Abstract
The innate immune response is essential for combating infectious disease. Macrophages and other cells respond to infection by releasing cytokines such as interleukin-1β (IL-1β), which in turn activate a well-described myeloid differentiation factor 88 (MYD88) -mediated, nuclear factor-κB (NF-κB) -dependent transcriptional pathway that results in inflammatory cell activation and recruitment1–4. Endothelial cells, which usually serve as a barrier to the movement of inflammatory cells out of the blood and into tissue, are also critical mediators of the inflammatory response5,6. Paradoxically, the same cytokines vital to a successful immune defense also have disruptive effects on endothelial cell-cell interactions and can trigger degradation of barrier function and dissociation of tissue architecture7–9. The mechanism of this barrier dissolution and its relationship to the canonical NF-κB pathway remains ill defined. Here we show that the direct, immediate, and disruptive effects of IL-1β on endothelial stability are NF-κB independent and are instead the result of signaling via the small GTPase, ADP-ribosylation factor 6 (ARF6), and its activator, ARF nucleotide binding site opener (ARNO). Moreover, we show that ARNO binds directly to the adaptor protein MYD88, and thus propose MYD88-ARNO-ARF6 as a proximal IL-1β signaling pathway distinct from that mediated by NF-κB (Supplementary Fig. 1). Finally, we show that SecinH3, an inhibitor of ARF guanine nucleotide-exchange factors (GEFs) such as ARNO, enhances vascular stability and significantly improves outcomes in animal models of inflammatory arthritis and acute inflammation.
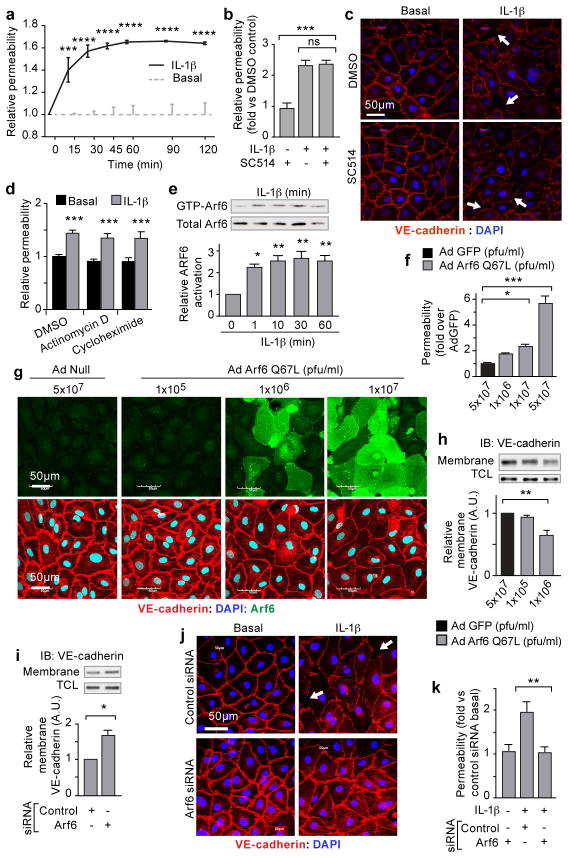
A defining characteristic of the cytokine-induced inflammatory response is the destabilization of endothelial barriers resulting in vascular permeability7–9. To dissect the pathway(s) involved in this tissue disruption, we treated cultured monolayers of human dermal microvascular endothelial cells (HMVEC-D) with IL-1β, and detected an increase in endothelial permeability within 15 minutes (Fig. 1a). The canonical IL-1β pathway involves ligand-stimulated activation of interleukin-1 receptor (IL-1R), which recruits the adaptor protein MYD88 to its cytoplasmic tail10. The subsequent signaling cascade through IL-1 receptor-associated kinase (IRAK) results in the phosphorylation of IκBα by inhibitor of kappa B kinase (IKK) leading to translocation of NF-κB to the nucleus and the eventual transcription of target genes that promote inflammatory cell responses3,4. To test the involvement of this pathway in IL-1β-induced vascular permeability, cells were treated with the IKK inhibitor, SC-51411. Although SC-514 prevented IL-1β-induced nuclear localization of NF-κB, it was unable to rescue either IL-1β-induced permeability or disruption of VE-cadherin surface localization (Fig. 1b, c and Supplementary Fig. 2a–c). We also asked if IL-1β-induced vascular permeability required other known MYD88 mediated downstream signaling mechanisms, including extracellular signal-regulated kinase (ERK1/2), p38, and c-Jun N-terminal kinase (JNK)12,13. Although ERK1/2, p38, and JNK were activated by IL-1β stimulation of endothelial cells, small molecule inhibitors of each of these pathways were unable to prevent IL-1β-induced vascular permeability or IL-1β-induced disruption of VE-cadherin cell surface localization (Supplementary Fig. 2d–h). Although specific NF-κB targets such as vascular endothelial growth factor A (VEGF-A), cyclooxygenase-2 (COX-2) and the COX-2 product progstaglandin-E2 (PGE2) are modulated by IL-1β, their activation had no effect on IL-1β-induced endothelial permeability (Supplementary Fig. 3a–e)3,14,15. Finally, treatment with Actinomycin D or Cycloheximide effectively inhibited transcription and translation (respectively) of NF-κB targets, but did not blunt IL-1β-induced permeability (Fig. 1d; Supplementary Fig. 3f–g). These data strongly support a role for the immediate and destabilizing effects of IL-1β on endothelial stability via signaling pathways independent of NF-κB, transcription, and translation.
Figure 1. Immediate effects of IL-1β are NF-κB independent and ARF6 dependent.
Monolayers of HMVEC-D stimulated with IL-1β assayed for: (a) permeability to horse-radish peroxidase (HRP) over time; (b) permeability to HRP following two hours treatment with SC514; (c) immunofluorescent localization of VE-cadherin; (d) permeability to HRP after thirty minutes treatment with Actinomycin-D, or Cycloheximide. (e) IL-1β-stimulated HMVEC-D lysates precipitated with GST-GGA3 and immunoblotted for ARF6. HMVEC-D monolayers infected with Ad-GFP or Ad-ARF6-Q67L: (f) permeability; (g) VE-cadherin (red) localization, ARF6 expression (green). Membrane fractions from (h) adenoviral-vector infected or (i) ARF6 siRNA-treated HMVEC-D immunoblotted for VE-cadherin. ARF6 siRNA-treated HMVEC-D stimulated with IL-1β: (j) VE-cadherin (arrows=disrupted VE-cadherin cell-surface localization) (k) permeability. N≥3; error bars=SEM. *p<0.05, **p<0.01, ***p<0.001.
IL-1β can disrupt VE-cadherin cell surface localization by promoting endocytic internalization9. We hypothesized that IL-1β might utilize ARF6, a known regulator of adherens protein localization16,17. Indeed, IL-1β activated ARF6 in HMVEC-D within one minute, a response accompanied by increased endocytosis of VE-cadherin within five minutes and an increase in monolayer permeability within 15 minutes (Fig. 1a, e and Supplementary Fig. 4a, b). IL-1β treatment did not affect total VE-cadherin mRNA or protein levels (Supplementary Fig. 4c, d). Adenoviral-mediated over-expression of constitutively active ARF6 (ARF6-Q67L)18 elicited a dose-dependent increase in endothelial permeability, as well as a disruption of VE-cadherin cell-surface localization (Fig. 1f, g and Supplementary Fig. 4e, f). A similar dose-dependent loss of total VE-cadherin was also observed, likely through internalization and subsequent degradation (Supplementary Fig. 4g). Interestingly, at lower doses of adenovirus where permeability was still induced, loss of total VE-cadherin was not observed, but a dose-dependent loss of cell-surface VE-cadherin occurred (Fig. 1f–h and Supplementary Fig. 4g). Moreover, siRNA knockdown of ARF6 enhanced VE-cadherin cell surface localization, prevented IL-1β-induced disruption of VE-cadherin, and prevented IL-1β-induced-endothelial permeability (Fig. 1i–k and Supplementary Fig. 4h, i). Collectively, these data link ARF6 as a critical regulator of VE-cadherin trafficking by controlling cell-surface localization and the immediate and disruptive effects of IL-1β-induced vascular permeability.
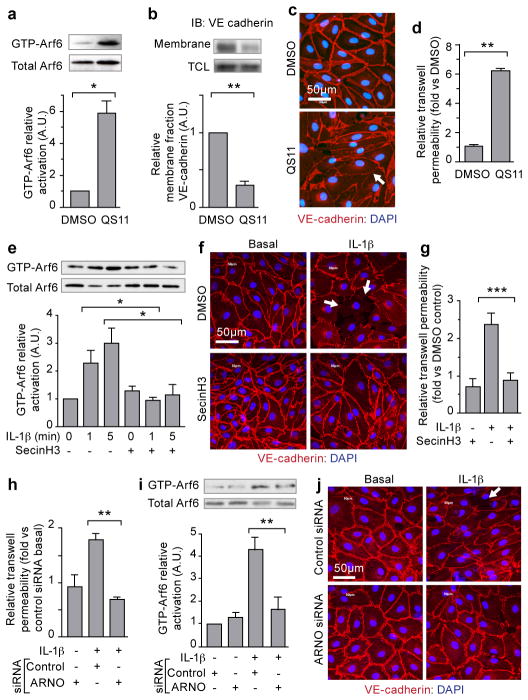
The ARF6 activation state is decreased by interaction with GTPase-activating proteins (GAPs) and increased by interaction with guanine nucleotide exchange factors (GEFs). Consistent with this, treatment of endothelial cells with the ARF-GAP inhibitor, QS11, evoked an increase in ARF6-GTP, a decrease in VE-cadherin cell-surface localization, and increased permeability (Fig. 2a–d and Supplementary Fig. 5a, b)19. We noted that a class of ARF-GEFs, the cytohesins, is highly expressed in multiple types of endothelial cells (Supplementary Fig. 5c). Accordingly, treatment of HMVECs with SecinH3, a cytohesin-inhibitor, significantly increased endothelial cell-surface localization of VE-cadherin (Supplementary Fig. 5d–f)20. Importantly, SecinH3 inhibited IL-1β-induced ARF6-GTP, as well as IL-1β-induced disruption of VE-cadherin cell-surface localization and endothelial permeability (Fig. 2e–g and Supplementary Fig. 5g–h). To determine which GEF might be uniquely involved, we used siRNA to knockdown Cytohesin-1, ARNO (cytohesin-2), Cytohesin-3, and GEP-100 (Supplementary Fig. 6a), and found that only siRNA targeting ARNO completely blocked IL-1β-induced endothelial permeability and phenocopied the knockdown of ARF6 (Fig. 2h and Supplementary Fig. 6 b). Furthermore, ARNO siRNA inhibited IL-1β-induced ARF6-GTP formation, inhibited IL-1β-induced disruption of VE-cadherin cell-cell contacts, and inhibited IL1-β-induced internalization of surface VE-cadherin (Fig. 2i, j, Supplementary Fig. 6c,g); all of these effects were rescued by expression of siRNA-resistant ARNO (Supplementary Fig. 6d–g). In cells treated with ARNO siRNA, viral expression of siRNA-resistant ARNO, but not viral expression of siRNA-resistant ARNO-E156K (carrying a mutation in the SEC7-domain), rescued the disruption of IL-1β-induced ARF6-GTP formation and permeability (Supplementary Fig. 6d–e)21. These data demonstrate that ARNO is a critical ARF-GEF necessary for IL-1β-induced activation of ARF6 and subsequent induction of vascular permeability, but does not rule out the role of other GEF-family members in similar responses in different cell types or in response to different cytokines.
Figure 2. Inhibition of ARF-GAPs and ARF-GEFs affect ARF6 activation and VE-cadherin localization.
HMVEC-D treated with QS11: (a) whole-cell lysates precipitated with GST-GGA3 and immunoblotted for ARF6; b) membrane fractions immunoblotted for VE-cadherin. (c) immunofluorescence for VE-cadherin (red); (d) permeability to HRP. HMVEC-D treated with IL-1β and SecinH3: (e) immunoblotting for ARF6; (f) Immunofluorescence for VE-cadherin (red); (g) permeability to HRP. HMVEC-D treated with anti-ARNO siRNA and stimulated with IL-1β: (h) permeability to HRP; (i) GST-GGA3 precipitation and ARF6 immunoblotting; (j) immunofluorescence of VE-cadherin (red) (Arrow=less cell-surface VE-cadherin. N≥3; error bars=SEM. *p<0.05, **p<0.01, ***p<0.001.
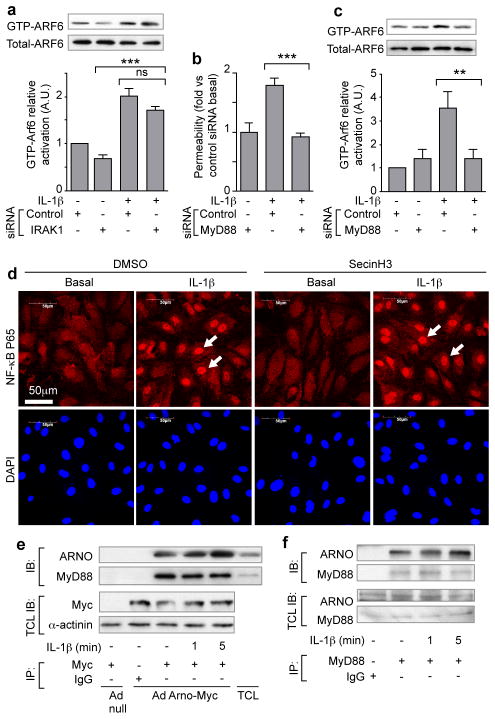
The signaling components in the NF-κB pathway downstream of IL-1β-induced activation of IL-1R are well characterized. Although the inhibition the NF-κB pathway at the level of IRAK by siRNA did not inhibit IL-1β-induced ARF6 activation, siRNA knockdown of MYD88 inhibited both IL-1β-induced permeability and ARF6-GTP activation, suggesting a bifurcation of IL-1β-induced signaling at the point of MYD88 (Fig. 3a–c and Supplementary Fig. 7a, b). The proposed bifurcation was further verified by the pharmacological uncoupling of the two pathway arms: although SecinH3 blunted IL-1β-induced permeability, it did not significantly inhibit IL-1β-induced NF-κB nuclear localization or NF-κB-dependent expression or localization of cell-surface adhesion molecules (Fig. 3d and Supplementary Fig. 7c, d). Furthermore, SecinH3 was unable to inhibit IL-1β-induced polymorphonuclear leukocyte rolling and adherence under shear stress on an endothelial monolayer (Supplementary Fig. 7e). Mechanistic support for this novel signaling arm was provided by the demonstration of an interaction between MYD88 and ARNO by co-immunoprecipitation in both over-expression and endogenous settings (Fig. 3e, f). Our hypothesis that ARNO is the critical GEF in IL-1β-induced permeability in endothelial cells was further strengthened by our inability to detect an interaction between MYD88 and other potentially relevant ARF-GEFS including cytohesin-1, cytohesin-3 and GEP-100 (Supplementary Fig. 7f, g).
Figure 3. The immediate IL-1β-induced permeability pathway diverges at MYD88.
(a) IRAK1 siRNA treated HMVEC-D, stimulated with IL-1β, subjected to GTP-ARF6 pull down and immunoblotted for ARF6. MYD88 siRNA-treated HMVEC-D stimulated with IL-1β: (b) permeability; (c) ARF6 activation. (d) NF-κB p65 immunofluorescence of HMVEC-D stimulated with IL-1β and SecinH3. (Arrow=nuclear localization). (e) Cell lysates from Ad ARNO-Myc infected HMVEC-D immunoprecipitated with anti-Myc antibodies and immunoblotted with anti-MYD88 antibodies. (f) Lysates from HMVEC-D immunoprecipitated with anti-MYD88 antibodies and immunoblotted with anti-ARNO antibodies. N≥3, error bars=SEM, * p<0.05, ** p<0.01, *** p<0.001.
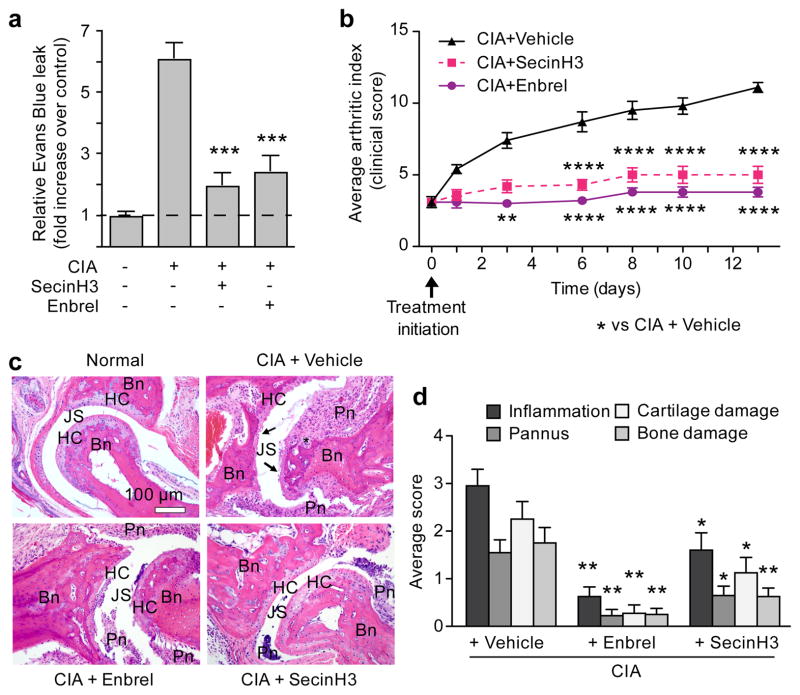
An effective therapeutic strategy to combat numerous inflammatory conditions is to target pro-inflammatory cytokines proximal to the NF-κβ pathway. However, this strategy can result in undesired pleiotrophic effects. We asked if targeting a single arm in this pathway - the one mediated by Arno-Arf6 - could inhibit acute or chronic inflammation in vivo in two animal models of inflammation. The first model we tested was rheumatoid arthritis, a disease characterized by a dysregulated cytokine response causing excessive inflammation and tissue damage and treated therapeutically in humans with the anti-cytokine TNFR:Fc fusion protein, etanercept (Enbrel®)22–25. A standard animal model of arthritis through which a tumor necrosis factor receptor (TNFR) fusion approach has been proven effective is collagen-induced arthritis (CIA)24–26. Exposure of animals to the cytohesin inhibitor SecinH3 after the onset of CIA-induced arthritis reduced vascular permeability in the joints, but had no effect on global cytokine levels at 24 hours after treatment initiation (Fig. 4a and Supplementary Fig. 8a, b). Additionally, a significant inhibition in the increase in arthritic index (AI), comparable to that achieved by treatment with Enbrel, was observed. The arthritic index (AI) is a scoring system determined by the number of digits or joints that are edematous or erythematous. The significance of our findings was verified by histologic scoring of inflammation, pannus development, cartilage damage, and bone damage (Fig. 4c, d). A similar effect of SecinH3 was confirmed in a second model of inflammation, the Carrageenan Air Pouch model. Six hours after an inflammatory stimulus, a time at which substantial inflammation was induced in the positive control mice, treatment with SecinH3 decreased exudate volume as well as leukocyte concentration in the exudates (Supplementary Fig. 8c, d). Collectively, these data identify MYD88-ARNO-ARF6 as a valid target for inflammatory conditions confirming a relevant role for manipulation of this pathway in vivo to modulate inflammatory processes and in the treatment of disease.
Figure 4. Inhibition of ARF-GEFs decreases CIA-induced vascular permeability and arthritis.
(a) Arthritis-induced vascular permeability in the joint measured by Evans Blue leak seven days after treatment initiation in the presence of SecinH3 or Enbrel. N=14 per group. (b) Chronological arthritic assessment. N=10/group. (c) H&E staining of sections through joints; Bn=bone, HC=hyaline cartilage, JS=joint space, Pn=inflammed pannus, *cartilage & bone loss, arrows=eroded cartilage. (d) Summary of histological changes of inflammation, pannus, cartilage, and bone damage of indicated treatment. Control, N=5; Enbrel and SecinH3, N=10; error bars=SEM. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 vs disease group.
Chronic inflammation causes tissue destruction through dysregulated cytokine release, inflammatory cell recruitment, and vascular permeability; yet each of these mechanisms has critical roles in many physiologic processes including the immune response5,7. We have identified a novel pathway that uncouples cytokine effects on vascular stability from other critical functions of the canonical NF-κB transcription program (Supplementary Fig. 1). Our model suggests the potential for inhibition of vascular leak without modulation of immune-cell adhesion or other critical NF-κB-dependent responses.
The activation of many inflammatory cytokine receptors disrupts cell-cell interactions, precipitating tissue edema and destruction5,7,9,27. Toll-like receptors and the interleukin receptor also utilize MYD88 and the mechanism described here may well apply3,10. Interestingly, TNFR1 does not utilize MYD88 yet still activates ARF6-GTP following stimulation (Supplementary Fig. 9). Whether ARNO or another ARF-GEF binds directly to TNFR1 or its adaptor protein TRADD is unknown as is the possibility ARFGEF-ARF6-cadherin serves as a common signaling module exploited by multiple cytokines. Though this study focused on the endothelium, the concept of cytokine receptor-ARF-Cadherin may also apply to the epithelial barrier which expresses these constituents and is also compromised by cytokines including IL-1β16.
Inhibition of this novel vascular stability pathway, which is aimed at enhancing the host’s resilience to the cytokine response, shows effects commensurate to those of class-leading drugs that target cytokines upstream of NF-κB and seek to blunt the cytokine response of the immune system outright. This approach may be particularly useful in arthritis where the current medical therapy can render a patient immunocompromised and susceptible to reactivation of infectious disease such as tuberculosis28. Application of these findings to other diseases characterized by excessive acute or chronic inflammatory states including sepsis, Crohn’s disease, ulcerative colitis, scleroderma and psoriasis should also be considered9,29,30.
Methods Summary
Transwell permeability
HMVEC-D cells were seeded on 1.0 μm Costar transwell inserts coated with fibronectin. Cells were grown to confluency and treated with SecinH3 for 3 hours or MAPK/NF-κB/transcription/translation inhibitors for 30 minutes followed by treatment with 10ng/ml IL-1β. Alternatively, cells were infected with Ad-GFP or Ad-ARF6Q67L adenovirus for 48 hours. siRNA knockdown was performed as described in supplementary methods and cells were treated with IL-1β 72 hours after the second siRNA transfection. Two hours later, horseradish peroxidase (HRP) was added to the top chamber at a final concentration of 100μg/ml. Medium was removed after 60 minutes from the lower chamber. For time-course transwell assays and transcription/translation inhibitor experiments (Figure 1a,c–d), HRP was added to the insert at the same time as IL-1β. Transwell inserts were moved to fresh wells after each timepoint, and the concentration of HRP in the bottom chamber was measured for monolayer permeability. HRP was assessed using media samples obtained from the lower chamber incubated with 0.5 mM of guaiacol and 0.6 mM H2O2. Spectrophotometric analysis of absorbance at 490 nm provided a quantitative evaluation of the amount of HRP that crossed the membrane. Data are presented as mean ± SEM of at least three independent experiments performed in quadruplicate.
A detailed description of all methods is provided in Supplementary Information
Supplementary Material
Acknowledgments
We thank D. Lim and T. Mleynek for graphical assistance. We thank G. Zimmerman and J. Kaplan for critical reading of the manuscript. We thank S. Odelberg for critical reading of the manuscript and statistical analysis. We thank J. Ling for help with immunostaining. We thank R. Campbell and A. Weyrich for providing primary human blood cells. We thank C. Rodesch and the University of Utah Cell Imaging/Fluorescence Facility as well as the University of Utah Flow Cytometry Facility. We thank M.P. Revelo for help with pathology. D.Y.L. and his laboratory were funded by grants from the NHLBI, Burroughs Wellcome Fund, Juvenile Diabetes Research Foundation, NIAID Rocky Mountain Regional Center of Excellence in Biodefense and Emerging Infectious Disease, the American Asthma Foundation, and the Department of Defense. D.Y.L. is the HA and Edna Benning Endowed Professor of Medicine and Cardiology.
Footnotes
Author Contributions
W.Z., N.R.L., C.C.G., and D.Y.L. were responsible for project conceptualization, experimental design, data analysis, and manuscript preparation. W.Z., C.C.G., C.T.D, Z.T., L.K.S., D.S.S., and J.G., performed and collected data for in vitro experiments. N.R.L. collected data for in vivo experiments. C.C.G. developed software techniques for immunofluorescence analysis. M.C.P.S. performed flow cytometry experiments. Z.T. and K.R.T. made constructs and adenoviruses. A.H.G. provided histology and pathology expertise. D.Y.L. was responsible for funding the project.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Reprints and permissions information is available at www.nature.com/reprints. The authors declare competing financial interests. The University of Utah has filed intellectual property concerning targeting signaling pathways to treat inflammatory arthritis. The University of Utah has licensed technology to Navigen, a biotechnology company owned in part by the University of Utah Research Foundation. N.R.L. and W.Z. are paid consultants for Navigen, and D.Y.L. is a co-founder of Navigen.
References
- 1.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nature reviews. Immunology. 2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 2.Collins T, et al. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 3.Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290:L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- 4.RothwARF DM, Karin M. The NF-kappa B activation pathway: a paradigm in information transfer from membrane to nucleus. Sci STKE. 1999:RE1. doi: 10.1126/stke.1999.5.re1. [DOI] [PubMed] [Google Scholar]
- 5.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nature reviews Immunology. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 6.Muller WA. Leukocyte-endothelial cell interactions in the inflammatory response. Lab Invest. 2002;82:521–533. doi: 10.1038/labinvest.3780446. [DOI] [PubMed] [Google Scholar]
- 7.Royall JA, et al. Tumor necrosis factor and interleukin 1 alpha increase vascular endothelial permeability. Am J Physiol. 1989;257:L399–410. doi: 10.1152/ajplung.1989.257.6.L399. [DOI] [PubMed] [Google Scholar]
- 8.West XZ, et al. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature. 2010;467:972–976. doi: 10.1038/nature09421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.London NR, et al. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci Transl Med. 2010;2:23ra19. doi: 10.1126/scitranslmed.3000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu J, Mohan C. Toll-like receptor signaling pathways--therapeutic opportunities. Mediators Inflamm. 2010;2010:781235. doi: 10.1155/2010/781235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kishore N, et al. A selective IKK-2 inhibitor blocks NF-kappa B-dependent gene expression in interleukin-1 beta-stimulated synovial fibroblasts. J Biol Chem. 2003;278:32861–32871. doi: 10.1074/jbc.M211439200. [DOI] [PubMed] [Google Scholar]
- 12.Matthews JS, O’Neill LA. Distinct roles for p42/p44 and p38 mitogen-activated protein kinases in the induction of IL-2 by IL-1. Cytokine. 1999;11:643–655. doi: 10.1006/cyto.1998.0478. [DOI] [PubMed] [Google Scholar]
- 13.Martin MU, Wesche H. Summary and comparison of the signaling mechanisms of the Toll/interleukin-1 receptor family. Biochim Biophys Acta. 2002;1592:265–280. doi: 10.1016/s0167-4889(02)00320-8. [DOI] [PubMed] [Google Scholar]
- 14.Kaltschmidt B, Linker RA, Deng J, Kaltschmidt C. Cyclooxygenase-2 is a neuronal target gene of NF-kappaB. BMC Mol Biol. 2002;3:16. doi: 10.1186/1471-2199-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 16.Palacios F, Price L, Schweitzer J, Collard JG, D’Souza-Schorey C. An essential role for ARF6-regulated membrane traffic in adherens junction turnover and epithelial cell migration. The EMBO journal. 2001;20:4973–4986. doi: 10.1093/emboj/20.17.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Souza-Schorey C, Li G, Colombo MI, Stahl PD. A regulatory role for ARF6 in receptor-mediated endocytosis. Science. 1995;267:1175–1178. doi: 10.1126/science.7855600. [DOI] [PubMed] [Google Scholar]
- 18.Riley KN, Maldonado AE, Tellier P, D’Souza-Schorey C, Herman IM. Betacap73-ARF6 interactions modulate cell shape and motility after injury in vitro. Mol Biol Cell. 2003;14:4155–4161. doi: 10.1091/mbc.E02-11-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Q, et al. Small-molecule synergist of the Wnt/beta-catenin signaling pathway. Proc Natl Acad Sci U S A. 2007;104:7444–7448. doi: 10.1073/pnas.0702136104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hafner M, et al. Inhibition of cytohesins by SecinH3 leads to hepatic insulin resistance. Nature. 2006;444:941–944. doi: 10.1038/nature05415. [DOI] [PubMed] [Google Scholar]
- 21.Beraud-Dufour S, et al. A glutamic finger in the guanine nucleotide exchange factor ARNO displaces Mg2+ and the beta-phosphate to destabilize GDP on ARF1. The EMBO journal. 1998;17:3651–3659. doi: 10.1093/emboj/17.13.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arend WP. Cytokine imbalance in the pathogenesis of rheumatoid arthritis: the role of interleukin-1 receptor antagonist. Semin Arthritis Rheum. 2001;30:1–6. doi: 10.1053/sarh.2001.23693. [DOI] [PubMed] [Google Scholar]
- 23.Szekanecz Z, Koch AE. Vascular involvement in rheumatic diseases: ‘vascular rheumatology’. Arthritis Res Ther. 2008;10:224. doi: 10.1186/ar2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Berg WB, Joosten LA, Helsen M, van de Loo FA. Amelioration of established murine collagen-induced arthritis with anti-IL-1 treatment. Clin Exp Immunol. 1994;95:237–243. doi: 10.1111/j.1365-2249.1994.tb06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joosten LA, Helsen MM, van de Loo FA, van den Berg WB. Anticytokine treatment of established type II collagen-induced arthritis in DBA/1 mice. A comparative study using anti-TNF alpha, anti-IL-1 alpha/beta, and IL-1Ra. Arthritis Rheum. 1996;39:797–809. doi: 10.1002/art.1780390513. [DOI] [PubMed] [Google Scholar]
- 26.Wooley PH, Dutcher J, Widmer MB, Gillis S. Influence of a recombinant human soluble tumor necrosis factor receptor FC fusion protein on type II collagen-induced arthritis in mice. J Immunol. 1993;151:6602–6607. [PubMed] [Google Scholar]
- 27.London NR, Whitehead KJ, Li DY. Endogenous endothelial cell signaling systems maintain vascular stability. Angiogenesis. 2009;12:149–158. doi: 10.1007/s10456-009-9130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keane J, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 29.Lee WL, Slutsky AS. Sepsis and endothelial permeability. N Engl J Med. 2010;363:689–691. doi: 10.1056/NEJMcibr1007320. [DOI] [PubMed] [Google Scholar]
- 30.Silva LC, Ortigosa LC, Benard G. Anti-TNF-alpha agents in the treatment of immune-mediated inflammatory diseases: mechanisms of action and pitfalls. Immunotherapy. 2010;2:817–833. doi: 10.2217/imt.10.67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.