Abstract
Background
Salmonella enterica is a frequent cause of bloodstream infection (BSI) in Asia but few data are available from Cambodia. We describe Salmonella BSI isolates recovered from patients presenting at Sihanouk Hospital Centre of Hope, Phnom Penh, Cambodia (July 2007–December 2010).
Methodology
Blood was cultured as part of a microbiological prospective surveillance study. Identification of Salmonella isolates was performed by conventional methods and serotyping. Antibiotic susceptibilities were assessed using disk diffusion, MicroScan and E-test macromethod. Clonal relationships were assessed by Pulsed Field Gel Electrophoresis; PCR and sequencing for detection of mutations in Gyrase and Topoisomerase IV and presence of qnr genes.
Principal Findings
Seventy-two Salmonella isolates grew from 58 patients (mean age 34.2 years, range 8–71). Twenty isolates were identified as Salmonella Typhi, 2 as Salmonella Paratyphi A, 37 as Salmonella Choleraesuis and 13 as other non-typhoid Salmonella spp. Infection with human immunodeficiency virus (HIV) was present in 21 of 24 (87.5%) patients with S. Choleraesuis BSI. Five patients (8.7%) had at least one recurrent infection, all with S. Choleraesuis; five patients died. Overall, multi drug resistance (i.e., co-resistance to ampicillin, sulphamethoxazole-trimethoprim and chloramphenicol) was high (42/59 isolates, 71.2%). S. Typhi displayed high rates of decreased ciprofloxacin susceptibility (18/20 isolates, 90.0%), while azithromycin resistance was very common in S. Choleraesuis (17/24 isolates, 70.8%). Two S. Choleraesuis isolates were extended spectrum beta-lactamase producer.
Conclusions and Significance
Resistance rates in Salmonella spp. in Cambodia are alarming, in particular for azithromycin and ciprofloxacin. This warrants nationwide surveillance and revision of treatment guidelines.
Author Summary
Salmonella enterica is a bacterium that causes important morbidity and mortality worldwide, especially in tropical low-resource settings. Over the past two decades, increasing rates of resistance for the commonly available oral antibiotics have been reported in Salmonella spp., especially from South(east) Asia. As microbiology laboratories are extremely scarce in Cambodia, data on the presence and resistance of Salmonella spp. in this country are limited. The authors describe the different types and antibiotic resistance of 72 Salmonella isolates from blood cultures sampled in 58 adult Cambodian patients with fever. The most common serovars were Salmonella Typhi and Salmonella Choleraesuis. The latter serovar causes illness in pigs, and may occasionally infect humans through contact with contaminated animals or environments, especially those with decreased immunity. The authors noted resistance for the first line oral antibiotics in nearly three quarters of all Salmonella isolates. In addition, 90% of all S. Typhi had decreased susceptibility for ciprofloxacin, while around 70% of S. Choleraesuis showed resistance to azithromycin. These results seriously limit the treatment options for typhoid fever and other invasive Salmonella infections and warrant nationwide surveillance of antibiotic resistance. This is the first report to describe such high rates of azithromycin resistance in Salmonella enterica.
Introduction
Salmonella enterica is an important cause of morbidity and mortality worldwide [1], [2]. Salmonella enterica serovar Typhi is the etiologic agent of typhoid fever while non-typhoid Salmonella spp. (NTS) are associated with gastroenteritis and invasive infections in children, the elderly and immune compromised [3]. Both S. Typhi and NTS are among the most frequent pathogens causing bloodstream infections (BSI) in tropical low-resource settings [4]. The highest incidence of Salmonella infections worldwide occurs in Asia [1], [5], mainly in South and Southeast Asia, where isolates show high rates of antibiotic resistance [6], [7]. Although fluoroquinolones are drugs of choice to treat invasive Salmonella infections, decreased susceptibility to ciprofloxacin (DCS) is increasing quickly worldwide [2]. Azithromycin and ceftriaxone have been recommended as treatment alternatives for typhoid fever in case of DCS [8]–[10].
Little is known about the epidemiology and the extent of antibiotic resistance in invasive human Salmonella infections in Cambodia.
As part of a microbiological surveillance study on the causes of BSI in Cambodian adults and an antibiotic stewardship program, we aimed to assess the antibiotic resistance patterns of invasive salmonellosis in this population.
Methods
Study setting and period
Sihanouk Hospital Centre of HOPE (SHCH) is a 40-bed non-government referral hospital in Phnom Penh, Cambodia. Microbiological services were installed in 2005. From July 2007 until June 2011 a prospective BSI surveillance program was carried out.
Patients and blood culture sampling
From all adult patients presenting with signs of the Systemic Inflammatory Response Syndrome (SIRS) [11], venous blood (2×10 ml) was drawn for culture with registration of demographic and clinical data. Patients were identified with a unique hospital number. Blood was cultured in home-made Brain Heart Infusion broth bottles (BIO-RAD, Berkeley, California) (July 2007–March 2009) and from April 2009 onward in BacT/ALERT culture bottles (bioMérieux, Marcy l'Etoile, France). Blood cultures were incubated for 7 days at 35°C and daily monitored for growth by visual inspection of the broth or the chromogenic growth indicator respectively. As part of standard patient care, isolates were identified by conventional biochemical tests and assessed for antibiotic susceptibility by disk diffusion. Isolates were stored at −70°C on porous beads in cryopreservative (Microbank, Pro-Lab Diagnostics, Richmond Hill, Canada).
Microbiological work-up of isolates
Isolates identified as Salmonella spp. at SHCH were retrieved from −70°C, checked for purity and further worked up at the Institute of Tropical Medicine (Antwerp, Belgium) and the Scientific Institute of Public Health (Brussels, Belgium). Serotyping was carried out by slide agglutination with commercial antisera according to the Kauffmann-White scheme [12].
Clonal relationships were assessed by pulsed field gel electrophoresis (PFGE) according to the PulseNet Europe protocol [13]. Genomic DNA was digested with XbaI restriction enzymes (New-England Biolab, Leusden, Netherlands), S. Braenderup H9812 was used as a size marker. Profiles were analyzed using the Dice coefficient [14] and the unweighted-pair group method using average linkages, with a tolerance of 1%.
For the compilation of the resistance data, only the first isolate per BSI episode (defined as a 14-day period following the first day of BSI diagnosis) was considered. Recurrent infections were defined as a new BSI episode with an identical Salmonella serovar at least 14 days after the former isolate and after appropriate treatment of the patient. Recurrent isolates were considered as duplicate isolates and not compiled into the resistance overview; their resistance data were considered separately.
Antibiotic susceptibilities were assessed by disk diffusion (using Neo-Sensitabs™, Rosco Diagnostica, Taastrup, Denmark) and MicroScan (Combo 42, Siemens Healthcare Diagnostics, Deerfield, USA). Minimal inhibitory concentrations (MIC) for nalidixic acid (NA), ciprofloxacin, chloramphenicol and azithromycin were determined using the E-test macromethod (bioMérieux).
Breakpoints were those defined by the Clinical Laboratory Standards Institute [15]; intermediately resistant isolates were considered as resistant. DCS was defined according to European Committee on Antimicrobial Susceptibility testing (EUCAST) guidelines, i.e. a MIC-value for ciprofloxacin >0.064 µg/mL [16]. Multidrug resistance (MDR) was defined as co-resistance to the first line antibiotics ampicillin, chloramphenicol and sulphamethoxazole-trimethoprim (SMX-TMP). For azithromycin and Enterobacteriaceae, no breakpoints have been published. EUCAST mentions treatment of S. Typhi infections with a MIC≤16 µg/mL and a recent publication proposed 16 µg/ml as ‘epidemiological cutoff’ value for wild type Salmonella spp. [17]. Detection and identification of ESBL producing bla genes was performed by a commercial multiplex ligation PCR microarray CT 101 (Check-Points Health BV, Wageningen, The Netherlands) [18].
Screening for mutations in the quinolone resistance-determining region (QRDR) was performed by amplification of a fragment of the gyrA, gyrB, and parC genes containing the QRDR as previously described [19] and sequencing of the fragments on a CEQ 2000 DNA sequencer (Beckman Coulter, High Wycombe, United Kingdom), using the DTSC-2 method. The sequences were compared and analyzed by Genestream software (Institut de Génétique Humaine, Montpellier, France). The presence of the plasmid-mediated quinolone resistance qnr genes (qnrA, qnrB, and qnrS) was determined using PCR [20]
Statistical analysis
Data were entered in Access and Excel databases (Microsoft Corporation, Redmond, Washington, USA). Risk factors were assessed by univariate analysis using the Χ2 with STATA software (Statacorp, College Station, Texas). Differences were considered statistically significant at p-values<0.05.
Results
Demographic and clinical data
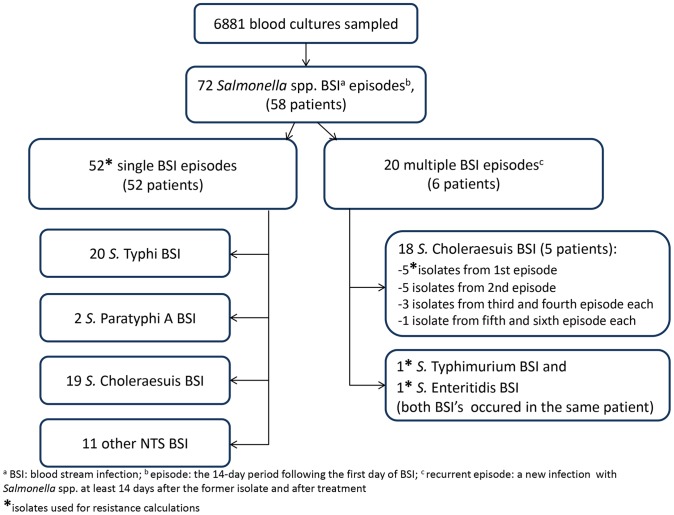
From 6881 blood cultures drawn during the study period, 72 non-duplicate Salmonella enterica isolates were recovered from 58 adult patients, representing 11.5% of all clinically significant organisms (CSO). These isolates were recovered from 59 first BSI episodes and 13 recurrent episodes (Figure 1). The serovars included S. Choleraesuis (n = 37; 51.4%) and S. Typhi (n = 20; 27.8%) followed by S. Enteritidis (n = 7; 9.7%), S. Typhimurium (n = 4; 5.6%), S. Paratyphi A (n = 2; 2.8%), S. London and S. Amsterdam (n = 1; 1.4% each).
Figure 1. Flow chart of blood stream infection (BSI) episodes, patients and corresponding serovars.
The mean age of patients with Salmonella BSI was 34.2 years (range 8–71), 51.7% were women. They came from at least 10 different provinces, mainly the greater Phnom Penh area (n = 11; 19.0%) and Kandal province (n = 7; 12.1%). The majority of Salmonella BSI occurred during the rainy months April to November (n = 57; 79.1%); no apparent other temporal or geographical clustering was noted.
Co-morbidity was present in 36 (62.1%) patients, mainly human immunodeficiency virus (HIV) infection (n = 32; 55.2%); we also noted systemic lupus erythematodes (n = 2; 3.4%), thalassemia and valvular heart disease (one patient each). For 13 HIV-infected patients, Salmonella BSI was the indicator disease for HIV-infection; only three HIV-patients were on antiretroviral treatment at the time of the BSI. The median CD4-cell count was 22 per microliter (range 2–253), concurrent opportunistic infections (OI) included tuberculosis (n = 6) and cryptococcal meningitis (n = 3). Of note, S. Choleraesuis was the most common pathogen in HIV-infected patients (21/32, 65.6%) whereas S. Typhi was predominantly recovered from HIV-negative patients (19/26, 73.1%). Of the 24 patients with S. Choleraesuis BSI, 12 (50.0%) presented with fever, six (25.0%) with abdominal pain and diarrhea and five (20.8%) with dyspnea and dry cough.
Patients were treated empirically with either ceftriaxone, amoxicillin-clavulanic acid or ciprofloxacin (or subsequent administration of these antibiotics) for a mean duration of 11.7 days (range 1–21). Additional treatment for HIV-related OI included SMX-TMP, fluconazole and tuberculostatic drugs.
A total of five patients infected with Salmonella spp. (8.6%) had one or more recurrent BSI episodes with the same serovar, all S. Choleraesuis (Figure 1). The mean interval to recurrence was 4.5 weeks (range 2–10 weeks). One HIV-patient had a S. Typhimurium BSI eight months after being treated for S. Enteritidis BSI.
Five patients (8.6%) died. Four of them had been infected by S. Choleraesuis and one by S. Typhimurium. All were HIV-infected with advanced immune depression; at least three of them suffered from concurrent life-threatening opportunistic infections (tuberculosis n = 2, cryptococcal meningitis n = 1). The median duration between the diagnosis of Salmonella BSI and death was 24 days (range 13–61 days)
PFGE
For S. Choleraesuis, three different PFGE profiles were obtained, of which Xb-Chol-1 was predominant (86.5%), including all 13 recurrent isolates (data not shown). The PFGE profiles of ‘first’ and ‘recurrent’ isolates were identical per patient. No association between a particular PFGE profile and resistance profile was observed.
All S. Typhi isolates had a similar PFGE profile (i.e.Xb-Ty-1) whereas S. Enteritidis and S. Typhimurium presented with two and three different profiles respectively.
Antibiotic resistance
Antibiotic resistance data as assessed for the 59 ‘first’ (i.e. non-recurrent) isolates are shown in Table 1. Of note, very high rates of MDR were seen in S. Typhi (15/20 isolates, 75.0%) and S. Choleraesuis (22/24 isolates, 91.7%) and to a lesser extent in other NTS (5/13 isolates, 38.5%).
Table 1. Antibiotic resistance in 59 Salmonella isolates (first BSI episode only), SHCH 2007–2011.
| resistant isolates | ||||
| (%) | (n) | |||
| Antibiotic | S. Typhi (n = 20) | S. Choleraesuis (n = 24) | other NTS (n = 13) | S. Paratyphi A (n = 2) |
| Multi drug resistancea | 75,0 | 91,7 | 38,5 | 0/2 |
| Fluoroquinolone resistance | ||||
| Nalidixic acid | 90,0 | 33,3 | 38,5 | 0/2 |
| Decreased ciprofloxacin susceptibility (DCS)b | 90,0 | 20,8 | 53,8 | 0/2 |
| High level ciprofloxacin resistancec | 0,0 | 0,0 | 7,7 | 0/2 |
| Second line antibiotics | ||||
| Azithromycind | 5,0 | 70,8 | 15,4 | 0/2 |
| Cefotaxime | 0,0 | 4,2 | 0,0 | 0/2 |
| Combined resistance | ||||
| MDR+DCS | 70,0 | 16,7 | 23,1 | 0/2 |
| MDR+DCS+Azithromycin | 0,0 | 4,2 | 7,7 | 0/2 |
| Reserve antibiotics | ||||
| Meropenem | 0,0 | 0,0 | 0,0 | 0/2 |
| Tigecyclin | 0,0 | 0,0 | 0,0 | 0/2 |
| Fosfomycin | 0,0 | 0,0 | 0,0 | 0/2 |
co-resistance to ampicillin+SMX-TMP+chloramphenicol;
MIC ciprofloxacin >0.064 µg/ml, see text for details;
MIC ciprofloxacin ≥4 µg/ml;
MIC azithromycin >16 µg/ml;
not included: 1 isolate S. Choleraesuis from recurrent infection, ESBL producing.
DCS was particularly present among S. Typhi isolates, with MIC50 and MIC90 of 0.25 µg/mL and 0.38 µg/mL respectively (Table 2). Thirty-one (88.6%) out of 35 DCS isolates displayed resistance to NA, with mutations in gyrA at either position 83 (n = 24) or 87 (n = 3) (Table 3). One S. Typhimurium displayed full resistance to ciprofloxacin (MIC 6 µg/mL) confined to two mutations in gyrA (Ser83→Phe and Asp87→Asn) and one in parC (Ser80→Arg). Of note, four isolates (all NTS) displayed DCS but were NA susceptible: no mutations in gyrA or parC were observed; in two of them presence of qnrS1 was detected. In 22 of 24 S. Choleraesuis and in all S. Paratyphi A we detected a parC mutation in position 57, regardless of susceptibility patterns.
Table 2. Distribution of minimal inhibitory concentration (MIC) for ciprofloxacin in 59 Salmonella isolates (first BSI episode only).
| MIC ciprofloxacin (µg/ml)a | |||||||||||||||
| Serovar (n) | 0.004 | 0.006 | 0.008 | 0.012 | 0.016 | 0.032 | 0.064 | 0.094 | 0.125 | 0.19 | 0.25 | 0.38 | 6 | MIC 50 | MIC 90 |
| S. Choleraesuis (24) | 2 | 5 | 5 | 2 | 2 | - | - | 3 | 4 | 1 | - | . | - | 0.012 | 0.125 |
| S. Paratyphi A (2) | - | - | - | 1 | 1 | - | - | - | - | - | - | - | - | NA | NA |
| S. Typhi (20) | - | 1 | - | 1 | - | - | - | - | 1 | 4 | 10 | 3 | - | 0.25 | 0.38 |
| other NTS (13) | - | - | 3 | - | - | - | 1 | 1 | 2 | 2 | 1 | 2 | 1 | 0.125 | 0.38 |
NA, not applicable.
resistance breakpoint 0.064 µg/ml.
Table 3. Mutations in Gyrase and Topoisomerase and presence of qnr genes, according to serovar and resistance phenotype in 59 Salmonella spp.
| Resistance phenotype | MIC ciprofloxacin | Serovars | n isolates | gyrA | gyrB | parC | qnr |
| NaS CipS (n = 24) | 0.004–0.064 | S. Typhi | 2 | Glu133→Glyb (n = 2) | - | - | - |
| S. Paratyphi A | 2 | - | - | Thr57→Serc (n = 2) | - | ||
| S. Choleraesuis | 16 | - | - | Thr57→Ser (n = 16) | - | ||
| other NTS | 4 | Ile125→Serd (n = 1) | - | - | - | ||
| NaS DCS (n = 4) | 0.125–0.38 | other NTS | 4 | - | - | Thr57→Ser (n = 1) | S1 (n = 2) |
| NaR DCS (n = 31) | 0.094–0.38 | S. Typhi | 18 | Ser83→Phe/Glu133→Gly (n = 18) | - | - | - |
| S. Choleraesuisa | 8 | Ser83→Phe (n = 2) | - | Thr57→Ser (n = 7) | - | ||
| Ser83→Tyr (n = 2) | - | - | - | ||||
| Asp87→Gly (n = 1) | - | - | - | ||||
| Asp87→Tyr (n = 1) | - | - | - | ||||
| other NTS | 4 | Ser83→Ile (n = 2) | - | - | S1 (n = 1) | ||
| Asp87→Tyr (n = 1) | - | - | - | ||||
| NaR CipR (n = 1) | 6 | S. Typhimurium | 1 | Ser83→Phe/Asp87→Asn | - | Ser80→Arg | - |
NaS, nalidixic acid susceptible; CipS, ciprofloxacin susceptible; NaR, nalidixic resistant; DCS, decreased ciprofloxacin susceptibility.
co-presence of Ser 83(gyrA) and Thr57 (parC) mutations in 4 isolates;
Glu133→Gly: silent mutation;
Thr57→Ser: silent mutation;
Ile125→Ser: silent mutation.
MIC levels for azithromycin were particularly high in S. Choleraesuis isolates, with MIC50 and MIC 90 as high as 32 and 128 µg/ml respectively (Table 4).
Table 4. Distribution of minimal inhibitory concentration (MIC) for azithromycin in 59 Salmonella isolates.
| MIC azithromycin (µg/ml)a | |||||||||||||||||
| Serovar (n) | 1.5 | 2 | 3 | 4 | 6 | 8 | 12 | 16 | 24 | 32 | 48 | 64 | 96 | 128 | >256 | MIC 50 | MIC 90 |
| S. Choleraesuis (24) | 1 | 1 | 3 | 2 | - | - | - | - | - | 6 | 1 | 3 | 2 | 3 | 2 | 32 | 128 |
| S. Paratyphi A (2) | - | - | - | 1 | 1 | - | - | - | - | - | - | - | - | - | NA** | NA | |
| S. Typhi (20) | - | 1 | 6 | 10 | 2 | - | - | - | - | - | - | - | 1 | - | - | 4 | 6 |
| other NTS (13) | 1 | 2 | 3 | 4 | 1 | - | 1 | - | - | - | - | 1 | - | - | - | 4 | 12 |
NA, not applicable.
epidemiological cutoff point 16 µg/ml.
In the successive isolates from patients with recurrent Salmonella BSI, no differences in resistance patterns were noted, except in one S. Choleraesuis (recovered 23 days after the first S. Choleraesuis BSI episode), having acquired ESBL. Presence of ESBL was also detected in another patient with S. Choleraesuis infection. Both ESBL-positive isolates carried bla CTX-M genes, The former was confirmed as CTX-M group 9 and displayed also MDR and azithromycin resistance (MIC 32 µg/mL). In the latter (CTX-M group 1), we observed additional DCS (MIC 0.125 µg/mL).
Discussion
We described the serovar distribution and antibiotic susceptibility of 72 Salmonella enterica BSI isolates from Cambodian adults, and noted a predominance of S. Typhi and S. Choleraesuis. Besides MDR, S. Typhi in particular displayed high rates of DCS, while S. Choleraesuis was associated with advanced HIV-infection and remarkably high azithromycin resistance rates.
Our findings have several limitations. The study describes Salmonella BSI mainly in adults. As Salmonella spp. is an important pediatric pathogen in tropical low-resource settings [1], [3], data on its invasive infections in children are essential to complement the epidemiological picture of salmonellosis in Cambodia. Next, our clinical hospital data did not allow calculations of incidence and/or the true burden of disease because the population denominator and referral pattern were not known. In addition, the presence of an HIV-treatment center in the hospital may have led to a patient selection bias. In spite of these limitations our data shed new light on invasive Salmonella infections in Cambodia.
In HIV-negative patients, S. Typhi was the most common serovar, with very high rates of MDR (75.0%) and DCS (90.0%). This confirms earlier trends from Cambodia as noted by Kasper and coworkers in 2009 [21] describing 56% of MDR and 80% DCS in S. Typhi. The presence of MDR and DCS has been observed in other Asian countries, albeit with important differences. A survey on typhoid fever in five countries [22] revealed MDR rates as variable as 65% in Pakistan, 22% in Vietnam, 7% in India and 0% in China/Indonesia whereas rates of NA resistant S. Typhi (NARST) ranged similarly between 57–59% (India, Pakistan), 44% (Vietnam) and 0% (China, Indonesia). Since the early 1990's, Southern Vietnam has been particularly mentioned as a regional ‘typhoid resistance hotspot’ with NARST/DCS rates as high as 90–98% [10], [23]. The geographical location of Cambodia in the vicinity of this regional ‘hotspot’ may be one of the explanations for the high rates of DCS among our patients with typhoid fever, given the intense cross-border traffic between the two countries. In addition, the uncontrolled use of ciprofloxacin and other antibiotics and the limited access to safe water and sanitation services [24] probably add to selection and spread of MDR and DCS isolates.
In Vietnam, the Ser83→Phe substitution in gyrA was described as the predominant underlying resistance mechanism for DCS [23]. We observed this mutation also in all S. Typhi isolates with combined DCS and NA resistance and to a lesser extent in S. Choleraesuis and other NTS. According to the Cambodian National Treatment Guidelines [25] ciprofloxacin is the first choice treatment for presumed typhoid fever with ceftriaxone as alternative. Given the failure risk of a treatment course with ciprofloxacin for invasive salmonellosis with DCS as high as 36% [26], we think the empiric treatment of typhoid fever with ciprofloxacin should be abandoned in Cambodia. Alternatives could be azithromycin for uncomplicated cases and ceftriaxone for hospitalized patients. Gatifloxacin proved to be a safe, cheap and effective alternative treatment in Nepal [27] and Vietnam [28], but it is not widely distributed in Cambodia, and caution remains regarding its use in the elderly and in a setting with increasing rates of MDR tuberculosis.
In addition, these data and their subsequent therapeutic challenges urge the need for more and better yet affordable diagnostic microbiology in Cambodia. More and adequately working microbiology laboratories across the country are essential for the improvement of clinical care and for surveillance of bacterial resistance.
Among HIV-infected patients, S. Choleraesuis was the most common serovar. It is a zoonotic pathogen causing paratyphoid in pigs and is an emerging cause of invasive infections in immune compromised patients in Southeast and Eastern Asia [29]. The prevalence of S. Choleraesuis was not yet described in Cambodia in swine nor in humans but it is a well-known pathogen in neighboring Thailand [30], [31].
All isolates in patients with recurrent S. Choleraesuis BSI had PFGE profiles which were identical to the first isolate, which is suggestive for relapse rather than for reinfection although the small number of pulsotypes and the limited discriminatory power of PFGE using XbaI [32] should be taken into account. Given the context of advanced HIV-infection, relapse is the more likely interpretation [33]
Most S. Choleraesuis isolates (70.8%) had azithromycin MIC-values exceeding 16 µg/mL. To our knowledge, this has not yet been described in a series of clinical Salmonella isolates from a single setting. Of note, also one S. Typhi and S. Enteritidis isolate displayed high azithromycin MIC-values. This contrasts with the low azithromycin MIC data for S. Typhi reported from Vietnam (MIC90 8–16 µg/mL [10], [34]), India and Egypt (MIC90 8 µg/mL [35], [36]). Azithromycin MIC-values up to 64 µg/mL in S. Typhi and Paratyphi A from India were recently described [37], and a Finnish study revealed azithromycin MIC-values ≥32 µg/mL in 1.9% of 1237 NTS isolates; half of them were isolated after travel to Thailand [38]. While considering the azithromycin resistance ‘epidemiological cutoff’ of 16 µg/mL [17], azithromycin resistance apparently presents an emerging problem as treatment failures have been described [39].
Possible mechanisms of azithromycin resistance include the presence of specific resistance genes (e.g. mphA, mphB, ermB), a mutation in rlpD or rlpV, or the acquisition of an efflux pump [40]. In Cambodia, generic azithromycin can be purchased over the counter of private clinics and pharmacies; local prices vary between 1 to 5 US $ per tablet. It is commonly used for respiratory tract infections, and often prescribed when all other treatments have failed (personal communication Thong Phe). No local data about macrolide use in animals are available, but a recent report from Vietnam showed that antibiotics such as macrolides, lincomycin, colistin, and aminoglycosides are actually used in livestock [41].
As the above mentioned azithromycin resistance in our study is most prevalent in S. Choleraesuis, our findings may firstly affect empiric treatment choices for fever and presumed BSI in HIV-infected patients. Given the complex resistance patterns in S. Choleraesuis, neither ciprofloxacin nor azithromycin appear to be safe choices; the most likely alternative in the Cambodian setting is probably a third generation cephalosporin. However, in two S. Choleraesuis isolates the presence of ESBL was found. Extensive antibiotic resistance, including ESBL has been reported before for S. Choleraesuis in East Asia [42], [43]. Even though ESBL prevalence in Salmonella enterica is still low compared to the very high rates in community-acquired Escherichia coli and Klebsiella pneumoniae isolates in the same study population [44], this is a very worrisome trend, as the potential for transmission of resistance genes is expected.
These results warrant further surveillance of resistance in invasive bacterial pathogens and Salmonella spp. in particular in Cambodia. More in depth research of the causes and molecular mechanisms of this in vitro measured azithromycin resistance are needed. In addition, integrated research on the human and veterinary epidemiology of S. Choleraesuis in Cambodia is essential for better understanding of the disease dynamics and planning of public health interventions.
Conclusions
S. Typhi and S. Choleraesuis are both common Salmonella serovars causing BSI in Cambodian adults; S. Choleraesuis closely associated with advanced HIV-disease. DCS and azithromycin resistance are very high in S. Typhi and S. Choleraesuis respectively, while presence of ESBL is emerging. Human salmonellosis has become a difficult-to-treat infection in Cambodia requiring close surveillance and public health attention.
Supporting Information
STROBE checklist.
(DOC)
Acknowledgments
The authors wish to thank the patients and staff at SHCH for their dedicated collaboration in the study, Ms. Diane Stessens for administrative help and Ms. Marleen Verlinden, Veerle Lejon and Hilde De Boeck for many hours of support at the bench.
Funding Statement
This work was supported by Project 2.08 of the third Framework Agreement between the Belgian Directorate General of Development Cooperation (http://diplomatie.belgium.be/en/policy/development_cooperation/) and the Institute of Tropical Medicine, Antwerp (Belgium). Erika Vlieghe was supported by the “Secundaire Onderzoeks Financiering ITG type A” (SOFI-A) grant of the Institute of Tropical Medicine, Antwerp (Belgium). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Crump JA, Luby SP, Mintz ED (2004) The global burden of typhoid fever. Bull World Health Organ 82: 346–353. [PMC free article] [PubMed] [Google Scholar]
- 2. Crump JA, Mintz ED (2010) Global trends in typhoid and paratyphoid Fever. Clin Infect Dis 50: 241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morpeth SC, Ramadhani HO, Crump JA (2009) Invasive non-Typhi Salmonella disease in Africa. Clin Infect Dis 49: 606–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reddy EA, Shaw AV, Crump JA (2010) Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis 10: 417–432 S1473-3099(10)70072-4 [pii];10.1016/S1473-3099(10)70072-4 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basnyat B, Maskey AP, Zimmerman MD, Murdoch DR (2005) Enteric (typhoid) fever in travelers. Clin Infect Dis 41: 1467–1472. [DOI] [PubMed] [Google Scholar]
- 6. Parry CM, Threlfall EJ (2008) Antimicrobial resistance in typhoidal and nontyphoidal salmonellae. Curr Opin Infect Dis 21: 531–538. [DOI] [PubMed] [Google Scholar]
- 7. Su LH, Chiu CH, Chu C, Ou JT (2004) Antimicrobial resistance in nontyphoid Salmonella serotypes: a global challenge. Clin Infect Dis 39: 546–551. [DOI] [PubMed] [Google Scholar]
- 8. Effa EE, Bukirwa H (2008) Azithromycin for treating uncomplicated typhoid and paratyphoid fever (enteric fever). Cochrane Database Syst Rev CD006083 [DOI] [PubMed] [Google Scholar]
- 9. Frenck RW Jr, Mansour A, Nakhla I, Sultan Y, Putnam S, et al. (2004) Short-course azithromycin for the treatment of uncomplicated typhoid fever in children and adolescents. Clin Infect Dis 38: 951–957. [DOI] [PubMed] [Google Scholar]
- 10. Parry CM, Ho VA, Phuong lT, Bay PV, Lanh MN, et al. (2007) Randomized controlled comparison of ofloxacin, azithromycin, and an ofloxacin-azithromycin combination for treatment of multidrug-resistant and nalidixic acid-resistant typhoid fever. Antimicrob Agents Chemother 51: 819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, et al. (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 31: 1250–1256 10.1097/01.CCM.0000050454.01978.3B [doi]. [DOI] [PubMed] [Google Scholar]
- 12.Grimont PAD aWF-X2 (2007) Antigenic Formulae of the Salmonella Serovars (9th edition).
- 13.CDC (2012) PulseNet. http://www.cdc.gov/pulsenet/.
- 14. Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, et al. (1995) Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33: 2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CLSI (1-1-2012) CLSI M100-S22. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-second Informational Supplement. CLSI document M100-S22. Clinical and Laboratory Standards Institute. ISBN: 1562387162. 28[1]. 1-1-2010. Wayne, PA: Clinical and Laboratory Standards Institute.
- 16.EUCAST (2012) European Committee on Antimicrobial Susceptibility Testing Breakpoint tables for interpretation of MICs and zone diameters. Version 1.3, January 5, 2011.
- 17. Sjolund-Karlsson M, Joyce K, Blickenstaff K, Ball T, Haro J, et al. (2011) Antimicrobial susceptibility to azithromycin among Salmonella enterica isolates from the United States. Antimicrob Agents Chemother 55: 3985–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Endimiani A, Hujer AM, Hujer KM, Gatta JA, Schriver AC, et al. (2010) Evaluation of a commercial microarray system for detection of SHV-, TEM-, CTX-M-, and KPC-type beta-lactamase genes in Gram-negative isolates. J Clin Microbiol 48: 2618–2622 JCM.00568-10 [pii];10.1128/JCM.00568-10 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Griggs DJ, Gensberg K, Piddock LJ (1996) Mutations in gyrA gene of quinolone-resistant Salmonella serotypes isolated from humans and animals. Antimicrob Agents Chemother 40: 1009–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cavaco LM, Hasman H, Xia S, Aarestrup FM (2009) qnrD, a novel gene conferring transferable quinolone resistance in Salmonella enterica serovar Kentucky and Bovismorbificans strains of human origin. Antimicrob Agents Chemother 53: 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kasper MR, Sokhal B, Blair PJ, Wierzba TF, Putnam SD (2010) Emergence of multidrug-resistant Salmonella enterica serovar Typhi with reduced susceptibility to fluoroquinolones in Cambodia. Diagn Microbiol Infect Dis 66: 207–209 S0732-8893(09)00365-4 [pii];10.1016/j.diagmicrobio.2009.09.002 [doi]. [DOI] [PubMed] [Google Scholar]
- 22. Ochiai RL, Acosta CJ, novaro-Holliday MC, Baiqing D, Bhattacharya SK, et al. (2008) A study of typhoid fever in five Asian countries: disease burden and implications for controls. Bull World Health Organ 86: 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chau TT, Campbell JI, Galindo CM, Van Minh HN, Diep TS, et al. (2007) Antimicrobial drug resistance of Salmonella enterica serovar typhi in asia and molecular mechanism of reduced susceptibility to the fluoroquinolones. Antimicrob Agents Chemother 51: 4315–4323 AAC.00294-07 [pii];10.1128/AAC.00294-07 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO (2012) Cambodia Health Profile. http://www.who.int/gho/countries/khm.pdf.
- 25.Kingdom of Cambodia, Ministry of Health (1-1-1999) Clinical and Therapeutic Guideline for Referral Hospitals. Second edition, November 1999.
- 26. Parry CM, Vinh H, Chinh NT, Wain J, Campbell JI, et al. (2011) The influence of reduced susceptibility to fluoroquinolones in Salmonella enterica serovar Typhi on the clinical response to ofloxacin therapy. PLoS Negl Trop Dis 5: e1163 10.1371/journal.pntd.0001163 [doi];PNTD-D-10-00171 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arjyal A, Basnyat B, Koirala S, Karkey A, Dongol S, et al. (2011) Gatifloxacin versus chloramphenicol for uncomplicated enteric fever: an open-label, randomised, controlled trial. Lancet Infect Dis 11: 445–454 S1473-3099(11)70089-5 [pii];10.1016/S1473-3099(11)70089-5 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dolecek C, Tran TP, Nguyen NR, Le TP, Ha V, et al. (2008) A multi-center randomised controlled trial of gatifloxacin versus azithromycin for the treatment of uncomplicated typhoid fever in children and adults in Vietnam. PLoS One 3: e2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chiu CH, Su LH, Chu C (2004) Salmonella enterica serotype Choleraesuis: epidemiology, pathogenesis, clinical disease, and treatment. Clin Microbiol Rev 17: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hendriksen RS, Bangtrakulnonth A, Pulsrikarn C, Pornruangwong S, Noppornphan G, et al. (2009) Risk factors and epidemiology of the ten most common Salmonella serovars from patients in Thailand: 2002–2007. Foodborne Pathog Dis 6: 1009–1019. [DOI] [PubMed] [Google Scholar]
- 31. Yodprom R, Pathanapitoon K, Kunavisarut P, Ausayakhun S, Wattananikorn S, et al. (2007) Endogenous endophthalmitis due to Salmonella choleraesuis in an HIV-positive patient. Ocul Immunol Inflamm 15: 135–138. [DOI] [PubMed] [Google Scholar]
- 32. Hsueh PR, Teng LJ, Tseng SP, Chang CF, Wan JH, et al. (2004) Ciprofloxacin-resistant Salmonella enterica Typhimurium and Choleraesuis from pigs to humans, Taiwan. Emerg Infect Dis 10: 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gordon MA, Banda HT, Gondwe M, Gordon SB, Boeree MJ, et al. (2002) Non-typhoidal salmonella bacteraemia among HIV-infected Malawian adults: high mortality and frequent recrudescence. AIDS 16: 1633–1641. [DOI] [PubMed] [Google Scholar]
- 34. Chinh NT, Parry CM, Ly NT, Ha HD, Thong MX, et al. (2000) A randomized controlled comparison of azithromycin and ofloxacin for treatment of multidrug-resistant or nalidixic acid-resistant enteric fever. Antimicrob Agents Chemother 44: 1855–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Butler T, Sridhar CB, Daga MK, Pathak K, Pandit RB, et al. (1999) Treatment of typhoid fever with azithromycin versus chloramphenicol in a randomized multicentre trial in India. J Antimicrob Chemother 44: 243–250. [DOI] [PubMed] [Google Scholar]
- 36. Girgis NI, Butler T, Frenck RW, Sultan Y, Brown FM, et al. (1999) Azithromycin versus ciprofloxacin for treatment of uncomplicated typhoid fever in a randomized trial in Egypt that included patients with multidrug resistance. Antimicrob Agents Chemother 43: 1441–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Capoor MR, Rawat D, Nair D, Hasan AS, Deb M, et al. (2007) In vitro activity of azithromycin, newer quinolones and cephalosporins in ciprofloxacin-resistant Salmonella causing enteric fever. J Med Microbiol 56: 1490–1494. [DOI] [PubMed] [Google Scholar]
- 38. Gunell M, Kotilainen P, Jalava J, Huovinen P, Siitonen A, et al. (2010) In vitro activity of azithromycin against nontyphoidal Salmonella enterica. Antimicrob Agents Chemother 54: 3498–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Molloy A, Nair S, Cooke FJ, Wain J, Farrington M, et al. (2010) First report of Salmonella enterica serotype paratyphi A azithromycin resistance leading to treatment failure. J Clin Microbiol 48: 4655–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Phuc Nguyen MC, Woerther PL, Bouvet M, Andremont A, Leclercq R, et al. (2009) Escherichia coli as reservoir for macrolide resistance genes. Emerg Infect Dis 15: 1648–1650 10.3201/eid1510.090696 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.[Anonymous] (2011) GARP Vietnam report: http://www.cddep.org/publications/situation_analysis_antibiotic_use_and_resistance_vietnam; accessed November 2nd, 2011.
- 42. Chiu CH, Su LH, Chu C, Chia JH, Wu TL, et al. (2004) Isolation of Salmonella enterica serotype choleraesuis resistant to ceftriaxone and ciprofloxacin. Lancet 363: 1285–1286 10.1016/S0140-6736(04)16003-0 [doi];S0140673604160030 [pii]. [DOI] [PubMed] [Google Scholar]
- 43. Su LH, Teng WS, Chen CL, Lee HY, Li HC, et al. (2011) Increasing ceftriaxone resistance in Salmonellae, Taiwan. Emerg Infect Dis 17: 1086–1090 10.3201/eid/1706.101949 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vlieghe E, Phe T, De Smet B, engchhun H, Kham C, Lynen L, et al.. (2011) Bloodstream Infection among Cambodian Adults: Key Pathogens and Resistance Patterns. Abstract presented at the 51th ICAAC, September 18–21 2011, Chicago, US.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
STROBE checklist.
(DOC)