Abstract
Vasectomy is the most common urological procedure in the United States with 18% of men having a vasectomy before age 45. A significant proportion of vasectomized men ultimately request vasectomy reversal, usually due to divorce and/or remarriage. Vasectomy reversal is a commonly practiced but technically demanding microsurgical procedure that restores patency of the male excurrent ductal system in 80–99.5% of cases and enables unassisted pregnancy in 40–80% of couples. The discrepancy between the anastomotic patency rates and clinical pregnancy rates following vasectomy reversal suggests that some of the biological consequences of vasectomy may not be entirely reversible in all men. Herein we review what is known about the biological sequelae of vasectomy and vasectomy reversal in humans, and provide a succinct overview of the evaluation and surgical management of men desiring vasectomy reversal.
Keywords: fertility, vasectomy reversal, vasovasostomy, vasoepididymostomy
Introduction
Worldwide, it is estimated that approximately 6% of married couples rely solely on vasectomy for contraception.1 In the United States, the prevalence of vasectomy among married couples is 7% to 10%, and increases with age such that 18% of men have undergone a vasectomy by age 45.2 More than 500,000 vasectomies are performed per year,3 and a significant proportion of infertility evaluations are performed on men with previous vasectomies who are interested in regaining fertility.4 The popularity of vasectomy reversal parallels the increasing divorce rate in the United States. Recent surveys suggest that 6–10% of vasectomized men ultimately sought consultation for reversal.3,5
Vasectomy reversal began with the work of Edward Martinat from the University of Pennsylvania. Martin’s first attempt at vasectomy reversal, a vasoepididymostomy, resulted in the birth of a full-term infant.6 Later, the procedure was popularized by Quiby and his assistant Vincent J. O’Conor, a pioneer in vasovasostomy, who reported a patency rate of 64%, but with unknown pregnancy rates.7 Despite the technical challenges, low success rates and political as well as religious ramifications that co-existed with vasectomy reversal, the procedure grew more popular in conjunction with vasectomy itself.
The goal of vasovasostomy and vasoepididymostomy is to bypass the obstruction of the male excurrent ductal system that results from vasectomy, thereby enabling unassisted reproduction. Although these procedures are commonly utilized in clinical practice, our understanding of vasal and epididymal physiology after vasectomy and vasectomy reversal in humans remains somewhat limited. In this review, we provide an overview of the pathophysiologic effects of vasectomy and vasectomy reversal on the human male excurrent ductal system. We also provide a brief overview of the clinical evaluation and surgical management of men desiring vasectomy reversal.
Effects of Vasectomy and Vasectomy Reversal in Humans
Vasectomy is performed by transection of the vas deferens with suture, clips, cautery or a combination of these in the scrotal portion of the vas. This transection disrupts the mucosal, muscular, and adventitial components of the vas deferens, including the autonomic nerves that mediate vasal secretory function and peristalsis. Vasal obstruction results in increased intraluminal pressures within the testicular remnant of the vas deferens. The increased pressure may have physiologic effects on epithelial cell morphology, cellular ultrastructure, and gene expression in the vas deferens and epididymis. Moreover, sperm cannot traverse the intentionally obstructed vasal lumen, and as such they accumulate and die within the testicular remnant of the vas deferens and the epididymis. A resultant local inflammatory response occurs in reaction to dying sperm, which has significant downstream sequelae, including a systemic cellular and humoral immunologic response that may impair testicular and sperm function.The clinical importance of this response is not clear in humans. Vasal transection and occlusion cause significant, independent pathophysiologic sequelae that may or may not be reversible by microsurgical bypass of vasal and/or epididymal obstruction during vasectomy reversal in humans.
Perhaps the most relevant study on the effects of vasal transection during vasectomy on vasal innervation was conducted by Dixon et al. in 1987.8 This group utilized immunohistochemical staining and electron microscopy to evaluate the intramural autonomic innervation of the human vas deferens after vasectomy. Vasal segments were harvested during vasectomy reversal and compared with nonobstructed vasal segments acquired at the time of initial vasectomy. They found that there were marked decreases in the noradrenergic innervation of the testicular vasal remnants in previously vasectomized men. These findings imply that vasal peristalsis, which is mediated by sympathetic autonomic activity, may be irreversibly impaired after vasectomy unless significant regeneration of autonomic nerve fibers occurs in the months and years following vasectomy reversal. Unfortunately, no studies have adequately assessed the regenerative capacity of vasal intramural nerves in humans after vasovasostomy or vasoepididymostomy.
Despite the paucity of anatomic and histologic data in the literature concerning vasal nerve recovery after vasectomy reversal, a study by Shafik et al. did provide further insight regarding vasal autonomic nerve function after vasectomy and vasectomy reversal.9 Shafik utilized transcutaneous electrovasography (EVG) to record the velocity, frequency and amplitude of nerve conduction in the vas deferens in 22 healthy men, 20 vasectomized men, and 18 men after vasectomy reversal. In normal, fertile men there was minimal temporal or individual variability in vasal conduction frequency, amplitude and velocity. In contrast, vasectomized patients exhibited lower conduction frequency and amplitude in the testicular vasal remnant and irregular, described as aberrant “vasoarrhythmic” conduction patterns. One to seven years after vasectomy reversal 7 of 22 patients had successfully conceived. Interestingly, 4 of these 7 patients had a normal electrovasographic evaluation during follow-up while 3 had decreased conduction frequencies and amplitudes but did not exhibit any vasoarrhythmia. This is in contrast to the 11 patients who failed to conceive, all of whom demonstrated electrovasographic evidence of vasoarrhythmia. Notably, the likelihood of abnormal vasal conduction studies was correlated with the interval of vasal obstruction prior to vasectomy reversal. This study suggests that nerve conduction recovery may be variable after vasectomy reversal, and seems to depend upon the interval of vasal obstruction.
Significant changes also occur in epithelial cell ultra-structure within the vas deferens after vasectomy, most of which are thought to result from changes in the intraluminal pressure after vasal ligation (increased pressure in the testicular vasal remnant and decreased pressure in the abdominal vasal remnant). Andonian et al. documented this phenomenon by comparing the ultra-structural features of the abdominal and testicular vasal remnants after vasectomy (harvested at the time of vasectomy reversal) to vasal segments harvested from fertile men undergoing vasectomy.10 Transmission electron microscopic analysis of vasal segments from healthy fertile men revealed the presence of many apical cytoplasmic protrusions from epithelial principle cells into the vasal lumen. Some of these protrusions remained attached to the principle cells by a stalk, whereas others were self-contained within the lumen of the vas deferens, suggesting a secretory process. The cytoplasmic protrusions, termed “apical blebs,” contain ribosomes and endoplasmic reticulum. Interestingly, these investigators observed a marked reduction in the number of apical blebs within the testicular remnants of the vas deferens in vasectomized patients undergoing vasectomy reversal. In addition, they observed dramatic luminal narrowing, epithelial cell flattening, reduction in organelle density, and absence of apical blebs on the abdominal vasal remnant. These findings are suggestive of de-differentiation of vasal epithelium within the abdominal vasal remnant in the absence of contact with seminal plasma. Whether or not these ultra-structural changes are clinically relevant and reversible with vasovasostomy or vasoepididymostomy remains to be determined.
Morphological changes are also apparent in the human epididymides after vasectomy. Older studies of cellular morphology and ultra-structure in the epididymides of vasectomized animals have demonstrated vacuolization and increases in the number and size of lysosomes within epididymal epithelial cells11,12 as well as segmental thinning of the epithelial lining of the vas deferens and epididymis near sites of luminal distension.13 In humans, dilatation of the entire epididymal tubule has been documented, with the most pronounced increase in luminal diameter noted in the cauda. Moreover, the height of the epididymal epithelium is altered by vasectomy. In normal men, maximal epididymal height occurs in the corpus of the epididymis. After vasectomy, however, the maximal height of the epididymal epithelium occurs in the caput.14 Alternations in the height of the epithelial cell layer in the epididymis after vasectomy suggest the presence of complex molecular biological effects of vasectomy on gene expression, as epithelial cellular volume and height are thought to be indicative of underlying RNA translational and protein secretory activities.
Indeed, recent analyses of the human epididymal transcriptome using microarrays have confirmed that vasectomy causes significant alterations in epididymal gene expression. Sullivan et al. characterized the epididymal transcriptomes within each region of the epididymis in both normal and vasectomized men.15 Cluster analysis of nearly 3000 genes demonstrated that expression of 1363 genes did not differ based on vasectomy status, whereas 911 genes were expressed only in normal epididymides, and 660 genes were only expressed after vasectomy. Interestingly, three of the differentially expressed genes have well-established roles in sperm maturation during epididymal transit (NPC2, CRISP1, and DCXL).
Unfortunately, no studies have directly examined the impact of vasectomy reversal on gene expression in epididymal fluid or tissue, as the only candidates for such a study would be the rare patients who desire a vasectomy subsequent to successful vasectomy reversal. However, RNA and protein detection studies in semen after vasectomy reversals have suggested that some of the alterations in epididymal gene expression that result from vasectomy may not be reversible.15 The clinical significance of such studies remains to be determined.
Vasectomy with subsequent vasectomy reversal may also be associated with detectable alterations in sperm DNA integrity. Sperm DNA integrity testing has emerged as a valuable measure of sperm quality that is predictive of natural conception, pregnancy outcomes after intrauterine insemination, and pregnancy loss after in vitro fertilization cycles.16,17 The most commonly utilized assay is the sperm chromatin structure assay (SCSA), which is a flow cytometric method that sorts sperm according to their susceptibility to DNA strand breaks upon exposure to a denaturant.
A study by Smit et al. sperm looked at DNA fragmentation with the SCSA in ejaculated semen after vasectomy reversal in 70 men. They demonstrated that sperm DNA fragmentation was increased in the vasectomy reversal patients when compared with proven fertile controls (30% vs. 15%, p < 0.001). The increase in sperm DNA fragmentation was correlated with lower sperm concentrations, lower sperm motility, and a lower percentage of morphologically normal sperm.18 Interestingly, however, there was no relationship between sperm DNA fragmentation and the likelihood of pregnancy after vasectomy reversal. Though the clinical significance of sperm DNA integrity testing after vasectomy reversal remains unclear, this supports the notion that vasectomy likely causes a myriad of molecular biological sequelae, including sperm DNA damage, which may be irreversible in some cases.
Other factors have been isolated and suggested to be associated with infertility after vasectomy reversal, including antisperm antibodies,19,20 granuloma formation21and persistent mechanical partial obstruction,22 which may occur despite partial patency and sperm in the ejaculate. Epididymal function, as discussed above, has been widely studied, as has epididymal dysfunction, which is believed by many to be one of the major factors contributing to infertility after vasectomy reversal when post-surgical patency has been established by demonstrating sperm in the ejaculate. Proteins isolated in epididymal fluid harvested at the time of vasectomy reversal, such as GTPase proteins in the Ras/RAB family and Syntenins, likely play a critical in sperm maturation23 and irreversible changes in protein synthesis despite microsurgical vasovasostomy or vasoepididymostomy may play a large role in infertility despite patency after vasectomy reversal.24,25
Preoperative Considerations
Vasectomized patients desiring biological paternity have two options: vasectomy reversal and sperm retrieval for in vitro fertilization with intracytoplasmic sperm injection (IVF/ICSI). Generally, vasectomy reversal is more cost-effective26 and is the favored approach when patients want multiple children, the female partner has normal fertility, and the obstructive interval is short. Nonetheless, both approaches yield acceptable reproductive outcomes, but the ultimate decision is made by each individual couple based upon their goals, social and religious beliefs, and the costs of each treatment.
The preoperative evaluation of men who select vasectomy reversal for fertility restoration should include a directed history, physical examination, and additional testing in some cases. The clinical history should focus on the patient’s history of previous conception, prior inguinal surgery, prior attempts at vasectomy reversal, and the fertility history of the female partner. In particular, the most important prognostic factors with respect to success following vasectomy reversal are duration since vasectomy and female partner’s age.27 Interestingly, pregnancy rates are higher if the patient has had a child with the same partner as opposed to men having a different partner.28
On physical exam, the size and consistency of each testis should be documented. Smaller and soft testes could suggest impaired spermatogenesis. An indurated, irregular epididymis often predicts secondary epididymal obstruction. Delicate palpation of the testicular end of the vas may reveal a sperm granuloma, which is associated with a better prognosis for restored fertility following vasectomy reversal. The sperm granulomas contain multiple epithelialized channels filled with sperm, thus tremendously increasing the surface area available for the absorption of sperm and vasal fluid. The granuloma acts ro prevent the build up of pressure in the epididymis. This prevents epididymal micro-rupture, which may result in further obstruction.29 Documentation of prior inguinal or scrotal incisions is critical, as such prior surgeries guide pre-operative planning and could indicate other sites of obstruction.
In additional to physical exam, we advocate that preoperative semen analysis be performed to confirm azoospermia. If complete sperm with tails are found on semen analysis, sperm are certain to be found in the vas on at least one side. The utility of serum testing for antisperm antibodies (ASA) is not established, however roughly 60% of vasectomized patients may have ASA,30 which may corroborate the diagnosis of obstruction and the presence of active spermatogenesis.31 If soft testes are palpated, determination of the serum follicle stimulating hormone (FSH) level is clinically valuable, as elevated FSH levels indicate impaired spermatogenesis and correlate with a poor prognosis for unassisted reproduction following vasectomy reversal. Some experts advocate routine testicular biopsy prior to vasectomy reversal to confirm the presence of normal sperm production, but this can be performed (if necessary) at the time of microsurgical reconstruction and subjects the patient to an additional procedure.
Intraoperative Considerations
Vasectomy reversal should be performed by an experienced microsurgeon using optical magnification provided by an operating microscope. General anesthesia is preferred, although the procedure may be performed with any anesthetic including local or regional anesthesia. Successful vasectomy reversal requires bypass of all obstructions within the male excurrent ductal system, which occur by definition at the vasectomy sites but may also occur secondarily within the epididymis after vasectomy. The likelihood of secondary epididymal obstruction is higher with increasing duration of the time since the vasectomy.32,33 In cases of simple vasal obstruction at the vasectomy site, vasectomy reversal is accomplished by vasovasostomy in which continuity is re-established between the testicular and abdominal remnants of the vas deferens. However, vasoepididymostomy is required in cases of secondary epididymal obstruction, during which the abdominal remnant of the vas deferens is anastomosed to the epididymal tubule on the testicular side of the secondary epididymal obstruction.
Intraoperative identification of secondary epididymal obstruction is thus critical during vasectomy reversal to determine the optimal microsurgical procedure. The key to intraoperative identification of secondary epididymal obstruction necessitating vasoepididymostomy is microscopic analysis of fluid collected from the remnant of the vas deferens on the testicular side of the vasectomy site. This fluid is easily collected after vasal transection proximal and distal to the vasectomy site, and is routinely performed in one of the early surgical steps of vasectomy reversal.
Cytological evaluation of the vasal fluid is performed by placing a drop of fluid from the testicular vasal remnant on a slide and diluting it with a drop of saline. The presence of any complete sperm with tails indicates patency of the male excurrent ductal system on the testicular side of the vasal transection (from which the fluid was collected), and therefore the absence of epididymal obstruction. In this case, vasovasostomy should be performed. If no sperm are seen, epididymal obstruction must be present and vasoepididymostomy is required. Borderline cases in which rare sperm parts are found are handled variably by different experts based on their experience and discussion of such cases is beyond the scope of this review.
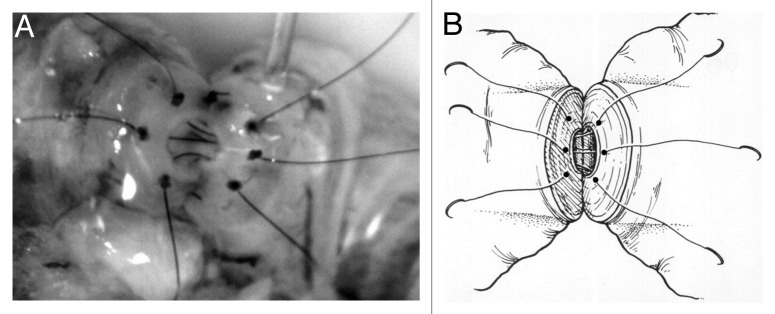
Vasovasostomy is performed by re-approximation of the vasal mucosal, muscular and adventitial layers with microsutures in one or multiple layers. The best reported results of vasovasostomy are from a series utilizing a 4-layer anastomosis in which the mucosal layer is re-approximated with 10–0 nylon sutures (20 µm in diameter), the muscular and adventitial layers are re-approximated with 9–0 nylon sutures (30 µm in diameter), and the vasal sheath is re = approximated with 8–0 nylon sutures (See Figure 1A and B).34
Figure 1. (A) Schematic representing re-approximation of vasal mucosa as part of a 4-layer closure using 10–0 nylon sutures with the microdot technique. (B) Intraoperative vasovasostomy repair – note the microdots spaced approximately 20 µm in diameter.
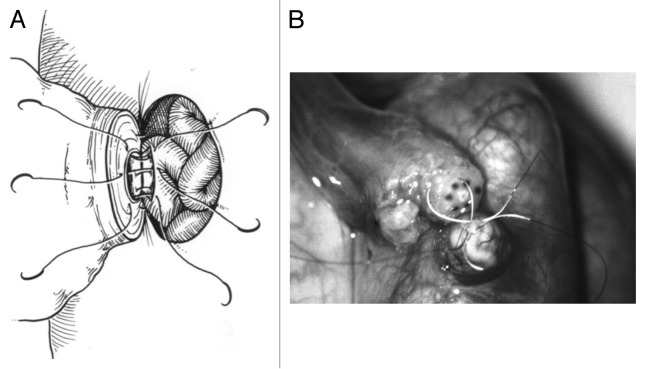
Vasoepididymostomy is a more technically challenging procedure than vasovasostomy due to the fragility and very small luminal diameter of the epididymal tubule. Several anastomotic techniques, including variants of end-to-end, end-to-side, and intussuscepted end-to-side vasoepididymostomies have been utilized, with the best reported results seen with a technique called longitudinal intussuscepted vasoepididymostomy.35 This technique uses two 10–0 sutures to intussuscept a longitudinally incised segment of the epididymal tubule into the lumen of the abdominal vasal remnant. The intussusception is then reinforced with multiple 9–0 and 8–0 sutures (See Figure 2A and B).
Figure 2. (A) Schematic demonstrating a vasoepididymostomy using a longitudinal intussuscepted technique with 10–0 sutures to intussuscept a longitudinally incised segment of the epididymal tubule into the lumen of the abdominal vasal remnant. The intussusception is then reinforced with multiple 9–0 and 8–0 sutures. (B) Intraoperative vasoepididymostomy using 10–0 sutures.
Outcomes of Vasectomy Reversal
Technical and reproductive outcomes of vasovasostomy and vasoepididymostomy are generally excellent when performed by experienced microsurgeons. Anastomotic patency, defined as the presence of sperm in the ejaculate after surgery, is achievable in the vast majority of patients after vasovasostomy (up to 99.5%) and vasoepididymostomy (up to 80%). Pregnancy rates, however, are significantly lower than reported technical success rates and occur in approximately 50% of couples after vasovasostomy and 30% of couples after vasoepididymostomy within one year of vasectomy reversal.36
Conclusions
Vasectomy reversals are common procedures for fertility restoration in previously vasectomized men. These procedures are technically challenging but yield excellent technical and acceptable reproductive outcomes. The discrepancy between anastomotic patency rates and pregnancy rates after vasectomy reversal suggests that some of the morphologic, ultra-structural, and molecular biological sequelae of vasectomy may be irreversible. Elucidation of these phenomena remains challenging for several reasons, most notably the very limited access that researchers have to human testicular, epididymal and vasal tissue after vasectomy and vasectomy reversal. Nonetheless, the recent application of microarray technology to the study of epididymal function after vasectomy has begun to elucidate changes in gene expression. Further insight into the pathophysiologic sequelae of vasectomy and vasectomy reversal in humans is necessary to enable development of novel diagnostic and therapeutic approaches for use in vasectomized men seeking biological paternity through natural conception.
Footnotes
Previously published online: www.landesbioscience.com/journals/spermatogenesis/article/22591
References
- 1.Lee R, Li PS, Goldstein M, Tanrikut C, Schattman G, Schlegel PN. A decision analysis of treatments for obstructive azoospermia. Hum Reprod. 2008;23:2043–9. doi: 10.1093/humrep/den200. [DOI] [PubMed] [Google Scholar]
- 2.Monoski MA, Li PS, Baum N, Goldstein M. No-scalpel, no-needle vasectomy. Urology. 2006;68:9–14. doi: 10.1016/j.urology.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Barone MA, Hutchinson PL, Johnson CH, Hsia J, Wheeler J. Vasectomy in the United States, 2002. J Urol. 2006;176:232–6, discussion 236. doi: 10.1016/S0022-5347(06)00507-6. [DOI] [PubMed] [Google Scholar]
- 4.Wood S, Montazeri N, Sajjad Y, Troup S, Kingsland CR, Lewis-Jones DI. Current practice in the management of vasectomy reversal and unobstructive azoospermia in Merseyside & North Wales: a questionnaire-based survey. BJU Int. 2003;91:839–44. doi: 10.1046/j.1464-410X.2003.04227.x. [DOI] [PubMed] [Google Scholar]
- 5.Pile JM, Barone MA. Demographics of vasectomy--USA and international. Urol Clin North Am. 2009;36:295–305. doi: 10.1016/j.ucl.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Kim HH, Goldstein M. History of vasectomy reversal. Urol Clin North Am. 2009;36:359–73. doi: 10.1016/j.ucl.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 7.O’Conor VJ. Anastomosis of the vas deferens after purposeful division for sterility. J Urol. 1948;59:229–33. doi: 10.1016/S0022-5347(17)69369-8. [DOI] [PubMed] [Google Scholar]
- 8.Dixon JS, Gilpin CJ, Gilpin SA, Gosling JA, Grant JF. The effects of vasectomy on the autonomic innervation of the human vas deferens. J Urol. 1987;137:1014–6. doi: 10.1016/s0022-5347(17)44349-7. [DOI] [PubMed] [Google Scholar]
- 9.Shafik A. Electrovasography in normal and vasectomized men before and after vasectomy reversal. Int J Androl. 1996;19:33–8. doi: 10.1111/j.1365-2605.1996.tb00432.x. [DOI] [PubMed] [Google Scholar]
- 10.Andonian S, Jarvi K, Zini A, Hermo L. Ultrastructural features of the vas deferens from patients undergoing vasectomy and vasectomy reversal. J Androl. 2002;23:691–701. [PubMed] [Google Scholar]
- 11.Flickinger CJ, Herr JC, Caloras D, Sisak JR, Howards SS. Inflammatory changes in the epididymis after vasectomy in the Lewis rat. Biol Reprod. 1990;43:34–45. doi: 10.1095/biolreprod43.1.34. [DOI] [PubMed] [Google Scholar]
- 12.Kumar BV, Shipstone AC, Setty BS. Effect of vasectomy on the ultrastructure of epididymal epithelium in rhesus monkey. Int J Fertil. 1990;35:180–91. [PubMed] [Google Scholar]
- 13.McGinn JS, Sim I, Bennett NK, McDonald SW. Observations on multiple sperm granulomas in the rat epididymis following vasectomy. Clin Anat. 2000;13:185–94. doi: 10.1002/(SICI)1098-2353(2000)13:3<185::AID-CA5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Légaré C, Thabet M, Picard S, Sullivan R. Effect of vasectomy on P34H messenger ribonucleic acid expression along the human excurrent duct: a reflection on the function of the human epididymis. Biol Reprod. 2001;64:720–7. doi: 10.1095/biolreprod64.2.720. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan R, Legare C, Thabet M, Thimon V. Gene expression in the epididymis of normal and vasectomized men: what can we learn about human sperm maturation? J Androl. 2011;32:686–97. doi: 10.2164/jandrol.110.012575. [DOI] [PubMed] [Google Scholar]
- 16.Spanò M, Bonde JP, Hjøllund HI, Kolstad HA, Cordelli E, Leter G, The Danish First Pregnancy Planner Study Team Sperm chromatin damage impairs human fertility. Fertil Steril. 2000;73:43–50. doi: 10.1016/s0015-0282(99)00462-8. [DOI] [PubMed] [Google Scholar]
- 17.Zini A, Jamal W, Cowan L, Al-Hathal N. Is sperm DNA damage associated with IVF embryo quality? A systematic review. J Assist Reprod Genet. 2011;28:391–7. doi: 10.1007/s10815-011-9544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smit M, Wissenburg OG, Romijn JC, Dohle GR. Increased sperm DNA fragmentation in patients with vasectomy reversal has no prognostic value for pregnancy rate. J Urol. 2010;183:662–5. doi: 10.1016/j.juro.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Naz RK, Deutsch J, Phillips TM, Menge AC, Fisch H. Sperm antibodies in vasectomized men and their effects on fertilization. Biol Reprod. 1989;41:163–73. doi: 10.1095/biolreprod41.1.163. [DOI] [PubMed] [Google Scholar]
- 20.Bohring C, Krause W. Differences in the antigen pattern recognized by antisperm antibodies in patients with infertility and vasectomy. J Urol. 2001;166:1178–80. doi: 10.1016/S0022-5347(05)65941-1. [DOI] [PubMed] [Google Scholar]
- 21.Carey PO, Howards SS, Flickinger CJ, Herr JC, Gallien TN, Caloras D, et al. Effects of granuloma formation at site of vasovasostomy. J Urol. 1988;139:853–6. doi: 10.1016/s0022-5347(17)42661-9. [DOI] [PubMed] [Google Scholar]
- 22.Carbone DJ, Jr., Shah A, Thomas AJ, Jr., Agarwal A. Partial obstruction, not antisperm antibodies, causing infertility after vasovasostomy. J Urol. 1998;159:827–30. doi: 10.1016/S0022-5347(01)63744-3. [DOI] [PubMed] [Google Scholar]
- 23.Thimon V, Frenette G, Saez F, Thabet M, Sullivan R. Protein composition of human epididymosomes collected during surgical vasectomy reversal: a proteomic and genomic approach. Hum Reprod. 2008;23:1698–707. doi: 10.1093/humrep/den181. [DOI] [PubMed] [Google Scholar]
- 24.Légaré C, Verville N, Sullivan R. Vasectomy influences expression of HE1 but not HE2 and HE5 genes in human epididymis. J Androl. 2004;25:30–43. doi: 10.1002/j.1939-4640.2004.tb02756.x. [DOI] [PubMed] [Google Scholar]
- 25.Légaré C, Boudreau L, Thimon V, Thabet M, Sullivan R. Vasectomy affects cysteine-rich secretory protein expression along the human epididymis and its association with ejaculated spermatozoa following vasectomy surgical reversal. J Androl. 2010;31:573–83. doi: 10.2164/jandrol.109.009860. [DOI] [PubMed] [Google Scholar]
- 26.Pavlovich CP, Schlegel PN. Fertility options after vasectomy: a cost-effectiveness analysis. Fertil Steril. 1997;67:133–41. doi: 10.1016/S0015-0282(97)81870-5. [DOI] [PubMed] [Google Scholar]
- 27.Fuchs EF, Burt RA. Vasectomy reversal performed 15 years or more after vasectomy: correlation of pregnancy outcome with partner age and with pregnancy results of in vitro fertilization with intracytoplasmic sperm injection. Fertil Steril. 2002;77:516–9. doi: 10.1016/S0015-0282(01)03219-8. [DOI] [PubMed] [Google Scholar]
- 28.Chan PT, Goldstein M. Superior outcomes of microsurgical vasectomy reversal in men with the same female partners. Fertil Steril. 2004;81:1371–4. doi: 10.1016/j.fertnstert.2003.09.066. [DOI] [PubMed] [Google Scholar]
- 29.Boorjian S, Lipkin M, Goldstein M. The impact of obstructive interval and sperm granuloma on outcome of vasectomy reversal. J Urol. 2004;171:304–6. doi: 10.1097/01.ju.0000098652.35575.85. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto M, Hibi H, Miyake K. The incidence of antisperm antibodies in patients with seminal tract obstructions. Nagoya J Med. Sci Mar. 1996;59:25–9. [PubMed] [Google Scholar]
- 31.Lee R, Goldstein M, Ullery BW, Ehrlich J, Soares M, Razzano RA, et al. Value of serum antisperm antibodies in diagnosing obstructive azoospermia. J Urol. 2009;181:264–9. doi: 10.1016/j.juro.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Silber SJ. Vasectomy and its microsurgical reversal. Urol Clin North Am. 1978;5:573–84. [PubMed] [Google Scholar]
- 33.Belker AM, Thomas AJ, Jr., Fuchs EF, Konnak JW, Sharlip ID. Results of 1,469 microsurgical vasectomy reversals by the Vasovasostomy Study Group. J Urol. 1991;145:505–11. doi: 10.1016/s0022-5347(17)38381-7. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein M, Li PS, Matthews GJ. Microsurgical vasovasostomy: the microdot technique of precision suture placement. J Urol. 1998;159:188–90. doi: 10.1016/S0022-5347(01)64053-9. [DOI] [PubMed] [Google Scholar]
- 35.Chan PT, Brandell RA, Goldstein M. Prospective analysis of outcomes after microsurgical intussusception vasoepididymostomy. BJU Int. 2005;96:598–601. doi: 10.1111/j.1464-410X.2005.05691.x. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein M, Tanrikut C. Microsurgical management of male infertility. Nat Clin Pract Urol. 2006;3:381–91. doi: 10.1038/ncpuro0524. [DOI] [PubMed] [Google Scholar]