Abstract
Objective
Low heart rate variability (HRV) has been identified as a strong predictor of a large number of diseases, reflecting the vital role of autonomic nervous system in maintaining health. It has been hypothesized that the gender differences in autonomic modulation may explain the gender differences in morbidity and/or mortality rate. This study aims to compare the autonomic balance of males with that of females based on short-term HRV analysis.
Materials and Methods
This cross sectional case-control study involved eighty males matched with seventy-six females. The age M±SD is 28.5±5.4 years in males and 27.3±5.6 years in females. Biocom 3000 ECG recorder was used for studying HRV. Data was analyzed using SPSS Software (v.17), screening studied variables for significant differences in the means between the groups was performed using unpaired t test. Mean heart rate (MHR) was introduced as a covariate in the statistical analysis of HRV using general linear model.
Results
All short-term HRV (5-min) time domain indices, total power (TP), very low frequency (VLF), low frequency (LF) and high frequency (HF) components of frequency domains were significantly higher in males except MHR, which was significantly higher in females (P<0.05 for all).
Conclusion
Global autonomic activity was higher in males. In contrast, females have higher heart rate compared with males.
Keywords: heart rate variability, gender, autonomic
Introduction
Experimental evidences suggest that autonomic markers such as heart rate variability (HRV), blood pressure variability (BPV) and baroreflex sensitivity (BRS) are useful methods in evaluating the autonomic modulation. HRV measures the variations of the time intervals between consecutive heartbeats, which vary under control of the autonomic nervous system. When the parasympathetic system is dominant, the heartbeat intervals oscillate with higher frequencies. When sympathetic arousal occurs, lower frequency oscillations take place. (1)
For descriptive statistical methods, the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology recommends several measures.(2) The most common are the time domain and the frequency domain methods. The time domain method is commonly used for 24-hour data recordings. However, according to the Task force, short-term recordings (e.g. 5-min length) can also be analyzed. Frequency domain method is used for both short and long-term recordings, however, it should be preferred to the time domain method when short-term recordings are investigated. (2)
In the time domain method, mean heart rate (MHR), mean heart beat interval (MNN), the square root of variance of RR intervals (SDNN) and square root of the mean squared differences of successive RR intervals (RMSSD). SDNN reflects all cyclic components of the variability in recorded series of RR intervals. RMSSD is an estimate of high-frequency variations in short-term RR recordings and therefore reflects parasympathetic regulation of the heart. (2)
Frequency domain parameters include total Power (TP), very Low Frequency (VLF), Low Frequency (LF), High Frequency (HF), Normalized Low Frequency (LF Norm), Normalized High Frequency (HF Norm) and LF/HF Ratio. TP reflects overall autonomic activity, however, the physiological explanation of the VLF component is less defined. (2, 3) The LF power is modulated by both sympathetic and parasympathetic outflows as well as by other factors, including baroreceptor activity. (3, 4)
Normalized Low Frequency (LF Norm) is the ratio between absolute value of the Low Frequency and difference between total power and very low frequency. Normalized High Frequency (HF Norm) is the ratio between absolute value of the High Frequency and difference between total power and very low frequency. The advantage of LF Norm and LF Norm is that they minimize the effect of changes in very low frequency power and emphasizes changes in sympathetic and parasympathetic regulation respectively. LF/HF Ratio signifies the overall balance between sympathetic and parasympathetic systems. (2)
Low HRV has been identified as a strong indicator of risk related to adverse events in healthy individuals and diseased patients, reflecting the vital role that autonomic nervous system played in maintaining health. (1,5–9) Raemakers et al hypothesized that there are gender differences in autonomic modulation making women at lower risk to develop cardiovascular diseases.(10) Other studies of gender differences in autonomic regulation demonstrate that women have significantly greater parasympathetic input to cardiac regulation than do age-matched men. (11.12) In contrast, the results of Umetani et al (13) revealed lower HRV in female compared with male subjects at age less than 30 years old, decreased gender differences at age between 30 – 50 years old and no gender differences at age above 50 years old. Therefore, previous studies examining gender differences in healthy populations report contradictory findings. This study aims to compare the autonomic balance of males with that of females based on short-term HRV analysis.
Materials and Methods
The studied subjects were a part of a larger study intended to detect autonomic modulation in asthmatic patients based on short-term HRV. The study involved fifty-six healthy subjects recruited mainly from university students and employees and one hundred asthmatic patients selected from chest clinics of teaching hospitals in Khartoum state – Sudan. Patients with past medical history suggestive of other chronic respiratory diseases apart from asthma, diabetes mellitus, hypertension, heart diseases or any illness that may alter heart rate were excluded from the study. The ages of both groups range between 20 – 40 years. Gender differences were evaluated in each group separately. In addition, all studied subjects are further subgrouped according to gender. The outcome of regrouping was eighty males and seventy-six females. The ages, asthma duration and severity were not statistically different between males and female at the time of examination (table-1).
Table-1.
Gender differences of age, asthma duration, asthma control test (ACT) score14 and the national asthma education and prevention program (NAEPP) Class15
| Female (M±SD) | Male (M±SD) | P | |
|---|---|---|---|
| Age | 27.3±5.6 | 28.5±5.4 | 0.161 |
| Asthma duration | 11.2±8.3 | 11.9±9.2 | 0.675 |
| ACT score | 14.7±4.8 | 15.5±4.2 | 0.381 |
| NAEPP Class | 2.3±0.9 | 2.5±0.8 | 0.265 |
M±SD = mean ± standard deviation
P = statistical significance of unpaired t-test
Biocom 3000 ECG recorder (Heart Rhythm Scanner - Version 2.0 - Biocom Technologies – U.S.A) was used for studying HRV. Volunteers were advised to lie down (in supine position) and breathe comfortably. Clean ECG signals and absence of movement artifacts were ensured before starting ECG recording. Heart Rhythm Scanner automatically finishes the trial session once its time expires (5 minutes). After recording the session, the ECG data was reviewed for abnormal ECG readings. The software is capable of doing an automatic search for various types of abnormal ECG recording irrelevant to their cause. This is based on the standard statistical procedure of exclusion of rough artifacts from the data series. Abnormal ECG readings were deleted and the software were allowed to calculate HRV parameters from the rest of the raw data.
Statistical evaluation was performed using the Microsoft Office Excel (Microsoft Office Excel for windows; 2003) and SPSS (SPSS for windows 11.5; Chicago, IL). Screening studied variables for significant differences in the means between the groups was performed using Student two-tailed, unpaired T-test.
In physiological studies comparing HRV in different well-defined groups, the differences between underlying heart rate should also be properly acknowledged. (2) Therefore, heart rate (MHR) was introduced as a covariate in the statistical analysis of HRV using general linear model.
Results
Testing for gender difference (after controlling for MHR) in short-term HRV (5-min) within studied groups revealed the following:
In the control group, all short-term HRV time domain indices showed no significant gender differences (Table 2). However, in the frequency domain, TP, LF and HF component were significantly higher in males (P ≤ 0.05 for all).
In asthmatics (table-3), all short-term HRV time domain indices are higher in males except MHR, which was significantly higher in females (P ≤ 0.02 for all).
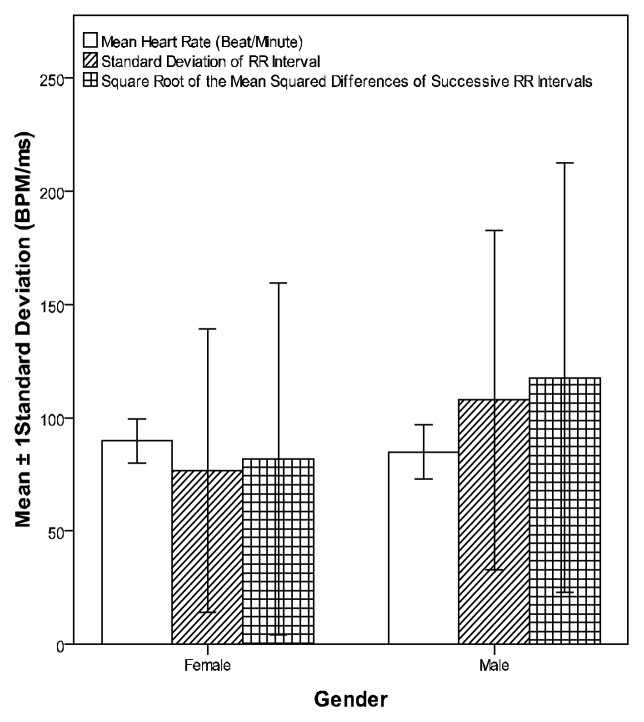
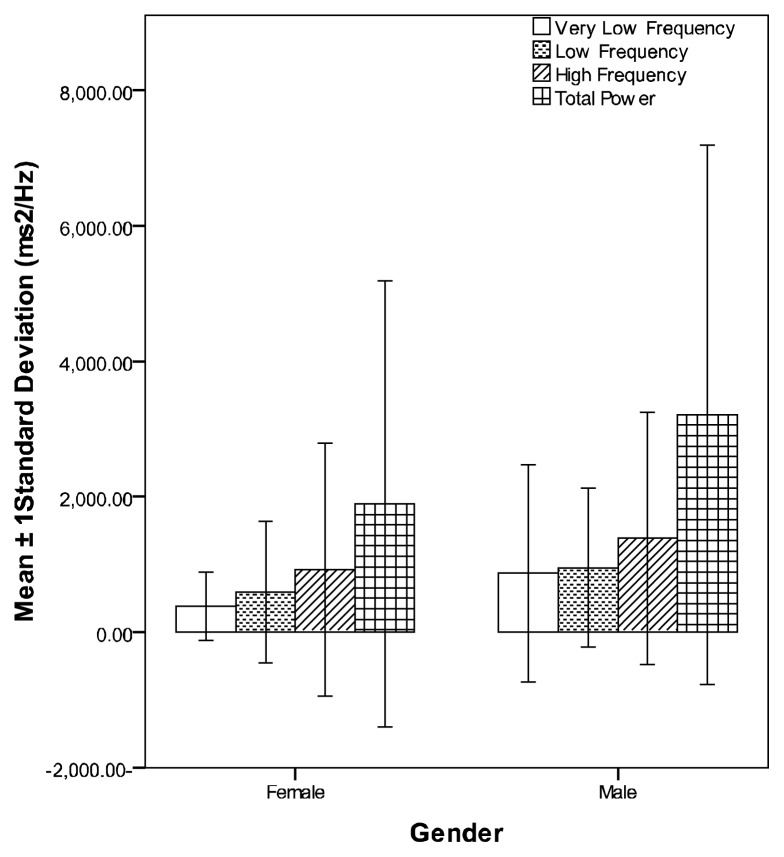
Comparing males with females of the whole sample (control and test groups), all short-term HRV time domain indices, TP, VLF, LF and HF components of frequency domains were significantly higher in males (table-4) except MHR, which was significantly higher in females (P ≤ 0.01 for all).
Table-2.
Gender differences of HRV among healthy subjects
| Female (M±SD) | Male (M±SD) | P1 | P2 | |
|---|---|---|---|---|
| MHR | 86.5±9.1 | 82.3±12.4 | 0.159 | - |
| MNN | 701.9±81.4 | 744.2±108.3 | 0.105 | - |
| SDNN | 64.4±45.1 | 90.6±65.1 | 0.086 | 0.089 |
| RMSSD | 71.7±64.3 | 95.0±84.1 | 0.249 | 0.192 |
| TP | 1108.1±1509.8 | 2771.9±3599.0 | 0.028* | 0.019* |
| VLF | 263.9±236.5 | 893.2±1836.1 | 0.078 | 0.081 |
| LF | 319.7±404.4 | 820.0±956.4 | 0.014* | 0.009* |
| HF | 524.5±919.7 | 1094.0±1547.4 | 0.100 | 0.049* |
| LF Norm | 49.9±18.0 | 55.5±16.6 | 0.234 | 0.213 |
| HF Norm | 50.1±18.1 | 44.5±16.6 | 0.234 | 0.213 |
| LF/HF | 1.3±1.1 | 1.7±1.2 | 0.329 | 0.247 |
Significant difference
M±SD = mean ± standard deviation
P1 = Not adjusted to MHR.
P2 = adjusted to MHR.
Table-3.
Gender differences of HRV among asthmatic subjects
| Female (M±SD) | Male (M±SD) | P1 | P2 | |
|---|---|---|---|---|
| MHR | 91.7±9.7 | 86.4±11.9 | 0.016* | - |
| MNN | 661.8±70.6 | 708.6±104.6 | 0.010* | - |
| SDNN | 83.8±70.2 | 117.1±78.9 | 0.028* | 0.014* |
| RMSSD | 87.6±84.6 | 130.0±98.6 | 0.024* | 0.006* |
| TP | 2350.3±3932.2 | 3438.9±4190.8 | 0.183 | 0.100 |
| VLF | 448.4±596.7 | 859.4±1484.0 | 0.077 | 0.074 |
| LF | 753.9±1255.5 | 1019.4±1277.9 | 0.298 | 0.183 |
| HF | 1147.9±2222.8 | 1541.7±2008.6 | 0.356 | 0.179 |
| LF Norm | 52.8±16.3 | 47.4±17.8 | 0.120 | 0.154 |
| HF Norm | 46.5±16.4 | 52.6±17.8 | 0.120 | 0.154 |
| LF/HF | 1.5±1.1 | 1.7±2.8 | 0.606 | 0.522 |
Significant difference
M±SD = mean ± standard deviation
P1 = Not adjusted to MHR.
P2 = adjusted to MHR.
Table 4.
Gender differences of HRV among all studied subjects.
| Female(M±SD) | Male(M±SD) | P1 | P2 | |
|---|---|---|---|---|
| MHR | 89.8±9.7 | 85.0±12.1 | 0.007* | - |
| MNN | 676.6±76.8 | 721.0±106.6 | 0.003* | - |
| SDNN | 76.7±62.5 | 107.8±75.0 | 0.006* | 0.002* |
| RMSSD | 81.8±77.7 | 117.7±94.8 | 0.011* | 0.002* |
| TP | 1892.6±3297.6 | 3205.5±3983.4 | 0.027* | 0.009* |
| VLF | 380.4±501.3 | 871.2±1604.4 | 0.012* | 0.011* |
| LF | 594.0±1044.6 | 949.6±1173.0 | 0.047* | 0.017* |
| HF | 918.2±1868.8 | 1385.0±1862.5 | 0.12 | 0.034* |
| LF Norm | 51.7±16.9 | 50.2±17.7 | 0.596 | 0.663 |
| HF Norm | 47.8±17.0 | 49.8±17.7 | 0.485 | 0.569 |
| LF/HF | 1.4±1.1 | 1.7±2.4 | 0.452 | 0.337 |
Significant difference
M±SD = mean ± standard deviation
P1 = Not adjusted to MHR.
P2 = adjusted to MHR.
Figures 1 and 2 compare means and standard deviations of time and frequency domains HRV indices of studied subjects respectively
Figure 1.
Means and standard deviations of the time domain HRV indices of all studied subjects
Figure 2.
Means and standard deviations of the frequency domain HRV indices of all studied subjects
Discussion
Short-term HRV analysis was used to compare the autonomic modulations of males with that of females. The results showed that none of the heart rate variability parameters was higher in women. The TP, LF and HF components were significantly higher in males of the control group while SDNN and RMSSD were significantly higher in asthmatic males. There were significant differences in the mean of almost all HRV parameters when gender difference was assessed in the whole sample (table-4). In the later comparison, although the studied subjects (males and females) were each a mix of normal and asthmatic patients, the ages, asthma duration and severity were not statistically different between the two genders at the time of examination (table-1). Therefore the males and females are still well matched when the whole sample was considered for the comparison.
Raemakers et al (10) hypothesized that there are gender differences in autonomic modulation making women at lower risk to develop cardiovascular diseases. Their results revealed higher SDNN, RMSSD and all frequency domain parameters, except for HF Norm, in men. However, following adjustment for MHR, SDNN showed no significant gender difference. Gender differences were confined to age categories of less than 40 years of age. Other studies of gender differences in autonomic regulation demonstrate that women have significantly greater HF spectral power than do age-matched men. (11, 12) Indications of enhanced parasympathetic input to cardiac regulation appear to be greater in women even during periods of cardiac stress; i.e., in response to brief coronary occlusions. (16)
In the present study, the parasympathetic indices that showed gender difference (RMSSD and HF power) was higher in males. Although RMSSD and HF component significantly correlated with the other parasympathetic index HF Norm, the later fails to show gender difference. This was also true for LF Norm.
Findings of present study confirm implications suggested by Umetani et al (16) that gender difference in HRV is secondary to higher levels of parasympathetic indices in men in the < 50 years old subjects. Umetani et al studied age and gender effects on the normal range of time domain heart rate variability (HRV) over nine decades in healthy subjects. Their results revealed at age <30 years, HRV for all measures was lower in female compared with male subjects. Gender differences decreased at age >30 years and disappeared at age >50 years. Umetani et al results are further supported by the fact that age range of present study (20–40 years old) shows no correlation between age and any of HRV indices.
The mechanisms underlying the gender difference in cardiac autonomic function are not clear. Kuo et al propose that middle-aged women and men have a more dominant parasympathetic and sympathetic regulation of heart rate, respectively. The gender-related difference in parasympathetic regulation diminishes after age 50 years, whereas a significant time delay for the disappearance of sympathetic dominance occurs in men. (11) This indicates that the decline in autonomic balance is probably related to the expected decrement in sex hormones later in life. (13)
Females have higher heart rate compared with males (P = 0.007 for MHR and P = 0.003 for MNN). This finding is comparable to that reported by Agelink et al study, which proved that women up to the age of 55 years have a higher resting heart rate compared with men. (17) The LF component of HRV is lower in females compared with males (P = 0.047). Other studies of gender differences in autonomic regulation demonstrate significantly greater parasympathetic activity in women than do age-matched me. (11, 12) These facts confuse the understanding of higher heart rates in females. This is because dominance of parasympathetic over sympathetic activity is expected to decrease heart rate in females. Based on previous studies, the following may explain high heart rate in females: first, Blood pressure is relatively lower in females compared with males probably secondary to lower level of angiotensin II in the former (18) and therefore inhibition by baroceptors. However, Baroreflex responsiveness is proved to be attenuated in middle-aged women compared with men. (19, 20) Second, the effects of sex hormones. (12, 21)
It is worth mentioning that possible causes of gender differences in HRV were not one of the primary parameters targeted in the current study. Gender differences in blood pressure, (18) body mass index, (22, 23) sex hormones, (12, 21), hemoglobin level, (24, 25) and some cytokines (21) are well established and may give clues regarding gender differences in HRV. Additional studies are needed to explore possible explanations of gender differences in HRV.
Conclusion
Previous studies examining gender differences in healthy populations, report mixed findings possibly due to examining different age groups and a failure to control confounding factors that might affect cardiovascular control. In the present study, males were well matched with females as regarding age and asthma severity. This is important because both age (11–17) and bronchial asthma (26–28) is known to affect HRV. Moreover, all major diseases that may affect HRV were excluded in studied subjects, making effects of possible confounding factors on autonomic modulation unlikely in this study. Results were interesting and revealed significantly higher mean in male in all studied time domain parameters and most of frequency domain indices. In contrast to many previous studies, the present study proved that RMSSD and HF power were higher in 20–40 years old males, which contradicts the hypothesis that the gender differences in autonomic modulation make males at higher risk to develop cardiovascular diseases. (10)
Acknowledgement
During this work, I have collaborated with many colleagues, for whom I have great regard, and I wish to extend my warmest thanks to Dr. Amal M. Saeed and Dr. Ramaze F. Elhakeem.
References
- 1.Vanderlei LC, Pastre CM, Hoshi RA, Carvalho TD, Godoy MF. Basic notions of heart rate variability and its clinical applicability. Rev Bras Cir Cardiovasc. 2009 Jun;24(2):205–17. doi: 10.1590/s0102-76382009000200018. [DOI] [PubMed] [Google Scholar]
- 2.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 3.Ori Z, Monir G, Weiss J, Sayhouni X, Singer DH. Heart rate variability. Frequency domain analysis. Cardiol Clin. 1992 Aug;10(3):499–537. [PubMed] [Google Scholar]
- 4.Moak JP, Goldstein DS, Eldadah BA, Saleem A, Holmes C, Pechnik S, Sharabi Y. Supine low-frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Cleve Clin J Med. 2009 Apr;76(Suppl 2):S51–9. doi: 10.3949/ccjm.76.s2.11. [DOI] [PubMed] [Google Scholar]
- 5.Gehi A, Mangano D, Pipkin, Browner W, Whooley M. Depression and heart rate variability in patients with stable coronary heart disease: findings from the Heart and Soul Study. Arch Gen Psychiatry. 2005;62(6):661–666. doi: 10.1001/archpsyc.62.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schroeder E, Liao D, Chambless L, Prineas R, Evans G, Heiss G. Hypertension, Blood Pressure, and Heart Rate Variability. The Atherosclerosis Risk in Communities (ARIC) Study. Hypertension. 2003;42:1106–1111. doi: 10.1161/01.HYP.0000100444.71069.73. [DOI] [PubMed] [Google Scholar]
- 7.Bernardi L, Ricordi L, Lazzari P, Solda P, Calciati A, Ferrari M, et al. Impaired circulation modulation of sympathovagal modulation of sympathovagal activity in diabetes. Circulation. 1992;86:1443–1452. doi: 10.1161/01.cir.86.5.1443. [DOI] [PubMed] [Google Scholar]
- 8.Ranpuria R, Hall M, Chan C, Unruh Mark. Heart rate variability (HRV) in kidney failure: measurement and consequences of reduced. HRV Nephrology Dialysis Transplantation. 2008;23(2):444–449. doi: 10.1093/ndt/gfm634. [DOI] [PubMed] [Google Scholar]
- 9.Wen T, Kwe W, Yang I, Yang T. Relationship between electrolytes and heart rate variability parameters in end-stage renal failure patients before and after hemodialysis. Anatol J Cardiol. 2007;7(1):142–4. [PubMed] [Google Scholar]
- 10.Raemakers D, Ector H, Aubert A, Rubens A, Van de Werf F. Heart rate variability and heart rate in healthy volunteers. Is the female autonomic nervous system cardioprotective? Eur Heart J. 1998;19:1334–1341. doi: 10.1053/euhj.1998.1084. [DOI] [PubMed] [Google Scholar]
- 11.Kuo T, Lin T, Yang C, Li C, Chen C, Chou P. Effect of aging on gender differences in neural control of heart rate. Am J Physiol Heart Circ Physiol. 1999;277:H2233–H2239. doi: 10.1152/ajpheart.1999.277.6.H2233. [DOI] [PubMed] [Google Scholar]
- 12.Rossy L, Thayer J. Fitness and gender-related differences in heart period variability. Psychosomatic Medicine. 1998;60:6, 773–781. doi: 10.1097/00006842-199811000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Umetani K, Singer D, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol. 1998;31:593–601. doi: 10.1016/s0735-1097(97)00554-8. [DOI] [PubMed] [Google Scholar]
- 14.Schatz M, Sorkness C, Li J, Marcus P, Murray J, Nathan R, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117:549–556. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 15.National Asthma Education Program. Guidelines for the diagnosis and management of asthma. 2007. available in: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf.
- 16.Airaksinen K, Ikeheimo M, Linnaluoto M, Tahvanainen K, Huikuri H. Gender differences in autonomic and hemodynamic reactions to abrupt coronary occlusion. J Am Coll Cardiol. 1998;31:301–306. doi: 10.1016/s0735-1097(97)00489-0. [DOI] [PubMed] [Google Scholar]
- 17.Agelink M, Malessa R, Baumann B, Majewski T, Akila F, Zeit T, et al. Standardized tests of heart rate variability: normal ranges obtained from 309 healthy humans, and effects of age, gender, and heart rate. Clinical Autonomic Research. 2001;11:99–108. doi: 10.1007/BF02322053. [DOI] [PubMed] [Google Scholar]
- 18.Xue B, Johnson A, Hay M. Sex differences in angiotensin II- induced hypertension. Braz J Med Biol Res. 2007;40(5):727–734. doi: 10.1590/s0100-879x2007000500018. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka M, Kimura T, Goyagi T, Saikai T, Tanaka S, Kazuhiko Suzuki, et al. Gender differences in baroreflex response and heart rate variability in anaesthetized humans. British Journal of Anaesthesia. 2004;92:831–835. doi: 10.1093/bja/aeh143. [DOI] [PubMed] [Google Scholar]
- 20.Huikuri H, Pikkujamsa S, Airaksinen K, Ikaheimo M, Rantala A, Kauma H, et al. Sex-Related Differences in Autonomic Modulation of Heart Rate in Middle-aged Subjects. 1996;94:122–125. doi: 10.1161/01.cir.94.2.122. [DOI] [PubMed] [Google Scholar]
- 21.Connor M, Motivala S, Valladares Olmstead R, Irwin M. Sex differences in monocyte expression of IL-6: role of autonomic mechanisms. Am J Physiol Regul Integr Comp Physiol. 2007;293:145–151. doi: 10.1152/ajpregu.00752.2006. [DOI] [PubMed] [Google Scholar]
- 22.Guízar J, Ahuatzin R, Amador N, Sánchez G, Romer G. Heart autonomic function in overweight adolescents. Indian Pediatr. 2005 May;42(5):464–9. [PubMed] [Google Scholar]
- 23.Gentile C, Orr J, Davy B, Davy K. Modest weight gain is associated with sympathetic neural activation in nonobese humans. Am J Physiol Regulatory Integrative Comp Physiol. 2007;292:1834– 1838. doi: 10.1152/ajpregu.00876.2006. [DOI] [PubMed] [Google Scholar]
- 24.Furuland H, Linde T, Englund A, Wikström B. Heart rate variability is decreased in chronic kidney disease but may improve with hemoglobin normalization. 2008;21(1):45–52. [PubMed] [Google Scholar]
- 25.Gehi A, Ix J, Shlipak M, Pipkin SS, Whooley MA. Relation of anemia to low heart rate variability in patients with coronary heart disease (from the Heart and Soul study) Am J Cardiol. 2005;95(12):1474–7. doi: 10.1016/j.amjcard.2005.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du J, He J, Wang Y. A study of heart rate variability in asthma. 2001;24:744–5. [PubMed] [Google Scholar]
- 27.Kazuma N, Otsuka K, Matsuoka I, Murata M. Heart rate variability during 24 hours in asthmatic children. 1997;14:597–606. doi: 10.3109/07420529709001450. [DOI] [PubMed] [Google Scholar]
- 28.Korematsu S. Autoregressive analysis of variability in heart rate and blood pressure in asthmatic children--difference of severity. Arerugi. 1995;44(9):1140–9. [PubMed] [Google Scholar]