cell migration.
DISCUSSION
Our data show that RhoD, via binding to FILIP1 and WHAMM, can control actin dynamics and, thereby, cell attachment and cell migration. To date, very few RhoD-binding proteins have been described. One of these proteins is the diaphanous-related formin splice variant of Dia2 known as hDia2C, which specifically binds RhoD and has a role in the motility of endosomal vesicles (Gasman et al., 2003). Also, Rif has been found to bind diaphanous-related formins, in this case mDia1 and mDia2 (Pellegrin and Mellor, 2005; Fan et al., 2010; Goh et al., 2011; Gorelik et al., 2011). In addition, RhoD has been found to interact with the Semaphorin receptors PlexinA1 and PlexinB1 and components of the TGF-β signaling pathway; however, the physiological significance of these interactions is not known (Zanata et al., 2002; Barrios-Rodiles et al., 2005; Tong et al., 2007; Hota and Buck, 2009). Given the limited knowledge about RhoD-binding protein, our data significantly increase our understanding of the mechanisms of action and function of RhoD.
We observed that RhoD activity, in addition to triggering filopodia-like extensions, also induced the formation of dense bundles of thin actin fibers. This implies that RhoD activity alone is sufficient to induce a shift in the actin organization into bundles of F-actin. Our observation that the expression of a dominant-negative variant of RhoD results in stress fiber dissolution and peripheral membrane ruffling suggests that RhoD activity is not only sufficient, but also required, to form F-actin bundles. RhoD has been observed to induce stress fiber and focal adhesion dissolution (Murphy et al., 1996), and this was suggested to be caused by the ability of RhoD to counteract RhoA-dependent stress fiber formation (Tsubakimoto et al., 1999). In agreement with this notion, we found that the RhoD-induced actin bundles were accompanied by a reduced number of extended parallel stress fibers. The RhoD-induced stress fiber dissolution was significantly suppressed in fibroblasts treated with siRNA targeting WHAMM. Taken together with the finding that WHAMM has Arp2/3-binding motifs and stimulates actin polymerization (Campellone et al., 2008), our data indicate that the actin nucleation–promoting activity of WHAMM is required for RhoD-dependent stress fiber dissolution. However, the suppression was not complete, which could be due to functional compensation by the WHAMM-like protein JMY (Zuchero et al., 2009). We found that RhoD activity decreases cell attachment and that knockdown of RhoD increased cell attachment, presumably due to the increased focal adhesion size. WHAMM knockdown alone did not affect cell attachment. Together, these observations suggest that WHAMM would act downstream of RhoD in a pathway that controls cell attachment. We postulate that FILIP1 has a similar role in cell attachment, although via a separate FLNa-dependent pathway.
FILIP1 was originally identified as an FLNa-binding protein (Nagano et al., 2002). In the original study, it was suggested that FILIP1 has a role in the regulation of the degradation of FLNa in a calpain-dependent manner. This study indicated that FILIP1 expression was associated with reduced lamellipodia formation and migration of COS7 cells. FLNa has an important role in the organization of actin networks, and it binds to preexisting actin filaments to form orthogonal lattices of filaments (Zhou et al., 2010). Studies in FLNa−/− mice indicate that neuronal and endothelial cells isolated from these mice appear to have normal F-actin structures and cell motility (Feng et al., 2006; Hart et al., 2006). In contrast, studies by Vadlamudi et al. (2002) showed that FLNa is needed for PAK1-dependent lamellipodia formation. Importantly, Baldassarre et al. (2009) showed that FLN is required for cell migration, since cells that lack both FLNa and FLNb had reduced cell migratory capacity. Hence there are several lines of evidence suggesting roles for FILIP1 and FLNa in controlling cell migration.
RhoD activation has been suggested to be a suppressor of cell migration. A study by Tsubakimoto et al. (1999) described a decrease in cell migration in fibroblasts expressing the constitutively active RhoD/G26V, when studied in a phagokinetic track assay. Murphy et al. (2001) made a similar observation in RhoD/G26V-expressing endothelial cells. These observations contradict our results, and the effects might be ascribed to the major effects that RhoD and Rif have on actin dynamics. Our results show that RhoD and WHAMM are required for the ability to perform directed migration in the wound closure assay. Although it is possible that both overactivity and underactivity of RhoD-dependent pathways affect cell migration in a negative manner, the most plausible conclusion of our results is that RhoD and its effectors stimulate the ability of cells to move. In line with this concept, the WHAMM-like protein JMY was shown to have a positive effect on cell migration when overexpressed in U2OS cells (Zuchero et al., 2009). Similarly, FILIP1-L and LUZP might also have roles in the regulation of cell migration (Cheung et al., 2001; Hsu et al., 2008; Kwon et al., 2008).
Rho GTPase proteins can control one cell function via various mechanisms of actions, as supported by a number of observations. For example, RhoA-induced stress fiber assembly is mediated both by mDia-induced actin polymerization and ROCK-mediated focal adhesion and F-actin stabilization, whereas Cdc42 induces the actin polymerization required for filopodia via both N-WASP–Arp2/3 and PAK1–LIMK and cofilin (Jaffe and Hall, 2005). Our results suggest that RhoD and its effectors control the coordination of Arp2/3- and FLNa-dependent mechanisms to regulate the actin filament system, cell adhesion, and cell migration.
We speculate that this use of different pathways has the benefit of providing both a more potent induction of a Rho GTPase–induced effect on cell behavior, as well as more options to control these effects. Given the importance of Rho GTPases in fundamental cellular processes, both potent and well-controlled effects by these proteins are likely of fundamental importance for cell behavior.
MATERIALS AND METHODS
Yeast two-hybrid screen
The Saccharomyces cerevisiae strain Y190 (genotype; MATa, gal4-542, gal80-538, his3, trp1-901, ade2-101, ura3-52, leu2-3, 112, URA3::GAL1-LacZ, Lys2::GAL1-HIS3cyhr) was transformed with a cDNA that encodes human RhoD/G26V fused to the GAL4 DNA-binding domain (GAL4DB) in the pYTH9 vector (Aspenström and Olson, 1995). This RhoD construct harbored cysteine-to-serine mutations in its CAAX box, since we reasoned that this would facilitate the nuclear translocation of RhoD during the screening procedure. This GAL4DB-RhoD/G26V–expressing yeast strain was used to screen a cDNA library from human mammary glands.
Antibodies, reagents, and constructs
The following antibodies were used: mouse anti-Myc (9E10; Covance, Princeton, NJ); mouse anti-FLAG monoclonal (M2), rabbit anti-RhoD, rabbit anti-WHAMM, mouse anti–α-tubulin (Sigma-Aldrich, St. Louis, MO); rabbit anti-Myc (Santa Cruz Biotechnology, Santa Cruz, CA); rabbit anti-GFP (Invitrogen, Carlsbad, CA); mouse monoclonal anti-FLNa (Millipore, Temecula, CA); rabbit anti-Arp3A (a generous gift from L. Machesky, The Beatson Institute, Glasgow, UK); tetramethyl rhodamine isothiocyanate (TRITC)-conjugated anti-mouse, and aminomethylcoumarin acetate (AMCA)-conjugated anti-rabbit (Jackson ImmunoResearch Laboratories, West Grove, PA); Alexa Fluor 488–conjugated anti-mouse and anti-rabbit (Invitrogen, Carlsbad, CA). Alexa Fluor 488–conjugated phalloidin (Invitrogen) and TRITC-conjugated phalloidin (Sigma-Aldrich) were used to visualize filamentous actin. Yellow fluorescent protein–tagged rat FILIP1 was a generous gift from Makoto Sato (University of Fukui, Japan). Human WHAMM (KIAA1971) was purchased from the Kazusa Institute (Chiba, Japan). FILIP1 and WHAMM full-length cDNAs and deletion mutants were generated by PCR and subcloned into the pRK5-FLAG vector and/or the pGEX-2T vector. The murine RhoD variants were subcloned into the pRK5Myc vector. The Myc-tagged Cdc42/Q61L, Rac1/Q61L, RhoA/Q63L, and Rif/Q77L in the pRK5 vector have been described before (Aspenström et al., 2004).
Cell culture, transfection, and immunoprecipitation
Human embryonic kidney 293T (HEK293T) cells, BJ human foreskin fibroblasts stably transfected with hTERT, and SV40 large T antigen (BJ/SV40T) cells, and green monkey COS-1 cells were cultured in DMEM supplemented with 10% (vol/vol) fetal bovine serum (FBS) and 1% (vol/vol) penicillin–streptomycin. Porcine aortic endothelial cells stably transfected with the human platelet-derived growth factor β receptor (PAE/PDGFRβ cells) were cultured in HAM's F12 medium supplemented with 10% (vol/vol) FBS and 1% (vol/vol) penicillin–streptomycin. All cell lines were cultured at 37°C in an atmosphere of 5% CO2. The cells were transfected using Lipofectamine (Invitrogen) or JetPEI reagents (PolyPlus Transfection, Illkirch, France), according to the protocols provided by the manufacturers.
For immunoprecipitation, the transiently transfected cells were lysed on ice in Triton X-100 lysis buffer (20 mM HEPES, pH 7.5, 100 mM NaCl, 1% Triton X-100, 10% glycerol, 5 mM EDTA, 1% aprotinin) 48 h posttransfection. The cell lysates were centrifuged for 15 min at 4°C, and the supernatants were incubated with the primary antibodies for 1 h at 4°C, after which the immunoprecipitates were collected on protein G-Sepharose (GE Healthcare, Waukesha, WI), for 1 h at 4°C. The beads were washed three times with Triton X-100 lysis buffer and subjected to SDS–PAGE, and the proteins were subsequently transferred onto nitrocellulose (Hybond C; GE Healthcare). Western blotting analyses were performed with the antibodies as specified in the figure legends; this was followed by horseradish peroxidase–conjugated anti-mouse or anti-rabbit antibodies (GE Healthcare). The proteins on the Western blots were revealed using Luminol immunoblotting reagent (Santa Cruz Biotechnology).
Knockdown of RhoD and WHAMM expression was induced by transfecting the BJ/SV40T cells with RhoD-directed siRNAs (esiRNA; Sigma-Aldrich) or with WHAMM-directed siRNAs (#1 and #2), RhoD siRNA#2, or a nontargeting siRNA (Ambion-Life Technology, Carlsbad, CA) using the SilentFect transfection reagent (Bio-Rad, Hercules, CA). The cells were incubated for 48 h posttransfection before being processed for the various assays.
Immunocytochemistry
The cells were seeded onto coverslips in six-well plates, fixed in 3% paraformaldehyde in phosphate-buffered saline (PBS) for 25 min at 37ºC, and then washed with PBS. The cells were permeabilized in 0.2% Triton X-100 in PBS for 5 min, washed with PBS, and blocked in 5% FBS in PBS for 30 min at room temperature. The primary and secondary antibodies were diluted in PBS containing 5% FBS. The cells were incubated with the primary antibodies and secondary antibodies for 1 h each, with washes in PBS between the incubations. The coverslips were then mounted on microscopy slides using Fluoromount-G (Southern Biotechnology, Birmingham, AL), photographed using a Zeiss AxioCAM MRm digital camera connected to a Zeiss AxioVert 40 CFL microscope, and processed with the AxioVision software (Zeiss, Jena, Germany). The cellular effects induced by ectopic expression were determined by microscopy analysis. At least 100 cells were scored for each transfection condition. Statistical analyses using Student's t test throughout the study were based on experiments that had been repeated at least three times.
Protein production and GST pull-down assays
GST-tagged fragments of FILIP1, WHAMM, RhoD, or GST alone were expressed in Escherichia coli and purified on glutathione-Sepharose beads (GE Healthcare). The pull-down assays were performed essentially as described previously (Aspenström et al., 2004).
Wound closure assay
For the wound closure assay, cells were seeded in six-well plates. The following day, siRNAs were transfected using SilentFect. The cells reached confluency over the next 48 h, and wounds were made in the confluent monolayers with a Gilson P200 pipette plastic tip. Two to three spots along the wound were marked with a pen under the plate. The wounded areas were photographed directly after the wounding (0 h) and again after 20 h with a Zeiss AxioVert 40 CFL microscope using a 10× objective. The cells that had moved into the wounded areas were counted on the photographs. The field of view was 0.603 mm2. The experiment was repeated five times and data from two to three wounds were analyzed for each condition.
Cell survival was determined by the calcein AM viability assay (Cells were washed three times with PBS and then treated with 1 mM calcein AM in PBS for 50 min at room temperature; this was followed by analysis of the fluorescence intensity at excitation 490 and emission 520 on a fluorescence plate reader.
Cell adhesion assay
For the adhesion assay, cells were seeded in six-well plates and, the following day, the cells were transfected with siRNAs as described above. After 48 h, the cells were trypsinized and seeded on coverslips precoated with serum. The cells were allowed to adhere for 30 min, 1 h, or 2 h. The cells were then washed with PBS to remove nonadhered cells and fixed in 3% paraformaldehyde for 25 min. The coverslips were mounted and photographed with a Zeiss AxioVert 40 CFL microscope using a 10× objective. Cells attaching to the coverslips under the different conditions were counted on the photographs. The data shown represent quantifications from 5 to 10 random sites at the coverslips and were normalized to the amount of cells attaching at the initial time point.
Supplementary Material
Acknowledgments
We are grateful for technical assistance with the viability assay provided by Thomas Helleday and Helge Gad, Stockholm University, Sweden. P.A. was supported by grants from the Ludwig Institute, Karolinska Institutet, Swedish Cancer Society (09 0368), and the Swedish Research Council (K2007-67X-15378-03-3 and K2010-66P-2). A.K.B.G. was supported by a Postdoctoral Fellowship from the Swedish Research Council and grants from the Karolinska Institutet Alex and Eva Wallström Foundation, the O. E. and Edla Johansson Foundation, the Magn. Bergvall Foundation, and the Swedish Society for Medical Research.
Abbreviations used:
- AMCA
aminomethylcoumarin acetate
- CortBP2
cortactin-binding protein 2
- FBS
fetal bovine serum
- FILIP1
filamin A–interacting protein
- FLNa
filamin A
- GAL4DB
GAL4 DNA-binding domain
- GAP
GTPase-activating protein
- GDI
guanine nucleotide disassociation inhibitors
- GEF
guanine nucleotide exchange factor
- PBS
phosphate-buffered saline
- siRNA
small interfering RNA
- SMC
structural maintenance of chromosome
- TRITC
tetramethyl rhodamine isothiocyanate
- WHAMM
WASp homologue associated with actin Golgi membranes and microtubules
Footnotes
*These authors contributed equally to this study.
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E12-07-0555) on October 19, 2012.
REFERENCES
- Aspenström P, Fransson Å, Saras J. The Rho GTPases have diverse effects on the organization of the actin filament system. Biochem J. 2004;377:327–337. doi: 10.1042/BJ20031041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspenström P, Olson MF. Yeast two-hybrid system to detect protein-protein interactions with Rho GTPases. Methods Enzymol. 1995;256:228–241. doi: 10.1016/0076-6879(95)56027-0. [DOI] [PubMed] [Google Scholar]
- Aspenström P, Ruusala A, Pacholsky D. Taking the Rho GTPases to the next level: the cellular function of the atypical Rho GTPases. Exp Cell Res. 2007;313:3673–3679. doi: 10.1016/j.yexcr.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Baldassarre M, Razinia Z, Burande CF, Lamsoul I, Lutz PG, Calderwood DA. Filamins regulate cell spreading and initiation of cell migration. PLoS One. 2009;4:e7830. doi: 10.1371/journal.pone.0007830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios-Rodiles M, et al. High-throughput mapping of a dynamic signaling network in mammalian cells. Science. 2005;307:1621–1625. doi: 10.1126/science.1105776. [DOI] [PubMed] [Google Scholar]
- Boureux A, Vignal E, Faure S, Fort P. Evolution of the Rho family of ras-like GTPases in eukaryotes. Mol Biol Evol. 2007;24:203–216. doi: 10.1093/molbev/msl145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campellone KG, Webb NJ, Znameroski EA, Welch MD. WHAMM is an Arp2/3 complex activator that binds microtubules and functions in ER to Golgi transport. Cell. 2008;134:148–161. doi: 10.1016/j.cell.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P, Simins K, Zerial M. The complexity of the Rab and Rho GTP-binding protein subfamilies revealed by a PCR cloning approach. Gene. 1992;112:261–264. doi: 10.1016/0378-1119(92)90387-5. [DOI] [PubMed] [Google Scholar]
- Cheung J, Petek E, Nakabayashi K, Tsui LC, Vincent JB, Scherer SW. Identification of the human cortactin-binding protein-2 gene from the autism candidate region at 7q31. Genomics. 2001;78:7–11. doi: 10.1006/geno.2001.6651. [DOI] [PubMed] [Google Scholar]
- Ellis S, Mellor H. The novel Rho-family GTPase Rif regulates coordinated actin-based membrane rearrangements. Curr Biol. 2000;10:1387–1390. doi: 10.1016/s0960-9822(00)00777-6. [DOI] [PubMed] [Google Scholar]
- Fan L, Pellegrin S, Scott A, Mellor H. The small GTPase Rif is an alternative trigger for the formation of actin stress fibers in epithelial cells. J Cell Sci. 2010;123:1247–1252. doi: 10.1242/jcs.061754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Chen MH, Moskowitz IP, Mendonza AM, Vidali L, Nakamura F, Kwiatkowski DJ, Walsh CA. Filamin A (FLNA) is required for cell-cell contact in vascular development and cardiac morphogenesis. Proc Natl Acad Sci USA. 2006;103:19836–19841. doi: 10.1073/pnas.0609628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad KBA, Aspenström P. Rif proteins take to the RhoD: Rho GTPases at the crossroads of actin dynamics and membrane trafficking. Cell Signal. 2010;22:183–189. doi: 10.1016/j.cellsig.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Gasman S, Kalaidzidis Y, Zerial M. RhoD regulates endosome dynamics through Diaphanous-related formin and Src kinase. Nat Cell Biol. 2003;5:195–204. doi: 10.1038/ncb935. [DOI] [PubMed] [Google Scholar]
- Goh WI, Sudhaharan T, Lim KB, Sem KP, Lau CL, Ahmed S. Rif-mDia1 interaction is involved in filopodium formation independent of Cdc42 and Rac effectors. J Biol Chem. 2011;286:13681–13694. doi: 10.1074/jbc.M110.182683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik R, Yang C, Kameswaran V, Dominguez R, Svitkina T. Mechanisms of plasma membrane targeting of formin mDia2 through its amino terminal domains. Mol Biol Cell. 2011;22:189–201. doi: 10.1091/mbc.E10-03-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AW, Morgan JE, Schneider J, West K, McKie L, Bhattacharya S, Jackson IJ, Cross SH. Cardiac malformations and midline skeletal defects in mice lacking filamin A. Hum Mol Genet. 2006;15:2457–2467. doi: 10.1093/hmg/ddl168. [DOI] [PubMed] [Google Scholar]
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- Hota PK, Buck M. Thermodynamic characterization of two homologous protein complexes: association of the semaphoring receptor plexin-B1 Rho GTPase binding domain with Rnd1 and active Rac1. Protein Sci. 2009;18:1060–1071. doi: 10.1002/pro.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C-Y, Chang NC, Lee MW, Lee KH, Sun DS, Lai C, Chang AC. LUZP deficiency affects neural tube closure during brain development. Biochem Biophys Res Commun. 2008;376:466–471. doi: 10.1016/j.bbrc.2008.08.170. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Kwon M, et al. Functional characterization of Filamin A interacting protein 1-like, a novel candidate for antivascular cancer therapy. Cancer Res. 2008;68:7332–7341. doi: 10.1158/0008-5472.CAN-08-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C, Saffrich R, Grummt M, Gournier H, Rybin V, Rubino M, Auvinen P, Lütcke A, Parton RG, Zerial M. Endosome dynamics regulated by a Rho protein. Nature. 1996;384:427–432. doi: 10.1038/384427a0. [DOI] [PubMed] [Google Scholar]
- Murphy C, Saffrich R, Olivo-Marin JC, Giner A, Ansorge W, Fotsis T, Zerial M. Dual function of RhoD in vesicular movement and cell motility. Eur J Cell Biol. 2001;80:391–398. doi: 10.1078/0171-9335-00173. [DOI] [PubMed] [Google Scholar]
- Nagano T, Yoneda T, Hatanaka Y, Kubota C, Murakami F, Sato M. Filamin A- interacting protein (FILIP) regulates cortical cell migration out of the ventricular zone. Nat Cell Biol. 2002;4:495–501. doi: 10.1038/ncb808. [DOI] [PubMed] [Google Scholar]
- Pellegrin S, Mellor H. The Rho family GTPase Rif induces filopodia through mDia2. Curr Biol. 2005;15:129–133. doi: 10.1016/j.cub.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Rottner K, Stradal TE. Actin dynamics and turnover in cell motility. Curr Opin Cell Biol. 2011;23:569–578. doi: 10.1016/j.ceb.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Tong Y, Chugha P, Hota PK, Alviani RS, Li M, Tempel W, Shen L, Park H-W, Buck M. Binding of Rac1, Rnd1, and RhoD to a novel Rho GTPase interaction motif destabilizes dimerization of the Plexin-B1 effector domain. J Biol Chem. 2007;282:37215–37224. doi: 10.1074/jbc.M703800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubakimoto K, Matsumoto K, Abe H, Ishii J, Amano M, Kaibuchi K, Endo T. Small GTPase RhoD suppresses cell migration and cytokinesis. Oncogene. 1999;18:2431–2440. doi: 10.1038/sj.onc.1202604. [DOI] [PubMed] [Google Scholar]
- Vadlamudi RK, Li F, Adam L, Nguyen D, Ohta Y, Stossel TP, Kumar R. Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat Cell Biol. 2002;4:681–690. doi: 10.1038/ncb838. [DOI] [PubMed] [Google Scholar]
- Zanata SM, Hovatta I, Rohm B, Püschel AW. Antagonistic effects of Rnd1 and RhoD GTPases regulate receptor activity in Semaphorin 3A-induced cytoskeletal collapse. J Neurosci. 2002;22:471–477. doi: 10.1523/JNEUROSCI.22-02-00471.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou AX, Hartwig JH, Akyürek LM. Filamins in cell signaling, transcription and organ development. Trends Cell Biol. 2010;20:113–123. doi: 10.1016/j.tcb.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Zuchero JB, Coutts AS, Quinlan ME, Thangue NB, Mullins RD. p53-cofactor JMY is a multifunctional actin nucleation factor. Nat Cell Biol. 2009;11:451–459. doi: 10.1038/ncb1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.