Abstract
Opioid-induced hyperalgesia (OIH) is a paradoxical increase in pain perception that may manifest during opioid treatment. For morphine, the metabolite morphine-3-glucuronide (M3G) is commonly believed to underlie this phenomenon. Here, in three separate studies, we empirically assess the role of M3G in morphine-induced hyperalgesia. In the first study, CD-1 mice injected with morphine (15 mg/kg subcutaneously) after pretreatment with the opioid receptor antagonist naltrexone (NTX) (15 mg/kg) showed tail withdrawal latency reductions indicative of hyperalgesia (2.5 ± 0.1 s at t = 30 min, P < 0.001 versus baseline). In these mice, the morphine/M3G concentration ratios versus effect showed a negative correlation (rp = −0.65, P < 0.001), indicating that higher morphine relative to M3G concentrations are associated with increased OIH. In the second study, similar hyperalgesic responses were observed in mice lacking the multidrug resistance protein 3 (MRP3) transporter protein (Mrp3−/− mice) in the liver and their wild-type controls (FVB mice; latency reductions: 3.1 ± 0.2 s at t = 30 min, P < 0.001 versus within-strain baseline). In the final study, the pharmacokinetics of morphine and M3G were measured in Mrp3−/− and FVB mice. Mrp3−/− mice displayed a significantly reduced capacity to export M3G into the systemic circulation, with plasma M3G concentrations just 7% of those observed in FVB controls. The data confirm previous literature that morphine causes hyperalgesia in the absence of opioid receptor activation but also indicate that this hyperalgesia may occur without a significant contribution of hepatic M3G. The relevance of these data to humans has yet to be demonstrated.
INTRODUCTION
Opioid-induced hyperalgesia (OIH) is the paradoxical increase in pain perception that may become manifest during opioid treatment for acute and chronic pain (1–4). OIH is characterized by increased sensitivity to painful stimuli (hyperalgesia) and nonpainful stimuli (allodynia) and compromises adequate pain treatment (1–4). Morphine is the prototypical μ-opioid receptor (MOR) agonist, acts primarily at this receptor subtype and is considered the gold standard for treatment of moderate to severe pain (1). In humans, morphine is metabolized in the liver by UDP-glucuronosyl transferase (UGT) into two major metabolites: M3G (60–70%) and morphine-6-glucuronide (M6G, 5–10%) (5,6). The larger proportion to M3G metabolism is because glucuronidation at the aromatic hydroxyl group (that is, the third C atom) is easier than at the alicyclic hydroxyl group (that is, the sixth C atom) (7). For this reason, most rodents, including rats, do not form M6G but only form M3G (7). The two metabolites also have distinct actions. Whereas M6G has proven analgesic actions, there is evidence that M3G is pronociceptive (5). For example, a systemic M3G injection increases pain sensitivity in mice, and relatively small intracerebroventricular, intrathecal or systemic M3G doses in rats evoke a general state of neuroexcitation with agitation to innocuous touch (8–12). In cancer patients treated with morphine, a cerebrospinal fluid ratio of M3G/M6G concentrations of <1 coincides with effective analgesia, whereas a ratio >1 is associated with ineffective analgesia (13). We interpret these data to suggest a role for M3G not only in ineffective analgesia but also in morphine-induced hyperalgesia.
Importantly, M3G effects are not diminished by the general opioid antagonist naloxone (14,15), indicating that M3G does not act via opioid receptor activation. Consistent with the hypothesis that M3G contributes to morphine-induced hyperalgesia, there is ample evidence that the opioid receptors are not involved in OIH. Morphine and other MOR agonists, such as fentanyl, induce OIH during systemic blockade of the opioid receptors with the nonselective opioid receptor antagonist naltrexone (NTX) (16–19). Furthermore, OIH induced by morphine and fentanyl is observed in triple knockout mice completely lacking μ-, κ- and δ-opioid receptors and their subtypes (17,19). Thus, M3G is widely believed to underlie hyperalgesia consequent to morphine exposure.
The aim of the current study was to empirically assess the putative role of M3G in morphine-induced hyperalgesia. To that end, we injected outbred CD-1 mice with morphine during treatment with NTX or saline and related plasma concentrations of morphine and M3G to the observed pharmacodynamic effects on a standard thermal nociceptive assay (tail-withdrawal test). Next, we compared the pharmacodynamic effects of morphine in mice lacking the multidrug resistance protein 3 (MRP3) with their wild-type FVB controls during systemic NTX exposure. MRP3 is a protein involved in transporting glucuronidated substances, such as M3G, from the cytoplasm of the hepatocytes into the systemic circulation (20). MRP3-deficient mice (Mrp3−/−) consequently have low and no detectable M3G concentrations in plasma and brain, respectively (20). Our hypothesis is that M3G formed in the liver plays a major role in morphine-induced hyperalgesia with (a) a significant correlation between M3G plasma concentrations and magnitude of hyperalgesia and (b) absence of hyperalgesia in Mrp3−/− mice treated with morphine and NTX. Only when both assumptions are met are we able to accept our hypothesis.
MATERIALS AND METHODS
Animals
Experiments were performed after approval of the protocol by the local Animal Ethics Committee. Adult male CD-1 mice were purchased from Charles River (Maastricht, the Netherlands). Mrp3−/−and FVB mice were generated in the laboratory (20); the Mrp3−/− mice have a FVB genetic background. The animals received water and food ad libitum and were housed in groups in individually ventilated, constant-temperature cages and kept in rooms with 12-h light/12-h dark cycles (lights on/lights off at 7:00 AM/PM). The experimental studies were performed in accordance with institutional guidelines and the guidelines of the International Association for the Study of Pain (21).
Nociceptive Assay
Nociception was assessed by measuring tail withdrawal latencies (TWLs) from a hot water bath as described previously (17,22). TWLs were obtained in triplicate (at 30-s intervals) with a cutoff value of 30 s to prevent tissue damage to the tail. Bath temperature was set to 47.5 ± 0.2°C to obtain a pretreatment TWL between 9 and 11 s to avoid possible floor effects in hyperalgesic mice. The TWLs were averaged and recorded into a digital datasheet for further analysis. All experiments were performed near mid-photophase to reduce circadian effects.
Drugs
Morphine hydrochloride 3·H2O (MOR) at a solution of 20 mg/mL was obtained from the local hospital pharmacy. NTX hydrochloride powder was purchased from Sigma-Aldrich (Zwijndrecht, the Netherlands) and dissolved in saline (0.9% NaCl) to obtain a 20 mg/mL solution.
Study Design
Study 1
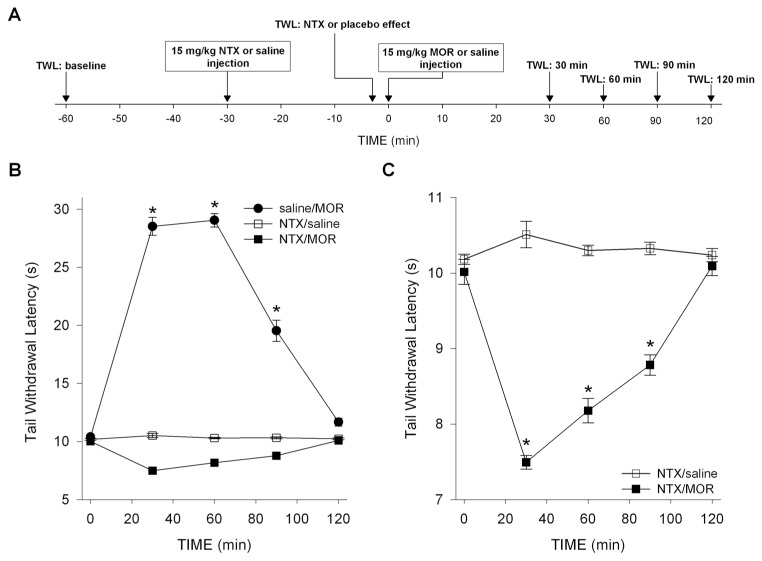
Morphine and M3G pharmacokinetics and morphine pharmacodynamics were tested in 72 CD-1 mice. The effect of morphine in CD-1 mice during treatment with NTX or saline was assessed using the tail-withdrawal test as described above. Each animal was tested once. The groups were randomly divided in three sets of 24 animals that received subcutaneous injections of 15 mg/kg NTX, followed 30 min later by 15 mg/kg morphine (NTX/MOR), saline followed 30 min later by 15 mg/kg morphine (saline/MOR) and 15 mg/kg NTX followed 30 min later by saline (NTX/ saline), respectively. TWLs were obtained 30 min before NTX, 3 min before the MOR or saline drug injections (NTX/ placebo effect) and at times 30, 60, 90 and 120 min after these injections (MOR/saline effect; see Figure 1A for an illustration of the study design). Each group was subdivided in four subgroups of six animals with different times for sacrificing and extraction of blood for pharmacokinetics (PK) analysis (at t = 30, 60, 90 and 120 min, directly after the nociceptive test).
Figure 1.
Design (A) and pharmacodynamic results of study 1 (B, C): effect of saline and NTX pretreatment on morphine-(MOR) or saline-induced changes in TWL in CD-1 mice. (A) Timing of TWL measurements and drug injections. (B) Saline pretreatment caused morphine-induced analgesia. Saline/MOR versus NTX/saline: P < 0.001; *P < 0.001 versus baseline. (C) NTX pretreatment caused morphine-induced hyperalgesia. The combination NTX/saline had no effect on the measured latencies. NTX/MOR versus NTX/saline: P < 0.001; *P < 0.001 versus baseline. Values are means ± SEM.
Study 2
Morphine pharmacodynamics was tested in Mrp3−/− and FVB mice. Mice of each strain were tested either after the subcutaneous injection of 15 mg/kg morphine, 30 min after 15 mg/kg NTX pretreatment (NTX/ MOR) or after the subcutaneous injection of saline, 30 min after 15 mg/kg NTX pretreatment (NTX/saline) (n = 6/strain/ condition). The injections and nociceptive testing protocols were identical to those in study 1.
Study 3
Morphine and M3G pharmacokinetics were assayed in Mrp3−/− mice (n = 10) and FVB mice (n = 9). Four Mrp3−/− and five FVB mice were injected with 15 mg/kg subcutaneous morphine and 30 min later were sacrificed to extract blood for morphine and M3G plasma concentration measurements. The remaining mice were used for urine collection and determination of morphine and M3G concentrations in urine collected for 24 h after 15 mg/kg morphine injection. To that end, the mice were kept in a metabolic cage, and their food was removed 24 h before morphine injection.
Analysis of Morphine and M3G Concentrations
In study 1, plasma proteins of all plasma samples were precipitated with 700 μL acetonitrile, 100 μL of 1 mmol/L zinc sulfate and an adequate amount of internal standards. A total of 200 μL of the supernatant was transferred in a glass tube and dried; the residues were reconstituted in 100 μL of 0.1 % (v/v) formic acid in water. Then, 20 μL of the sample was injected by an Ultimate 3000 autosampler (Dionex, Amsterdam, the Netherlands) and pumped on a 3-μm, 120Å, 50 × 2.1 mm YMC pack ODS-AQ column (YMC Inacom, Overberg, the Netherlands). The eluent was monitored by a Quattro microAPI tandem mass spectrometer (Waters, Etten-Leur, the Netherlands). Peak areas of reaction ions from morphine, M3G, M6G and the internal standards were obtained in the multiple reaction mode and integrated by data software Masslynx 4.1 (Waters, Etten-Leur, the Netherlands). All analytes were measured in one run. In study 3, morphine and M3G concentrations in urine and plasma were determined as described by Rook et al.(23).
Statistical Analysis
Behavioral data of studies 1 and 2 were analyzed by two-way repeated-measure analysis of variance for the main effect with a post hoc Tukey test for treatment and time. Morphine and M3G concentrations obtained in study 3 were compared between genotypes with the Mann-Whitney U test (plasma) and two-tailed t test (urine). All statistical tests were performed using SigmaPlot version 12 for Windows (Systat Software, Chicago, IL, USA). P values <0.05 were considered significant. Data are expressed as mean ± standard error of the mean (SEM).
RESULTS
Study 1
TWL baseline values were 10.1 ± 0.05 s (NTX/MOR), 10.3 ± 0.05 s (NTX/saline) and 10.2 ± 0.08 s (saline/MOR). These values did not differ significantly from values obtained after NTX or saline injections (as measured just before morphine or saline injections). The effects of MOR or saline treatments on TWL are given in Figures 1B and C. No effect on TWL was observed from the combination NTX/ saline. Morphine combined with saline produced profound antinociception during the entire testing period (increase in TWL at t = 30 min: 19.1 ± 0.6 s, P < 0.001 versus baseline; main effect: P < 0.001 versus NTX/saline). In contrast, combining morphine with NTX decreased TWL by 2.5 ± 0.1 s at t = 30 min compared with baseline values (P < 0.001). Hyperalgesia persisted during the entire testing period (main effect: P = 0.004 versus NTX/saline and P < 0.001 versus saline/ MOR).
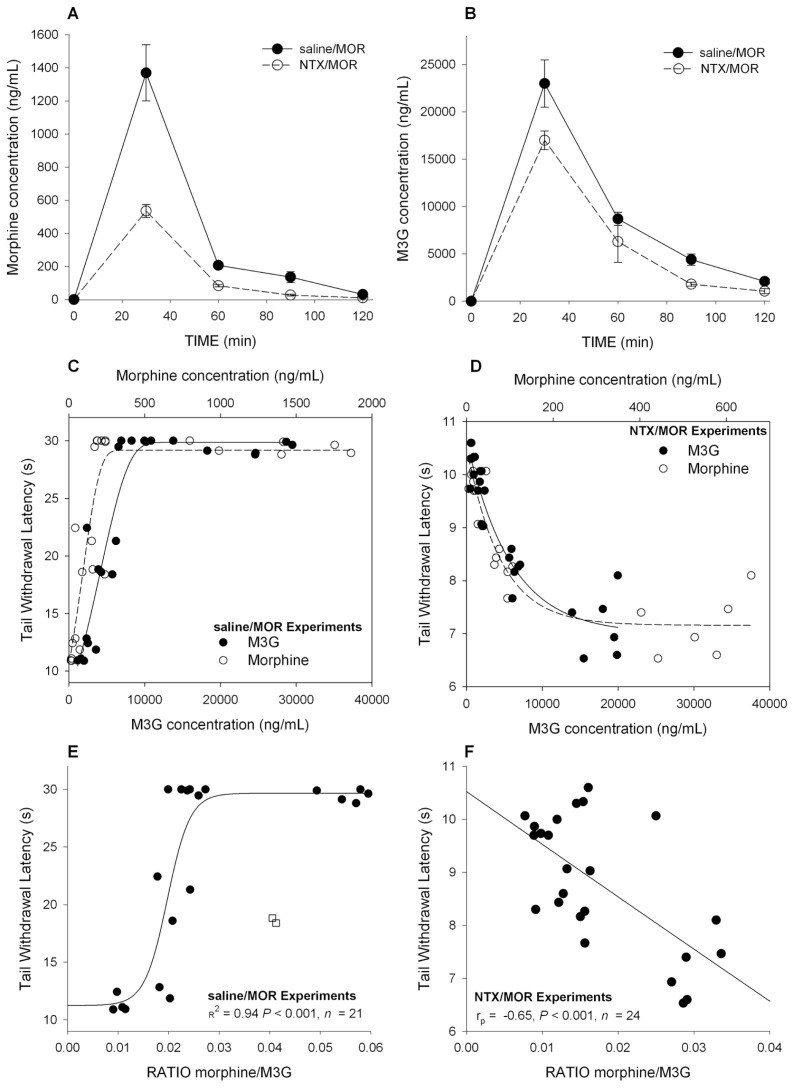
NTX pretreatment had an impact on both morphine and M3G PK, causing a reduction in maximum concentrations (Maximum plasma concentration (CMAX); Figures 2A, B). After saline/MOR, morphine and M3G CMAX (reached at t = 30 min) were 1,364 ± 170 ng/mL and 23.2 ± 2.5 μg/mL, respectively. After NTX/MOR, morphine and M3G CMAX (reached at t = 30 min) were 535 ± 40 ng/mL (P = 0.002 versus MOR/saline) and 18 ± 1 μg/mL (P = 0.002 versus MOR/saline), respectively.
Figure 2.
Morphine and M3G pharmacokinetics and pharmacodynamics in CD-1 mice (study 1). (A) Mean morphine plasma concentrations versus time in mice treated with saline followed by morphine (saline/MOR) and mice treated with NTX followed by morphine (NTX/MOR). (B) Mean M3G plasma concentrations versus time in mice treated with saline followed by morphine (saline/MOR) and mice treated with NTX followed by morphine (NTX/MOR). (C) Individual morphine (open circle) and M3G (closed circle) plasma concentrations after saline pretreatment versus effect (TWL). To guide the eye, a sigmoid function is fitted through the two data sets. (D) Individual morphine (open circle) and M3G (closed circle) plasma concentrations after NTX pretreatment versus effect (TWL). To guide the eye, an exponential function is fitted through the two data sets. (E) The ratio of the plasma concentrations morphine and M3G (morphine/M3G) versus TWL for animals treated with saline/MOR. There is a clear positive correlation for ratios <0.03 ( rp = 0.72). At ratios >0.03, no further increase in TWL was observed because of the preset cutoff value of 30 s. The line through the data is a sigmoid fitted to the whole data set (R2 = 0.94). Two outliers (open squares) were not taken into account in the analysis. Each data point is one morphine/M3G measurement obtained in one mouse. PK samples were obtained at t = 30, 60, 90 and 120 min after morphine injection. (F) The ratio of the plasma concentrations morphine and M3G (morphine/M3G) versus TWL for animals treated with NTX/MOR. A negative correlation was present with rp = −0.65. Each data point is one morphine/M3G measurement obtained in one mouse. PK samples were obtained at t = 30, 60, 90 and 120 min after morphine injection.
In Figures 2C and D, the pharmacokinetic–pharmacodynamic (PK–PD)relationships between morphine, M3G and TWL are given for animals treated with saline/MOR and those treated with NTX/MOR. This PK-PD analysis shows that analgesic TWLs correlated well with morphine and M3G plasma concentrations, with linear dose-dependent increases in TWL until the cutoff value of 30 s was reached (Figure 2C). Similarly, hyperalgesic TWLs correlated well with morphine and M3G plasma concentrations with dose-dependent decreases in TWLs, which were well described by an exponential function (Figure 2D). The morphine/M3G concentration ratios versus effect (TWL) are given in Figure 2F for animals treated with NTX/MOR, showing a negative correlation (Pearson correlation coefficient, rp = −0.65, P < 0.001), indicating that higher morphine levels relative to M3G concentrations are associated with increased hyperalgesia.
Study 2
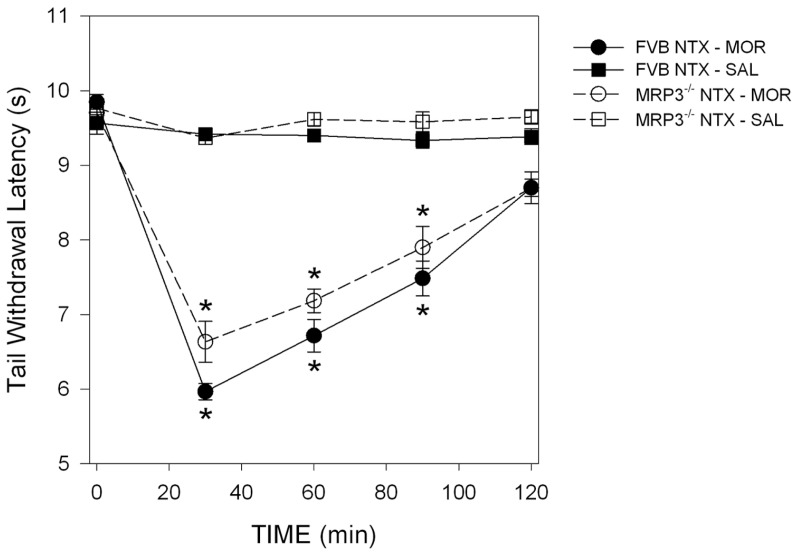
The effects of treatment on TWL in FVB and Mrp3−/− mice are given in Figure 3. NTX/saline was without effect on TWL (P = 0.44) in FVB and Mrp3−/− mice. In contrast, NTX/MOR caused hyperalgesia in both strains. In FVB mice, TWL decreased by 3.9 ± 0.2 s at t = 30 min (P < 0.001 versus baseline), and hyperalgesia was present throughout the test period (treatment effect: P < 0.001 versus NTX/ saline treatment). Similarly, in Mrp3−/−mice, TWL decreased by 3.1 ± 0.2 s at t = 30 min (P < 0.001 versus baseline) and hyperalgesia was present throughout the test period (treatment effect: P < 0.001 versus NTX/saline treatment; strain comparison: P = 0.10).
Figure 3.
Pharmacodynamic results of study 2. Effect of NTX pretreatment on morphine or saline induced changes in TWL in FVB and Mrp3−/− mice. NTX pretreatment caused morphine-induced hyperalgesia in both genotypes. The combination NTX/saline had no effect on the measured latencies. Treatment effect (NTX/MOR versus NTX/saline) is shown in FVB mice (P < 0.001) and Mrp3−/− mice (P < 0.001). Strain comparison, P = 0.10. *P < 0.001 versus baseline. Values are means ± SEM. t = 0 is the time of the morphine or saline subcutaneous injection.
Study 3
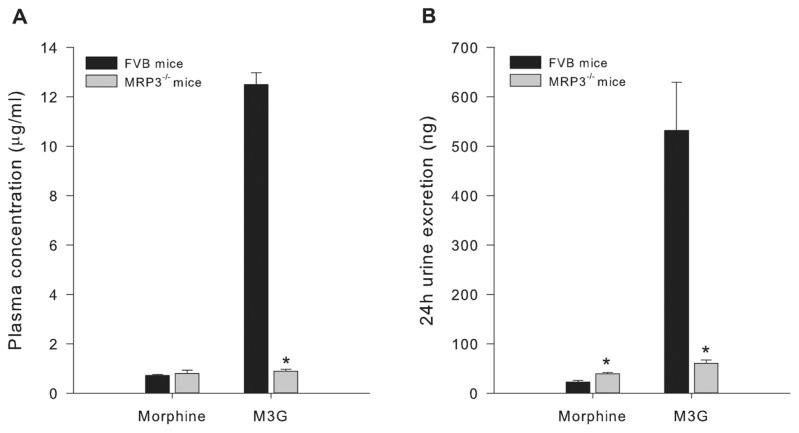
Morphine plasma concentrations 30 min after 15 mg/kg morphine did not differ between Mrp3−/− and FVB mice. In contrast, Mrp3−/− mice generated M3G plasma levels, just 7% of those measured in FVB mice: 0.9 ± 0.08 μg/mL at t = 30 min versus 12.5 ± 0.5 μg/mL (P = 0.03; Figure 4). The data also show that Mrp3−/− mice excreted more morphine in their 24-h urine than FVB mice (MRP3−/−39.2 ± 2.8 μg/24 h versus FVB 22.6 ± 3.3 μg/24 h, P = 0.003), whereas M3G excretion was significantly reduced by 88% in MRP3−/− (60.3 ± 6.9 μg/24 h) relative to FVB (532 ± 97 μg/24 h; P = 0.004) mice.
Figure 4.
Pharmacokinetic results of study 3. (A) Morphine and M3G plasma concentration in FVB and Mrp3−/− mice. (B) The 24-h morphine and M3G excretion in FVB and Mrp3−/− mice. Values are means ± SEM. *P < 0.05 versus FVB mice.
DISCUSSION
Opioids are widely used in the management of moderate to severe pain. Development of hyperalgesia was acknowledged as an important factor in the limitation of efficacy of opioid therapy in some patients. Elucidating the mechanism of hyperalgesia and identifying compounds that prevent or reverse its manifestation might result in better opioid-based interventions for treatment of pain. Over the years, several mechanisms of OIH have been proposed, including the accumulation of the pronociceptive morphine metabolite M3G (8–12), activation of the N-methyl-d-aspartate receptor (NMDAR) by morphine or M3G (16–19) and activation of TLR4 receptors by morphine or M3G (24,25). These mechanisms may not be mutually exclusive. This study focused on the possible involvement M3G in the development of OIH after morphine treatment and assessed further whether the MOR is the molecular site of OIH.
The first set of experiments that we performed showed that OIH is rapidly induced in CD-1 mice injected with morphine after pretreatment with high-dose NTX, a nonselective antagonist of the opioid receptors (Figures 1B, C). These data indicate that OIH is exposed by blocking the opioid receptors to morphine and subsequent analgesia, which confirm earlier findings showing that morphine and the phenylpiperidines (for example, fentanyl) induce OIH via non-opioid receptor–related pathways (16–19,26). Whether these hyperalgesia or excitatory pathways are activated by morphine or its major metabolite in rodents (M3G) is unknown. There is evidence from experimental and clinical studies that M3G has excitatory properties and hence may be involved in OIH after morphine treatment (8–12). To explore this further, we measured morphine and M3G concentrations in plasma of the CD-1 mice. Depending on the treatment, both compounds showed a high degree of correlation to either analgesia after saline/MOR (Figure 2C) or hyperalgesia after NTX/MOR (Figure 2D). The MOR/M3G concentration ratios versus effect (TWL), obtained in mice treated with NTX/MOR, show a negative correlation (Figure 2E), indicating that with relative greater morphine than M3G concentrations, the magnitude of hyperalgesia increases. This favors a role for morphine rather than M3G in the induction of OIH in our model.
An interesting observation in study 1 is that NTX pretreatment affected morphine’s pharmacokinetics, causing lower morphine and M3G concentrations compared with saline pretreated animals (Figures 2A, B). NTX enhances morphine glucuronidation (27), which may be explained by induction of the liver UGT enzyme system and possibly causes some greater excretion of M3G via gut and kidney. Because of these effects, the MOR/M3G concentration ratios were smaller in NTX relative to saline-pre-treated animals. Assuming a role for morphine in OIH, we may have underestimated the absolute magnitude of OIH in the NTX-pretreated animals. Irrespective, the relationship between the MOR/M3G ratios versus TWLs will not be affected. Hence, we argue that the influence of NTX on morphine’s pharmacokinetics did not affect the outcome of our studies.
We further explored the role of M3G by testing the effect of NTX/MOR in mice lacking the MRP3 gene. The MRP3 gene product, a multidrug resistance transporter present on the sinusoidal membrane of the hepatocyte, is involved in the efflux of glucuronides, including M3G, from the hepatocyte into the bloodstream (20). Mice that lack the MRP3 gene have a greatly reduced capacity to export M3G into the systemic circulation and consequently M3G accumulates in the liver, as the metabolic process in the liver (converting morphine into M3G) remains intact. Indeed, M3G pharmacokinetic analysis in Mrp3−/−mice showed low amounts of M3G in plasma (7% of control) and urine (12% of control; Figure 4) after a morphine injection. We did not measure M3G in brain tissue in the current study, but we previously were unable to detect any M3G in brain tissue of Mrp3−/− mice, 30 min after injection of the identical 15 mg/kg morphine dose injection here (20). This result suggests that the low plasma concentrations of M3G do not result in detectable concentrations of M3G in the brain of Mrp3−/− mice (see below).
The pharmacodynamic data obtained in the Mrp3−/− mice showed that, like FVB controls, they manifest morphine- induced hyperalgesia (after NTX/MOR) of substantial and significant magnitude of the same duration (Figure 3). Hence, morphine-hyperalgesia developed despite low M3G concentrations in urine, plasma and brain. These data suggest that morphine rather than M3G is the main cause of OIH in this model. Indeed, in humans and mice, after systemic administration, the morphine-glucuronides cross the blood-brain barrier poorly or not at all (5,6,20), suggesting that morphine is more likely involved in OIH than M3G. For example, in wild-type mice, after systemic M3G administration, brain concentrations remain low at levels more than 50 times less than those observed in the rest of the system (liver, plasma) (20). Nonetheless, we cannot exclude the possibility that some morphine is metabolized at central sites into M3G. Supportive data, however, suggest that these brain M3G levels are low, and, consequently, this pathway will be of minor importance (28). Further brain morphine and M3G concentration response studies are needed to quantify a possible role of central morphine metabolism on morphine-induced hyperalgesia. Irrespective, it is unlikely that M3G from the hepatic metabolism of morphine played a role in the observed morphine-induced hyperalgesia in Mrp3−/− mice.
On the basis of the present and ample previous findings (16–19,26), it is increasingly clear that opioid receptor activation is not required for development of OIH but that other signaling pathways are involved. One possibility is the excitatory glutamatergic pronociceptive pathway via activation of NMDAR (16,18,19). Activation of the NMDAR enhances signal transmission in the pain circuitry from spinal cord to the cortex leading to allodynia and hyperalgesia (29,30). A direct role of the NMDAR in OIH has been suggested (16,18,19). It is possible that morphine, or its metabolite M3G, interacts directly or indirectly with the NMDAR (31). Indeed, the neuroexcitatory effects of M3G are mediated by NMDAR receptor activity (32), and NMDA receptor antagonists dose-dependently reduce M3G symptoms, including enhanced nociception (8). Yet another possibility to explain OIH is through opioid-induced glial cell activation, causing neuroinflammation and consequently pronociception, including opposition of acute and chronic analgesia, opioid analgesic tolerance and also OIH. Recent studies indicate involvement of nonclassical opioid receptors, including the toll-like receptor 4 (TLR4) on glia cells in this process. Both morphine and M3G display significant TLR4 activity (24,25).
CONCLUSION
The role of M3G in morphine-induced hyperalgesia was extensively investigated through a series of experiments that involved both pharmacodynamic and pharmacokinetic measurements. We confirm the presence of OIH in mice injected with morphine after pretreatment with NTX and show for the first time the manifestation of morphine-induced hyperalgesia in mice lacking the MRP3 protein, despite the very low plasma M3G concentrations in these animals due to the inability to release M3G from hepatocytes. Collectively, these data suggest that morphine itself is responsible for inducing hyperalgesia through non-opioidergic pathways and that hepatic M3G is not involved in morphine hyperalgesia. The relevance of these murine data to humans has yet to be demonstrated.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349:1943–53. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- 2.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–87. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 3.van Elstraete AC, Sitbon P, Trabold F, Mazoit X, Benhamou D. A single dose of intrathecal morphine induces long-lasting hyperalgesia: the protective effect of prior administration of ketamine. Anesth Analg. 2005;101:1750–6. doi: 10.1213/01.ANE.0000184136.08194.9B. [DOI] [PubMed] [Google Scholar]
- 4.Yaksh TL, Harty GJ, Onofrio BM. High dose of spinal morphine produce a nonopiate receptor–mediated hyperesthesia: clinical and theoretic implications. Anesthesiology. 1986;64:590–7. doi: 10.1097/00000542-198605000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Christrup LL. Morphine metabolites. Acta Anaesthesiol Scand. 1997;41:116–22. doi: 10.1111/j.1399-6576.1997.tb04625.x. [DOI] [PubMed] [Google Scholar]
- 6.Sarton E, et al. Sex differences in morphine analgesia: an experimental study in healthy volunteers. Anesthesiology. 2000;93:1245–54. doi: 10.1097/00000542-200011000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Lötsch J. Pleiotropic effects of morphine-6β-glucuronide. Anesthesiology. 2009;110:1209–10. doi: 10.1097/ALN.0b013e3181a1075b. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett SE, Cramond T, Smith MT. The excitatory effects of morphine-3-glucuronide are attenuated by LY274614, a competitive NMDA receptor antagonist, and by midazolam, an agonist at the benzodiazepine site on the GABAA receptor complex. Life Sci. 1994;54:687–94. doi: 10.1016/0024-3205(94)00552-4. [DOI] [PubMed] [Google Scholar]
- 9.Labella FS, Pinsky C, Havlicek V. Morphine derivatives with diminished opiate receptor potency show enhanced central excitatory activity. Brain Res. 1979;174:263–71. doi: 10.1016/0006-8993(79)90849-7. [DOI] [PubMed] [Google Scholar]
- 10.Gong QL, Hedner J, Bjorkman R, Hedner T. Morphine-3-glucuronide may functionally antagonize morphine-6-glucuronide induced antinociception and ventilatory depression in the rat. Pain. 1992;48:249–55. doi: 10.1016/0304-3959(92)90065-J. [DOI] [PubMed] [Google Scholar]
- 11.Woolf CJ. Intrathecal high dose morphine produces hyperalgesia in the rat. Brain Res. 1981;209:491–5. doi: 10.1016/0006-8993(81)90176-1. [DOI] [PubMed] [Google Scholar]
- 12.Yaksh TL, Harty GJ, Onofrio BM. High dose of spinal morphine produce a nonopiate receptor-mediated hyperesthesia: clinical and theoretic implications. Anesthesiology. 1986;64:590–7. doi: 10.1097/00000542-198605000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Dennis GC, et al. Analgesic responses to intrathecal morphine in relation to CSF concentrations of morphine-3,β-glucuronide and morphine-6,β-glucuronide. Life Sci. 1999;64:1725–31. doi: 10.1016/s0024-3205(99)00110-1. [DOI] [PubMed] [Google Scholar]
- 14.Lipkowski AW, Carr DB, Langlade A, Osgood PF, Szyfelbein SK. Morphine-3-glucuronide: silent regulator of morphine actions. Life Sci. 1994;55:149–54. doi: 10.1016/0024-3205(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 15.Ekblom M, Gardmark M, Hammarlund-Udenaes M. Pharmacokinetics and pharmacodynamics of morphine-3-glucuronide in rats and its influence on the antinociceptive effect of morphine. Biopharm Drug Disp. 1993;14:1–11. doi: 10.1002/bdd.2510140102. [DOI] [PubMed] [Google Scholar]
- 16.Juni A, Klein G, Kest B. Morphine hyperalgesia in mice is unrelated to opioid activity, analgesia, or tolerance: evidence for multiple diverse hyperalgesic systems. Brain Res. 2006;1070:35–44. doi: 10.1016/j.brainres.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 17.Juni A, Klein G, Pintar JE, Kest B. Nociception increases during opioid infusion in opioid receptor triple knock-out mice. Neuroscience. 2007;147:439–44. doi: 10.1016/j.neuroscience.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 18.Juni A, Klein G, Kowalczyk B, Ragnauth A, Kest B. Sex differences in hyperalgesia during morphine infusion: effect of gonadectomy and estrogen treatment. Neuropharmacology. 2008;54:1264–70. doi: 10.1016/j.neuropharm.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 19.van Dorp E, et al. Morphine-6β-glucuronide rapidly increases pain sensitivity independently of opioid receptor activity in mice and humans. Anesthesiology. 2009;110:1356–63. doi: 10.1097/ALN.0b013e3181a105de. [DOI] [PubMed] [Google Scholar]
- 20.Zelcer N, et al. Mice lacking multidrug resistance protein 3 show altered morphine pharmacokinetics and decreased antinociception by morphine-6-glucuronide. Proc Natl Acad Sci U S A. 2005;102:7274–9. doi: 10.1073/pnas.0502530102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 22.Swartjes M, Morariu A, Niesters M, Aarts L, Dahan A. Non-selective and NR2B-selective NMDA receptor antagonists produce antinociception and long-term relief of allodynia in acute and neuropathic pain. Anesthesiology. 2011;115:165–74. doi: 10.1097/ALN.0b013e31821bdb9b. [DOI] [PubMed] [Google Scholar]
- 23.Rook EJ, Hillebrand MJX, Rosing H, van Ree JM, Beijnen JH. The quantitative analysis of heroin, methadone and their metabolites and the simultaneous detection of cocaine, acetylcodeine and their metabolites in human plasma by high-performance liquid chromatography coupled with tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;824:213–21. doi: 10.1016/j.jchromb.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, et al. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci U S A. 2012;109:6325–30. doi: 10.1073/pnas.1200130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutchinson MR, et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24:83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waxman AR, Arout C, Caldwell M, Dahan A, Kest B. Acute and chronic fentanyl administration causes hyperalgesia independently of opioid receptor activity in mice. Neurosci Lett. 2009;462:68–72. doi: 10.1016/j.neulet.2009.06.061. [DOI] [PubMed] [Google Scholar]
- 27.Antonilli L, Petecchia E, Capriolo D, Badiani A, Nencini P. Effect of repeated administrations of heroin, naltrexone, methadone, and alcohol on morphine glucuronidation in the rat. Psychopharmacology. 2005;182:58–64. doi: 10.1007/s00213-005-0030-7. [DOI] [PubMed] [Google Scholar]
- 28.Wahlström A, Winblad B, Bixo M, Rane A. Human brain metabolism of morphine and naloxone. Pain. 1988;35:121–7. doi: 10.1016/0304-3959(88)90219-9. [DOI] [PubMed] [Google Scholar]
- 29.Chizh BA. Low dose ketamine: a therapeutic and research tool to explore N-methyl-D-aspartate (NMDA) receptor-mediated plasticity in pain pathways. J Psychopharmacol. 2007;21:259–71. doi: 10.1177/0269881105062484. [DOI] [PubMed] [Google Scholar]
- 30.Sigtermans M, et al. Ketamine produces effective and long-term pain relief in patients with Complex Regional Pain Syndrome Type 1. Pain. 2009;145:304–11. doi: 10.1016/j.pain.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 31.Sánchez-Blázquez P, Rodríguez-Munoz M, Garzón J. Mu-opioid receptors transiently activate the Akt-nNOS pathway to produce sustained potentiation of PKC-mediated NMDAR-CaMKII signaling. PLoS One. 2010;5:311278. doi: 10.1371/journal.pone.0011278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hemstapat K, Monteith GR, Smith D, Smith MT. Morphine-3-glucuronide’s neuro-excitatory effects are mediated via indirect activation of N-methyl-D-aspartic acid receptors: mechanistic studies in embryonic cultured hippocampal neurons. Anesth Analg. 2003;97:494–505. doi: 10.1213/01.ANE.0000059225.40049.99. [DOI] [PubMed] [Google Scholar]