Abstract
Lipopolysaccharide (LPS) is ubiquitous in the environment. Inhalation of LPS has been implicated in the pathogenesis and/or severity of several lung diseases, including pneumonia, chronic obstructive pulmonary disease and asthma. Alveolar macrophages are the main resident leukocytes exposed to inhaled antigens. To obtain insight into which innate immune pathways become activated within human alveolar macrophages upon exposure to LPS in vivo, we conducted a study in eight healthy humans, in which we instilled sterile saline into a lung segment by bronchoscope, followed by instillation of LPS into the contralateral lung. Six hours later, a bilateral bronchoalveolar lavage was performed and whole-genome transcriptional profiling was done on purified alveolar macrophages, comparing cells exposed to saline or LPS from the same individuals. LPS induced differential expression of 2,932 genes in alveolar macrophages; 1,520 genes were upregulated, whereas 1,440 genes were downregulated. A total of 26 biological functions were overrepresented in LPS-exposed macrophages; 44 canonical pathways affected by LPS were identified, among which the genes associated with the role of pattern recognition receptors in recognition of bacteria and viruses represented the top pathway. Other pathways included cellular immune response, signaling by tumor necrosis factor (receptor) family members, cytokine signaling and glucocorticoid receptor signaling. These results reveal for the first time a large number of functional pathways influenced by the biologically relevant challenge provided by LPS administered into the airways. These data can assist in identifying novel targets for therapeutic intervention in pulmonary diseases associated with LPS exposure, including pneumonia, asthma and chronic obstructive pulmonary disease.
INTRODUCTION
Lipopolysaccharide (LPS) is a proinflammatory constituent of the gram-negative bacterial cell wall (1). LPS is ubiquitous in the environment and is often present in high concentrations in organic dust, waste, air pollution and cigarette smoke (2). Inhalation of LPS has been implicated in the pathogenesis and/or severity of several lung diseases, including chronic obstructive pulmonary disease and asthma (3). In addition, pulmonary effects of LPS contribute to the acute lung inflammation that accompanies gram-negative pneumonia (4). LPS initiates an innate immune response in cells through stimulation of the Toll-like receptor (TLR)-4–myeloid differentiation protein 2 (MD2) receptor complex (1,5).
Our laboratory and others have used the model of bronchial instillation of LPS in healthy volunteers to investigate the induction of inflammation and coagulation in the human lung (6–10). Administration of LPS into a lung subsegment results in a brisk influx of neutrophils into the bronchoalveolar space, accompanied by local release of proinflammatory cytokines and chemokines as well as local activation of the coagulation system (6–10). Moreover, LPS activates alveolar macrophages in vivo, as reflected by enhanced expression of 10 different mRNAs encoding proinflammatory cytokines and chemokines (9). The current study was conducted to obtain detailed insight into which innate immune pathways become activated within human alveolar macrophages upon exposure to LPS in vivo. Therefore, we performed whole-genome profiling of purified alveolar macrophages harvested 6 h after an intrabronchial challenge with LPS, by using macrophages obtained from a contralateral lung segment challenged with normal saline as controls.
MATERIALS AND METHODS
Subjects
Eight nonsmoking men (age 21.3 ± 0.6 years, mean ± standard error of the mean [SEM]) were recruited by advertising. Screening, consisting of a questionnaire, physical examination, routine blood and urine investigation (including drug screen), electrocardiogram and spirometry, did not reveal any abnormality. Smoking was ruled out by history. The subjects were part of a simultaneous study investigating the inflammatory response upon bronchial instillation of LPS or lipoteichoic acid in the human lung (9). The study was approved by the institutional ethics and research committees of the Academic Medical Center, University of Amsterdam. Written informed consent was obtained from all subjects before study enrollment.
Study Design
Ten milliliters of sterile saline was instilled into a lung subsegment (either the right middle lobe or lingula) followed by instillation of LPS (in 10 mL saline) from Escherichia coli (U.S. Pharmacopeial Convention, Lot G, Bureau of Biologics, U.S. Food and Drug Administration, Rockville, MD, USA) into the contralateral lung, by using a flexible video bronchoscope. The subjects were randomized to left or right lungs for saline or LPS instillation. The subjects received LPS at a dose of 4 ng/kg body weight. A bilateral bronchoalveolar lavage was performed 6 h postchallenge; seven successive 20-mL aliquots of prewarmed 0.9% saline were instilled in the saline-challenged subsegment of the lung, and each was aspirated immediately with low suction. This procedure was repeated in the LPS-challenged subsegment of the contralateral lung. In each subject, both bronchoscopies were performed by the same experienced pulmonologist.
Isolation of Alveolar Macrophages
Alveolar macrophages were isolated from bronchoalveolar lavage fluid (BALF) by using CD71 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) as described (9). Briefly, BALF was immediately centrifuged for 10 min at 300g at 4°C to pellet the cells. Cells were resuspended in ice-cold sterile phosphate-buffered saline, supplemented with 0.5% bovine serum albumin and 2 mmol/L ethylenediaminetetraacetic acid (EDTA), pH 7.4, and were passed over a 40-μm cell strainer to obtain a single cell suspension. Subsequently, cells were incubated for 15 min with CD71 microbeads (Miltenyi Biotec) at 4°C and separated by magnetic sorting using an autoMACS (Miltenyi Biotec). Purity of alveolar macrophages was determined by Giemsa-stained cytospins; CD71+ cells obtained from the saline-treated lung segment were 99.9 ± 0.1% alveolar macrophages (mean ± SEM); CD71+ cells obtained from the LPS-treated lung segment were 94.9 ± 1.5% alveolar macrophages. After isolation, alveolar macrophages were dissolved in RNeasy lysis buffer (Qiagen) and stored at −80°C until use for RNA isolation.
Microarray Analyses
RNA was isolated using an RNeasy Mini Kit (Qiagen). The quality of RNA was determined with the Bioanalyzer® 2100 (Agilent Technologies, Waldbronn, Germany), following the manufacturer’s protocol. cDNA was synthesized from 50 ng total RNA using the WT-Ovation™ System (NuGEN, San Carlos, CA, USA) powered by Ribo-SPIA™ technology. Fragmented cDNA was end-labeled with a biotin-conjugated nucleotide analog (DLR-1a; Affymetrix, Santa Clara, CA, USA) by using terminal transferase (Roche Diagnostics, Mannheim, Germany). Fragmented and labeled cDNA was hybridized for 18 h at 50°C in a hybridization solution containing 7% dimethyl sulfoxide. Hybridization was performed using GeneChip® Human Genome U133 Plus 2.0 arrays (Affymetrix), containing 54,675 probe sets corresponding to 38,500 identified genes. After washing, chips were stained with streptavidin-phycoerythrin according to the Affymetrix EukGE-WS2v4 protocol using the Fluidic FS450 station. The microarrays were read with the GeneChip Scanner 3000 (Affymetrix). Affymetrix Gene-Chip Operating Software, version 1.4 (GCOS), was used to manage Affymetrix GeneChip array data and to automate the control of GeneChip fluidics stations and scanners.
Data Analysis
Data processing
Expression data were generated using the robust multi-array average (RMA) method (11) implemented in the Affy package of the Bioconductor microarray analysis environment (http://www.bioconductor.org). The RMA method consists of three steps: background adjustment, quantile normalization (12) and probe set summary of the log-normalized data applying a median polishing procedure. Before the analysis of differentially expressed genes, a filter was applied to minimize the technical noise. In both groups, the genes with an expression level under a threshold value corresponding to the overall median value from all microarrays were removed. A linear model for microarray, as implemented in the LIMMA package available on the Bioconductor environment, was selected to identify differentially expressed genes (13). This approach was based on a moderated paired t test that allows taking into account patient effects in the linear model. The Benjamini and Hocherg (B-H) approach was used to correct for multiple testing with a level of significance fixed at 0.01. Thus, genes with a corrected p value <0.01 were considered differentially expressed. Spotfire Decision Site 8.2.1 for Functional Genomics software (Spotfire, Gothenburg, Sweden) was used to generate and visualize the hierarchical clusters of the samples, measuring similarity of gene expression and across arrays. Principal component analysis was performed by using the Partek® Genomics Suite™ (Partek, St. Louis, MO, USA).
Functional Enrichment and Canonical Pathway Analyses
Functional enrichment and canonical pathway analyses were performed to define overrepresentation of biological functional categories and canonical pathways of the selected genes. Functional and canonical pathway analyses of specific genes coming from statistical analysis were performed by using Ingenuity Pathway Analysis (Ingenuity Systems, Redwood City, CA, USA). The B-H multiple testing correction p value test was used to calculate the p value for determining the probability that each biological function or canonical pathway assigned to the dataset was due to chance alone. A p value <0.01 was applied in all calculations, and the Human Genome U133 Plus 2.0 array was used as the reference when ranking the statistical significance of functions or canonical pathways.
All supplementary materials are available online at www.molmed.org.
RESULTS
Clinical Signs
As reported earlier (9), intrabronchial LPS administration was associated with a modest rise in body temperature (0.7 ± 0.2°C) without clear symptoms.
Differential Gene Expression Induced by LPS
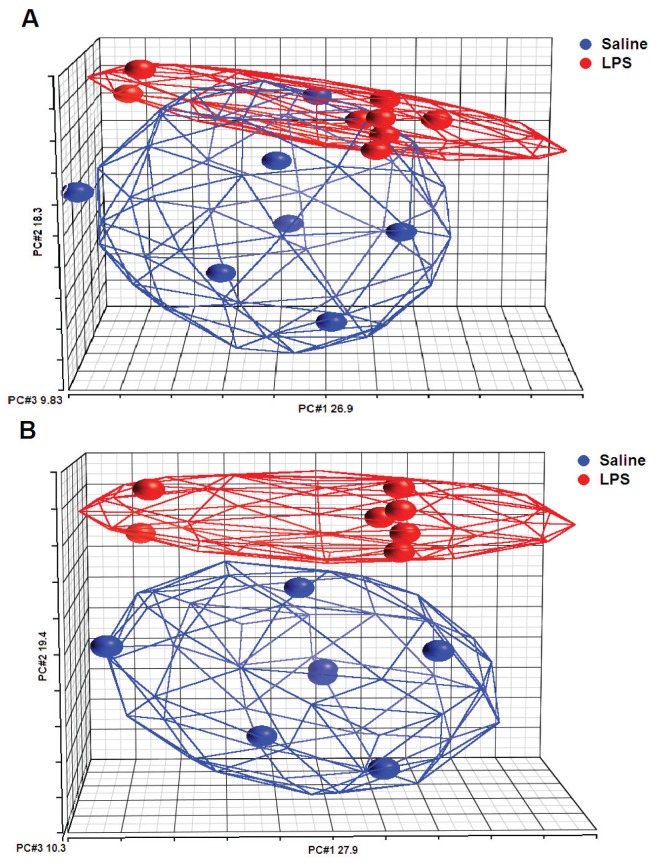
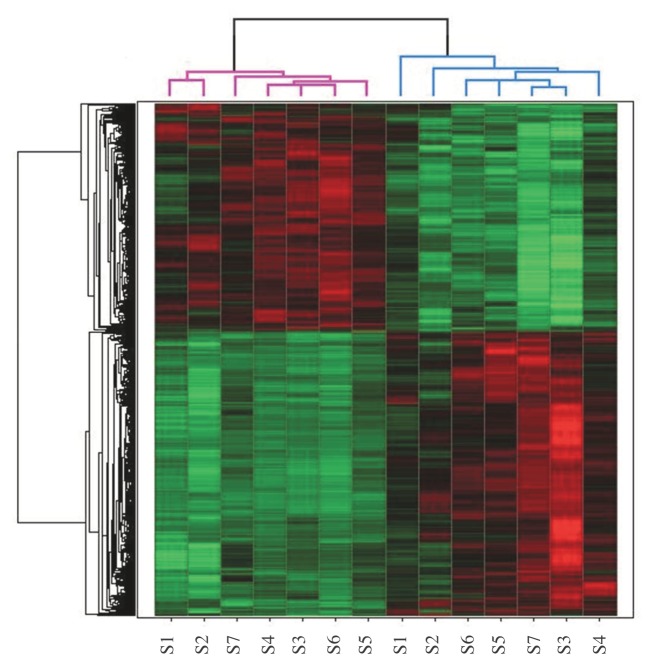
To investigate the effect of LPS instillation in the human lung in vivo on gene expression in alveolar macrophages, we conducted a study in which eight healthy subjects were challenged with LPS into a lung subsegment via a bronchoscope; a contralateral lung subsegment, challenged with saline, served as an intra-individual control. We previously determined mRNA expression of 40 targeted genes encoding inflammatory mediators in alveolar macrophages by multiplex ligation-dependent probe amplification; 10 of these genes were found to be upregulated (9). This analysis revealed one outlier among the eight LPS-challenged study subjects, who did not show the clear inflammatory gene expression, as detected in the other seven subjects. Here, we first performed principal component analysis to examine the relationship between LPS and saline sample profiles, by using data generated by whole genome arrays. This analysis clearly identified the discordant subject previously detected by multiplex ligation-dependent probe amplification (Figure 1A). Principal component analysis after exclusion of this single subject revealed that LPS and saline samples were clearly separated, describing overall changes in apparent gene expression (Figure 1B). On the basis of these analyses and because the primary objective of the current study was to identify pathways induced by LPS in alveolar macrophages in vivo, we excluded this discordant subject from the current analysis. The most likely explanation for this discordant subject is a technical error (that is, lavage of an adjacent lung subsegment during the second bronchoscopy). In the remaining seven subjects, among the 54,675 probes sets evaluated, we identified 4,807 probe sets (2,932 unique genes) that were differentially expressed in alveolar macrophages harvested from the lung subsegments challenged either with LPS or with normal saline (p value <0.01). A total of 2,669 probe sets (1,520 unique genes) were upregulated by LPS (Supplementary Table S1), whereas 2,138 probe sets (1,440 unique genes) were downregulated (Supplementary Table S2). The hierarchical cluster diagram demonstrated that expression profiles obtained after LPS and saline instillation are distinctly different (Figure 2). To validate the data generated by microarray analysis, we compared the expression of a number of genes with our previously reported mRNA expression levels measured by multiplex ligation-dependent probe amplification (9). In this earlier investigation, we reported that bronchial instillation of LPS resulted in enhanced alveolar macrophage expression of mRNAs encoding the proinflammatory mediators interleukin (IL)-6, IL-8, IL-1α, IL-1β, IL-1RA, monocyte chemoat-tractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1α and MIP-1β; moreover, LPS upregulated the expression of mRNAs encoding proinflammatory signaling factors IκBα and nuclear factor (NF)-κB subunit 1 (9). All of these changes were also detected in the current microarray analysis (Supplementary Table S1).
Figure 1.
Principal component (PC) analysis mapping of the samples from the whole microarray data. The color indicates the different groups (blue: saline samples; red: LPS samples) and ellipsoids are drawn around each group. (A) From the eight subjects, the total variation explained by PCs 1, 2 and 3 is 55%. Overlap is formed around saline and LPS samples. (B) From the seven subjects, the total variation explained by PCs 1, 2 and 3 is 57.5%. Clear clusters are formed around saline and LPS samples.
Figure 2.
Unsupervised hierarchical clustering of gene expression profiles in alveolar macrophages obtained from seven individuals (S) subject to saline instillation into a lung subsegment and LPS into the contralateral lung. The hierarchical clustering analysis clearly distinguished the expression patterns between saline (purple dendrogram) and LPS (blue dendrogram) samples. Each row represents a gene; each column shows the expression of 4,807 probe sets (2,932 unique genes) expressed by each sample. Red indicates genes that are expressed at higher levels, and green indicates genes that are expressed at lower levels.
Functional Clusters Induced by LPS
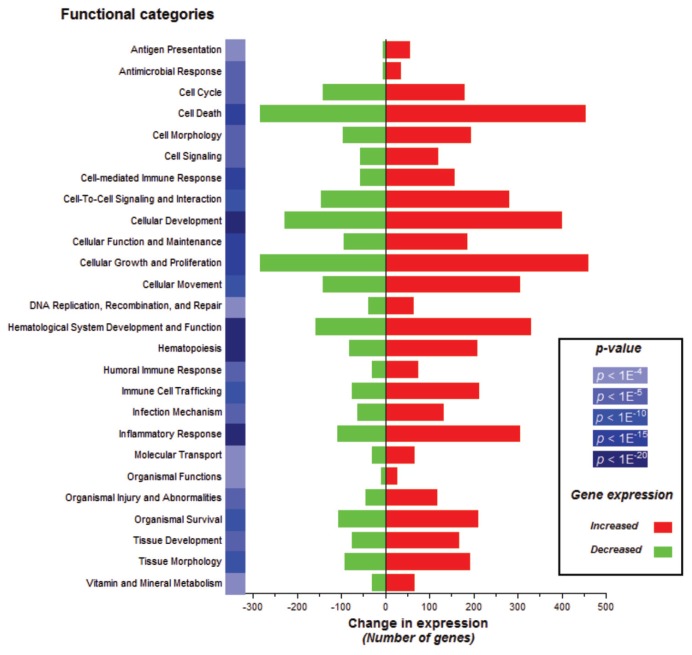
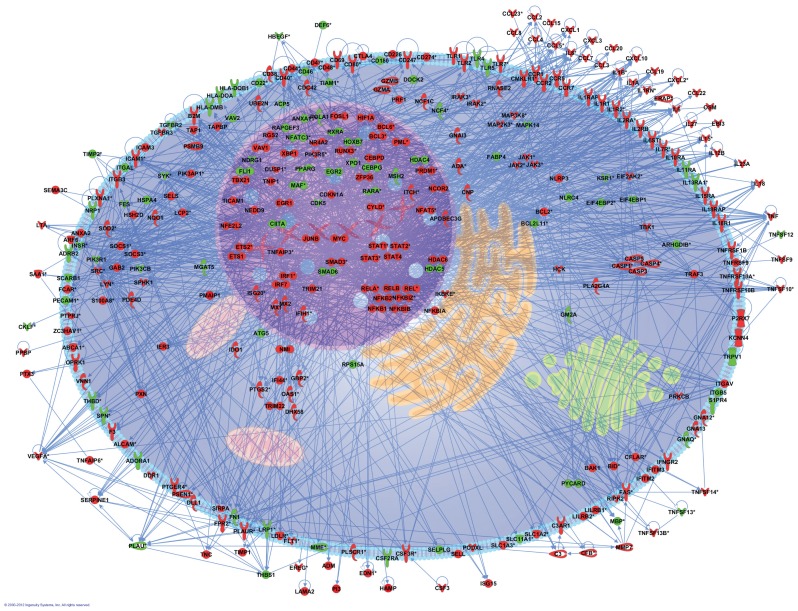
To obtain insight into the biological processes elicited by in vivo LPS administration into the human lung, the list of differentially expressed genes was submitted to the Ingenuity Pathway Analysis. The 2,932 genes were subjected to gene function enrichment analysis to determine which main functional categories were affected by LPS. A total of 26 biological functions were identified at the 0.01 significance level to be overrepresented (Figure 3). One of the most significant related functions identified (p value <1E−20) was represented by genes implicated in the inflammatory response in which eight genes were validated by multiplex ligation-dependent probe amplification (9). It was found that this main functional category represented the following subprocesses (with enrichment scores in parentheses): immune response (1.48E−22), inflammation (4.35E−12 to 2.18E−4), activation (4.48E−11 to 2.53E−9), cell movement (1.34E−9 to 8.32E−8), infiltration (4.37E−7), accumulation (7.97E−7 to 1.19E−4), chemo-taxis (6.87E−7 to 1.17E−4) and recruitment (1.21E−5 to 2.14E−4). To better understand the inflammatory response mechanisms implicated in macrophages after stimulation by LPS, we proposed a cell model composed of 331 genes presenting direct interactions (Figure 4).
Figure 3.
Selected gene classifications according to biological functions. Significant regulated genes (t test false discovery rate [FDR] 0.01; n = 2,932 unique genes) from LPS samples versus saline samples were groups according to the biological functions (p < 0.01) in over- or underexpression output list. The change in expression for each function is depicted in red for upregulation and green for downregulation.
Figure 4.
Pathways analysis of representative genes involved in inflammatory response. An inflammatory cell model was constructed from 331 genes involved in inflammatory response. The intensity of the node color indicated the degree of upregulation (red) or downregulation (green).
Functional Pathways Induced by LPS
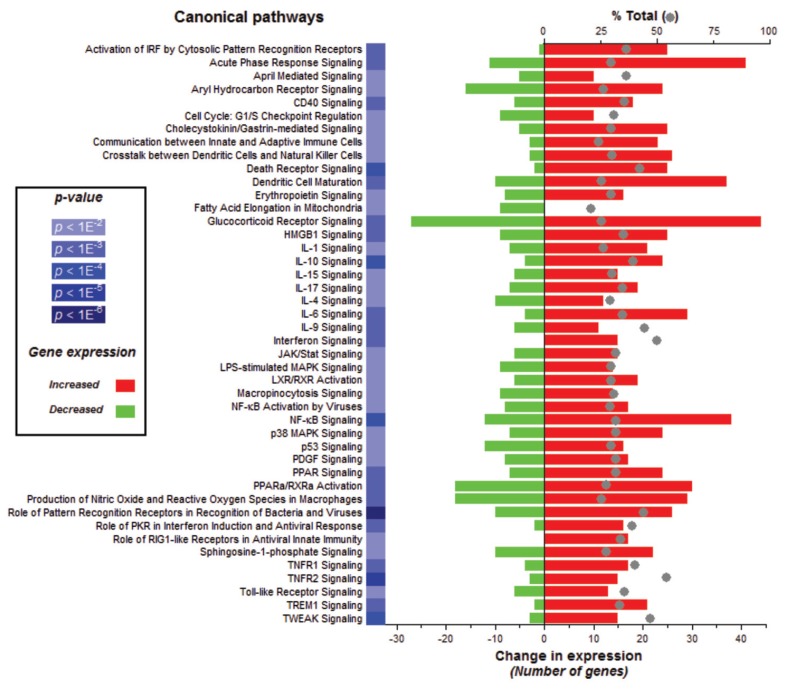
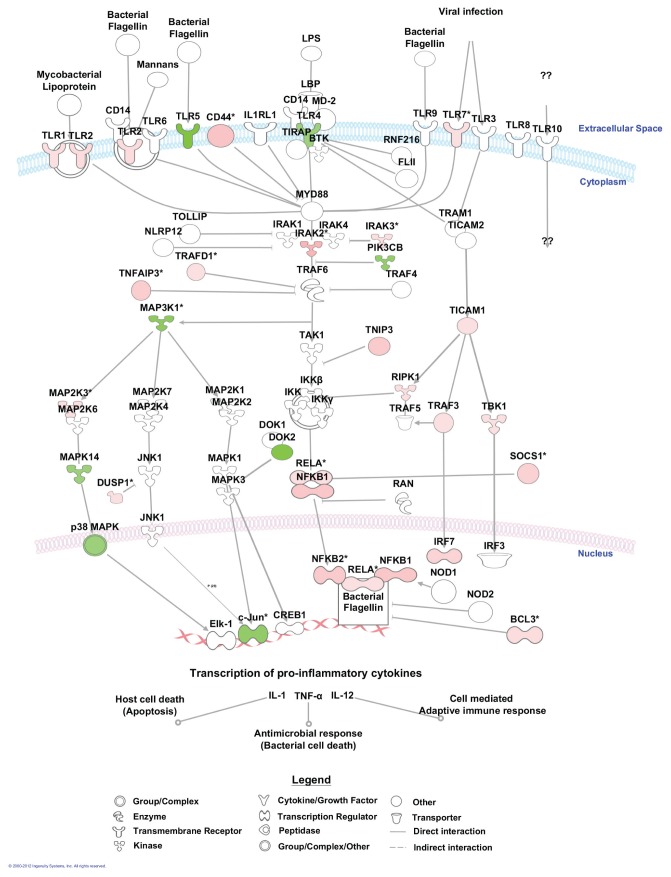
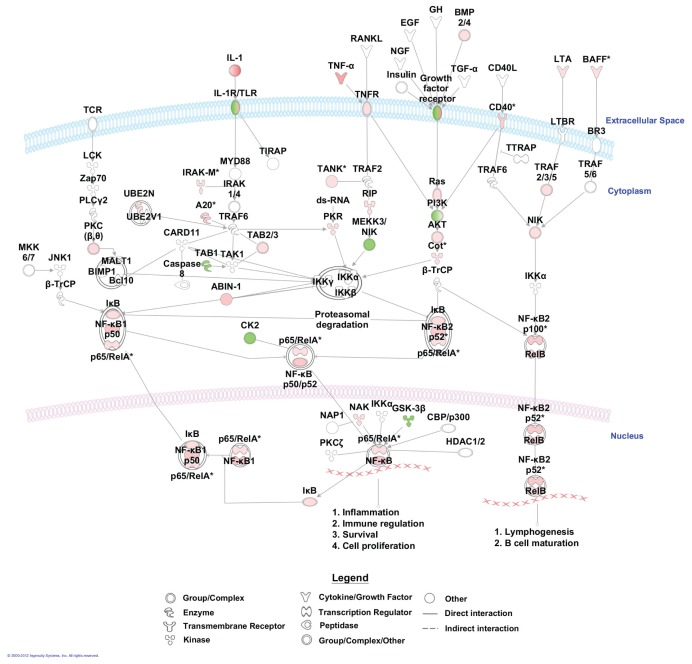
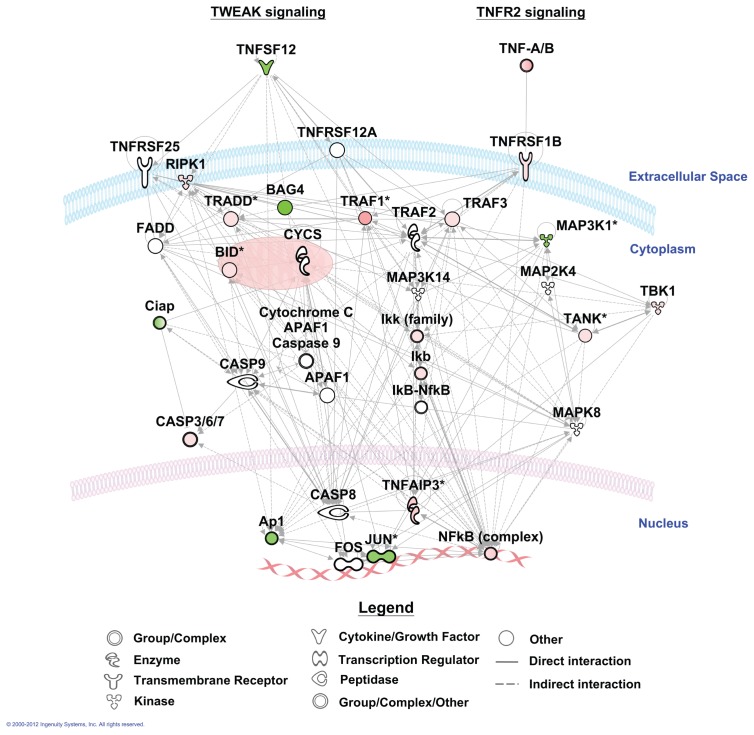
To further elucidate the effect of LPS on alveolar macrophage gene expression, we conducted pathway analyses. There were 44 canonical pathways with a B-H multiple testing correction p value <0.01 (Figure 5). The genes associated with the role of pattern recognition receptors in recognition of bacteria and viruses were the top pathway (p value 3.16E−10). The genes associated with these two top pathways and more specifically those linked to TLR signaling are mapped to the pathway (Figure 6). Moreover, NF-κB signaling, the central node in the connecting genes related to inflammation and immune response, was highly enriched (p value 1.23E−7; Figure 7). To summarize, pathways corresponding to pathogen-influenced signaling (role of pattern recognition receptors in recognition of bacteria and viruses, TLR signaling, production of nitric oxide and reactive oxygen species in macrophages, role of protein kinase R (PKR) in interferon induction and antiviral response, role of retinoic acid–inducible gene 1 (RIG1)-like receptors in antiviral innate immunity, LPS-stimulated mitogen-activated protein kinase [MAPK] signaling), cellular immune response (NF-κB, macropinocytosis, high mobility group protein box 1 [HMGB1], triggering receptor expressed on myeloid cells 1 [TREM1] and interferon signaling), cytokine signaling (IL-1, IL-4, IL-6, IL-9, IL-10, IL-15 and IL-17 signaling) and inflammation (TNF receptor type II [TNFR2] and TWEAK signaling [Figure 8], glucocorticoid receptor signaling, signaling, CD40 signaling, liver X receptor [LXR]/retinoid X receptor [RXR] activation) were prominently represented.
Figure 5.
Selected gene classifications according to biological canonical pathways. Significant regulated genes (t test false discovery rate [FDR] 0.01; n = 2,932 unique genes) from LPS samples versus saline samples were grouped according to the canonical pathways (p < 0.01) in over- or underexpression output list. The change in expression for each pathway is depicted in red for upregulation and green for downregulation. The percent of total (displayed with a gray ball) is based on the number of significantly changed genes out of the total number of genes assigned to each canonical pathway. PPAR, peroxisome proliferator–activated receptor. IRF, IFN regulatory factor; PDGF, platelet-derived growth factor.
Figure 6.
Part of the top canonical pathway affected by in vivo LPS administration into the human lung. From the pathway, for the role of pattern recognition receptors in recognition of bacteria and viruses, we focused specifically on TLR signaling. The intensity of colors indicates the degree of upregulation (red) or downregulation (green).
Figure 7.
Mapping of significant genes to NF-κB signaling. Genes colored in red are upregulated, and genes colored in green are downregulated.
Figure 8.
Mapping of significant genes to TWEAK and TNF receptor type 2 signaling. Genes colored in red are upregulated, and genes colored in green are downregulated.
DISCUSSION
Genome-wide transcriptional profiling affords a comprehensive insight into the effect of a disease or biological challenge in humans. Because comorbidity, disease severity and therapeutic interventions influence the expression of genes during disease, several investigators have used experimental inflammation models in healthy humans to study alterations in gene expression profiles under controlled conditions. In particular, a number of studies have examined the effect of intravenous LPS administration on genome-wide gene expression profiles in peripheral blood leukocytes in humans (14–17). Our laboratory previously analyzed the expression of 40 genes implicated in inflammation in alveolar macrophages from healthy human subjects challenged with LPS via a bronchoscope (9). The current investigation is the first to examine gene expression profiles in human alveolar macrophages exposed to LPS in vivo by using an unbiased approach. We found that 6 h after LPS administration into a lung subsegment, 2,932 genes were differentially expressed in alveolar macrophages when compared with cells harvested from the contralateral lung from the same individuals. A total of 1,520 genes were upregulated by LPS, whereas 1,440 genes were downregulated. A total of 26 biological functions were overrepresented in LPS-exposed macrophages, and 44 canonical pathways affected by LPS were identified, among which the genes associated with the role of pattern recognition receptors in recognition of bacteria and viruses represented the top pathway.
Of the many types of macrophages found in the body, alveolar macrophages are in contact with external stimuli most frequently. As such, alveolar macrophages play a key role in immune surveillance of the lung. LPS is a relevant biological stimulus to study the pathogenesis of inflammatory lung disease, considering its ubiquitous presence in the environment and its presumptive role in many different pulmonary disorders, including acute and chronic obstructive lung disease and pneumonia (2–4). The present data were obtained at only one time point after LPS instillation; the invasive procedure required to challenge the airways of healthy humans in vivo and to harvest alveolar macrophages precluded a more detailed kinetic analysis. The 6-h time point was used previously by our laboratory and other laboratories to evaluate the early inflammatory response to LPS in the healthy human lung (6,9,10,18,19). To the best of our knowledge, only one previous study examined the kinetics of inflammatory responses after bronchial LPS instillation, harvesting BALF 2, 6, 24 or 48 h after LPS administration (6). This investigation showed that proinflammatory cytokine and chemokine levels peak 6 h after LPS, whereas at later time points, most inflammatory mediators return to baseline and mostly undetectable levels within the bronchoalveolar space (6). In this first 6-h period, neutrophil influx is initiated, peaking after 24 h. The 6-h time point used in the current investigation is primarily based on this earlier kinetic study (6). Although we consider it likely that this time point is most representative of the early inflammatory response to LPS in the bronchoalveolar space, we cannot exclude that some responses will be missed and/or are representative for resolution of inflammation. In our opinion, our data cannot be compared with an earlier study reporting the kinetics of gene expression profile changes in whole blood leukocytes after intravenous LPS injection (14–17): inflammatory changes, including proinflammatory cytokine release, occur much faster in the intravenous challenge model, and gene expression profiles were examined in unseparated whole blood leukocytes with a strongly variable composition depending on the time point after LPS. Considering that LPS stimulates macrophages by interacting with MD2 and subsequently activating TLR4 (5), our results provide insight into acute TLR4-specific activation of alveolar macrophages in their natural environment.
A previous investigation examined whole genome gene expression profiles in neutrophils harvested from blood and the bronchoalveolar space of human volunteers exposed to LPS by bronchoscopic instillation 16 h earlier (20). Clear gene expression differences were apparent between air space and circulating neutrophils: approximately 15% of expressed genes had altered expression levels, including broad increases in inflammatory-and chemotaxis-related genes, as well as antiapoptotic pathways, suggesting that neutrophils sequestered in the lung become fundamentally different from those resident in the circulation. Whereas this latter study focused on genomic changes in human neutrophils induced by alveolar transmigration after local administration of LPS, we determined the influence of intrabronchial LPS on gene expression in the main resident leukocyte within the bronchoalveolar space, the alveolar macrophage. To the best of our knowledge, whole genome gene expression profiling has not been done on purified resident lung cells previously. One study compared protein markers of lung inflammation in BALFs obtained from healthy subjects challenged with LPS via a bronchoscope (that is, similar to the challenge used in our study) and from patients with acute respiratory distress syndrome by using proteomics (21). It was noted that acute respiratory distress syndrome patients had protein expression patterns similar to those observed 6 h after intrabronchial LPS administration (21), suggesting that similar pathways become activated in these two conditions and that the model used here may have relevance for human disease.
Among canonical pathways altered by LPS, the most significant effect was seen on the pathway implicated in recognition of bacteria and viruses by pattern recognition receptors. The vast majority of genes examined in the arrays demonstrated enhanced expression, indicating that LPS stimulates gene pathways in alveolar macrophages facilitating pathogen recognition early after entering the bronchoalveolar space. Likewise, LPS clearly affected multiple genes connected with NF-κB activation. In accordance, innate immune recognition is a major biological function of alveolar macrophages in various conditions (22).
Of considerable interest, TWEAK (tumor necrosis factor [TNF]-like weak inducer of apoptosis) signaling was among the most clearly LPS-induced canonical pathways. Indeed, the expression of >50% of all genes involved in TWEAK signaling included in the microarray was altered by LPS, the majority of which was upregulated. This percentage was even higher for genes implicated in signaling of TNF receptor type II, which at least in part relates to overlapping signaling cascades upon activation of TWEAK and TNF receptor type II (see Figure 8). TWEAK (also known as TNFSF12, APO3L and CD255) and its receptor Fn14 (fibroblast growth factor-inducible 14, also known as TNFRSF12A, TWEAKR and CD266) are members of the TNF and TNF receptor super families, respectively (23). The TWEAK–Fn14 axis interaction regulates many cellular activities including proliferation, migration, differentiation, apoptosis, angiogenesis and inflammation (23). Of note, mRNA expression of TWEAK was reduced in LPS exposed alveolar macrophages (Supplementary Table S2), which contrasts with data obtained in THP-1 monocytic cells in vitro, in which LPS was reported to induce TWEAK mRNA expression (24). Considering the predominant enhancement of TWEAK signaling, these data suggest that our analysis (conducted 6 h after LPS instillation) may have missed an earlier upregulation of TWEAK gene expression. TWEAK induced various proinflammatory mediators in THP-1 cells, including IL-6, MCP-1, IL-8 and matrix metalloproteinase 9 (MMP-9) (25), whereas TWEAK stimulated human bronchial epithelial cells to produce IL-8 and granulocyte-macrophage colony-stimulating factor (GM-CSF) through Fn14 (26). Although the functional role of TWEAK-Fn14 in the lung in vivo has not been studied thus far, these results suggest that TWEAK/Fn14 interaction may play a role in airway inflammatory responses. In accordance with this hypothesis, in injury models involving other organs, the TWEAK-Fn14 axis indeed seems to play an important beneficial role in tissue repair after acute injury (23). Of note, the expression of TNFRSF1B (the type II TNF receptor) was upregulated upon LPS exposure, which differs from findings on reduced expression of this receptor on circulating monocytes upon intravenous LPS challenge (27).
CONCLUSION
Alveolar macrophages represent the major resident hematopoietic cell in the bronchoalveolar space. Together with respiratory epithelial cells, alveolar macrophages orchestrate the immediate response to pathogens and pollutants that reach the lower respiratory tract. Here, we report for the first time the effects of locally instilled LPS on whole genome gene expression profiles in alveolar macrophages in vivo, revealing a large number of functional pathways influenced by this clinically relevant challenge. Our data can assist in identifying novel targets for therapeutic intervention in pulmonary diseases associated with LPS exposure, including pneumonia, asthma and chronic obstructive pulmonary disease.
Supplemental Data
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol. 2003;2:169–76. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- 2.Rylander R. Endotoxin in the environment: exposure and effects. J Endotoxin Res. 2002;8:241–52. doi: 10.1179/096805102125000452. [DOI] [PubMed] [Google Scholar]
- 3.Michel O. Role of lipopolysaccharide (LPS) in asthma and other pulmonary conditions. J Endotoxin Res. 2003;9:293–300. doi: 10.1179/096805103225002539. [DOI] [PubMed] [Google Scholar]
- 4.Mizgerd JP. Acute lower respiratory tract infection. N Engl J Med. 2008;358:716–27. doi: 10.1056/NEJMra074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishii KJ, Koyama S, Nakagawa A, Coban C, Akira S. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe. 2008;3:352–63. doi: 10.1016/j.chom.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 6.O’Grady NP, et al. Local inflammatory responses following bronchial endotoxin instillation in humans. Am J Respir Crit Care Med. 2001;163:1591–8. doi: 10.1164/ajrccm.163.7.2009111. [DOI] [PubMed] [Google Scholar]
- 7.Nick JA, et al. Recombinant human activated protein C reduces human endotoxin-induced pulmonary inflammation via inhibition of neutrophil chemotaxis. Blood. 2004;104:3878–85. doi: 10.1182/blood-2004-06-2140. [DOI] [PubMed] [Google Scholar]
- 8.van der Poll T, Levi M, Nick JA, Abraham E. Activated protein C inhibits local coagulation after intrapulmonary delivery of endotoxin in humans. Am J Respir Crit Care Med. 2005;171:1125–8. doi: 10.1164/rccm.200411-1483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoogerwerf JJ, et al. Lung inflammation induced by lipoteichoic acid or lipopolysaccharide in humans. Am J Respir Crit Care Med. 2008;178:34–41. doi: 10.1164/rccm.200708-1261OC. [DOI] [PubMed] [Google Scholar]
- 10.Hoogerwerf JJ, et al. Activation of coagulation and inhibition of fibrinolysis in the human lung on bronchial instillation of lipoteichoic acid and lipopolysaccharide. Crit Care Med. 2009;37:619–25. doi: 10.1097/CCM.0b013e31819584f9. [DOI] [PubMed] [Google Scholar]
- 11.Irizarry RA, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–64. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 12.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–93. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 13.Smyth GK. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004;3:Article3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 14.Calvano SE, et al. A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–7. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 15.Talwar S, et al. Gene expression profiles of peripheral blood leukocytes after endotoxin challenge in humans. Physiol Genomics. 2006;25:203–15. doi: 10.1152/physiolgenomics.00192.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haimovich B, et al. A novel model of common Toll-like receptor 4- and injury-induced transcriptional themes in human leukocytes. Crit Care. 2010;14:R177. doi: 10.1186/cc9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotz KT, et al. Clinical microfluidics for neutrophil genomics and proteomics. Nat Med. 16:1042–7. doi: 10.1038/nm.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maris NA, et al. Activation of coagulation and inhibition of fibrinolysis in the lung after inhalation of lipopolysaccharide by healthy volunteers. Thromb Haemost. 2005;93:1036–40. doi: 10.1160/TH04-08-0492. [DOI] [PubMed] [Google Scholar]
- 19.Maris NA, et al. Antiinflammatory effects of salmeterol after inhalation of lipopolysaccharide by healthy volunteers. Am J Respir Crit Care Med. 2005;172:878–84. doi: 10.1164/rccm.200503-451OC. [DOI] [PubMed] [Google Scholar]
- 20.Coldren CD, et al. Functional and genomic changes induced by alveolar transmigration in human neutrophils. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1267–76. doi: 10.1152/ajplung.00097.2006. [DOI] [PubMed] [Google Scholar]
- 21.de Torre C, Ying SX, Munson PJ, Meduri GU, Suffredini AF. Proteomic analysis of inflammatory biomarkers in bronchoalveolar lavage. Proteomics. 2006;6:3949–57. doi: 10.1002/pmic.200500693. [DOI] [PubMed] [Google Scholar]
- 22.Opitz B, van Laak V, Eitel J, Suttorp N. Innate immune recognition in infectious and non-infectious diseases of the lung. Am J Respir Crit Care Med. 2010;181:1294–309. doi: 10.1164/rccm.200909-1427SO. [DOI] [PubMed] [Google Scholar]
- 23.Winkles JA. The TWEAK-Fn14 cytokine-receptor axis: discovery, biology and therapeutic targeting. Nat Rev Drug Discov. 2008;7:411–25. doi: 10.1038/nrd2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chacon MR, et al. Expression of TWEAK and its receptor Fn14 in human subcutaneous adipose tissue: relationship with other inflammatory cytokines in obesity. Cytokine. 2006;33:129–37. doi: 10.1016/j.cyto.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Kim SH, et al. TWEAK can induce proinflammatory cytokines and matrix metallopro-teinase-9 in macrophages. Circ J. 2004;68:396–9. doi: 10.1253/circj.68.396. [DOI] [PubMed] [Google Scholar]
- 26.Xu H, et al. TWEAK/Fn14 interaction stimulates human bronchial epithelial cells to produce IL-8 and GM-CSF. Biochem Biophys Res Commun. 2004;318:422–7. doi: 10.1016/j.bbrc.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 27.van der Poll T, et al. Endotoxin induces downregulation of tumor necrosis factor receptors on circulating monocytes and granulocytes in humans. Blood. 1995;86:2754–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.