Abstract
Background/Aims
Resveratrol has been demonstrated to be protective in the cardiovascular system. The aim of this study was to assess the effects of resveratrol on hydrogen peroxide (H2O2)-induced increase in late sodium current (I Na.L) which augmented the reverse Na+-Ca2+ exchanger current (I NCX), and the diastolic intracellular Ca2+ concentration in ventricular myocytes.
Methods
I Na.L, I NCX, L-type Ca2+ current (I Ca.L) and intracellular Ca2+ properties were determined using whole-cell patch-clamp techniques and dual-excitation fluorescence photomultiplier system (IonOptix), respectively, in rabbit ventricular myocytes.
Results
Resveratrol (10, 20, 40 and 80 µM) decreased I Na.L in myocytes both in the absence and presence of H2O2 (300 µM) in a concentration dependent manner. Ranolazine (3–9 µM) and tetrodotoxin (TTX, 4 µM), I Na.L inhibitors, decreased I Na.L in cardiomyocytes in the presence of 300 µM H2O2. H2O2 (300 µM) increased the reverse I NCX and this increase was significantly attenuated by either 20 µM resveratrol or 4 µM ranolazine or 4 µM TTX. In addition, 10 µM resveratrol and 2 µM TTX significantly depressed the increase by 150 µM H2O2 of the diastolic intracellular Ca2+ fura-2 fluorescence intensity (FFI), fura-fluorescence intensity change (△FFI), maximal velocity of intracellular Ca2+ transient rise and decay. As expected, 2 µM TTX had no effect on I Ca.L.
Conclusion
Resveratrol protects the cardiomyocytes by inhibiting the H2O2-induced augmentation of I Na.L.and may contribute to the reduction of ischemia-induced lethal arrhythmias.
Introduction
Despite intensive research has been conducted in recent years, cardiac arrhythmias remain a serious problem. Late sodium current (I Na.L) has been recognized as an important factor contributing to the abnormal repolarization in ischemic and failured hearts [1]. I Na.L plays an important role in determining the action potential duration (APD) [2] and the alteration of the intracellular Na+ concentration ([Na+]i) [3], [4]. It has also been reported that hypoxia increased I Na.L in rat ventricular myocytes [4], and the increase in Na+ inflow during hypoxia increased [Na+]i which in turn rose the intracellular Ca2+ concentration ([Ca2+]i) via the Na+-Ca2+ exchanger (NCX) resulting in a Na+-dependent intracellular Ca2+ overload induced by I Na.L [5], [6], [7]. An increase in [Ca2+]i caused cardiac arrhythmias and irreversible cell damage [8]. Furthermore, increased I Na.L caused arrhythmic activity and contractile dysfunction [9], [10]. Therefore, inhibition of I Na.L is considered to be a new potential target for therapeutic intervention in patients with myocardial ischaemia and heart failure [10]–[14].
Resveratrol (trans-3, 4′, 5-trihydroxystilbene), a polyphenol in various vegetables and fruits, is abundant in grapes. The root extracts of Polygonum cuspidatum, a constituent of Chinese and Japanese folk medicine, is also a good source of resveratrol [15]. Sufficient clinical and epidemiological evidence showed that the consumption of red wine reduced the incidence of mortality and morbidity in patients with coronary heart disease [16]. Among all the evidence, the well-known one is now popularly termed as the “French paradox” [16], [17]. Resveratrol has been considered to be responsible for the cardiovascular benefits after moderate wine consumption [18]. It is speculated that resveratrol may act as an antioxidant, which modulates the vascular cell functions [19], inhibits platelet aggregation [20], and reduces lipoprotein oxidation [21], to serve as a cardioprotective agent. H2O2, a reactive oxygen species, is a by-product of oxidative metabolism in which energy activation and electron reduction are involved, and was enhanced during ischemia-reperfusion of the heart [22]. Excessive amount of H2O2 augmented I Na.L in ventricular myocytes [10], [23], but the reducing agents, e.g., dithiothreitol (DTT) and glutathione (GSH), reversed these changes induced by either H2O2 or hypoxia [24], [25]. Since resveratrol acts as an antioxidant [26], we presumed that it might inhibit the increase in I Na.L induced by H2O2.
To further clarify the pharmacological mechanisms and the scope of application of the agent, it is critical to determine the effect of resveratrol on I Na.L. Previous investigation showed that 50 µM of resveratrol reduced I Na.L in a recombinant expression system with the R1623Q LQT3 mutation [27]. To our knowledge, the effect of resveratrol on I Na.L in ventricular myocytes with increased H2O2 has not been reported. Therefore, this study was designed to address the impact of resveratrol on the Na+-dependent Ca2+ overload induced by H2O2-induced increase in I Na.L in ventricular myocytes, with the intention to shed some light on its potential clinical application in the future.
Materials and Methods
Isolation of Ventricular Myocytes
Adult New Zealand white rabbits (body weight 1.7–2 kg) of either sex were heparinized (2000 U) and anesthetized with ketamine (30 mg kg −1 i.v.) and xylazine (7.5 mg kg−1 i.m.). Hearts were excised rapidly and perfused retrogradely on a Langendorff apparatus for 5 min with a Ca2+-free Tyrode’s solution containing (in mM): NaCl 135, KCl 5.4, MgCl2 1, NaH2PO4 0.33, HEPES 10 and glucose 10 (pH 7.4, adjusted with NaOH), and then a Tyrode’s solution containing enzyme (collagenase type I, 0.1 g/l) and bovine serum albumin (BSA, 0.5 g/l) for 40–50 min. The perfusate was finally switched to KB solution containing (in mM): KOH 70, taurine 20, glutamic acid 50, KCl 40, KH2PO4 20, MgCl2 3, EGTA 0.5, HEPES 10, and glucose 10 (pH 7.4), for 5 min. All perfusates were bubbled with 100% O2 and maintained at 37°C. The left ventricles were then cut into small chunks and gently agitated in KB solution. The cells were filtered through nylon mesh and stored in KB solution at 25°C. The use of animals in this investigation was approved by the Institutional Animal Care and Use Committee of Wuhan University of Science and Technology and conformed to the "Guide for the Care and Use of Laboratory Animals" published by the National Institutes of Health (NIH publication no. 85-23, revised 1996) and the Guide for the Care and Use of Laboratory Animals of Hubei Province, China.
Protocol of Experiments
Isolated cells were perfused with Tyrode's solution saturated oxygenated with 100% O2 (control) and were then exposed to Tyrode's solution containing 300 µM H2O2 for 7 min. Next, isolated cells were perfused with Tyrode's solution containing both 300 µM H2O2 and one of the following, resveratrol (10, 20, 40 or 80 µM) for 10 min, 4 µM tetrodotoxin (TTX) for 10 min, or 4 µM ranolazine for 5 min.
Solutions and Drugs
To record I Na.L, the intracellular pipette solution contained (mM): CsCl 120, CaCl2 1, MgCl2 5, Na2ATP 5, TEACl 10, EGTA 11, HEPES 10 (pH 7.3). The extracellular solution contained (mM): NaCl 135, CsCl 70, CaCl2 1,MgCl2 1, CdCl2 0.05, glucose 5, HEPES 5 (pH 7.4). In addition, 1 µM nicardipine was used to block the L-type Ca2+ channels.
To record Na+-Ca2+ exchanger current (I NCX), the intracellular pipette solution contained (mM): NaCl 20, CaCl2 10, aspartic acid 50, MgCl2 3, EGTA 20, HEPES 10, MgATP 5 and CsOH 120 (pH 7.3). The bath solution contained (mM): NaCl 140,CaCl2 2, MgCl2 2, HEPES 5, and glucose 10 (pH 7.4). In addition, 20 µmol L−1 ouabain, 1 mmol L−1 BaCl2, 2 mmol L−1 CsCl and 1 µmol L−1 nicardipine were used to block the Na+-K+ pump, K+ channels and L-Ca2+ channels, respectively. I NCX was measured as the Ni2+ sensitive current that could be blocked by NiCl2 5.0 mmol L−1.
Krebs-Henseleit bicarbonate (KHB) buffer for intracellular Ca2+ fluorescence measurement, the bath solution contained (mM): NaCl 131, KCl 4, CaCl2 1, MgCl2 1, glucose 10, and HEPES 10 (pH 7.4).
To record L-type calcium current (I Ca.L), the intracellular pipette solution contained (mM): CsCl 80, CsOH 60, aspartic acid 40, CaCl2 0.65, HEPES 5, EGTA 10, MgATP 5 and disodium creatine phosphate 5 (pH 7.2). The bath solution contained (mM): NaCl 135, KCl 5.4, MgCl2 0.5, CaCl2 1.8, NaH2PO4 0.33, HEPES 10, glucose 10 (pH 7.4).
H2O2 was a product of Wuhan Zhongnan Chemical Reagent Co. (Wuhan, China). All other chemicals were purchased from Sigma. Stock solutions of drugs were prepared in water. Each of the stocks was diluted to the required concentrations in the external recording solution immediately before use.
Electrical Recordings
Experiments were performed at room temperature (22–24°C). Rabbit ventricular myocytes were placed into a recording chamber that was bathed with normal extracellular solution, in the absence and presence of drug (s), at a rate of 2 ml min−1. I Na.L, I NCX and I Ca.L were recorded in voltage clamp mode by using whole-cell patch-clamp techniques in rabbit ventricular myocytes. Patch electrodes were pulled with a two-stage puller (PP-830, Narishige Group, Tokyo, Japan). Their resistances were in the range of 1–1.5 MΩ. Capacitance and series resistances were adjusted to obtain minimal contribution of the capacitive transients. A 60% to 80% compensation of the series resistance was usually achieved without ringing. Currents were obtained with an EPC 9 amplifier (Heka Electronic, Lambrecht, Pfalz, Germany) and a Multiclamp 700B amplifier (Axon Instruments, Inc. USA), filtered at 2 kHz, digitized at 10 kHz, and stored on a computer hard disk for further analysis.
Intracellular Ca2+ Fluorescence Measurement
Myocytes were loaded with fura-2-AM (0.5 µM) for 10 min at 25°C, and fluorescence measurements were recorded with a dual excitation fluorescence photomultiplier system (Ionoptix). Myocytes were imaged through an Olympus IX-70 Fluor 40 × oil objective. The cells were field stimulated with a suprathreshold (150%) voltage and at a frequency of 0.5 Hz, 3-ms duration, using a pair of platinum wires placed on opposite sides of the chamber connected to a FHC stimulator (Brunswick, NE, USA). The polarity of the stimulatory electrodes was reversed frequently to avoid possible build up of electrolyte by-products. Cells were exposed to light emitted by a 75-W lamp and passed through either a 340- or 380-nm filter (bandwidths were ±15 nm) while being stimulated to contract at 0.5 Hz. Fluorescence emissions were detected between 480 and 520 nm by a photomultiplier tube after first illuminating the cells at 340 nm for 0.5 s then at 380 nm for the duration of the recording protocol (333 Hz sampling rate). The 360 excitation scan was repeated at the end of the protocol, and qualitative changes in intracellular Ca2+ level were inferred from the ratio of the fura-fluorescence intensity (FFI) at both wavelengths. Intracellular Ca2+ fluorescence measurements were assessed using the following indices: diastolic intracellular Ca2+ level (diastolic FFI) (340/380 ratio), electrically stimulated rise in intracellular Ca2+ (△FFI) (340/380 ratio), maximal velocity of Ca2+ rise and Ca2+ decay (340/380 ratio).
Data Analysis
Whole-cell recordings were analyzed using clampfit 9.0 (Axon Instruments, Inc.USA) and PulseFit (V8.74, HEKA). Figures were plotted by Origin (V7.0, OriginLab Co., MA, USA). All amplitudes of I Na.L were tested at 200 ms in depolarization testing pulse to eliminate the influence of transient sodium current (I Na.T). Statistical significance between two groups and multiple groups were evaluated by Student’s t-test and one-way analysis of variance (ANOVA), respectively. All values were expressed as mean ± SD, and the number of cells (n) in each group was given. P<0.05 was considered to be statistically significant.
Results
Effects of Resveratrol and TTX on I Na.L Under Normal Condition
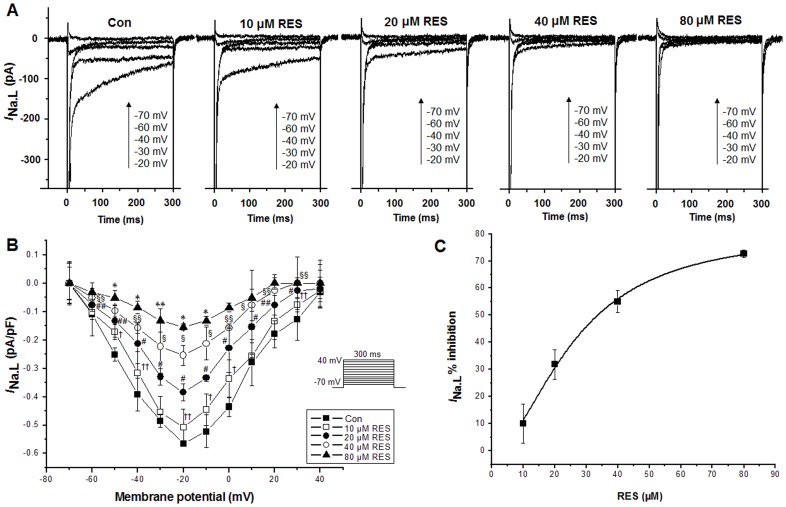
To identify I Na.L, the current was recorded first in the absence and then in the presence of 4 µM TTX with 300 ms voltage steps from a holding potential (HP) of −120 to −20 mV. The values of current recorded before (control condition) and after application of TTX were −0.400±0.050 and −0.154±0.038 pA pF−1 (n = 6, P<0.05 versus control), respectively, indicating that this TTX-sensitive current recorded was I Na.L. When I Na.L was recorded under normal condition using depolarizing pulses with a duration of 300 ms applied at 0.25 Hz from a HP of −120 mV in 10 mV increments between −70 and −20 mV, administration of 10, 20, 40 and 80 µM resveratrol resulted in decreased amplitudes of I Na.L in a concentration dependent manner (Figure 1A, 1B). Figure 1B showed the I-V relationship of I Na.L after the administration of 10, 20, 40 and 80 µM resveratrol, without a shift of the voltage at which the I Na.L amplitude was maximal (Figure 1B). Figure 1C shows the inhibition amounts of 10, 20, 40 and 80 µM resveratrol on the I Na.L with an IC50 of 34.442 µM.
Figure 1. Effects of resveratrol (RES) on I Na.L under control condition (Con) in rabbit ventricular myocytes.
A. 10, 20, 40 and 80 µM resveratrol decreased the amplitude of I Na.L in a concentration dependent manner. B. Effect of resveratrol (10, 20, 40 and 80 µM) on the current-voltage relationship. C. The inhibition amounts of 10, 20, 40 and 80 µM resveratrol on I Na.L. Values are expressed as mean ± SD, n = 8 cells/group. †P<0.01, ††P<0.05 versus control group; #P<0.01, ##P<0.05 versus 10 µM resveratrol group; §P<0.01, §§P<0.05 versus 20 µM resveratrol group; *P<0.01, **P<0.05 versus 40 µM resveratrol group.
Effects of Resveratrol, Ranolazine and TTX on the Increased I Na.L by H2O2
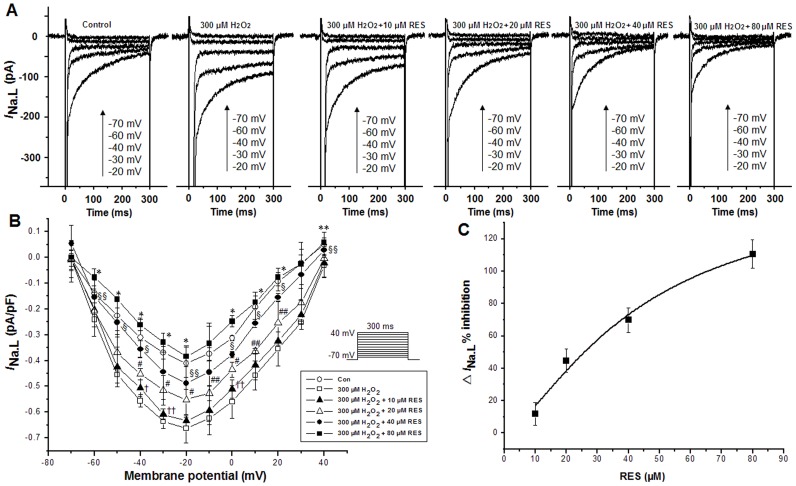
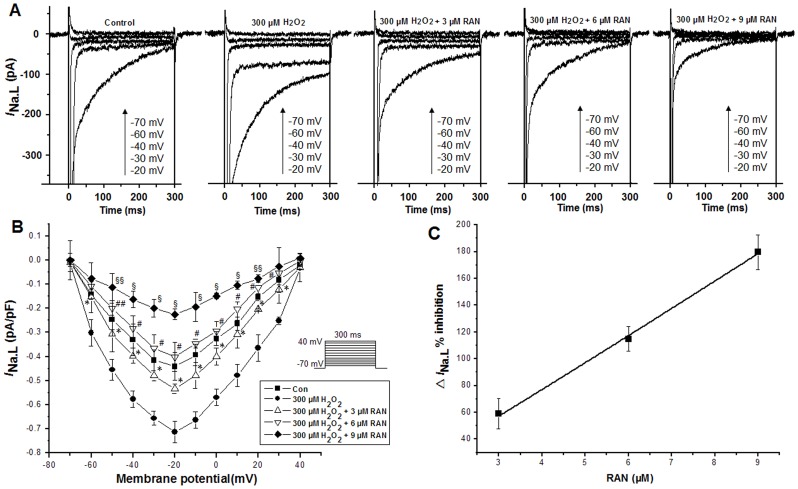
Currents were recorded using depolarizing pulses with a duration of 300 ms at a rate of 0.25 Hz from a HP of −120 mV, in 10 mV increments between −70 and −20 mV. Administration of resveratrol at concentrations of 10, 20, 40 and 80 µM resulted in a decrease in the amplitudes of I Na.L in a concentration dependent manner in myocytes exposed to H2O2 (Figure 2). H2O2 (300 µM) increased the amplitudes of I Na.L but 10, 20, 40 and 80 µM resveratrol decreased the amplitudes of I Na.L in the continued presence of H2O2 (Figure 2A). Shown in figure 2B are the I-V relationships of I Na.L after the sequential application of 300 µM H2O2, 10, 20, 40 and 80 µM resveratrol respectively, without a shift of the voltage at which the I Na.L amplitude was maximal. Figure 2C shows the inhibition amounts of 10, 20, 40 and 80 µM resveratrol on the △I Na.L (H2O2 induced increase in I Na.L) induced by 300 µM H2O2 with an IC50 of 26.192 µM. Ranolazine (3, 6 and 9 µM) attenuated the increased I Na.L in the presence of 300 µM H2O2 in a concentration dependent manner (Figure 3). Shown in figure 3B are the I-V relationships of I Na.L after the sequential application of 300 µM H2O2 in the absence and presence of 3, 6 and 9 µM ranolazine respectively, without a shift of the voltage at which the I Na.L amplitude was maximal. Figure 3C shows the inhibition amounts of 3, 6 and 9 µM ranolazine on the △I Na.L induced by 300 µM H2O2 with 300 ms voltage steps from a HP of −120 to −20 mV with an IC50 of 2.457 µM. TTX (4 µM) reversed the increased I Na.L caused by 300 µM H2O2. The values of I Na.L under the control conditions, after application of 300 µM H2O2 and 4 µM TTX were −0.323±0.087, −0.878±0.071(n = 6, P<0.05 versus control) and −0.258± −0.045 pA pF−1 (n = 6, P<0.05 versus H2O2), respectively.
Figure 2. Resveratrol inhibited the increase in I Na.L induced by 300 µM H2O2.
A. 10, 20, 40 and 80 µM resveratrol decreased the amplitudes of I Na.L in the presence of 300 µM H2O2 in a concentration dependent manner. B. The I-V relationships of I Na.L after the sequential application of 300 µM H2O2, 10, 20, 40 and 80 µM resveratrol. C. the inhibition amounts of 10, 20, 40 and 80 µM resveratrol on the △I Na.L induced by 300 µM H2O2. Values are expressed as mean ± SD, n = 8 cells/group. †P<0.01, ††P<0.05 versus H2O2 group; #P<0.01, ##P<0.05 versus 10 µM resveratrol group; §P<0.01, §§P<0.05 versus 20 µM resveratrol group; *P<0.01, **P<0.05 versus 40 µM resveratrol group.
Figure 3. Ranolazine (RAN) inhibited the increase in I Na.L caused by 300 µM H2O2.
A. 3, 6 and 9 µM ranolazine decreased the amplitudes of the increased I Na.L induced by 300 µM H2O2 in a concentration dependent manner. B. The I-V relationships of I Na.L after the sequential application of H2O2 alone, H2O2 plus 3, 6 and 9 µM ranolazine. C. the inhibition amounts of 3, 6 and 9 µM ranolazine on △I Na.L induced by 300 µM H2O2. Values are expressed as mean ± SD, n = 8 cells/group. *P<0.01 versus H2O2 group; #P<0.01, ##P<0.05 versus 3 µM ranolazine group; §P<0.01, §§P<0.05 versus 6 µM ranolazine group.
Effects of Resveratrol, Ranolazine and TTX on Increased Electrogenic I NCX by H2O2
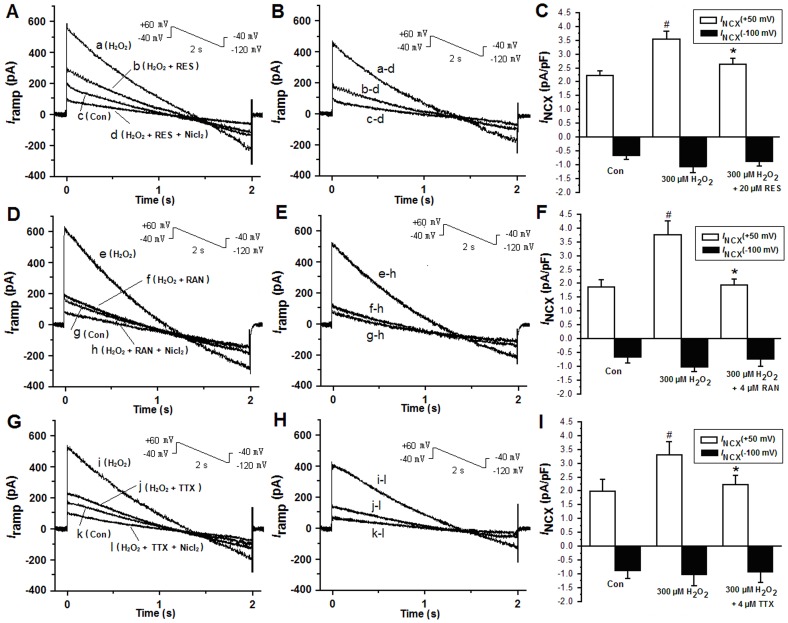
Electrogenic I NCX was measured to determine whether the reverse NCX was activated by the increase of I Na.L,. Membrane currents were elicited using ramp voltage-clamp pulses from a HP of −40 mV to +60 mV for 100 ms and then ramped to −120 mV over a period of 2 seconds (i.e. at 90 mV s−1) before returning to −40 mV. The current-time relationship was constructed from the declining slope of the ramp pulse (Figure 4A, 4B, 4D, 4E, 4G, 4H). Figure 4B, 4E, 4H shows the Ni2+-sensitive (NCX) current obtained by subtracting the data in the trace d, h or l from the data in the trace a, e, i, b, f, j, c, g or k in the panel 4A, 4D or 4G. Figure 4C, 4F, 4I are I NCX measured at voltage levels of +50 mV and −100 mV, respectively, as the Ni2+-sensitive current by subtracting the current recorded in the presence from that in the absence of 5 mM NiCl2.
Figure 4. A. The I-T (current-time relationship) curves were constructed from the negative slope region of a ramp voltage clamp, and were sequentially shown during no drug (control, trace c), H2O2 alone (trace a), H2O2 plus 20 µM resveratrol (trace b), and Ni2+ (5 mM) (trace d) in the continued presence of 300 µM H2O2.
B. Ni2+-sensitive I NCX was obtained by subtracting the data in the trace d from the data in trace a, b, and c in panel A. The enhanced reverse I NCX induced by H2O2 was restored by 20 µM resveratrol. C. Histograms show the mean current densities of I NCX obtained from B. Values are expressed as mean ± SD, n = 8 cells/group. #P<0.01 versus control group; *P<0.01 versus H2O2 group. D. Similar to A, the I-T (current-time relationship) curves were constructed, and were shown during no drug (control, trace g), H2O2 alone (trace e), H2O2 plus 4 µM RAN (trace f), and Ni2+ (5 mM) (trace h) in the continued presence of H2O2. E. Ni2+-sensitive I NCX were obtained by subtracting the data in trace h from the data in traces e, f and g in panel D. The reverse I NCX increased by H2O2 was restored by 4 µM RAN. F. Histograms show the mean current densities of I NCX obtained from E. Values are expressed as mean ± SD, n = 8 cells/group. #P<0.01 versus control group; *P<0.01 versus H2O2 group. G. Similar to A and D, the I-T (current-time relationship) curves were constructed, and were shown during no drug (control, trace k), H2O2 alone (trace i), H2O2 plus 4 µM TTX (trace j), and Ni2+ (5 mM) (trace l) in the continued presence of H2O2. H. Ni2+-sensitive I NCX were obtained by subtracting the data in trace l from the data in traces i, j and k in panel G. The reverse I NCX increased by H2O2 was restored by 4 µM TTX. I. Histograms show the mean current densities of I NCX obtained from H. Values are expressed as mean ± SD, n = 8 cells/group. #P<0.01 versus control group; *P<0.01 versus H2O2 group.
I NCX was recorded after 7 minutes of exposure of H2O2. The mean current density of the inward I NCX had little change, while the reverse I NCX increased significantly (n = 7, Figure 4B, 4C, 4E, 4F, 4H, 4I). 20 µM resveratrol, 4 µM ranolazine or 4 µM TTX diminished the increase of I NCX (n = 7, Figure 4B, 4C, 4E, 4F, 4H, 4I).
Effects of Resveratrol and TTX on Increased Intracellular Ca2+ Transient by H2O2
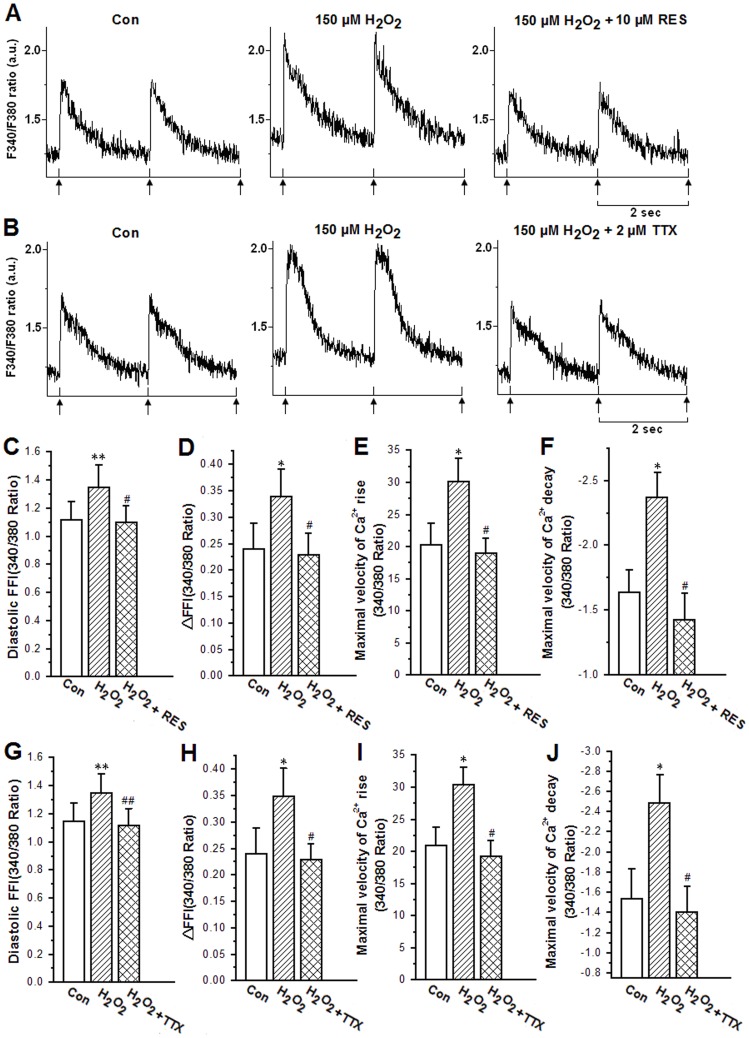
As shown earlier, resveratrol could reduce the increase in I Na.L and I NCX by H2O2, theoretically it should also decrease the increase in intracellular Ca2+ transients by H2O2. To minimize the cell contracture by 300 µM H2O2 due to the increase in the amplitude of calcium transients and diastolic calcium concentration, the concentration of H2O2 used was 150 µM. Cells were perfused with KHB solution for 5 min and then with KHB containing 150 µM H2O2 for 10 min. The diastolic intracellular Ca2+ fura-2 fluorescence intensity (FFI), fura-fluorescence intensity change (△FFI), maximal velocity of Ca2+ rise and Ca2+ decay were all enhanced by 150 µM H2O2 (Figure 5). However, 10 µM resveratrol reversed all these enhancements, as shown in Figure 5A, 5C, 5D, 5E, 5F. TTX (2 µM) also depressed these enhancements of the abovementioned parameters induced by 150 µM H2O2 (Figure 5B, 5G, 5H, 5I, 5J). These results indicated that both resveratrol (10 µM) and TTX (2 µM) could attenuate the H2O2-induced augmentations in diastolic Ca2+ concentration and calcium transients amplitude.
Figure 5. Effects of 10 µM resveratrol and 2 µM TTX on intracellular Ca2+ transient properties of adult rabbit ventricular myocytes in the presence of H2O2 (150 µM).
A, B. Representative recordings showing intracellular Ca2+ transients under different conditions; C, G. diastolic intracellular Ca2+ fura-2 fluorescence intensity (FFI); D, H. electrically stimulated increase in FFI (△FFI); E, I. maximal velocity of intracellular Ca2+ transient rise; F, J. maximal velocity of intracellular Ca2+ transient decay. Values are expressed as mean ± SD, n = 6–7 cells/group, **P<0.05, *P<0.01 versus control group; ##P<0.05; #P<0.01 versus H2O2 group.
Effects of TTX on I Ca.L
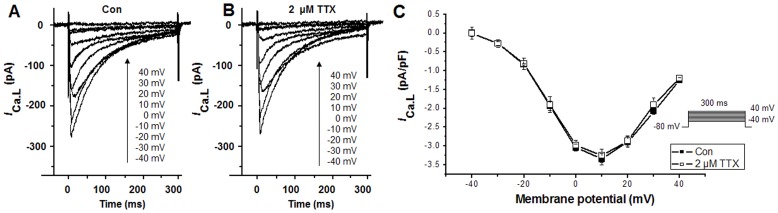
Recent evidence suggests a potential for TTX to inhibit L-type Ca2+ channels [28]. The results in this study showed that 2 µM TTX inhibited H2O2-induced augmentations in diastolic Ca2+ concentration and amplitude of calcium transients. To identify the effect of 2 µM TTX on intracellular Ca2+ was from its blocking of I Na.L but not the inhibition of L-type Ca2+ channels, I Ca.L was measured. The results indicated that at a low concentration (2 µM) TTX is relatively a selective I Na.L blocker. Using depolarizing pulses with a duration of 300 ms applied at 0.5 Hz from a HP of −80 mV, in 10 mV increments between −40 and +40 mV, I Ca.L was recorded in the absence (Figure 6A) and presence (Figure 6B) of 2 µM TTX. Figure 6C showed the effect of TTX (2 µM) application on the current-voltage relationship of I Ca.L. TTX at concentration of 2 µM had no effect on I Ca.L, accounting for that the effects of 2 µM TTX to inhibit H2O2-induced augmentations in diastolic Ca2+ concentration and amplitude of calcium transients were from its inhibition on I Na.L and subsequently the reverse I NCX.
Figure 6. Effects of 2 µM TTX on I Ca.L under control conditions in rabbit ventricular myocytes.
A. I Ca.L under control conditions. B. I Ca.L after the application of 2 µM TTX. C. Effects of TTX (2 µM) on current-voltage relationship of I Ca.L. Values are expressed as mean ± SD, n = 6 cells/group. P>0.05 versus control group.
Discussion
The mechanisms underlying the genesis of ischemia- and reperfusion-induced arrhythmias are notoriously complex and controversial. There has been an interest in the concept that oxygen free radicals play a role in the pathogenesis of myocardial ischemia and infarction. It has been reported that a burst of H2O2, an important reactive oxygen species, is generated in the myocardium during ischemia and reperfusion [29]–[33] and causes Ca2+ overload through many ways [34], [35], [36], [37]. For example, the activation of ryanodine receptors with H2O2 could also account for the increased cytosolic Ca2+ levels found with ROS production, which could account for the Ca2+ overload in cells [34]. Furthermore, the excessive amount of H2O2 could increase I Na.L in cardiomyocytes, subsequently leading to intracellular Ca2+ overload through reverse NCX (the Na+-dependent Ca2+ overload induced by I Na.L) and ultimately causing cell damage [10], [23], [38], [39]. Reducing agent, e.g., dithiothreitol (DTT) and reduced glutathione (GSH), could reverse the increased I Na.L by H2O2 and hypoxia [5], [24], [25]. Resveratrol, a natural antioxidant, has beneficial effects against coronary heart disease. Previous studies have shown that resveratrol effectively suppressed ischemia/reperfusion-induced arrhythmia [40], [41] and reduced both peak I Na and I Na.L in the R1623Q LQT3 mutation in a recombinant expression system [27]. But the effect of resveratrol on the increased I Na.L and reverse I NCX under proarrhythmic conditions (H2O2) in rabbit ventricular myocytes has not been investigated yet. The data from this study addressed the impact of resveratrol on the Na+-dependent Ca2+ overload.
In this study, I Na.L was increased by H2O2 (Figure 2, 3). Ranolazine attenuated the increased I Na.L by H2O2 in a concentration dependent manner (Figure 3) and 4 µM TTX attenuated the increased I Na.L increased by H2O2 as well. These data are consistent with other reports and our previous studies that the I Na.L inhibitors ranolazine and TTX significantly inhibited late I Na.L at clinical relevant concentrations [42], [43]. H2O2-induced intracellular Na+ and Ca2+ overload was associated with an enhanced I Na.L and therefore was attenuated by the I Na.L inhibitors ranolazine and TTX [10]. The I Na.L blocking agents may be effective in preventing arrhythmias by reducing [Na+]i load and subsequently the [Ca2+]i load. However, ranolazine has been suggested to inhibit the cardiac ryanodine receptor (IC50 = 10 µM) [44], which could also modulate intracellular Ca2+ levels. Ranolazine is currently approved as an antianginal agent that reduces the Na+-dependent Ca2+ overload via inhibition of the I Na.L and thus improves diastolic tone and oxygen handling during myocardial ischemia [7]. I Na.L is an important contributing factor to intracellular Ca2+ overload in the pathogenesis of myocardial ischemia and infarction. In rabbit ventricular myocytes, low concentrations of TTX (1.5–4.0 µM) did not alter the L-type Ca2+ current (Figure 6) and I Na.T [6], [25], [45], but obviously inhibited the I Na.L. Accordingly TTX was used to confirm the process of Na+-dependent Ca2+ overload induced by H2O2. The effect of resveratrol on I Na.L is similar to ranolazine and TTX. Resveratrol inhibited I Na.L in both normal and H2O2-treated cells in a concentration dependent manner (Figure 1, 2). This result is consistent with our previous studies that DTT and reduced glutathione could reverse the increase in I Na.L induced by either H2O2 or hypoxia [24], [25], indicating resveratrol may act as an antioxidant to eliminate the detrimental effects of H2O2 on I Na.L. Changes in redox potential or surface charge may account for some ionic current block [46], therefore it is possible that the antioxidant properties of resveratrol may contribute to the I Na.L inhibition observed in this study.
Recently, it has been reported that reverse I NCX was increased along with the increased I Na.L during hypoxia and was decreased along with the I Na.L inhibition by TTX in hypoxic ventricular myocytes, suggesting that the increased I Na.L contributed to the increase in the reverse I NCX [6]. In this study, 300 µM H2O2 increased the reverse I NCX while the inward I NCX was not affected obviously, whereas ranolazine or TTX attenuated the increase in the reverse I NCX significantly (Figure 4). Different from I Na.T, I Na.L can be blocked by a low concentration of ranolazine and TTX, and the consequent reduction of Na+ loading via the decrease of the I Na.L can prevent the increase in the reverse I NCX-induced intracellular Ca2+ accumulation [47]. Ranolazine (4 µM) and TTX (4 µM) decreased the reverse I NCX through the inhibition of I Na.L. Similarly, resveratrol (20 µM) attenuated the increase in the reverse I NCX by H2O2. Thus, we concluded that the effect of resveratrol to inhibit the increased reverse I NCX caused by H2O2 was from its inhibition of I Na.L.
In this study, 150 µM H2O2 significantly increased the amplitude of calcium transients and diastolic calcium concentration in the ventricular cell which could be reversed by TTX (2 µM). The intracellular Ca2+ overload caused by ROS was due to an increase in [Na+]i followed with an increase in Ca2+ influx via the reverse mode of the NCX [48]. Then the large entry of Ca2+ into the cell will cause intracellular Ca2+ overload [49], [50]. TTX also inhibited L-type Ca2+ channel with an IC50 value of 55±2 µM [28]. In this study in rabbit ventricular myocytes, 2 µM TTX inhibited I Na.L and restrained Ca2+ overload induced by H2O2 but not affected L-type Ca2+ channels (Figure 6), supporting that I Na.L played an important role in the genesis of Ca2+ overload induced by H2O2. TTX also reversed the increase in calcium transients amplitude and diastolic calcium concentration through inhibiting the increased I Na.L by H2O2. Resveratrol (10 µM) also restrained the increased calcium transients amplitude and the diastolic calcium concentration induced by H2O2 (150 µM). Therefore the effects of resveratrol on the Na+-dependent Ca2+ overload induced by enhanced I Na.L were similar to 2 µM TTX, suggesting that the reduction of Ca2+ overload by resveratrol may have similar mechanism to TTX, i.e., inhibition of I Na.L. Indeed, resveratrol has also been suggested to inhibit the ryanodine receptor-induced intracellular Ca2+ increase [51] which may account for the reduction of [Ca2+]i. The results in this study indicated that resveratrol reduced both I Na.L and reverse I NCX which was responsible for the reversal of intracellular Ca2+ overload in the presence of H2O2. Resveratrol may inhibit both the ryanodine receptor-induced intracellular Ca2+ overload and I Na.L-induced increase in reverse I NCX to attenuate the intracellular Ca2+ overload. Further research will be needed to clarify the contribution of the two pathways by resveratrol in the absence and presence of H2O2.
Conclusions
I Na.L is an important target for resveratrol to prevent or treat ventricular arrhythmias. I Na.L increased by H2O2 induces intracellular Ca2+ overload (the increased diastolic calcium concentration) through the increase in the reverse I NCX. The inhibitive effect of resveratrol on H2O2-induced I Na.L may reduce the concentration of [Na+]i, lower [Ca2+]i by attenuating reverse NCX to eliminate Ca+ overload, and ultimately inhibit the electrical abnormalities.
Acknowledgments
We thank Lin Wu, an Associate Professor of Beijing University, for revising the manuscript.
Funding Statement
This work was supported by the National Natural Science Foundation of China (81072637). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wasserstrom JA, Sharma R, O'Toole MJ, Zheng J, Kelly JE, et al. (2009) Ranolazine antagonizes the effects of increased late sodium current on intracellular calcium cycling in rat isolated intact heart. J Pharmacol Exp Ther 331: 382–91. [DOI] [PubMed] [Google Scholar]
- 2. Kiyosue T, Arita M (1989) Late sodium current and its contribution to action potential configuration in guinea pig ventricular myocytes. Circ Res 64: 389–97. [DOI] [PubMed] [Google Scholar]
- 3. Saint DA (2006) The role of the persistent Na (+) current during cardiac ischemia and hypoxia. J Cardiovasc Electrophysiol 17 Suppl 1S96–S103. [DOI] [PubMed] [Google Scholar]
- 4. Ju YK, Saint DA, Gage PW (1996) Hypoxia increases persistent sodium current in rat ventricular myocytes. J Physiol 497: 337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hammarström AK, Gage PW (2002) Hypoxia and persistent sodium current. Eur Biophys J 31: 323–30. [DOI] [PubMed] [Google Scholar]
- 6. Tang Q, Ma J, Zhang P, Wan W, Kong L, et al. (2012) Persistent sodium current and Na (+)/H (+) exchange contributes to the augmentation of the reverse Na (+)/Ca (2+) exchange during hypoxia or acute ischemia in ventricular myocytes. Pflugers Arch 463: 513–22. [DOI] [PubMed] [Google Scholar]
- 7. Sossalla S, Maier LS (2012) Role of ranolazine in angina, heart failure, arrhythmias, and diabetes. Pharmacol Ther 133: 311–23. [DOI] [PubMed] [Google Scholar]
- 8. Richardt G, Tölg R (1997) [Cellular sequelae of myocardial ischemia]. Z Kardiol 86 Suppl 123–32. [PubMed] [Google Scholar]
- 9. Undrovinas A, Maltsev VA (2008) Late sodium current is a new therapeutic target to improve contractility and rhythm in failing heart. Cardiovasc Hematol Agents Med Chem 6: 348–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L (2006) Blocking late sodium current reduces H2O2-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther 318: 214–22. [DOI] [PubMed] [Google Scholar]
- 11. Belardinelli L, Shryock JC, Fraser H (2006) Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart 92 Suppl 4iv6–iv14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hale SL, Leeka JA, Kloner RA (2006) Improved left ventricular function and reduced necrosis after myocardial ischemia/reperfusion in rabbits treated with ranolazine, an inhibitor of the late sodium channel. J Pharmacol Exp Ther 318: 418–23. [DOI] [PubMed] [Google Scholar]
- 13. Sossalla S, Wagner S, Rasenack EC, Ruff H, Weber SL, et al. (2008) Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts–role of late sodium current and intracellular ion accumulation. J Mol Cell Cardiol 45: 32–43. [DOI] [PubMed] [Google Scholar]
- 14. Saint DA (2008) The cardiac persistent sodium current: an appealing therapeutic target? British Journal of Pharmacology 153: 1133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen L, Han Y, Yang F, Zhang T (2001) High-speed counter-current chromatography separation and purification of resveratrol and piceid from Polygonum cuspidatum. J Chromatogr A 907: 343–6. [DOI] [PubMed] [Google Scholar]
- 16. Renaud S, de Lorgeril M (1992) Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 339: 1523–6. [DOI] [PubMed] [Google Scholar]
- 17. Constant J (1997) Alcohol, ischemic heart disease, and the French paradox. Coron Artery Dis 8: 645–9. [DOI] [PubMed] [Google Scholar]
- 18. Das DK, Sato M, Ray PS, Maulik G, Engelman RM, et al. (1999) Cardioprotection of red wine: role of polyphenolic antioxidants. Drugs Exp Clin Res 25: 115–20. [PubMed] [Google Scholar]
- 19. Wallerath T, Deckert G, Ternes T, Anderson H, Li H, et al. (2002) Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 106: 1652–8. [DOI] [PubMed] [Google Scholar]
- 20. Olas B, Wachowicz B, Saluk-Juszczak J, Zielinski T (2002) Effect of resveratrol, a natural polyphenolic compound, on platelet activation induced by endotoxin or thrombin. Thromb Res 107: 141–5. [DOI] [PubMed] [Google Scholar]
- 21. Frankel EN, Kanner J, German JB, Parks E, Kinsella JE (1993) Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet 341: 454–7. [DOI] [PubMed] [Google Scholar]
- 22. Kourie JI (1998) Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol 275: C1–24. [DOI] [PubMed] [Google Scholar]
- 23. Ma JH, Luo AT, Zhang PH (2005) Effect of hydrogen peroxide on persistent sodium current in guinea pig ventricular myocytes. Acta Pharmacol Sin 26: 828–34. [DOI] [PubMed] [Google Scholar]
- 24. Luo A, Ma J, Zhang P, Zhou H, Wang W (2007) Sodium channel gating modes during redox reaction. Cell Physiol Biochem 19: 9–20. [DOI] [PubMed] [Google Scholar]
- 25. Wang W, Ma J, Zhang P, Luo A (2007) Redox reaction modulates transient and persistent sodium current during hypoxia in guinea pig ventricular myocytes. Pflugers Arch 454: 461–75. [DOI] [PubMed] [Google Scholar]
- 26. Wood LG, Wark PA, Garg ML (2010) Antioxidant and anti-inflammatory effects of resveratrol in airway disease. Antioxid Redox Signal 13: 1535–48. [DOI] [PubMed] [Google Scholar]
- 27. Wallace CH, Baczkó I, Jones L, Fercho M, Light PE (2006) Inhibition of cardiac voltage-gated sodium channels by grape polyphenols. Br J Pharmacol 149: 657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hegyi B, Bárándi L, Komáromi I, Papp F, Horváth B, et al. (2012) Tetrodotoxin blocks L-type Ca(2+) channels in canine ventricular cardiomyocytes. Pflugers Arch 464: 167–74. [DOI] [PubMed] [Google Scholar]
- 29. Arroyo CM, Kramer JH, Leiboff RH, Mergner GW, Dickens BF, et al. (1987) Spin trapping of oxygen and carbon-centered free radicals in ischemic canine myocardium. Free Radical Bio Med 3: 313–6. [DOI] [PubMed] [Google Scholar]
- 30. Garlick PB, Davies MJ, Hearse DJ, Slater TF (1987) Direct detection of free radicals in the reperfused rat heart using electron spin resonance spectroscopy. Circ Res 61: 757–60. [DOI] [PubMed] [Google Scholar]
- 31. Zweier JL (1988) Measurement of superoxidederived free radicals in the reperfused heart: Evidence for a free radical mechanism of reperfusion injury. J Biol Chem 263: 1353–7. [PubMed] [Google Scholar]
- 32. Baker JE, Felix CC, Olinger GN, Kalyanaraman B (1988) Myocardial ischemia and reperfusion: Direct evidence for free radical generation by electron spin resonance spectroscopy. Proc Natl Acad Sci U S A 85: 2786–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bolli R, Patel BS, Jeroudi MO, Lai EK, McCay PB (1988) Demonstration of free radical generation in “stunned” myocardium of intact dogs with the use of the spin trap a-phenyl N-Tert-Butyl Nitrone. J Clin Invest 82: 476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boraso A, Williams AJ (1994) Modification of the gating of the cardiac sarcoplasmic reticulum Ca(2+)-release channel by H2O2 and dithiothreitol. Am J Physiol 267: H1010–6. [DOI] [PubMed] [Google Scholar]
- 35. Sato H, Takeo T, Liu Q, Nakano K, Osanai T, et al. (2009) Hydrogen peroxide mobilizes Ca2+ through two distinct mechanisms in rat hepatocytes. Acta Pharmacol Sin 30: 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith MA, Herson PS, Lee K, Pinnock RD, Ashford ML (2003) Hydrogen-peroxide-induced toxicity of rat striatal neurones involves activation of a non-selective cation channel. J Physiol 547 (Pt 2): 417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang KT, Pan SF, Chien CL, Hsu SM, Tseng YZ, et al. (2004) Mitochondrial Na+ overload is caused by oxidative stress and leads to activation of the caspase 3- dependent apoptotic machinery. FASEB J 18: 1442–4. [DOI] [PubMed] [Google Scholar]
- 38. Liu H, Cala PM, Anderson SE (2010) Na/H exchange inhibition protects newborn heart from ischemia/reperfusion injury by limiting Na+-dependent Ca2+ overload. J Cardiovasc Pharmacol 55: 227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Josephson RA, Silverman HS, Lakatta EG, Stern MD, Zweier JL (1991) Study of the mechanisms of hydrogen peroxide and hydroxyl free radical-induced cellular injury and calcium overload in cardiac myocytes. J Biol Chem 266: 2354–61. [PubMed] [Google Scholar]
- 40. Hung LM, Chen JK, Huang SS, Lee RS, Su MJ (2000) Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc Res 47: 549–55. [DOI] [PubMed] [Google Scholar]
- 41. Hung LM, Chen JK, Lee RS, Liang HC, Su MJ (2001) Beneficial effects of astringinin, a resveratrol analogue, on the ischemia and reperfusion damage in rat heart. Free Radic Biol Med 30: 877–83. [DOI] [PubMed] [Google Scholar]
- 42. Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, et al. (2004) Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation 110: 904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Undrovinas AI, Belardinelli L, Undrovinas NA, Sabbah HN (2006) Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol 17 Suppl 1S169–S177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Parikh A, Mantravadi R, Kozhevnikov D, Roche MA, Ye Y, et al. (2012) Ranolazine stabilizes cardiac ryanodine receptors: a novel mechanism for the suppression of early afterdepolarization and torsades de pointes in long QT type 2. Heart Rhythm 9: 953–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang S, Ma J, Zhang P, Luo A, Ren Z, et al.. (2012) Sophocarpine Attenuates the Na+-dependent Ca2+ Overload Induced by Anemonia Sulcata Toxin II-increased Late Sodium Current in Rabbit Ventricular Myocytes. J Cardiovasc Pharmacol. [Epub ahead of print]. [DOI] [PubMed]
- 46. Bhatnagar A, Srivastava SK, Szabo G (1990) Oxidative stress alters specific membrane currents in isolated cardiac myocytes. Circ Res 67: 535–549. [DOI] [PubMed] [Google Scholar]
- 47. Barry WH, Zhang XQ, Halkos ME, Vinten-Johansen J, Saegusa N, et al. (2010) Nonanticoagulant heparin reduces myocyte Na+ and Ca2+ loading during simulated ischemia and decreases reperfusion injury. Am J Physiol Heart Circ Physiol 298: H102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wagner S, Seidler T, Picht E, Maier LS, Kazanski V, et al. (2003) Na+-Ca2+ exchanger overexpression predisposes to reactive oxygen species-induced injury. Cardiovasc Res 60: 404–12. [DOI] [PubMed] [Google Scholar]
- 49. El-Ani D, Stav H, Guetta V, Arad M, Shainberg A (2011) Rapamycin (sirolimus) protects against hypoxic damage in primary heart cultures via Na+/Ca2+ exchanger activation. Life Sci 89: 7–14. [DOI] [PubMed] [Google Scholar]
- 50. Sun L, Ai J, Wang N, Zhang R, Li J, et al. (2010) Cerebral ischemia elicits aberration in myocardium contractile function and intracellular calcium handling. Cell Physiol Biochem 26: 421–30. [DOI] [PubMed] [Google Scholar]
- 51. Liu Z, Zhang LP, Ma HJ, Wang C, Li M, et al. (2005) Resveratrol reduces intracellular free calcium concentration in rat ventricular myocytes. Sheng Li Xue Bao 57: 599–604. [PubMed] [Google Scholar]