Abstract
OBJECTIVES:
This study evaluated plantar thermography sensitivity and specificity in diagnosing diabetic polyneuropathy using cardiac tests (heart rate variability) as a reference standard because autonomic small fibers are affected first by this disease.
METHODS:
Seventy-nine individuals between the ages of 19 and 79 years old (28 males) were evaluated and divided into three groups: control (n = 37), pre-diabetics (n = 13) and type 2 diabetics (n = 29). The plantar images were recorded at baseline and then minutes after a provocative maneuver (Cold Stress Test) using an infrared camera that is appropriate for clinical use. Two thermographic variables were studied: the thermal recovery index and the interdigital anisothermal technique. Heart rate variability was measured in a seven-test battery that included three spectral indexes (in the frequency domain) and four Ewing tests (the Valsalva maneuver, the orthostatic test, a deep breathing test, and the orthostatic hypotension test). Other classically recommended tests were applied, including electromyography (EMG), Michigan inventory, and a clinical interview that included a neurological physical examination.
RESULTS:
Among the diabetic patients, the interdigital anisothermal technique alone performed better than the thermal recovery index alone, with a better sensitivity (81.3%) and specificity (46.2%). For the pre-diabetic patients, the three tests performed equally well. None of the control subjects displayed abnormal interdigital anisothermal readouts or thermal recovery indices, which precluded the sensitivity estimation in this sample of subjects. However, the specificity (70.6%) was higher in this group.
CONCLUSION:
In this study, plantar thermography, which predominately considers the small and autonomic fibers that are commonly associated with a sub-clinical condition, proved useful in diagnosing diabetic neuropathy early. The interdigital anisothermal test, when used alone, performed best.
Keywords: Thermography, Diabetic Neuropathy, Cardiac Autonomic Neuropathy, Small Fibers Neuropathy
INTRODUCTION
Peripheral neuropathy is a common complication in diabetes mellitus (DM) that leads to serious functional disability; however, its evaluation has not been standardized (1-7). Electromyography with nerve conduction assessment is the neurophysiological test that confirms the distal symmetric polyneuropathy that is typical of diabetes, and it is the standard protocol in most centers that specialize in neuropathy (6-9). However, this test does not evaluate small fibers, is minimally invasive, requires expertise, and is time consuming (5,6). The clinical aspects, including validated inventories, are valued in specialized diabetes centers; nevertheless, the screening for subclinical cases has low sensitivity, as demonstrated by the absence of symptoms or signs of distal symmetrical polyneuropathy.
Small fiber neuropathy may occur at any stage of diabetes, including during pre-diabetes. It is often painful, even in the absence of abnormalities, which are measured using conventional standard neurophysiological tests. The discrepancy between clinical presentation and test results could delay the correct diagnosis and appropriate treatment (8,10-12). Many diagnostic methods can assess small fibers; however, no single test can make this diagnosis. A battery of sensitive, reproducible and specific tests covering the somatic and autonomic systems is recommended (5,11,12). These tests include cardiovascular monitoring, sudomotor testing, pupillary responses, quantitative sensory tests, laser evoked potentials and thermography (12).
Using such techniques has proven useful not only for diagnosis but also for guiding adequate therapy and optimizing the follow-up. Infrared computerized thermography is a method of visualization, documentation and measurement of the infrared rays along the human body. According to Stefan-Boltzmann, this emission is proportional to the temperature of the skin and is directly related to blood flow in the cutaneous vessels (13,14,15). This method offers some advantages over others because it is completely non-contact, is painless and does not generate inputs beyond the equipment itself.
In diabetes, vascular activity in the extremities, especially in the feet, may be indicative of autonomic neuropathy. Because vasomotor tone is regulated by fibers from the neurovegetative sympathetic system, its dysfunction could be associated with varying temperature patterns (16-19). Peripheral neurovegetative sympathetic nerve degeneration in advanced neuropathy damages the neurogenic control mechanisms that regulate capillary and arteriovenous (AV) shunt flow, which leads to an increase in the AV shunt in the feet of patients with diabetic neuropathy (20-23). These shunts are maintained in the constricted state by neurovegetative sympathetic tone. Losing this tone because of sympathetic neuropathy results in the shunt opening and the deviation of blood flow from the skin (20-23).
Because of the great vulnerability that small fibers have to metabolic changes associated with hyperglycemia, injuries to these fibers may present earlier and with less marked changes than sensory-motor neuropathies (24,25). Although diabetic neuropathies are classified as diabetic microangiopathic complications, it is now known that their pathophysiological mechanism is multifactorial, and there is sufficient evidence that small-fiber polyneuropathy and even cardiac autonomic neuropathy may precede diabetes (i.e., during the pre-diabetic condition) (26-29).
Cardiovascular reflex tests are standard for clinical autonomic evaluation because of their good sensitivity, specificity, and reproducibility; these tests are also noninvasive and well standardized. However, these tests require a well-trained evaluator and considerable evaluation time. Cardiovascular reflex tests can identify cardiac autonomic neuropathy even at the subclinical stage (29-36). This type of neuropathy can be subdivided into subclinical (predominantly functional and reversible) and clinical (structural neuronal changes are present). The subclinical subtype can only be diagnosed by tests and may occur as early as the time of the initial diabetes diagnosis. The clinical subtype is symptomatic and occurs during more advanced disease stages (35).
The autonomic nerves are affected in various clinical diabetic neuropathy subtypes. In the most common type (typical polyneuropathy: symmetric, distal, and predominantly sensory), there is a strong correlation between the progressive damage of the somatic and autonomic fibers. Indeed, 50% of diabetic patients with polyneuropathy have asymptomatic cardiac autonomic neuropathy, while 100% of patients with symptomatic cardiac autonomic neuropathy have polyneuropathy (3,37). The prevalence of cardiac autonomic neuropathy progressively increases in direct proportion to age, diabetes duration, and poor glucose control.
The aim of this study was to evaluate plantar thermography sensibility and specificity in diabetic patients with polyneuropathy at the diagnosis using cardiac tests (heart rate variability) as a reference standard because the autonomic small fibers are first affected, and there is a close relationship with cardiac and somatic autonomic nerve fibers in diabetes (18,23).
MATERIALS AND METHODS
This study was performed from March 2010 to August 2011. The principles of the Declaration of Helsinki (38) were applied, and all of the patients provided informed written consent. The study was approved by the Ethics Committee of the Hospital de Clínicas de Porto Alegre (HCPA), RS, Brazil, decision No. 09-446, January 2010.
Subjects
Seventy-nine individuals aged between 19 and 79 years (28 males) were evaluated and divided into three groups: controls (n = 37), pre-diabetics (n = 13), and type 2 diabetics (n = 29). The patients with pre-diabetes and diabetes were referred to the study from the Endocrinology and Pre-diabetes Out-clinic Unit of the Hospital de Clínicas de Porto Alegre (Porto Alegre, Brazil). Diabetes and pre-diabetes were defined according to the American Diabetes Association (ADA) criteria (9). The control group consisted of volunteers that did not fit the criteria for type 2 diabetes or pre-diabetes. Smokers and subjects with other conditions that could potentially cause neuropathies, such as hypothyroidism and alcoholism, or a condition in which the thermal plantar images could mimic neuropathy, such as lumbosacral radiculopathy, were excluded. Ischemic heart disease or stroke patients were also excluded because of the heart rate variability tests were used.
Data collection and instrumentation
The data collection was conducted at a controlled laboratory temperature of 23±0.5°C and a relative humidity of 50±5%. All of the tests occurred on the same day and lasted 1.5 hours per participant.
Clinical evaluation
Clinical data, including age, gender, body mass index, and arterial systolic and diastolic pressure, were collected on the evaluation day. The clinical diagnosis of distal symmetric polyneuropathy was assessed using the Michigan inventory (39), a neuropathy score that consists of an inspection of foot deformities or ulcers and a brief neurological examination. We used the Achilles reflex test, a vibration sensitivity test on the hallux dorsum with a 128 Hz tuning-fork and a test for tactile sensibility using 10 g of nylon monofilament (Sory®, Bauru-SP-Brazil) on the plantar aspect of the hallux. The Michigan inventory was considered to be positive for neuropathy when four or more points (of a possible ten) were scored.
Heart rate variability
Both the ADA and the American Academy of Neurology (AAN) (40,41) recommend the following protocol: three Ewing tests (the deep breathing test, Valsalva maneuver, and orthostatic test) at the time of a type 2 diabetes diagnosis and five years after a type 1 diabetes diagnosis and repeated annually thereafter. These three tests performed together have good reproducibility and specificity above 91%; the deep breathing and orthostatic tests have 93% sensitivity, and the Valsalva maneuver has 98% sensitivity. In addition, a spectral analysis was performed for heart rate variability, which is a result of sympathetic and parasympathetic balance at the sinus node. The method consists of seven parameters that are evaluated together, including a three-band spectral analysis of heart rate variability and four Ewing tests (the Valsalva maneuver, orthostatic test, deep breathing test and orthostatic hypotension test); this method has 98% sensitivity and 100% specificity (35).
To evaluate the presence of cardiac autonomic neuropathy, heart rate variability tests were performed. These tests comprised three spectral indices (in the frequency domain) and four Ewing tests (35), including the Valsalva maneuver, orthostatic test, deep breathing test, and orthostatic hypotension test. The electrocardiogram was recorded (particularly the QRS complex) using electrocardiography equipment (Neurosoft®, Ivanovo, Russia) and software that was created for heart rate variability analysis (Poly-Spectrum®). A questionnaire concerning autonomic dysfunction symptoms was given following the protocol described by Rolim et al. (35).
The presence of incipient cardiac autonomic neuropathy was defined as having two abnormal tests (98% specificity), and established neuropathy was diagnosed when three tests were abnormal (100% specificity) (35). In this study, we considered patients to be either positive or negative for cardiac autonomic neuropathy.
Plantar thermography
The plantar infrared images were recorded using three thermal infrared cameras (the PV320T Electrophysics, IRI 4010 IRYSIS, and T400 Flir) with thermal sensitivities of 0.08 and 0.01°C, a full-scale spectral range between 7-12 and 8-14 μ and a maximum error of 2%. The protocol for infrared images followed the recommendations of the American Academy of Thermology (42). Notably, caffeinated drinks or other vasoactive substances were suspended for at least eight hours prior to testing, and all prescribed medications were suspended 12 hours prior to testing.
The participants were acclimated for 15 minutes in the examination room by lying on a stretcher with bare legs and feet and no surface contact. The plantar infrared image was recorded at baseline followed by the provocative maneuvers using the cold stress test. This test consists of immersing the feet, which are protected with thin plastic, for 60 seconds in water at 15°C. After 10 minutes, a new plantar infrared image was recorded to evaluate the thermal recovery index. To calculate the recovery index, the average temperatures of 10 regions of interest with similar dimensions were used: the hallux, 1st, 3rd, and 5th metatarsal heads and heel on both soles, as shown in Equation 1.
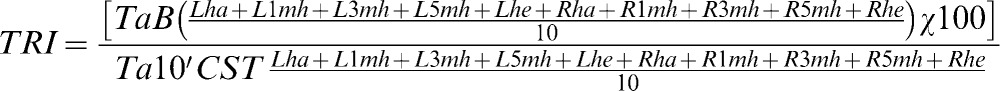 |
TRI: thermal recovery Index; TaB: the average basal temperature of the 10 ROI (°C); Ta10'CST: the average temperature 10 minutes after the cold stress test of the 10 ROI (°C); L: left foot; R: right foot; ha: hallux; 1 mh: 1st metatarsal head; 3 mh: 3rd metatarsal head; 5 mh: 5th metatarsal head; he: heel.
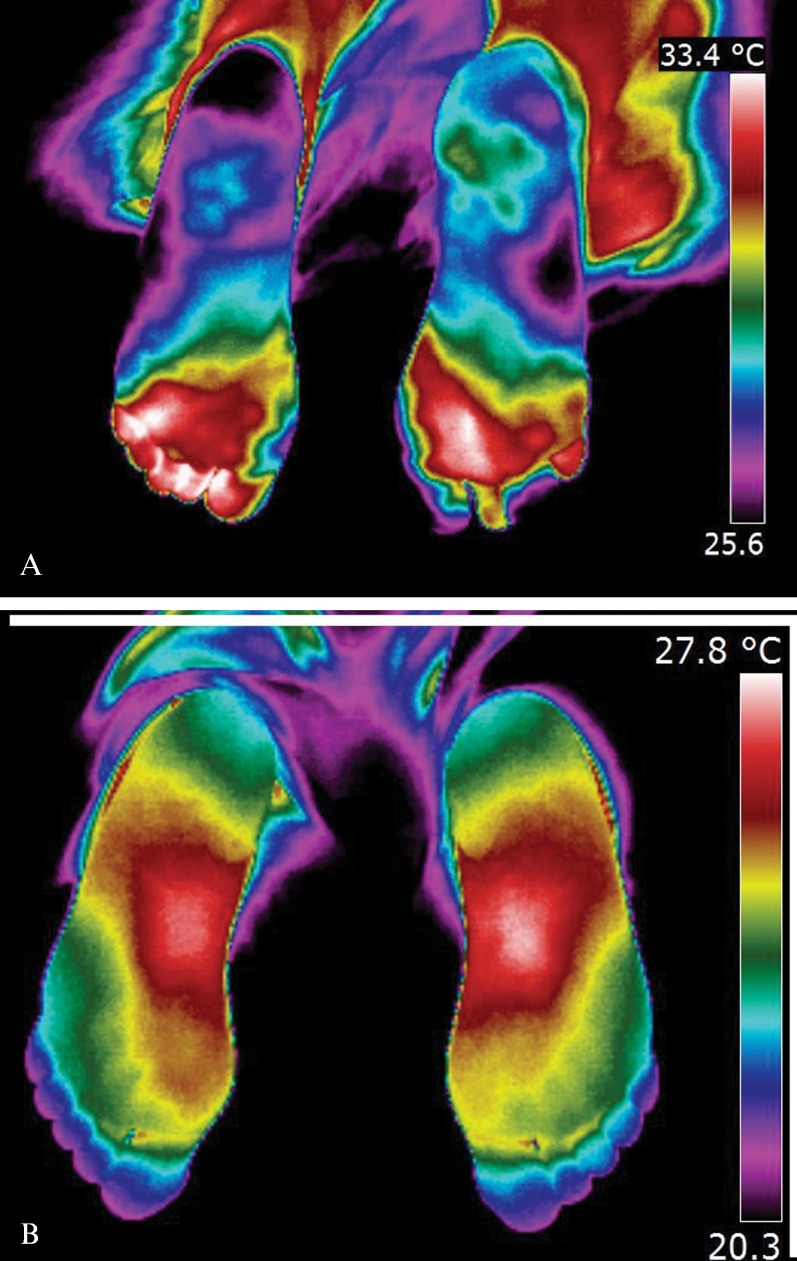
The interdigital anisothermal was assessed and was considered to be positive when thermal gradients (ΔT) ≥0.4°C existed between any of the toes 10 minutes after the cold stress test (41-49). Figure 1 shows two examples of the interdigital anisothermal test.
Figure 1.
(A) Plantar thermographic image in a diabetic patient, showing Interdigital Anisothermal (the white arrow shows the different colors in toes meaning ΔT ≥ 0.4°C). (B) Plantar thermographic image in a control subject, with regular thermal distribution, without Interdigital Anisothermal (color gradient of toes considered normal, ΔT < 0.4°C).
In this study, which is based on specific literature, an interdigital anisothermal result or a thermal recovery index of <90% or >100% after the cold stress test were considered (alone or in combination) to be positive for early neuropathy (small fibers and neurovegetative sympathetic fibers with vasomotor functions) (42-50).
Electromyography
The neuropathy screening protocol included the functional assessment of motor and sensory nerves of the four segments, as well as a myography using a needle electrode in suspected cases of axonal injury or root involvement (3,5-8). To record and analyze the data, a two channel electromyogram (Neurosoft®, Ivanovo, Russia) and dedicated software (NeuroMep®) were used. This method classifies the neuropathy into its various manifestations (i.e., mononeuropathies, multiple mononeuropathy and polyneuropathy). In this study, EMG was considered to be positive when it demonstrated distal symmetric polyneuropathy and was typical of diabetic neuropathy (5,7,51,52). The evaluator was blinded to the other test results.
Statistical analysis
The categorical variables were described by absolute and relative frequencies, and the continuous variables were described by the mean and standard deviation (SD) or the median and interquartile range. The cutoff point for infrared thermography, which was represented by the thermal recovery index in this study using heart rate variability as a reference standard, was obtained by creating a receiver operating characteristic (ROC) curve. The area under the curve was used to estimate the accuracy of the method. The Kappa coefficient was assessed to analyze the agreement between the thermographic methods versus heart rate variability. The association between the categorical variables was assessed using the chi-squared test or Fisheŕs exact test. The means were compared using Student's t-test or a one-way analysis of variance (ANOVA) with Tukey's post-hoc test. In asymmetric cases, a Mann-Whitney U-test was applied. A significance level of 5% was adopted, and all of the statistical analyses were performed using SPSS (Statistical Package for the Social Sciences) version 18.0.
RESULTS
The analyses were separated by group because the groups were not homogeneous, with the exception of gender. The control subjects were younger (p = 0.001) and had a lower body mass index than the pre-diabetic subjects (p<0.001). The controls also had lower blood pressure values than the diabetic and pre-diabetic subjects (systolic, p<0.001, diastolic, p = 0.012), as shown in Table 1.
Table 1.
Patient characteristics.
| Variables* | DM (n = 29) | Pre-DM (n = 13) | Control (n = 37) | p-value |
| Age (years) | 55.9±9.4b | 56.8±12.6b | 45.1±14.9a | 0.001 |
| Gender (Female/Male) | 20/9 | 10/3 | 21/16 | 0.350 |
| Time of diagnosis (years) | 5 (2-9) | 1 (1-2) | - | <0.001 |
| Body mass index (kg/m2) | 27.1±4.0ab | 30.0±2.8)b | 25.3(3.8)a | <0.001 |
| Systolic BP (mmHg) | 131.7±8.0b | 130.5±6.2b | 121.7(9.0)a | <0.001 |
| Diastolic BP (mmHg) | 85.9±7.9b | 83.5±6.3ab | 79.7(8.8)a | 0.012 |
BP: Blood Pressure. * Mean±standard deviation or median (25th percentile to 75th percentile). a,b Same letters do not differ by Tukey's post hoc test at the 5% significance level.
For the three glycemic statuses, the pre-DM group presented with the highest thermal recovery index and had abnormal interdigital anisothermal results. The DM group presented with the highest number of abnormal results from the neuropathy diagnosis tests (heart rate variability, Michigan inventory, and EMG), as reported in Table 2. Although we observed a greater number of positive results for neuropathy diagnosis, only 6.9% (n = 2) of the patients from the DM group presented with a positive Michigan inventory test, thus identifying clinical neuropathy by the loss of protective foot sensation.
Table 2.
Absolute and relative frequencies of neuropathy test results according to glycemic status.
| Test | DM (n = 29) | Pre-DM (n = 13) | Control (n = 37) | |||
| Abnormal | Normal | Abnormal | Normal | Abnormal | Normal | |
| TRI (%) | 22 (75.9) | 7 (24.1) | 10 (76.9) | 3 (23.1) | 14 (37.8) | 23 (62.2) |
| IDA (%) | 20 (69.0) | 9 (31.0) | 10 (76.9) | 3 (23.1) | 13 (35.1) | 24 (64.9) |
| TRI+AID (%) | 15 (51.7) | 14 (48.3) | 10 (76.9) | 3 (23.1) | 13 (35.1) | 24 (64.9) |
| HRV (%) | 16 (55.2) | 13 (44.8) | 5 (38.5) | 8 (61.5) | 3 (8.1) | 34 (91.9) |
| Michigan (%) | 2 (6.9) | 27 (93.1) | 0 (0) | 13 (100) | 0 (0.0) | 37 (100) |
| EMG (%) | 16 (55.2) | 13 (44.8) | 2 (15.4) | 11 (84.6) | 0 (0.0) | 37(100) |
TRI: thermal recovery index; IDA: interdigital anisothermal, HRV: heart rate variability, (Michigan inventory); EMG: electromyography.
Cardiac autonomic neuropathy (diagnosed using the heart rate variability method) was the reference used to estimate the sensitivity and specificity of the plantar infrared thermography. The interdigital anisothermal test and the thermal recovery index, alone or in combination, were used to identify the subjects with cardiac autonomic neuropathy in the three groups (Table 3). In the diabetic subjects, the interdigital anisothermal test alone performed better than the thermal recovery index, and both had better sensitivity (81.5%) and specificity (46.2%). For the pre-diabetic patients, all three tests performed equally well (sensitivity 80.0%, specificity 25.0%). None of the control subjects had abnormal results for the interdigital anisothermal test or the thermal recovery index, which would have precluded the sensitivity estimation in this sample of subjects. However, the specificity was higher in the control subjects (70.6%).
Table 3.
Comparison of the methods.
| Comparisons | % abnormal | Sensibility (%) | Specificity (%) |
| DM Group (n = 29) | |||
| HRV×TRI | 55.2×75.9 | 75.0 | 23.1 |
| HRV×IDA | 55.2×69.0 | 81.3 | 46.2 |
| HRV×both (TRI+IDA) | 55.2×51.7 | 56.3 | 53.8 |
| Pre-DM Group (n = 13) | |||
| HRV×TRI | 38.5×76.9 | 80.0 | 25.0 |
| HRV×IDA | 38.5×76.9 | 80.0 | 25.0 |
| HRV×both (TRI+IDA) | 38.5×76.9 | 80.0 | 25.0 |
| Control Group (n = 37) | |||
| HRV×TRI | 8.1×37.8 | - | 58.8 |
| HRV×IDA | 8.1×35.1 | - | 61.8 |
| HRV×both (TRI+IDA) | 8.1×27.0 | - | 70.6 |
HRV: heart rate variability; TRI: thermal recovery index, IDA: interdigital anisothermal.
Because the interdigital anisothermal test performed best, the clinical data regarding the results of this test are depicted in Table 4. In the pre-diabetic patients, the clinical data were significantly different between the subjects who presented or did not present with interdigital anisothermal results. The subjects in the pre-DM group who presented with an abnormal plantar thermography result were older than those who presented with a normal interdigital thermographic pattern.
Table 4.
Interdigital anisothermal clinical characteristics.
| Variables*) | DM (n = 29) | Pre-DM (n = 13) | Control (n = 37) | ||||||
| Abnormal | Normal | p-value | Abnormal | Normal | p-value | Abnormal | Normal | p-value | |
| Age (years) | 56.1±8.8 | 55.4±11.1 | 0.876 | 60.7±10.9 | 43.7±9.1 | 0.032 | 46.4±16.4 | 44.3±14.3 | 0.695 |
| Gender (Female/Male) | 14/6 | 6/3 | 1.000 | 9/1 | ½ | 0.108 | 8/5 | 12/12 | 0.744 |
| Time of diagnosis (years) | 5.5 (2.3-9.5) | 2 (1-10.5) | 0.216 | 1.5 (0.9-2.0) | 1 (1-1) | 0.469 | - | - | - |
| BMI (kg/m2) | 27.2±4.4 | 26.9±3.3 | 0.825 | 29.7±3.0 | 31.1±2.2 | 0.467 | 26.2±4.6 | 24.8±3.2 | 0.290 |
| SBP (mmHg) | 133.5±5.9 | 127.8±10.9 | 0.170 | 129.7±6.7 | 133.3±2.9 | 0.393 | 121.2±10.8 | 121.9±8.1 | 0.799 |
| DBP (mmHg) | 87.0±5.5 | 83.3±11.7 | 0.393 | 83.5±11.7 | 83.3±7.6 | 0.970 | 80.0±11.7 | 79.6±7.1 | 0.893 |
Mean (SD) or median (25th percentile to 75th percentile). BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure. Level of significance = 5%.
DISCUSSION
The presence of interdigital anisothermal using a simplified plantar thermographic study appears to be the most appropriate diagnostic test for the early diagnosis of neuropathy in the patients with DM and pre-DM. Because of its sensitivity and specificity, in addition to its convenience and timing, interdigital anisothermal assessed by plantar thermography seems to be most suitable for diabetic neuropathy screening programs. Its application is simple using the proposed protocol, and it is cost-effective because it requires no additional supplies.
It is important to identify diabetic neuropathy early to prevent secondary complications, such as neuropathic pain and diabetic foot (10,11,52,53). Unfortunately, because of economic concerns and the lack of technological resources, a consensus of international associations dedicated to treating and preventing diabetes has advocated only using clinical examination as a population screening method (56-59). In this study, the clinical application of a validated clinical inventory (Michigan inventory) did not identified neuropathy even in cases with positive EMG, which would certainly include late cases of neuropathy. Plantar thermography is a new, non-invasive method that can be included in neuropathy screening programs, thereby increasing the sensitivity for proper diagnosis.
The current method for the early diagnosis of diabetic neuropathy is a battery of tests, including methods capable of assessing thick and thin nerve fibers, particularly the autonomic nerve fibers (5,12). The two types of thermographic tests (thermal recovery index after the cold stress test and the interdigital anisothermal test) demonstrated differences in patterns between the three groups. These patterns reflect the sympathetic nerve fibers with vasomotor tone function and its effect on the skin temperature of the feet (15,17,18).
Another benefit provided by plantar thermography for chronic patients, such as those with DM, is the possibility of following the evolutionary aspects of the disease because thermography allows for functional imaging (60). Because of the strict control of risk factors, such as glycemic control, cutaneous vasomotor functionality of the feet can be monitored periodically using this method.
In this study, plantar thermography proved useful in the early diagnosis of diabetic neuropathy, particularly the small and autonomic fibers that are commonly associated with a sub-clinical condition. Future research studying the thermal recovery index for diabetes duration would be useful, particularly when comparing the density of nerve fibers using skin biopsy and Quantitative Sensitive Test (QST) to demonstrate a low sensitivity to heat. In addition, it would be interesting to compare the potential amplitude of the sural nerve, when present, with the thermographics variable studied here (interdigital anisothermal and thermal recovery index), as demonstrated by Shun et al. (61).
One limitation of this study is that it is a cross-sectional study that involves a sensitive test. Therefore, follow-up will be needed to assess the development of more advanced forms of neuropathy and diabetic complications.
ACKNOWLEDGMENTS
This work received financial support from CAPES and CNPq.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Dick PJ. Detection, characterization, and staging of polyneuropathy: assessed in diabetics. Muscle Nerve. 1988;11(1):21–32. doi: 10.1002/mus.880110106. [DOI] [PubMed] [Google Scholar]
- 2.Kahn R. Proceedings of a consensus development conference on standardized measures in diabetic neuropathy. Diabetes Care. 1992;15(8):1081–3. [PubMed] [Google Scholar]
- 3.Thomas PK. Classification, differential diagnosis and staging of diabetic peripheral neurophaty. Diabetes. 1997;46(Suppl 2):S54–7. doi: 10.2337/diab.46.2.s54. [DOI] [PubMed] [Google Scholar]
- 4.Boulton AJM. Guidelines for diagnosis and outpatient management of diabetic peripheral neuropathy. European Association for the Study of Diabetes, Neurodiab. Diabetes Metab. 1998;24(Suppl 3):S55–65. [PubMed] [Google Scholar]
- 5.England JD, Grosnseth GS, Franklin G, Carter GT, Kinsella LJ, Cohen JA, et al. Practice parameter Evaluation of distal symmetric polyneuropathy: role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review): report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation. Neurology. 2009;72(2):177–84. doi: 10.1212/01.wnl.0000336345.70511.0f. [DOI] [PubMed] [Google Scholar]
- 6.Perkins BA, Bril V. Diabetic neuropathy: a review emphasizing diagnostic methods. Clin Neurophysiol. 2003;114(7):1167–75. doi: 10.1016/s1388-2457(03)00025-7. [DOI] [PubMed] [Google Scholar]
- 7.Tesfaye S, Boulton AJM, Dick PJ, Freeman R, Horowitz M, Kempler P. Diabetic Neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–93. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claus D, Mustafa C, Vogel W, Herz M, Neundorfer B. Assessment of diabetic neuropathy: definition of norm and discrimination of abnormal nerve function. Musc Nerve. 1993;16(7):757–68. doi: 10.1002/mus.880160711. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association (ADA) Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2012;35(1):S11. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulton AMJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freemann R, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–62. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 11.Boulton AJM, Malik RA. Neuropathy of impaired glucose tolerance and its measurement. Diabetes Care. 2010;33(1):207–9. doi: 10.2337/dc09-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santiago S, Ferrer T, Espinosa ML. Neurophysiological studies of thin myelinated (A delta) and unmyelinated (C) fibers: application to peripheral neuropathies. Neurophysiol Clin. 2000;30(1):27–42. doi: 10.1016/S0987-7053(00)88865-6. [DOI] [PubMed] [Google Scholar]
- 13.Anbar M. Clinical thermal imaging today. IEEE Eng Med Biol. 1998;17(4):25–33. doi: 10.1109/51.687960. [DOI] [PubMed] [Google Scholar]
- 14.Balbinot LF. Brazil: Blucher Academic; 2009. Computerized thermography in the identification of myofascial trigger points. Chapter 3. [Google Scholar]
- 15.Vargas JVC, Brioschi ML, Dias FG, Parolin MB, Mulinari-Brener FA, Ordonez JC, et al. Normalized methodology for medical infrared imaging. Infrared Physics & Technology. 2009;52(1):42–7. [Google Scholar]
- 16.Fushimi H, Inque T, Yamada Y, Matsyama Y, Kubo M, Kameyama M. Abnormal vasoreaction of peripheral arteries to cold stimulus of both handskin diabetics. Diabetes Res Clin Pract. 1996;32(1-2):55–9. doi: 10.1016/0168-8227(96)01222-3. [DOI] [PubMed] [Google Scholar]
- 17.Langer L, Fagerberg SE, Johnsén C. Peripheral circulation in diabetes mellitus–a study with infrared termography. Acta Med Scand. 1972;191(1-2):17–20. [PubMed] [Google Scholar]
- 18.Sundkvist G, Almér LO, Lilja B. Autonomic neuropathy and toe circulation. A prospective study. Acta Med Scand. 1986;219(3):305–8. doi: 10.1111/j.0954-6820.1986.tb03316.x. [DOI] [PubMed] [Google Scholar]
- 19.Zooter H, Kerbl R, Gallistl S, Nitsche H, Borkenstein M. Rewarming index of the lower leg assessed by infrared thermography in adolescents with Type 1 Diabetes Mellitus. J Pediatr Endocrinol Metab. 2003;16(9):1257–62. doi: 10.1515/jpem.2003.16.9.1257. [DOI] [PubMed] [Google Scholar]
- 20.Uematsu S, Edwin DH, Jankel WR, Kozikowski J, Trattner M. Quantification of thermal asymmetry. Part 1: Normal values and reproducibility. Neurosurg. 1988;69(4):552–5. doi: 10.3171/jns.1988.69.4.0552. [DOI] [PubMed] [Google Scholar]
- 21.Flynn MD, Tooke JE. Microcirculation and the diabetic foot. Vasc Med Rev. 1990;1:121–38. [Google Scholar]
- 22.Vinik AI, Erbas T, Park TS, Stansberry KB, Scaneli JA, Pittenger GL. Dermal neurovascular dysfunction in type 2 diabetes. Diabetes Care. 2001;24(8):1468–75. doi: 10.2337/diacare.24.8.1468. [DOI] [PubMed] [Google Scholar]
- 23.Vinik AI, Erbas T, Park TS, Pierce KK, Stansberry KB. Methods for evaluation of peripheral neurovascular dysfunction. Diabetes Technol Ther. 2001;3(1):29–50. doi: 10.1089/152091501750220000. [DOI] [PubMed] [Google Scholar]
- 24.Albers JM, Hermann WH, Pop-Bususi R, Martin CL, Clearly P, Waberski B. Subclinical neuropathy at trial completion possible predictors of incident neuropathy. Diabetes Care. 2007;30(10):2613–18. doi: 10.2337/dc07-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ewing DJ, Campbell IW, Burt AA, Clarke BF. Vascular reflexes in diabetic autonomic neuropathy. Lancet. 1973;2(7842):1354–6. doi: 10.1016/s0140-6736(73)93323-0. [DOI] [PubMed] [Google Scholar]
- 26.Ewing DJ, Clarke BF. Hand skin blood flow in diabetic patients with autonomic neuropathy and microangiopathy. Diabetes Care. 1991;14(10):897–902. doi: 10.2337/diacare.14.10.897. [DOI] [PubMed] [Google Scholar]
- 27.Vinik AI. Complications of diabetes. Clin Cornerstone. 2005;5(2):38–52. [Google Scholar]
- 28.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic Autonomic Neuropathy. Diabetes Care. 2003;26(5):1553–79. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 29.Valensi P, Paries J, Attali JR. Cardiac autonomic neuropathy in diabetic patients: influence of diabetes duration, obesity, and microangiopathic complications–the French multicenter study. Metabolism. 2003;52(7):815–20. doi: 10.1016/s0026-0495(03)00095-7. [DOI] [PubMed] [Google Scholar]
- 30.Valensi P, Paries J, Lormeau B, Assad N, Attali JR. Cardiac parasympathetic changes: a new component of the insulin resistance syndrome. Diabetes. 1999;48(Suppl 1):A149. [Google Scholar]
- 31.Ewing DJ, Campbell IW, Clarke BF. The natural history of diabetic autonomic neuropathy. Q J Med. 1980;49(193):95–108. [PubMed] [Google Scholar]
- 32.San Antonio Conference. Report and recommendations of the San Antonio conference on diabetic neuropathy. Ann Neurol. 1988;24(1):99–104. doi: 10.1002/ana.410240120. [DOI] [PubMed] [Google Scholar]
- 33.Howorka K, Pumprla J, Schabmann A. Optimal parameters of short-term heart rate spectrogram for routine evaluation of diabetic cardiovascular autonomic neuropathy. J Auton Nerv Syst. 1998;69(2-3):164–72. doi: 10.1016/s0165-1838(98)00015-0. [DOI] [PubMed] [Google Scholar]
- 34.Task Force of the European Society of Cardiology and the American Society of Pacing Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93(5):1043–65. [PubMed] [Google Scholar]
- 35.Rolim LC, Sá JR, Chacra AR, Dib AS. Neuropatia autonômica cardiovascular diabética: fatores de risco, impacto clínico e diagnóstico precoce. Arq. Bras. Cardiol. 2008;90(4):e24–32. doi: 10.1590/s0066-782x2008000400014. [DOI] [PubMed] [Google Scholar]
- 36.Makimatila S, Sclenzka A, Mantysaari M, Bergholm R, Summanen P, Saar P, et al. Predictors of abnormal cardiovascular autonomic function measured by frequency domain analysis of heart rate variability and conventional tests in patients with type 1 diabetes. Diabetes Care. 2000;23(11):1686–93. doi: 10.2337/diacare.23.11.1686. [DOI] [PubMed] [Google Scholar]
- 37.Low PA, Vermino S, Suarez G. Autonomic dysfunction in peripheral nerve disease. Muscle Nerve. 2003;27(6):646–61. doi: 10.1002/mus.10333. [DOI] [PubMed] [Google Scholar]
- 38.World Medical Association. Note of Clarification in Paragraph 29 added by theWMA General Assembly. Washington, DC: 2002. Declaration of Helsinki: Ethical Principles for medical Research Involving Human Subjects, October, 2000. [Google Scholar]
- 39.Michigan Neuropathy Screening Instrument (MNSI) and Michigan Diabetic Neuropathy Score (MDNS) / http://www.measurementexperts.org/instrument/instrument_reviews.asp?detail = 66.
- 40.American Diabetes Association (ADA) and American Academy of Neurology (AAN) Consensus Statements of Medical Care in Diabetes-2008. Diabetes Care. 2008;31 Suppl 1;51:S12–S54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 41.American Diabetes Association (ADA) Position Statement. Diabetes Care. 2009;32(1):S35. [Google Scholar]
- 42.Bharara M, Cobb JE, Claremont DJ. Thermography and Thermometry in the assesment of diabetic neuropathic foot: a case for furthering the role of termal techniques. Int J Low Extrem Wounds. 2006;5(4):250–60. doi: 10.1177/1534734606293481. [DOI] [PubMed] [Google Scholar]
- 43.Papanas N, Papatheodorou K, Papazoglou D, Kotsiou S, Maltezos E. Association between foot temperature and sudomotor dysfunction in type 2 diabetes. J Diabetes Sci Technol. 2010;4(4):803–7. doi: 10.1177/193229681000400406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brioschi ML, Mehl A, Oliveira AGN, Freitas MAS, Macedo JF, Matias JEF, et al. Diabetic foot evaluation by infrared skin thermometry. Rev Med Paraná. 2007;65(1):33–41. [Google Scholar]
- 45.Bharara M, Cobb JE, Claremont DJ. Thermography and Thermometry in the assessment of diabetic neuropathic foot: a case for furthering the role of thermal techniques. Int J Low Extrem Wounds. 2006;5(4):250–60. doi: 10.1177/1534734606293481. [DOI] [PubMed] [Google Scholar]
- 46.Ring F. Thermal imaging today and its relevance to diabetes. J Diabetes Sci Technol. 2010;4(4):857–62. doi: 10.1177/193229681000400414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alberbaty M, Uematsu S. American Academy of Thermology. Georgetown University Medical Center; 1986. Medical Thermology. [Google Scholar]
- 48.Ring EFJ, Houdas Y. New York, , USA: Plenum Press; 1982. Human Body Temperature: its measurement and regulation. [Google Scholar]
- 49.Brioschi ML, Macedo JF, Macedo RAC. Skin thermography: new concepts. J Vasc Bras. 2003;2(2):151–60. [Google Scholar]
- 50.Herrick RT, Herrick SK. Thermography in the detection of carpal tunnel syndrome and other compressive neuropathies. J Hand Surg. 1987;12(5):943–9. doi: 10.1016/s0363-5023(87)80262-9. [DOI] [PubMed] [Google Scholar]
- 51.Souza A, Nery CAS, Marciano LHSC, Garbino JA. Avaliação da neuropatia periférica: correlação entre a sensibilidade cutânea dos pés, achados clínicos e eletroneuromiográficos. Acta Fisiatrica. 2005;12(3):87–93. [Google Scholar]
- 52.Rathmann W, Ziegler D, Jahnke M, Haastert B, Gries FA. Mortality in diabetic patients with cardiovascular autonomic neuropathy. Diabet Med. 1993;10:820–4. doi: 10.1111/j.1464-5491.1993.tb00173.x. [DOI] [PubMed] [Google Scholar]
- 53.Shaw JE, Zimmet PZ. The epidemiology of diabetic neuropathy. Diabetes Rev. 1999;7:245–52. [Google Scholar]
- 54.Ziegler D. Cardiovascular autonomic neuropathy: clinical manifestations and measurement. Diabetes Reviews. 1999;7:342–57. [Google Scholar]
- 55.Ziegler D, Zentai C, Perz S, Rathmann W, Haastert B, Meisinger C, et al. Selective contribution of diabetes and other cardiovascular risk factors to cardiac autonomic dysfunction in the general population. Exp Clin Endocrinol Diabetes. 2006;114:153–9. doi: 10.1055/s-2006-924083. [DOI] [PubMed] [Google Scholar]
- 56.Lira JR, Castro AA, Pitta GBB, Figueiredo LFP, Lage VMM, Junior FM. Prevalência de polineuropatia sensitivo-motora nos pés no momento do diagnóstico do diabetes melito. J Vasc Br. 2005;4(1):22–6. [Google Scholar]
- 57.Malerbi D, Franco LJ. The Brazilian Cooperative Group on the Study of Diabetes Prevalence. Multicenter Study of the Prevalence of diabetes mellitus and Impaired Glucose Tolerance in the urban Brazilian population aged 30-69 years. Diabetes Care. 1992;15(11):1509–16. doi: 10.2337/diacare.15.11.1509. [DOI] [PubMed] [Google Scholar]
- 58.Moreira RO, Leite NM, Cavalcanti F, Oliveira FJD. Diabetes Mellitus: Neuropatia. Projeto Diretrizes. Associação Médica Brasileira e Conselho Federal de Medicina. 2005 http://www.projetodiretrizes.org.br/4_volume/09-Diabetesm.pdf. [Google Scholar]
- 59.Olaleye D, Perkins BA, Bril V. Evaluation of three screening tests and a risk assessment model for diagnosing peripheral neuropathy in the diabetes clinic. Diabetes Research and Clinical Practice. 2001;54(2):115–28. doi: 10.1016/s0168-8227(01)00278-9. [DOI] [PubMed] [Google Scholar]
- 60.Sacharuk VZ, Lovatel GA, Ilha J, Marcuzzo S, Pinho AS, Xavier LL, et al. Thermographic evaluation of hind paw skin temperature and functional recovery of locomotion after sciatic nerve crush in rats. Clinics. 2011;66(7):1259–66. doi: 10.1590/S1807-59322011000700023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shun CT, Chang YC, Wu HP, Hsieh SC, Lin WM, Lin YH, et al. Skin denervation in type 2 diabetes: Correlations with diabetic duration and functional impairments. Brain. 2004;127(Pt 7):1593–605. doi: 10.1093/brain/awh180. [DOI] [PubMed] [Google Scholar]